Abstract
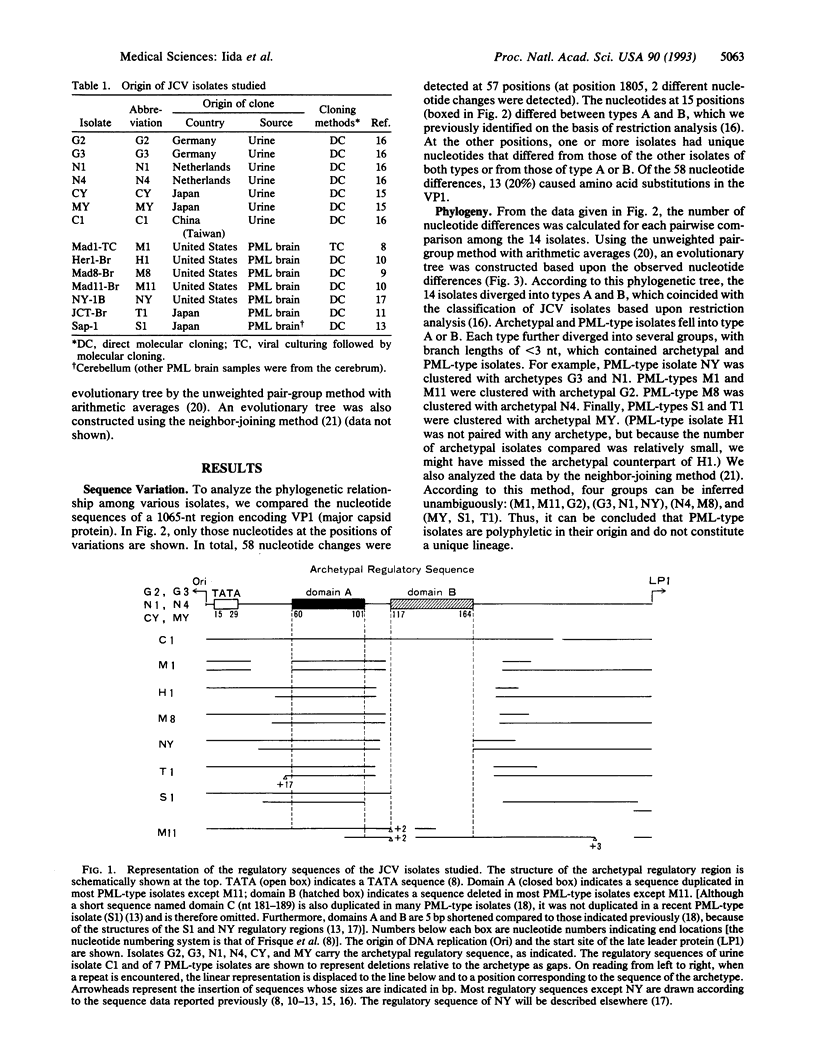
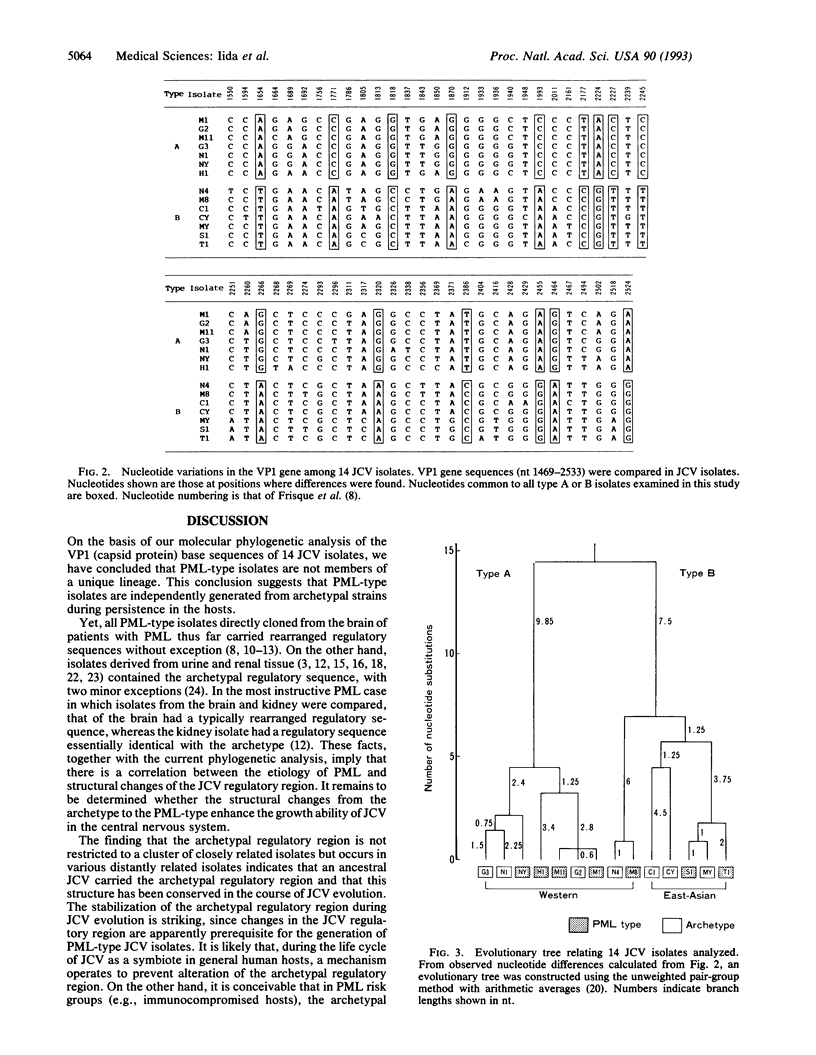
JC polyomavirus (JCV) DNAs from the urine of nonimmunocompromised individuals (designated archetypal isolates) regularly contain a regulatory sequence that may have generated various regulatory sequences of JCV isolates derived from the brain of patients with progressive multifocal leukoencephalopathy (PML). In this report, we constructed a phylogenetic tree for 14 isolates (7 archetypes and 7 PML types) from DNA sequence data on the VP1 (major capsid protein) gene. According to the phylogenetic tree, the 14 isolates diverged into types A and B, each of which contained archetypal and PML-type isolates. Each type further diverged into several groups containing archetypal and PML-type isolates. We conclude that PML-type isolates are polyphyletic in their origin and do not constitute a unique lineage. This conclusion suggests that PML-type JCV isolates are generated from archetypal strains during persistence in the hosts. Furthermore, the present phylogenetic analysis indicates that an ancestral JCV carried the archetypal regulatory sequence and that this structure has been conserved in the course of JCV evolution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesters P. M., Heritage J., McCance D. J. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983 Apr;147(4):676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- Chuke W. F., Walker D. L., Peitzman L. B., Frisque R. J. Construction and characterization of hybrid polyomavirus genomes. J Virol. 1986 Dec;60(3):960–971. doi: 10.1128/jvi.60.3.960-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörries K. Progressive multifocal leucoencephalopathy: analysis of JC virus DNA from brain and kidney tissue. Virus Res. 1984 Jan;1(1):25–38. doi: 10.1016/0168-1702(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Flaegstad T., Sundsfjord A., Arthur R. R., Pedersen M., Traavik T., Subramani S. Amplification and sequencing of the control regions of BK and JC virus from human urine by polymerase chain reaction. Virology. 1991 Feb;180(2):553–560. doi: 10.1016/0042-6822(91)90069-n. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Padgett B. L., Walker D. L. Comparison of infectious JC virus DNAs cloned from human brain. J Virol. 1983 Jan;45(1):299–308. doi: 10.1128/jvi.45.1.299-308.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber G., Dörries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988 May;62(5):1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz R. B., Eaton B. A., Kubik M. F., Latorra D., McGregor J. A., Dynan W. S. BK virus and JC virus shed during pregnancy have predominantly archetypal regulatory regions. J Virol. 1991 Aug;65(8):4515–4519. doi: 10.1128/jvi.65.8.4515-4519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., Foster G. C. Multiple JC virus genomes from one patient. J Gen Virol. 1984 Aug;65(Pt 8):1405–1411. doi: 10.1099/0022-1317-65-8-1405. [DOI] [PubMed] [Google Scholar]
