Abstract
The aim of the present study was to assess the clinicopathological features and prognostic factors of primary small gastrointestinal stromal tumors (GISTs) outside the stomach. The clinical data, clinicopathological features and prognostic factors of 20 patients with a pathologically-confirmed diagnosis of non-gastric GIST that were treated at Liaoning Cancer Hospital & Institute between July 2006 and December 2013 were retrospectively analyzed. In total, 15 patients were male and 5 were female, with a median age of 58 years (range, 44–82 years). A change in bowel habits was the original symptom of rectal small GISTs in 6 out of 8 patients, while patients with small GISTs in other locations demonstrated no overt symptoms and the lesions were detected by systematic examinations of other diseases or abdominal surgical procedures performed on other organs. In total, 19 patients out of the total 20 patients underwent surgery, and 1 patient with rectal GIST received continuous oral imatinib mesylate (400 mg once a day) instead of undergoing surgery. The mean diameter of tumors was 1.55±0.54 cm (range, 0.3–2.0 cm) and the median was 1.70 cm. The pathomorphology of the lesions was mainly spindle cell, and immunohistochemistry revealed the expression rate of cluster of differentiation (CD)117, CD34 and discovered on GIST-1 were 85, 80 and 70%. According to the mitosis index, small rectal GISTs were more frequent compared with other positions (P<0.05), while the frequency of small GISTs >1 cm in size was not significantly different from the frequency of small GISTs ≤1 cm in size (P=0.995). All 20 patients were followed up, with a median follow-up duration of 49.5 months (range, 10.5–94.4 months). At the end of the follow-up period, tumor recurrence occurred in 5 patients and 1 patient succumbed following progression. According to the analysis of the tumor sites, the RFS time of patients with small rectal GISTs was significantly different than the RFS time in patients with small GISTs in other positions. The clinical symptoms of non-gastric small GISTs were not evident and were challenging to detect. Small GISTs, regardless of size, possessed malignant potential and once detected, GISTs should be surgically resected. Lesions located in the rectum demonstrated an increased degree of malignancy and were more likely to recur. The tumor size and Ki67 index could not be considered as prognostic factors of non-gastric small GISTs.
Keywords: small gastrointestinal stromal tumors, surgery, prognosis, non-gastric
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract, with an incidence of 1–2 cases per 100,000 individuals worldwide. The leading site of invasion is the stomach, which accounts for ~45.0% of total invasion, followed by the small intestine, omentum, colorectum and esophagus (1–3). There is unified agreement that the principle treatment strategy for primary GISTs measuring >2 cm should be surgery if curative resection may be possible. A high mitotic count, non-gastric location, large size, rupture and insufficient adjuvant imatinib are considered to be factors independently associated with poor prognosis. Certain patients with primary GIST are cured by surgery alone, however, administration of adjuvant imatinib mesylate for at least 3 years is now recommended when the risk of recurrence is considered to be significant (4–5). Imatinib mesylate is the first choice for patients with recurrent, metastatic or unresectable GISTs. Patients receiving preoperative imatinib who exhibit a complete/partial response or stable disease based on the Choi criteria, may be candidates for surgery. Other patients without an indication of successful surgery should accept long-term imatinib treatment until progression, then change to second-line target agents or join clinical trials. Cytoreductive surgery only for recurrent, metastatic or unresectable GISTs is not recommended (6–8).
In general, there are no specific symptoms for early-stage GISTs, which leads to late treatment (9). The clinical presentation of GISTs is highly variable, according to the tumor site and size. The most frequent symptoms are anemia, weight loss, gastrointestinal bleeding, abdominal pain and mass-associated symptoms (10). With the development of endoscopy, particularly the application of endoscopic ultrasonography (EUS), small GISTs in the stomach, duodenum and esophagus are easy to be detected with more associated studies (11), while small GISTs in other sites of the body are challenging to detect, with a smaller number of associated studies. However, controversy remains for the surgical indications and timing of surgery for the treatment of small GISTs with a diameter <2 cm (12).
The present study retrospectively analyzed the clinical data of 20 patients with GISTs ≤2 cm in diameter that were located outside the stomach and diagnosed between July 2006 and December 2013, and discussed the clinicopathological features and prognostic factors. The study was approved by the Ethics Committee of Liaoning Cancer Hospital & Institute (Shenyang, China), and written informed consents were obtained from all patients.
Materials and methods
Patients
Between July 2006 and December 2013, 20 patients with non-gastric small GISTs were treated at the Liaoning Cancer Hospital & Institute (Shenyang, Liaoning, China). In total, 19 of these patients underwent surgery and the lesions were pathologically confirmed to be GISTs subsequent to surgery. The remaining 1 patient did not undergo surgery, but the diagnosis was pathologically confirmed by biopsy. Out of the 20 patients, 15 were male and 5 were female, with ages ranging between 44 and 82 years (median, 58 years). A change in bowel habits was the original symptom in 6 out of 8 patients diagnosed with rectal small GISTs, while small GISTs in other locations resulted in no overt symptoms and were detected during systematic examinations for other diseases or abdominal procedures performed on other organs. None of the patients possessed a history of familial GISTs.
