Abstract
Ionizing irradiation induces acute and chronic injury to tissues and organs. Applications of antioxidant therapies for the management of ionizing irradiation injury fall into three categories: (1) radiation counter measures against total or partial body irradiation; (2) normal tissue protection against acute organ specific ionizing irradiation injury; and (3) prevention of chronic/late radiation tissue and organ injury. The development of antioxidant therapies to ameliorate ionizing irradiation injury began with initial studies on gene therapy using Manganese Superoxide Dismutase (MnSOD) transgene approaches and evolved into applications of small molecule radiation protectors and mitigators. The understanding of the multiple steps in ionizing radiation-induced cellular, tissue, and organ injury, as well as total body effects is required to optimize the use of antioxidant therapies, and to sequence such approaches with targeted therapies for the multiple steps in the irradiation damage response.
Keywords: ionizing irradiation, antioxidants, oxidative stress, mitochondrial mechanisms of apoptosis
1. Introduction
Ionizing irradiation induces sequential steps of cellular, tissue, organ, and total body injury [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Within fractions of a second during ionizing irradiation exposure, water in cells is hydrolyzed and free radicals are generated, including superoxide and hydroxyl radical, which are detectable in experiments [16,17,18,19,20,21,22,23,24,25,26,27,28]. Sequential steps involve the generation of hydrogen peroxide [29]. At this stage (seconds), and for minutes, hours, and days after exposure, the management of oxidative stress from ionizing irradiation shares many steps common with other forms of injury, including: Hyperbaric oxygen injury, hypoxia, chemical toxin exposure, heat, ultraviolet irradiation, chemical toxicity, and infection [30,31,32,33]. Over days after irradiation, tissues and organs respond to a sequence of events culminating in an acute inflammatory response [1,30,31,34,35,36,37,38,39,40,41,42,43,44]. Somewhat unique to radiation injury is the subsequent latent period during which many injury markers, including histopathologic effects are undetectable [32,45,46,47,48,49,50,51,52,53,54,55]. Tissue volume, tissue type, and genetic factors control the onset of a final late/chronic radiation injury phase, which includes fibrosis and scarring [48].
In recent years, attempts to ameliorate ionizing irradiation injury have utilized reagents and therapeutics common to the treatment of other forms of tissue and organ injury [56]. Prominent among these attempts has been the approach to utilize antioxidant therapies. Initial experiments using free radical scavengers include N-Acetyl-Cysteine (NAC) or the supplement/replenishing of antioxidant stores by delivering glutathione. These methods demonstrated some success in tissue culture and animal models [57,58,59,60,61,62]. However, true breakthroughs occurred with the molecular cloning and expression of transgenes for antioxidant enzymes, prominently the superoxide dismutases [63,64,65,66,67]. Replenishment of superoxide enzymes by protein delivery was ineffective compared to delivery of transgene for protein production [63,64]. The relatively cumbersome nature of producing transgenes for gene therapy invigorated the development of small molecule antioxidants with properties similar to the transgene products. Prominent in this category was the development of Tempo (Figure 1), a nitroxide with the capacity to scavenge free radicals and, through cycling of the nitroxide to hydroxyl-amine, to neutralize multiple free radicals for each molecule of Tempo [59,68]. Finally, the role of mitochondria in the mechanism of irradiation apoptosis prompted the development of mitochondrial-targeted Tempo (GS-nitroxides and triphenylphosponium nitroxides) [29,68,69,70,71,72,73,74]. Initial success in animal models using mitochondrial-targeted antioxidants has uncovered multiple steps in the response to ionizing irradiation, which cannot be explained solely by individual cellular responses (nuclear-mitochondrial signaling and apoptosis). These realizations have led to new therapeutic approaches including the application of new drugs, with actions distinct from the antioxidant approach. Nevertheless, three categories of applications of mitochondrial-targeted antioxidants have gained prominence as potential therapeutic strategies toward the amelioration of irradiation injury.
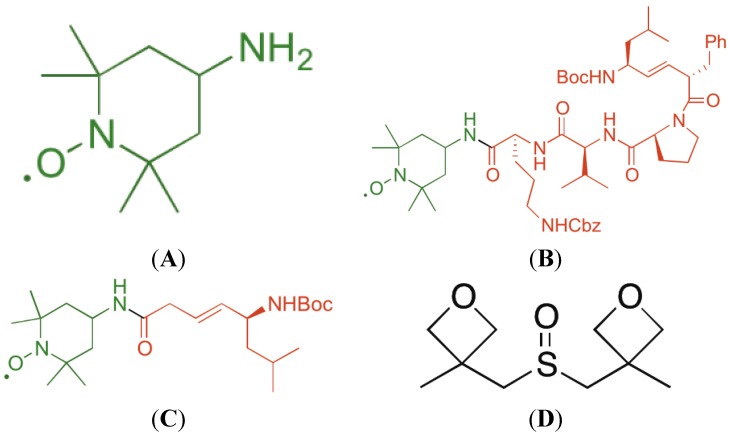
Figure 1.