- Martin J. D., King D. M., Slauch J. M., Frisque R. J. Differences in regulatory sequences of naturally occurring JC virus variants. J Virol. 1985 Jan;53(1):306–311. doi: 10.1128/jvi.53.1.306-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Jona M., Yasui K., Nagashima K. Genetic characterization of JC virus Tokyo-1 strain, a variant oncogenic in rodents. Virus Res. 1987 Apr;7(2):159–168. doi: 10.1016/0168-1702(87)90077-3. [DOI] [PubMed] [Google Scholar]
- Myers C., Frisque R. J., Arthur R. R. Direct isolation and characterization of JC virus from urine samples of renal and bone marrow transplant patients. J Virol. 1989 Oct;63(10):4445–4449. doi: 10.1128/jvi.63.10.4445-4449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. New human papovaviruses. Prog Med Virol. 1976;22:1–35. [PubMed] [Google Scholar]
- Rentier-Delrue F., Lubiniecki A., Howley P. M. Analysis of JC virus DNA purified directly from human progressive multifocal leukoencephalopathy brains. J Virol. 1981 May;38(2):761–769. doi: 10.1128/jvi.38.2.761-769.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Yogo Y., Furuta Y., Takada A., Irie T., Kasai M., Sano K., Fujioka Y., Nagashima K. Molecular characterization of a JC virus (Sap-1) clone derived from a cerebellar form of progressive multifocal leukoencephalopathy. Acta Neuropathol. 1992;83(2):105–112. doi: 10.1007/BF00308469. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Yogo Y., Kitamura T., Aso Y. Persistence of archetypal JC virus DNA in normal renal tissue derived from tumor-bearing patients. Virology. 1992 Feb;186(2):736–741. doi: 10.1016/0042-6822(92)90040-v. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Iida T., Taguchi F., Kitamura T., Aso Y. Typing of human polyomavirus JC virus on the basis of restriction fragment length polymorphisms. J Clin Microbiol. 1991 Oct;29(10):2130–2138. doi: 10.1128/jcm.29.10.2130-2138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Hara K., Iida T., Taguchi F., Tajima A., Kawabe K., Aso Y. Sequence rearrangement in JC virus DNAs molecularly cloned from immunosuppressed renal transplant patients. J Virol. 1991 May;65(5):2422–2428. doi: 10.1128/jvi.65.5.2422-2428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Ueki T., Aso Y., Hara K., Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990 Jun;64(6):3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