Treatment methods
In total, 19 patients underwent the R0 resection and no mortality or other serious complications occurred during the perioperative period. Out of these 19 patients, 7 patients possessed rectal GISTs, among which 3 lesions demonstrated transanal local excision, 2 lesions were excised using high anterior resection (HAR), 1 lesion was excised using Hartmann's procedure and 1 lesion was excised using Miles' procedure. In addition, 4 patients possessed small intestinal GISTs, which resulted in 3 patients undergoing bowel resection and 1 patient undergoing enucleation. Colon GISTs were identified in 4 patients, consisting of 3 lesions located in ascending colon, with 1 patient possessing a synchronous GIST of the descending colon, and 1 lesion located in the transverse colon. All these patients underwent radical colon resection. Peritoneal GISTs, which were located in the mesentery and omentum, were identified in 4 patients, all of whom underwent complete resection. All patients underwent R0 resection without receiving any targeted drugs or undergoing other treatments. The tumor of 1 patient diagnosed with rectal GIST was located in the Dentate line; therefore, the patient did not undergo Miles' procedure and was continuously orally administered with the targeted drug imatinib mesylate (400 mg once a day; Novartis, Basel, Switzerland).
Pathology and immunohistochemistry
All tissue samples underwent pathological examination. Firstly, the shape of the tumor cells was assessed according to hematoxylin and eosin staining. The GISTs were revealed to mainly be formed by spindle cells, with few formed by epithelioid cells or mixed cells. Immunohistochemical staining was then performed subsequent to the cells being identified as similar to GIST cells in morphology. The main detection index consisted of the expression of discovered on GIST-1 (DOG-1), cluster of differentiation (CD)117, CD34, α-smooth muscle actin (α-SMA), desmin and S-100, as well as the mitosis count in 50 high power fields (HPFs).
Subsequent to 2012, the Department of Pathology of the Liaoning Cancer Hospital & Institute added the detection of the Ki67 index following immunohistochemical staining to the assessment of GIST surgical specimens. As a result, the specimens obtained prior to 2012 lacked records of the Ki67 index, and immunohistochemical staining for Ki67 was therefore performed in the present study. The tissues were observed under the microscope with a ×40 object lens (CH-BI45-T; Olympus, Tokyo, Japan), and scoring for the expression of Ki67 was performed by counting at least 500 tumor cells in 50 HPFs. All brown-stained nuclei, regardless of the staining intensity, were considered to be positive for Ki67 expression. However, there may be certain errors as the specimens had been stored for a long period of time. Patients in this group did not undergo gene detection, as all lesions were smaller, with a good prognosis. The majority of patients were not administered with targeted agents and there was a low desire for gene detection.
National Institutes of Health (NIH) recurrence risk assessment
In accordance with the NIH risk stratification reported in the study by Joensuu (13) and the NCCN Task Force study (14), GISTs are divided into four recurrence risk stratifications, consisting of extremely low, low, moderate and high risk. The present GISTs were classified according to the NIH risk stratification.
Follow-up
Outpatient review and telephone calls were used to perform the follow-up and the last follow-up was August 1, 2014. The recurrence-free survival (RFS) time was calculated from the date of surgery to the date of clear relapse, metastasis or the end of follow-up.
Statistical analysis
IBM SPSS Statistics 19.0 software (IBM, Armonk, NY, USA) was used for the present statistical analyses. The data were expressed as the mean ± standard deviation. Categorical data were expressed as the rate or percentage and were analyzed using Fisher's exact test. The RFS time was calculated according to the Kaplan-Meier method. The log-rank test was used to compare the survival distributions. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathological features
The clinicopathological features of the 20 patients are reported in Table I. The mean tumor diameter was 1.55±0.54 cm (0.3–2.0 cm). In total, 6 GISTs were combined with other digestive system tumors, consisting of 4 GISTs in the small intestine and 2 GISTs in the enterocoelia, and were found during pre-operative examination or incidentally during surgery (Figs. 1 and 2). According to the analysis of cell morphology, 19 tumors consisted of spindle cells (95%), 1 tumor consisted epithelioid cells (5%) and mixed cell morphology was not observed. The results of immunohistochemical analysis revealed that the rates of CD117, CD34, DOG-1, S-100, α-SMA and desmin expression were 17/20 (85%), 16/20 (80%), 14/20 (70%), 6/20 (30%), 4/20 (20%) and 1/20 (5%), respectively. The rate of combined CD117, CD34 and DOG-1 expression was 40%. The mean Ki67 index subsequent to immunohistochemical staining was determined to be 4.65±2.23% (range, 1–10%), and 5% was considered to be a cutoff in the stratified statistics. In total, 13 tissues demonstrated a Ki67 index ≤5% and 7 tissues demonstrated an index >5%. The number of mitoses was observed in 50 HPFs, and 14 tissues were determined to possess a mitotic index of ≤5 mitoses per 50 HPFs and 6 patients were determined to possess a mitotic index of 6–10 mitoses per 50 HPFs. As all cases were non-gastric with a diameter ≤2 cm, according to the NIH risk stratification, regardless of tumor site and size, 14 tumors were classified as extremely low risk and 6 tumors were classified as moderate risk, which was the same result of the mitosis-based risk classification.