Structures of 4-Amino-Tempo (A); XJB-5-131 (B); JP4-039 (C); and MMS350 (D).
2. Experimental Section
2.1. Mice
The C57BL/6NHsd, FVB/N, 129/Sv, and Fanconi Anemia (FA) Fancd2−/− mice on both the 129/Sv and C567BL/6 genetic backgrounds have been described previously [75,76]. Mice were housed four per cage according to the Institutional IACUC Protocol, and fed standard laboratory chow and deionized water.
2.2. Tissue Culture Experiments
Techniques for long-term bone marrow culture, establishment of bone marrow stromal cell lines, and Interleukin 3 (IL-3) dependent hematopoietic progenitor cell lines have been published [36,77]. Methods for growing fresh bone marrow colony forming units CFU-GEMM have been published previously [78].
2.3. Manganese Superoxide Dismutase-Plasmid Liposomes
The techniques for production and administration of MnSOD-PL have been described previously [1,26]. Briefly, the MnSOD transgene was expressed in plasmid vector, the plasmid was grown according to published methods, and it was delivered in a liposome preparation of cationic liposomes by an intra-tracheal installation [1,2,3], intravenous route [79,80,81], intra-oral [40], or intraesophageal [6,82] route according to previous publications.
2.4. GS-Nitroxides and JP4-039
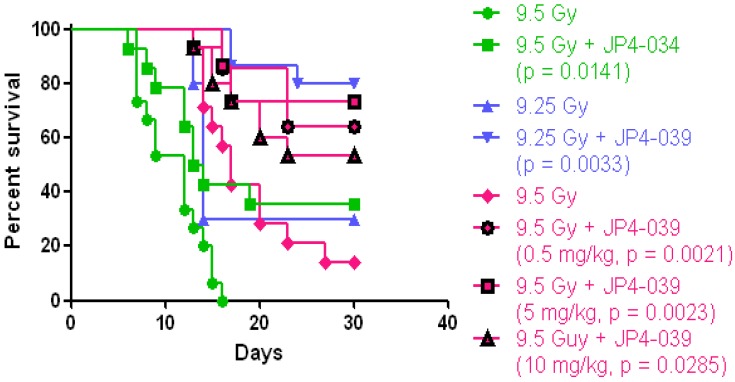
The mitochondrial-targeted nitroxide based on 4-Amino-Tempo (Figure 1) have been described in detail [42,68,83,84]. GS-nitroxide drugs were prepared by attachment of modified gramicidin S fragments to the nitroxide, thus generating mitochondrial targeted agents of various lengths (Figure 1A–C). These included the larger molecule XJB-5-131 [85], and a shorter analog, JP4-039, which have been shown to be radiation mitigators (Figure 2 and Figure 3) [68]. JP4-039 has been demonstrated to be effective as a total body radiation protector and mitigator [80,86] in both C57BL/6NHsd mice (Figure 3) and in C3H/HeN mice (Figure 4). The laboratory of Dr. Peter Wipf of the Department of Chemistry at the University of Pittsburgh synthesized XJB-5-131, JP4-039 as well as MMS350, a highly water soluble sulfoxide with a different mechanism of action for prevention of radiation damage.
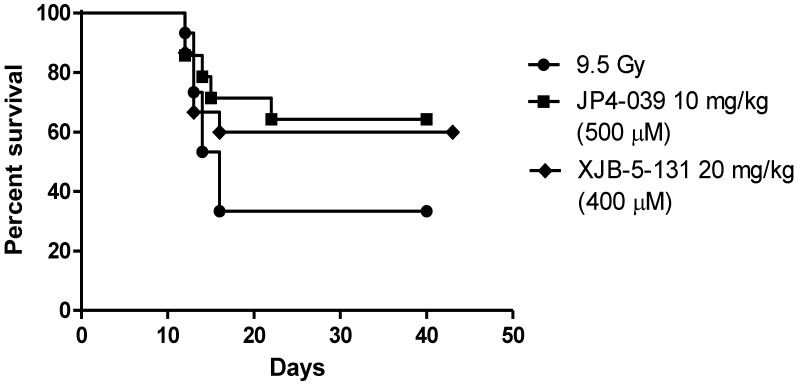
Figure 2.