Table I.
Clinicopathological characteristics of 20 patients with GISTs.
| Clinicopathological characteristics | Total, n (%) |
|---|---|
| Gender | |
| Male | 15 (75) |
| Female | 5 (25) |
| Age | |
| ≤58 years | 11 (55) |
| ﹥58 years | 9 (45) |
| Tumor size | |
| ≤1 cm | 7 (35) |
| 1–2 cm | 13 (65) |
| Tumor site | |
| Rectum | 8 (40) |
| Non-rectum | 12 (60) |
| Morphology | |
| Spindle | 19 (95) |
| Epithelioid | 1 (5) |
| Mixed | 0 (0) |
| Immunohistochemistry | |
| CD117 (+) | 17 (85) |
| CD34 (+) | 16 (80) |
| DOG-1 (+) | 14 (70) |
| Ki67 index | |
| ≤5% | 13 (65) |
| ﹥5% | 7 (35) |
| Mitotic index | |
| ≤5 per 50 HPFs | 14 (70) |
| >5 per 50 HPFs | 6 (30) |
| NIH risk stratificationa | |
| Very low | 14 (70) |
| Moderate | 6 (30) |
As the tumor site of the 20 cases was non-gastric, with diameter <2 cm, the NIH risk stratification, which only referred to mitotic index excluding tumor site and size, resulted in the same risk stratification as the statistical result of the mitotic index. GISTs, gastrointestinal stromal tumors; CD, cluster of differentiation; DOG-1, discovered on GIST-1; HPFs, high power fields; NIH, National Institutes of Health.
Figure 1.
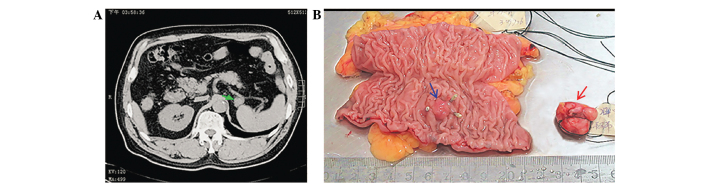
Patient with early-stage colorectal cancer, diagnosed by colonoscopy. (A) Abdominal computed tomography detected a small mass with a diameter of ~2 cm in the front of the spleen (green arrow), but no symptoms were exhibited by the patient. (B) The surgery removed colorectal cancer and the mass in the front of the spleen at the same time. The titanium clips marked the rectal tumor (blue arrow), which was diagnosed as a highly-differentiated adenocarcinoma, with a diameter of ~0.7 cm, that had infiltrated the submucosa, but had not metastasized to the lymph nodes. The lesion in the lower-right of the image was diagnosde as GIST (red arrow), and spindle cells were observed following hematoxylin and eosin staining, with 2 mitoses per 50 high power fields. The findings of immunohistochemical analysis were CD117(+), discovered on GIST-1(+) and CD34(+), with a Ki67 index of 10%, α-SMA(−), S-100(−) and desmin(+). GIST, gastrointestinal stromal tumor; CD, cluster of differentiation.
Figure 2.
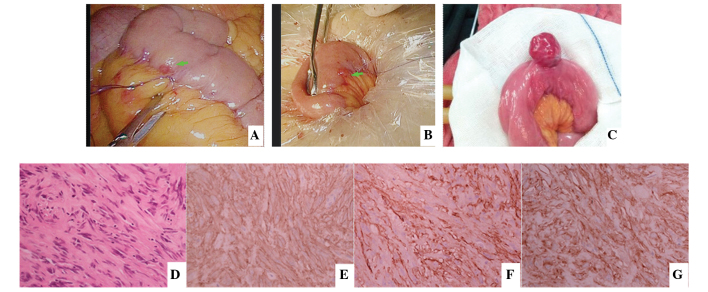
(A and B) An abnormal lesion was detected in the small intestine of a patient during the process of laparoscopic rectal surgery and suturing was used to mark the lesion first. The small intestine was then removed from umbilical incision to remove the 0.3-cm lesion, which was the smallest GIST identified in the present study. The diagnosis was confirmed by post-operative pathology and immunohistochemistry. (C) Small intestine GIST found when detecting the small intestine in open surgery. (D) Hematoxylin and eosin staining revealed spindle cell in morphology and immunohistochemical analysis was performed for the detection of (E) CD117(+), (F) CD34(+) and (G) discovered on GIST-1(+). Excluding (C), all images were obtained from the same patient. GIST, gastrointestinal stromal tumor; CD, cluster of differentiation.
Clinicopathological associations
The present patients consisted of 20 patients with non-gastric small GISTs, 8 lesions of the rectum and 12 lesions of the non-rectum, consisting of 4 in the colon, small intestine and enterocoelia, respectively. The GISTs were divided into rectal and non-rectal tumors, according to the site, and statistical analysis was performed. No differences were identified between the patient age, patient gender and Ki67 index in the rectal and non-rectal groups. However, there was a difference in the mitotic index, as mitosis was increased in the rectal small GISTs compared with non-rectal small GISTs (P=0.018; Table II). Stratified statistics were conducted according to the tumor size and literature (15), considering a 1 cm diameter as a cutoff. Of the 20 tumors, there were 7 tumors with a diameter ≤1 cm and 13 tumors with a diameter >1.0 and ≤2.0 cm. No difference was identified between the patient ages, patient genders, mitotic indices and Ki67 indices of patients with tumors ≤1 cm in diameter and those with tumors between >1.0 and ≤2.0 cm in diameter. Notably, in 7 tumors with a diameter ≤1 cm, 2 tumors demonstrated a mitotic index of >5 mitoses per 50 HPF (Table III).
Table II.
Association between the tumor site and characteristics of 20 patients diagnosed with GISTs, determined by the OR and corresponding 95% CI.
| Tumor site | ||||
|---|---|---|---|---|
| Characteristic | Rectum, n | Non-rectum, n | OR (95% CI) | P-value |
| Total cases | 8 | 12 | ||
| Age | ||||
| ≤58 years | 6 | 5 | 4.200 (0.590–30.100) | 0.197 |
| >58 years | 2 | 7 | ||
| Gender | ||||
| Male | 6 | 9 | 1.000 (0.130–7.890) | 1.000 |
| Female | 2 | 3 | ||
| Mitotic index | ||||
| ≤5 per 50 HPF | 3 (1a) | 11 | 0.056 (0.004–0.663) | 0.018 |
| 6–10 per 50 HPF | 5 (3a) | 1 (1a) | ||
| Ki67 index | ||||
| ≤5% | 4 | 9 | 0.330 (0.050–2.240) | 0.356 |
| >5% | 4 | 3 | ||
The distribution of 5 patients that experienced recurrence. OR, odds ratio; CI, confidence interval; GISTs, gastrointestinal stromal tumors; HPF, high power fields.
Table III.
Association between the tumor size and characteristics of 20 patients diagnosed with GISTs, determined by the OR and corresponding 95% CI.
| Tumor size | ||||
|---|---|---|---|---|
| Characteristic | ≤1.0 cm, n | >1.0 cm, n | OR (95% CI) | P-value |
| Total | 7 | 13 | ||
| Age | ||||
| ≤58 years | 4 | 7 | 1.14 (0.18–7.28) | 1.000 |
| >58 years | 3 | 6 | ||
| Gender | ||||
| Male | 6 | 9 | 2.67 (0.24–30.06) | 0.613 |
| Female | 1 | 4 | ||
| Mitotic index | ||||
| ≤5 per 50 HPF | 5 | 9 (1a) | 1.11 (0.15–8.37) | 0.999 |
| 6–10 per 50 HPF | 2 (2a) | 4 (2a) | ||
| Ki67 index | ||||
| ≤5% | 5 | 8 | 1.56 (0.26–11.37) | 1.001 |
| >5% | 2 | 5 | ||
The distribution of 5 patients that experienced recurrence. OR, odds ratio; CI, confidence interval; GISTs, gastrointestinal stromal tumors; HPF, high power fields.
Survival time and the association with clinicopathological factors
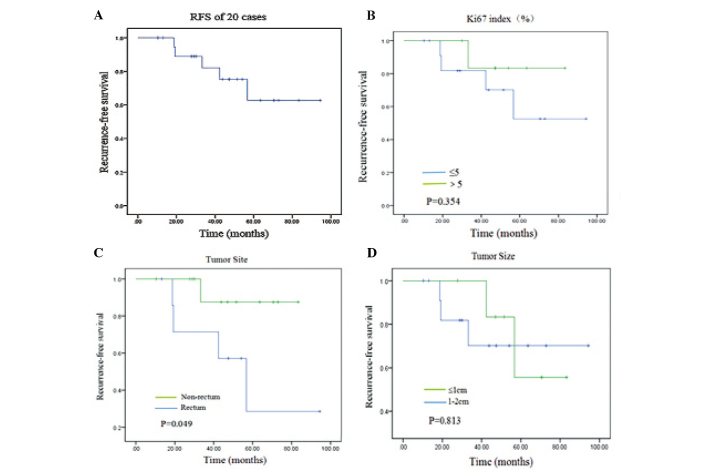
In total, 20 patients were followed up and the median follow-up time was 49.5 months (range, 10.5–94.4 months). At the end of follow-up, 5 patients had experienced tumor recurrence, 4 of which possessed rectal tumors and 1 possessed an enterocoelial tumor. Among these patients, 1 patient succumbed following progression, 1 patient succumbed to accidental death and 1 patient succumbed to heart disease. In addition, 1 patient with rectal GIST received imatinib mesylate continuously and is currently in a stable condition. Due to the low incidence of recurrence in the present study, the median RFS time could not be calculated. As there were few samples and cases of tumor progression and mortality, univariate and multivariate analyses could not be performed. Analyzing the RFS time according to the Ki67 index revealed no difference between Ki67 indices of ≤5% and >5% (P=0.354). The RFS time was analyzed between the rectum and non-rectum groups, which revealed a significant difference in the RFS time between the rectum and non-rectum groups (P=0.049). The RFS time was also analyzed according to the tumor size, and it was found that there was no difference in RFS time between tumors ≤1 cm in diameter and tumors between >1.0 and ≤2.0 cm in diameter (Fig. 3).