Effective radiation mitigation by two GS-nitroxide analogs (XJB-5-131 and JP4-039). Groups of 15 C57BL/6NHsd mice received total body irradiation, and then 24 h later intravenous administration of 100 μL of F14 liposomes containing either XJB-5-131 or JP4-039, standardized for equimolar concentration. The heavier molecular weight of XJB-5-131 requires larger quantities to achieve an equimolar concentration with JP4-039. There was equivalent radiation mitigation by both drugs.
Figure 3.
Effective mitigation of total body irradiation damage in C57BL/6NHsd mice by intravenous administration of JP4-039. Experiments shown are over a course of a year accounting for the TBI dose “drift” of the LD50/30. At 3 different time points during a single calendar year, experiments were carried out delivering JP4-039/F15 in 100 μL volume containing 20 mg/kg drug, to mice. In these experiments, the LD50/30 was noted to “drift” over the course of the year and could not be explained by changes in the Cesium-70 Gamma Cell irradiator, supplier of mice, age of mice, gender of mice (all were female), diet, or other factors in the animal care facility. In all experiments, despite the “drift” of the LD50/30, JP4-039 was an effective mitigator against total body irradiation.
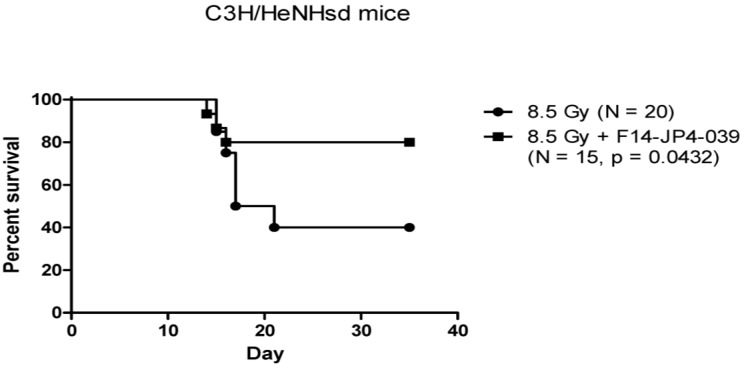
Figure 4.
Mitigation of total body irradiation damage to C3H/HeNHsd mice by intravenous JP4-039/F14 antioxidant small molecule therapy. Groups of C3H/HeNHsd mice (n = 15) received total body irradiation, and 24 h later intravenous administration of 100 μL of F14 liposomes containing 100 μg of JP4-039. Survival was quantitated, and there was a significant increase in survival in mice given JP4-039.
2.5. Assays for Antioxidant Stores
The Trolox assay for antioxidant stores has been published in detail and these methods have been previously described [75,76].
2.6. Assays for Apoptosis, Mitochondrial Content, and Mitochondrial Number
The methods for quantitation of ionizing irradiation effects on mitochondria have been published previously [28,87].
3. Results and Discussion
3.1. Antioxidant Therapies to Prevent and/or Mitigate Total Body Irradiation Injury
Two applications of antioxidant therapies in ionizing irradiation damage have recently been described. Protection against total body irradiation damage has been demonstrated with MnSOD-plasmid liposomes administered intravenously 24 h prior to total body irradiation [79,81]. In two model systems, one in which MnSOD-PL was given alone [79], and in another system supplemented with an antioxidant diet delivered after irradiation [81], improved survival of both male and female mice was demonstrated. The 24 h time point before irradiation was chosen based on previous studies that demonstrated a requirement for this time to get transgene into cells [66,88,89,90,91,92,93], facilitate nuclear migration, insertion of the plasmid into the nucleus, production of RNA for MnSOD, production of MnSOD, and then transport of the mitochondrial targeted SOD to the mitochondria, where radiation protection and mitigation actions were demonstrated [77,94,95,96]. Tissue culture studies demonstrated the requirement for mitochondrial targeting. In initial studies, cytoplasmic SOD1 (Cu/ZnSOD) was demonstrated to have little radioprotective or mitigation effect. However, when the mitochondrial targeting sequence from SOD2 (MnSOD) was added to Cu/ZnSOD, the molecule targeted the mitochondria and was radiation protective [36,49]. In contrast, when the mitochondrial targeting sequence was removed from the MnSOD transgene product, little radiation protection was seen, and gene product was concentrated in the cellular cytoplasm [49].
Antioxidant therapies to prevent total body irradiation damage were extended by the development of small molecule SOD mimics [17,18,19,42,70,97,98,99,100,101]. One strategy utilized in our laboratory was to increase the effectiveness of the nitroxide 4-Amino-Tempo (4-AT) by facilitating a mitochondrial enrichment [68]. Several GS-nitroxide variants were developed in the laboratory of Peter Wipf, Ph.D. [42,102,103]. Mitochondrial targeting was achieved by using a hemigramicidin analog attached to 4-Amino-Tempo [68]. Two different GS-nitroxides were compared for in vivo radiation mitigation, when delivered 24 h after total body irradiation. Figure 2 demonstrates the similar effectiveness of XJB-5-131, which shows a 300–600-fold mitochondrial concentration capacity, specifically in the inner mitochondrial membrane, compared to JP4-039, a molecule with a truncated mitochondrial targeting sequence, and a ca. 20-30-fold increased mitochondrial concentration. As shown in Figure 2, both molecules delivered in equimolar concentration 24 h prior to total body irradiation showed significant mitigation capacity.
The difference between radiation protection and mitigation has particular relevance for the Radiation Counter Measures Program of the National Institutes of Allergy and Infectious Disease (NIAID) of the National Institutes of Health (NIH) [89]. Delivery of a potent preventive drug prior to irradiation, which can target mitochondria and prevent the depletion of antioxidant stores has great relevance for first responders in an irradiation incident, where accumulation of radio-isotopes might be expected, or whether further exposure to photon or high linear energy transfer particle (neutron, proton) irradiation might occur. However, the radiation protection strategy is not relevant to victims of a radiation terrorist event or nuclear reactor damage in which irradiation exposure would take place prior to the administration of drugs. Delivery of an irradiation modifying drug 24 h or later after irradiation is considered “mitigation”, and thus the radiation mitigator properties have been a focus of the Radiation Counter Measure Program. Figure 2 demonstrates that both XJB-5-131 and JP4-039 were effective mitigators despite a significant difference in mitochondrial concentration capacity. This data argues that the difference between a 30-fold and 600-fold mitochondrial concentration capacity might not be relevant to a successful total body radiation mitigation. However, other studies have demonstrated the effectiveness of XJB-5-131 in crossing the blood brain barrier to ameliorate the toxicity of traumatic brain injury, while JP4-039 does not cross the blood brain barrier [85].
The mechanism of total body irradiation protection and mitigation by both gene therapy (MnSOD-PL), and small molecule antioxidants (GS-nitroxides) [16,42,57,58,77,79,83,94,95,96,97,104,105,106,107,108] may not be attributable solely to direct cellular effects. The bystander effect of both radiation injury and amelioration of irradiation damage by radiation mitigator drugs has been well documented in the radiobiology literature [76]. Most recently, using Fancd2−/− (129/Sv) mice, a bystander effect of partial body irradiation was demonstrated. Head and neck irradiation of mice demonstrated significant suppression of bone marrow at the distant femur site [76]. Application of a mitochondrial targeted antioxidant radiation protector/mitigator to the oral cavity/oropharynx, to be described in the next section, not only protected the treated tissue, but also suppressed the bystander effect of radiation damage. A positive bystander effect also applies to the application of radiation protectors and mitigators. Uptake and mitochondrial targeting of drug in a relatively small percentage of cells within an organ can confer organ radiation protection or mitigation [1,40,90]. This was demonstrated with esophageal delivery of hemagglutin-epitope tagged MnSOD transgene product, which, when delivered prior to irradiation conferred significant organ radioprotection, while the transgene product was only detectable in a small fraction of cells within that organ [39]. These data were confirmed in both lungs and esophagus as well as oral cavity organ specific radiation protection strategies (These data will be described in the next section regarding acute radiation injury to specific organs and targeted therapies using the antioxidant approach).
3.2. Antioxidant Radioprotection and Radiation Mitigation in Organ Specific/Localized Applications
The effectiveness of the antioxidant approach in both preventing and ameliorating irradiation injury was demonstrated in model systems utilizing both MnSOD-PL gene therapy [45], and also more recently with targeted GS-nitroxides [68].
Organ specific radioprotection using MnSOD-PL has been demonstrated in bladder [37], intestine [46], oral cavity/oropharynx [40,41], esophagus [6,109], lung [1,90], and in fetal mice in vivo [80]. In each of these systems, MnSOD-PL administration utilized a localized liposomal free system, Herpes Virus vector or adenovirus vector administered transgene. Organ specific radiation protection/mitigation was demonstrated in each model system in mice or rats utilizing radiobiological parameters of injury including histopathology, organ function, physiologic monitors (bladder measurement of water and urea transepithelial transfer), and by assays for specific cellular damage including the apotag assay for apoptosis, and electron microscopic evidence of cell death [17,18,19,59,98,99,110].