Figure 3.
RFS analysis and the factors affected by the clinicopathological characteristics of 20 patients with non-gastric small GISTs, determined by Kaplan-Meier analysis. (A) RFS in all patients. (B) Association between Ki-67 index (%) and RFS. No difference was found in RFS between Ki67 indices ≤5% and >5%, thus Ki-67 was not a prognostic indicator for small GISTs. (C) The RFS in the patients with rectal small GISTs and patients with non-rectal small GISTs was markedly different. The non-rectum group demonstrated a longer RFS compared with the rectum group (77.0±5.4 vs. 54.6±12.2 months; P=0.049) (D) No difference was found in the RFS between tumors <1 cm and those 1–2 cm in size. RFS, recurrence-free survival; GIST, gastrointestinal stromal tumors.
Discussion
Small GISTs have been the focus of previous studies, but the majority of small GISTs exhibit no symptoms, with the exception that small GISTs near the rectum may result in a change of bowel habits (9,11,15), which occurred in 6 of the 8 patients with rectal GISTs in the present study. Other enterocoelia small GISTs exhibited no compression on other tissues and organs, as the lesions were small in size, with infrequent symptoms of bleeding, necrosis and perforation, which was challenging to identify. The majority of GISTs reported in the literature are located in the stomach, and one of the most important reasons for this identification is the development of endoscopy and EUS (11,16). This technology increased the sensitivity of stomach and duodenum examination to small lesions. Several GISTs combined with other malignant tumors, which were mostly gastric cancer, have been incidentally detected during surgery or in the resected gastric specimen (11,15–17). Few individual cases of small GISTs in the intraperitoneal, colorectal and small intestine regions that were detected incidentally during surgery for another disease or during physical examination, which was similar to the identification of lesions in the present study, have been reported in the literature (18,19). Analysis of the histopathological type of the lesions revealed that spindle cells accounted for 95% of the present cases, and the rates of CD117, CD34 and DOG-1 expression were approximately equal to those in overt GISTs, which was consistent with pathological descriptions of small GISTs in the majority of the literature (20,21).
For the features and treatment of small GISTs, controversy remains on whether these small lesions represent the early stages of malignant GISTs or are hyperplastic proliferations of an entirely benign nature, that in certain cases may not even represent clonal neoplastic proliferation (12,22). The National Comprehensive Cancer Network (NCCN) recommends surgical resection for tumors >2 cm in diameter due to the malignant potential of these lesions, and tumors <2 cm in diameter may be conservatively followed up (14). Certain studies have identified poor prognostic features in small GISTs, since suspected small GISTs >1.4 cm in diameter with irregular margins, identified using EUS, were associated with significant progression. It has been suggested that this subgroup is monitored by a more intensive follow-up (16). Due to the limited number of reported cases in the literature, it is challenging to obtain results regarding the prognosis of small GISTs by numerous sampling tests. However, it is well known that the tumor size and mitotic index are the best prognostic indicators for determining the malignant potential of GISTs (23).
In the present study, although the small GISTs were ≤2 cm in size, the mitotic index of 6 small non-gastric GISTs was >5 mitoses per 50 HPF and the mitotic index of 2 out of 7 small GISTs (≤1 cm) was >5 per 50 HPF, which indicated the malignant potential and implied the necessity of surgical resection of small GISTs. No significant difference was identified between the mitotic index in the ≤1 cm diameter and 1–2 cm diameter groups, indicating that mitosis occurs in the early stage of disease, which is consistent with the findings of numerous studies in the literature (24,25). Gene detection was not performed in the present study, but a previous study by Corless et al (26) performed c-kit gene mutation testing on 13 small GISTs that were identified during autopsy or found incidentally, among which exon 11 (84.7%) was found to possess mutations of the c-kit gene. An associated study (20) revealed that even the smallest GIST (diameter, 0.2 cm) harbored mutations of the c-kit gene. These studies indicated that the mutation of c-kit or platelet-derived growth factor receptor (PDEGFR) was a critical event at an early stage of GIST development. From the aforementioned analysis, the present study hypothesized that all small non-gastric GISTs ≤2 cm in diameter, which demonstrate malignant potential and may eventually develop to overt GISTs, should be resected once diagnosed or incidentally identified. It is unnecessary to decide a cutoff, such as 1 or 1.4 cm, in small GISTs in order to predict the group that may possess an increased chance for recurrence, as certain previous studies have reported (24,27).