Application of GS-nitroxides as organ specific radioprotectors followed the work of MnSOD-PL mediated radiation protection, and utilized the same organ specific radiation damage systems. JP4-039, which demonstrated significant total body irradiation protection and mitigation properties (described in the last section) was highly effective when delivered in formulations designed to keep drug localized to a specific organ system [76]. JP4-039 was delivered in a liposomal emulsion containing Tween (F15), which was designed to keep the drug localized and in direct contact with cells exposed to the drug. The JP4-039/F15 formulation was a highly effective radiation protector and mitigator for esophagus and oral cavity/oropharynx [76,111]. In recent studies, JP4-039/F15 administration to both FA Fancd2−/− (129/Sv) and Fancd2−/− (C57BL/6) mice, as well as heterozygote and wild type litter mates showed significant protection and mitigation of damage to the oral cavity/oropharynx in both single fraction and fractionated irradiation [76,112]. In models of organ specific radiation protection/mitigation, principally designed for translational approaches in clinical radiotherapy, there has been great concern that antioxidant drugs would also protect tumors. Initial studies with MnSOD-PL as well as JP4-039/F15 demonstrated no protection of orthotopic lung tumors [9,41] or orthotopic head and neck cancer [96,112].
3.3. Antioxidant Therapy to Prevent Ionizing Radiation Late Effects
There are several components to ionizing irradiation-induced late effects [59]. It has been demonstrated that volume of tissue/organ irradiated, total dose of irradiation, and in the case of clinical radiotherapy, the fraction size of radiation, all contribute to the severity and time of onset of late effects [48]. In clinical radiotherapy, late effects are most critical in producing organ failure [79]. Kidney, liver, lung, and esophageal irradiation are known to induce fibrosis, which can limit the effectiveness of treatment due to late side effects [48,50]. In the case of total body irradiation, late effects are most prominent after relatively low doses of irradiation that, while suppressing natural blood counts, do not lead to acute radiation damage. The most prominent late effect is the induction of cancer [79]. The molecular mechanism of irradiation late effects has been the subject of intense investigation. Antioxidant approaches to prevent late effects of irradiation have gained significant interest in recent years. Oxidative stress has been shown to be a chronic component of irradiation damage to tissues and organs. These oxidative stress events are detected in the lung years after therapeutic lung irradiation [57]. In animal models, markers of antioxidant stress are detected in irradiated lungs months after irradiation[54]. A critical approach has been taken to utilize antioxidant therapies to ameliorate late effects. The administration of MnSOD-plasmid liposomes intravenously prior to irradiation has been shown to significantly improve acute survival measured by the lethal dose for 50% of mice at 30 days (LD50/30), but also to significantly ameliorate radiation late effects, prominently life shortening and carcinogenesis [79,113]. In a second series of experiments, mice given MnSOD-PL 24 h prior to irradiation were then placed on a novel antioxidant diet consisting of a wide variety of antioxidants and chemopreventive agents [81]. Under these conditions, surviving animals demonstrated significant further amelioration of radiation-induced life shortening. In both of these experiments, MnSOD-PL alone, or supplemented with an antioxidant diet, there was no increase in detectable cancers in mice surviving for prolonged periods after irradiation.
There is much evidence to support a difference in the molecular mechanism of acute irradiation damage compared to late effects [54,55,114]. The administration of radioprotective MnSOD-PL into the lungs prior to irradiation was significant in preventing acute radiation pneumonitis and death. However, in a mouse model that received MnSOD-PL 100 days after irradiation, when late effects (radiation fibrosis) began to appear, late effects were not ameliorated [47]. Studies with pulmonary irradiation in a fibrosis prone mouse strain (C57BL/6NHsd) compared to a fibrosis resistant mouse strain (C3H/HeNHsd) demonstrated a significant difference in radiation responses in the lungs with respect to the RT-PCR detected induction of RNA transcripts [55]. Fibrosis prone C57BL/6NHsd mice showed upregulation of TLR4 (Toll-Like Receptor 4) in the lung at the time of the initiation of fibrosis, while C3H/HeNHsd mice did not show upregulation of TLR4 [115].
Furthermore, the critical role of TGF-β in late irradiation fibrosis has been demonstrated in SMAD3−/− knockout mice [116,117], which demonstrated no irradiation-induced skin fibrosis, and no detectable radiation pulmonary fibrosis. Migration into the lungs of bone marrow stromal cell progenitors of lung fibroblasts has been shown to be a significant component of pulmonary fibrosis following irradiation. In an experiment in which wild type mice were chimeric for either SMAD3−/− fluorochrome labeled bone marrow stromal cells or wild type sex mismatched fluorochrome labeled bone marrow stromal cells, there were significant decreases in migratory capacity of the SMAD3−/− stromal cells into the lung contributing to radiation fibrosis [117]. Decreased motility of bone marrow stromal cells from SMAD3−/− mice was also characteristic of this genotype, suggesting further contribution to the fibrotic phenotype after irradiation of the motility of the stromal cells [116].