Though rectal GISTs are less common than non-rectal GISTs, accounting for ~5% of total GISTs (28), they remain the focus of studies, as the symptoms for rectal GISTs appear earlier compared with the symptoms of GISTs located in the enterocoelia. Rectal GISTs may also be detected by digital rectal examination, fiber colonoscopy and ultrasonic endoscopy (29). The principle of surgery for rectal GISTs is different from the principle for rectal cancer, as no lymph node dissection or TME resection is required, but a tumor-free resection margin and complete resection are necessary (30). Liu et al (31) reported the results of the surgical treatment of 21 patients with rectal GISTs and considered that mitosis, a positive resection margin and open surgery may be poor prognostic factors, with the DFS of the group that received open surgery being decreased compared with the group that received local excision group. It was suggested that for rectal GISTs located <5 cm from the anus, transanal resection should be performed. For larger lesions, initial adjuvant therapy of imatinib mesylate and then surgical treatment subsequent to a reduction in lesion size has been recommended. The findings of the study by Wang et al (32) demonstrated that this novel adjuvant therapy for rectum GISTs is a safe and effective therapy with a clear benefit for the local excision, in terms of feasibility, function preservation and safety.
Of the 20 patients in the present study, 8 possessed rectal GISTs. In total, 1 patient was continuously administered with imatinib mesylate instead of undergoing surgical resection, and 7 patients underwent surgical treatment without receiving adjuvant drugs. Recurrence occurred in 4 out of these 7 patients, including 2 patients that underwent transanal resections, 1 patient that underwent rectal anterior resection and 1 patient that underwent Hartmann's procedure. Due to the limited number of patients, the prognostic effect of various surgical methods was unable to be compared, but the malignant potential of rectal GISTs was determined to be increased compared with GISTs in other sites. Rectal GISTs were also easy to assess for local recurrence.
The mitotic index was compared between the rectum group, in which 5 lesions demonstrated >5 mitoses per 50 HPF, and the non-rectum group, in which 1 lesion demonstrated >5 mitoses per 50 HPF. This difference was statistically significant. This finding was consistent with certain findings in the literature (33,34), as rectal GISTs have been reported to possess comparatively higher mitotic activity, confirming a distinctively aggressive biology.
Investigation of the rectum group revealed that the RFS time of this group was significantly decreased compared with the RFS time of the non-rectum group. The NCCN Task Force (14) previously reported 111 rectal GISTs with a mitotic index of >5 mitoses per 50 HPF and ≤2 cm in size that demonstrated a recurrence rate of 54%. In the present study, there were 5 patients with a mitotic index of >5 mitoses per 50 HPFs in the rectal GISTs. Out of these patients, 3 developed recurrence (Table II), resulting in a 60% recurrence rate. However, due to the smaller sample size, there was little difference between the results of the current study and the NCCN guidelines, which indicated that the metastatic potential of rectal small GISTs was increased compared with the lesions at other sites. Surgical treatment was required once rectal GISTs were detected, and those patients with a mitotic index >5 per 50 HPFs should receive surgery combined with the administration of imatinib mesylate.
As one of the most important immunocytochemical markers of proliferation in tumors, the Ki67 index is already accepted as a clinical predictor of the prognosis of breast cancer or neuroendocrine tumors (35,36), but the criteria of the Ki67 index in GISTs is not well-defined yet. Zhao et al (37) detected the Ki67 index in 370 patients and hypothesized that the Ki67 index was an independent prognostic factor for the RFS time of patients with GISTs subsequent to analysis. Wang et al (38) reported the association between Ki67 and clinicopathological factors and hypothesized that Ki67 was associated with NIH risk stratification. At present, the function of the Ki67 index is valued in the clinic, so Ki67 detection is performed on GIST surgical specimens. In the present study, Ki67 detection was conducted for those specimens that had not undergone Ki67 detection previously. However, as the specimens had been stored for a long time, this detection may have resulted in errors. Also, due to the limited numbers of specimens, associations between Ki67 expression and factors including tumor size, site and mitosis were not identified. The RFS time of the patients with a Ki67 index ≤5% and those with an index >5% was not significantly different, which requires additional analysis by increasing the number of specimens assessed.