A recent advance in prevention of irradiation late effects has been the demonstration of a water-soluble dimethylsulfoxide analog, MMS350 (Figure 1), which, when administered to mice beginning 100 days after thoracic irradiation, significantly ameliorated fibrosis [54]. MMS350 has been shown to be easily administered in mL drinking water and is safely delivered to mice continuously after thoracic irradiation.
Effects of irradiation have been shown to be common to other forms of toxic substance induced fibrosis. In recent studies of liver fibrosis, the involvement of specific cytokine receptors has been shown to be mediated at the level of the endothelial cells [118,119]. Involvement of endothelial cells in radiation fibrosis is also supported by studies with the von Willebrand Factor (vWF−/− knockout mice), which showed reduced fibrosis [120]. TLR4−/− (knockout) mice also showed reduced radiation fibrosis [115]. While the molecular biologic response is complex, endothelial cells appear to be gate-keepers for the signaling determining whether tissues repair radiation damage by restoration of tissue function or demonstrate a damage signal that solicits migration from the bone marrow into that organ of bone marrow stromal cell progenitors of fibrosis and induces the late fibrotic phenotype [110].
4. Conclusions
There is much evidence to support an oxidative stress model for both acute ionizing irradiation effects and chronic oxidative stress mediated irradiation late effects. The initiation of therapeutic programs to combat radiation damage began with studies of transgene therapy utilizing mitochondrial targeted MnSOD-Plasmid Liposomes. These have been demonstrated in a recent clinical trial to be safe and effective in ameliorating acute irradiation toxicity to the esophagus [121]. MnSOD-PL therapy was shown to be effective in preventing radiation damage in total body experiments, and also in organ specific irradiation. The translation of this technology into clinical radiotherapy is in progress. A more recent development has been the use of MnSOD-mimic compounds and unnatural anti-antioxidants. XJB-5-131 and JP4-039 are enriched in mitochondria, and, consequently, seem to be particularly effective [122,123]. Triphenylphosphonium targeted nitroxides are also effective radiation protectors and mitigators [124]. Most recently, a highly water-soluble dimethylsulfoxide analog, MMS350, has been shown to be safe when administered in drinking water and prevents both the acute and chronic effects of total body irradiation or pulmonary irradiation [54]. Further studies will be required to optimize the delivery of specific agents designed to prevent irradiation-induced apoptosis. Perhaps even more important is the mounting evidence that other forms of irradiation damage are involved in cellular, tissue, and organ responses to radiation, including necroptosis, ferroptosis, and alteration in oxidative lipidomics of tissues [43,71,101]. This damage leads not only to localized tissue injury, but also effects migration into tissues of inflammatory cells, which, through the production of inflammatory mediators, exacerbate the radiation response. Oxidative stress remains a prominent factor in ionizing irradiation damage and the use of antioxidants in both the fundamental study of radiation countermeasures and in clinical radiotherapy appears to be warranted.
Acknowledgments
Supported by Research Grants U19A168021 NIAID/NIH and the Fanconi Anemia Research Foundation. This project used the UPCI Animal Facility that is supported in part by award P30CA047904.
Author Contributions
All authors designed, carried out, and interpreted results of experiments described in this article.
Conflicts of Interest
Joel Greenberger, Michael Epperly, Valerian Kagan, and Peter Wipf are the inventors of multiple patents related to the use of GS-nitroxides and MMS350 JP4-039 to treat ionizing irradiation injury.
References
- 1.Epperly M.W., Bray J.A., Kraeger S., Zwacka R., Engelhardt J., Travis E., Greenberger J.S. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 2.Zwacka R.M., Dudus L., Epperly M.W., Greenberger J.S., Engelhardt J.F. Redox gene therapy protects human IB-3 lung epithelial cells against ionizing radiation-induced apoptosis. Hum. Gene Ther. 1998;9:1381–1386. doi: 10.1089/hum.1998.9.9-1381. [DOI] [PubMed] [Google Scholar]
- 3.Epperly M.W., Bray J.A., Krager S., Berry L.A., Gooding W., Engelhardt J.F., Zwacka R., Travis E.L., Greenberger J.S. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int. J. Radiat. Oncol. Biol. Phys. 1999;43:169–181. doi: 10.1016/S0360-3016(98)00355-1. [DOI] [PubMed] [Google Scholar]
- 4.Epperly M.W., Travis E.L., Sikora C., Greenberger J.S. Magnesium superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: Modulation of irradiation-induced mRNA for IL-1, TNF-α, and TGF-β correlates with delay of organizing alveolitis/fibrosis. Biol. Blood Marrow Transplant. 1999;5:204–214. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- 5.Epperly M.W., Bray J.A., Esocobar P., Bigbee W.L., Watkins S., Greenberger J.S. Overexpression of the human MnSOD transgene in subclones of murine hematopoietic progenitor cell line 32D cl 3 decreases irradiation-induced apoptosis but does not alter G2/M or G1/S phase cell cycle arrest. Radiat. Oncol. Investig. Clin. Basic Res. 1999;7:331–342. doi: 10.1002/(SICI)1520-6823(1999)7:6<331::AID-ROI3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Stickle R.L., Epperly M.W., Klein E., Bray J.A., Greenberger J.S. Prevention of irradiation-induced esophagitis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat. Oncol. Investig. Clin. Basic Res. 1999;7:204–217. doi: 10.1002/(SICI)1520-6823(1999)7:4<204::AID-ROI2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Gorbunov N.V., Pogue-Geile K.L., Epperly M.W., Bigbee W.L., Draviam R., Day B.W., Wald N., Watkins S.C., Greenberger J.S. Activation of the nitric oxide synthase 2 pathway in the response of bone marrow stromal cells to high doses of ionizing radiation. Radiat. Res. 2000;154:73–86. doi: 10.1667/0033-7587(2000)154[0073:AOTNOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Epperly M.W., Sikora C., Defilippi S., Bray J., Koe G., Liggitt D., Luketich J.D., Greenberger J.S. Plasmid/liposome transfer of the human manganese superoxide dismutase (MnSOD) transgene prevents ionizing irradiation-induced apoptosis in human esophagus organ explant culture. Int. J. Cancer. 2000;90:128–137. doi: 10.1002/1097-0215(20000620)90:3<128::AID-IJC2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Epperly M.W., Defilippi S., Sikora C., Gretton J., Kalend K., Greenberger J.S. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Ther. 2000;7:1011–1018. doi: 10.1038/sj.gt.3301207. [DOI] [PubMed] [Google Scholar]
- 10.Epperly M.W., Epstein C.J., Travis E.L., Greenberger J.S. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese superoxide dismutase-plasmid/liposome (SOD2-PL) intratracheal gene therapy. Radiat. Res. 2000;154:365–374. doi: 10.1667/0033-7587(2000)154[0365:DPRROM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Epperly M.W., Gretton J.A., DeFilippi S.J., Sikora C.A., Liggitt D., Koe G., Greenberger J.S. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD-PL) gene therapy. Radiat. Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:MORICE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Epperly M.W., Travis E.L., Whitsett J.A., Epstein C.J., Greenberger J.S. Overexpression of manganese superoxide dismutase (MnSOD) in whole lung or alveolar type II (AT-II) cells of MnSOD transgenic mice does not provide intrinsic lung irradiation protection. Radiat. Oncol. Investig. 2001;96:11–21. doi: 10.1002/1097-0215(20010220)96:1<11::aid-ijc2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Bernard M.E., Kim H., Rajagopalan M.S., Stone B., Salimi U., Rwigema J.-C., Epperly M.W., Shen H., Goff J., Franicola D., et al. Repopulation of the irradiation damaged lung with marrow derived cells. In Vivo. 2012;26:9–18. [PMC free article] [PubMed] [Google Scholar]
- 14.Berhane H., Epperly M., Cao S., Goff J., Franicola D., Wang H., Greenberger J.S. Radioresistance of bone marrow stromal and hematopoietic progenitor cell lines derived from Nrf2−/− homozygous deletion recombinant negative mice. In Vivo. 2013;27:571–582. [PMC free article] [PubMed] [Google Scholar]