There are a few limitations of the present study. Firstly, no examination of the expression of the c-kit and PDEGFR genes was performed in the present patients. Secondly, additional studies should be performed with increased numbers of patients to investigate the association between clinicopathological features, including the Ki67 index, mitotic index and risk grade, and the prognosis. Thirdly, multicenter randomized controlled trials should be performed to compare biological behaviors, clinicopathological features and prognostic differences between small gastric and non-gastric GISTs and the significance of surgery in the treatment of small GISTs.
It is challenging to detect non-gastric small GISTs as the clinical symptoms are not evident, and the majority of these lesions are detected in procedures performed on other organs. Non-gastric GISTs, regardless of the size, may demonstrate mitotic change and recurrence to indicate malignant potential. Once this is detected, surgical resection is required. Rectal small GISTs with increased malignant potential and recurrence rates require more attention. At present, it is challenging to utilize the Ki67 index as a prognostic factor for the assessment of non-gastric small GISTs, and this requires additional investigation by increasing the number of specimens studied.
References
- 1.Huang H, Liu YX, Zhan ZL, Liang H, Wang P, Ren XB. Different sites and prognoses of gastrointestinal stromal tumors of the stomach: Report of 187 cases. World J Surg. 2010;34:1523–1533. doi: 10.1007/s00268-010-0463-y. [DOI] [PubMed] [Google Scholar]
- 2.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: An analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 3.Doğusoy G Bülbül. Turkish GIST Working Group: Gastrointestinal stromal tumors: A multicenter study of 1160 Turkish cases. Turk J Gastroenterol. 2012;23:203–211. [PubMed] [Google Scholar]
- 4.von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Casper ES, Conrad EU, III, DeLaney TF, Ganjoo KN, George S, et al. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw. 2014;12:853–862. doi: 10.6004/jnccn.2014.0080. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H, Eriksson M, Hall KS, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE, et al. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer. 2014;120:2325–2333. doi: 10.1002/cncr.28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen C, Chen H, Yin Y, Chen J, Zhang B, Chen Z, Chen J. Preoperative imatinib for patients with primary unresectable or metastatic/recurrent gastrointestinal stromal tumor. Clinics (Sao Paulo) 2014;69:758–762. doi: 10.6061/clinics/2014(11)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischof DA, Kim Y, Blazer DG, III, Behman R, Karanicolas PJ, Law CH, Quereshy FA, Maithel SK, Gamblin TC, Bauer TW, et al. Surgical management of advanced gastrointestinal stromal tumors: An international multi-institutional analysis of 158 patients. J Am Coll Surg. 2014;219:439–449. doi: 10.1016/j.jamcollsurg.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Ogata K, Mochiki E, Ojima H, Haga N, Fukuchi M, Aihara R, Ando H, Uchida N, Toyomasu Y, Suzuki M, et al. A multicenter long-term study of imatinib treatment for Japanese patients with unresectable or recurrent gastrointestinal stromal tumors. J Surg Oncol. 2014;110:942–946. doi: 10.1002/jso.23773. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Xue A, Fang Y, Shu P, Ling J, Hu J, Hou Y, Shen K, Qin J, Sun Y, et al. Clinicopathological features of small gastrointestinal stromal tumors. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18:338–341. (In Chinese) [PubMed] [Google Scholar]
- 10.Sorour MA, Kassem MI, Ghazal A-H, El-Riwini MT, Abu Nasr A. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 2014;12:269–280. doi: 10.1016/j.ijsu.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Nakajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527–1535. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY. ESMO Guidelines Working Group: Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):64–67. doi: 10.1093/annonc/mdp131. [DOI] [PubMed] [Google Scholar]
- 13.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. (quiz S42-S44).J Natl Compr Canc Netw. 2010;8(Suppl 2):S1–S41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi S, Gasparotto D, Toffolatti L, Pastrello C, Gallina G, Marzotto A, Sartor C, Barbareschi M, Cantaloni C, Messerini L, et al. Molecular and clinicopathologic characterization of gastrointestinal stromal tumors (GISTs) of small size. Am J Surg Pathol. 2010;34:1480–1491. doi: 10.1097/PAS.0b013e3181ef7431. [DOI] [PubMed] [Google Scholar]
- 16.Fang YJ, Cheng TY, Sun MS, Yang CS, Chen JH, Liao WC, Wang HP. Suggested cutoff tumor size for management of small EUS-suspected gastric gastrointestinal stromal tumors. J Formos Med Assoc. 2012;111:88–93. doi: 10.1016/j.jfma.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Agaimy A, Wünsch PH, Sobin LH, Lasota J, Miettinen M. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23:120–129. doi: 10.1053/j.semdp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Wang YP, Li Y, Song C. Metachronous multiple gastrointestinal stromal tumors and adenocarcinoma of the colon: A case report. Oncol Lett. 2014;8:1123–1126. doi: 10.3892/ol.2014.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agaimy A, Wünsch PH, Dirnhofer S, Bihl MP, Terracciano LM, Tornillo L. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: A clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am J Surg Pathol. 2008;32:867–873. doi: 10.1097/PAS.0b013e31815c0417. [DOI] [PubMed] [Google Scholar]
- 20.Chetty R. Small and microscopically detected gastrointestinal stromal tumours: An overview. Pathology. 2008;40:9–12. doi: 10.1080/00313020701716490. [DOI] [PubMed] [Google Scholar]
- 21.Abraham SC, Krasinskas AM, Hofstetter WL, Swisher SG, Wu TT. ‘Seedling’ mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol. 2007;31:1629–1635. doi: 10.1097/PAS.0b013e31806ab2c3. [DOI] [PubMed] [Google Scholar]
- 22.He YL. Consensus and controversy of surgical diagnosis and treatment for gastrointestinal stromal tumor. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:201–203. (In Chinese) [PubMed] [Google Scholar]
- 23.Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608–615. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Feng F, Li M, Sun L, Hong L, Cai L, Wang W, Xu G, Zhang H. Surgical resection should be taken into consideration for the treatment of small gastric gastrointestinal stromal tumors. World J Surg Oncol. 2013;11:273. doi: 10.1186/1477-7819-11-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MY, Park YS, Choi KD, Lee JH, Choi KS, Kim H, Song HJ, Lee GH, Jung HY, Kim JH, et al. Predictors of recurrence after resection of small gastric gastrointestinal stromal tumors of 5 cm or less. J Clin Gastroenterol. 2012;46:130–137. doi: 10.1097/MCG.0b013e31821f8bf6. [DOI] [PubMed] [Google Scholar]
- 26.Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567–1572. doi: 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vogelaere K, Van Loo I, Peters O, Hoorens A, Haentjens P, Delvaux G. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg Endosc. 2012;26:2339–2345. doi: 10.1007/s00464-012-2186-7. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: A clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121–1133. doi: 10.1097/00000478-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Sun LF, He JJ, Yu SJ, Xu JH, Wang JW, Li J, Song YM, Ding KF, Zheng S. Transsacral excision with pre-operative imatinib mesylate treatment and approach for gastrointestinal stromal tumors in the rectum: A report of two cases. Oncol Lett. 2014;8:1455–1460. doi: 10.3892/ol.2014.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson MJ, Fitzgerald JE, Strauss DC, Hayes AJ, Thomas JM, Messiou C, Fisher C, Benson C, Tekkis PP, Judson I. Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. Br J Surg. 2015;102:965–971. doi: 10.1002/bjs.9818. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Yan Z, Liao G, Yin H. Treatment strategy of rectal gastrointestinal stromal tumor (GIST) J Surg Oncol. 2014;109:708–713. doi: 10.1002/jso.23562. [DOI] [PubMed] [Google Scholar]
- 32.Wang JP, Wang T, Huang MJ, Wang L, Kang L, Wu XJ. The role of neoadjuvant imatinib mesylate therapy in sphincter-preserving procedures for anorectal gastrointestinal stromal tumor. Am J Clin Oncol. 2011;34:314–316. doi: 10.1097/COC.0b013e3181dea970. [DOI] [PubMed] [Google Scholar]
- 33.Dong C, Jun-Hui C, Xiao-Jun Y, Mei K, Bo W, Chen-Fe J, Wei-Li Y. Gastrointestinal stromal tumors of the rectum: Clinical, pathologic, immunohistochemical characteristics and prognostic analysis. Scand J Gastroenterol. 2007;42:1221–1229. doi: 10.1080/00365520701376507. [DOI] [PubMed] [Google Scholar]
- 34.Chen BX, Hao DM, Zhang ZX. Prognostic analysis of 40 cases with rectal gastrointestinal stromal tumor. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:263–265. (In Chinese) [PubMed] [Google Scholar]
- 35.Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol. 2013;66:512–516. doi: 10.1136/jclinpath-2012-201085. [DOI] [PubMed] [Google Scholar]
- 36.Koumarianou A, Chatzellis E, Boutzios G, Tsavaris N, Kaltsas G. Current concepts in the diagnosis and management of poorly differentiated gastrointestinal neuroendocrine carcinomas. Endokrynol Pol. 2013;64:60–72. [PubMed] [Google Scholar]
- 37.Zhao WY, Xu J, Wang M, Zhang ZZ, Tu L, Wang CJ, Lin TL, Shen YY, Liu Q, Cao H. Prognostic value of Ki67 index in gastrointestinal stromal tumors. Int J Clin Exp Pathol. 2014;7:2298–2304. [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Chen P, Liu XX, Zhao W, Shi L, Gu XW, Zhu CR, Zhu HH, Zong L. Prognostic impact of gastrointestinal bleeding and expression of PTEN and Ki-67 on primary gastrointestinal stromal tumors. World J Surg Oncol. 2014;12:89. doi: 10.1186/1477-7819-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]