- 15.Kanter D., O’Brien M.B., Shi X.-H., Chu T., Mishima T., Beriwal S., Epperly M.W., Wipf P., Greenberger J.S., Sadovsky Y. The impact of ionizing radiation on placental trophoblasts. Placenta. 2014;35:85–91. doi: 10.1016/j.placenta.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belikova N.A., Jiang J., Tyurina Y.Y., Zhao Q., Epperly M.W., Greenberger J., Kagan V.E. Cardiolipin specific peroxidase reactions of cytochrome c in mitochondria during irradiation induced apoptosis. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:176–185. doi: 10.1016/j.ijrobp.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Tyurin V.A., Tyurina Y.Y., Kochanek P.M., Hamilton R., DeKosky S.T., Greenberger J.S., Bayir H., Kagan V.E. Methods in Enzymology. Volume 442. Elsevier, Inc.; Waltham, MA, USA: 2008. Chapter 19, oxidative lipidomics of programmed cell death; pp. 375–393. [DOI] [PubMed] [Google Scholar]
- 18.Kagan V.E., Bayir A., Bayir H., Stoyanovsky D., Borisenko G.G., Tyurina Y.Y., Wipf P., Atkinson J., Greenberger J.S., Chapkin R.S., et al. Mitochondria-targeted disruptors and inhibitors of cytochrome c/cardiolipin peroxidase complexes: A new strategy in anti-apoptotic drug discovery. Mol. Nutr. Food Res. 2009;53:104–114. doi: 10.1002/mnfr.200700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan V.E., Bayir H.A., Belikova N.A., Kapralov O., Tyurina Y.Y., Tyurin V.A., Jiang J., Stoyanovsky D.A., Wipf P., Kochanek P., et al. Cytochrome c/cardiolipin relations in mitochondria: A kiss of death. Free Radic. Biol. Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belikova N.A., Jiang J., Stoyanovsky D.A., Greenberger J.S., Kagan V.E. Mitochondria-targeted (2-hydroxyamino-vinyl)-triphenyl-phosphonium releases NO and protects mouse embryonic cells against irradiation-induced apoptosis. FEBS Lett. 2009;583:1945–1950. doi: 10.1016/j.febslet.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyurin V.A., Tyurina Y.Y., Jung M.-Y., Tungekar M.A., Wasserloos K.J., Bayir H., Greenberger J.S., Kochanek P.M., Shvedova A.A., Pitt B., et al. Mass-spectrometric analysis of hydroperoxy- and hydroxy-derivatives of cardiolipin and phosphatidylserine in cells and tissues induced by proapoptotic and pro-inflammatory stimuli. J. Chromatogr. B. 2009;877:2863–2879. doi: 10.1016/j.jchromb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagan V.E., Wipf P., Stoyanovsky D., Greenberger J.S., Borisenko G., Belikova N.A., Yanamala N., Samhan Arias A.K., Tungekar M.A., Jiang J., et al. Mitochondrial targeting of electron scavenging antioxidants: Regulation of selective oxidation vs. random chain reactions. Adv. Drug Deliv. Rev. 2009;61:1375–1385. doi: 10.1016/j.addr.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoyanovsky D.A., Vlasova I.I., Belikova N.A., Kapralov A., Tyurin V., Greenberger J.S., Kagan V.E. Targeting and activation of NO donors in mitochondria. Peroxidase metabolism of (2-hydroxyamino-vinyl)-triphenyl-phosphonium by cytochrome c releases NO and protects cells from apoptosis. FEBS Lett. 2009;583:2000–2005. doi: 10.1016/j.febslet.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belikova N.A., Glumac A., Rafikov R., Jiang J., Greenberger J.S., Kagan V.E., Bayir H. Radioprotection by short-term oxidative preconditioning: Role of manganese superoxide dismutase. FEBS Lett. 2009;583:3437–3442. doi: 10.1016/j.febslet.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyurin V.A., Tyurina Y.Y., Ritov V.B., Lysytsya A., Amoscato A.A., Kochanek P.M., Hamilton R., DeKosky S.T., Greenberger J.S., Bayir H., et al. Oxidative lipidomics of apoptosis: Quantitative assessment of phospholipid hydroperoxides in cells and tissues. Mol. Biol. 2010;610:353–374. doi: 10.1007/978-1-60327-029-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epperly M.W., Lai S.M., Mason N., Lopresi B., Dixon T., Franicola D., Niu Y., Wilson W.R., Kanai A.J., Greenberger J.S. Effectiveness of combined modality radiotherapy of orthotopic human squamous cell carcinomas in Nu/Nu mice using Cetuximab, Tirapazamine, and MnSOD-plasmid liposome gene therapy. In Vivo. 2010;24:1–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Tyurina Y.Y., Tyurin V.A., Kapralova V.I., Wasserloos K., Mosher M., Epperly M., Greenberger J., Pitt B.R., Kagan V.E. Oxidative lipidomics of γ-irradiation induced lung injury: Mass-spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiat. Res. 2011;175:610–621. doi: 10.1667/RR2297.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyurina Y.Y., Poloyac S.M., Tyurin V.A., Kapralov A.A., Jiang J., Anthonymuthus T.S., Kapralova V.I., Vikulina A.S., Jung M.-J., Epperly M.W., et al. A mitochondrial pathway for biosynthesis of lipid mediators. Nat. Chem. 2014;6:542–552. doi: 10.1038/nchem.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoyanovsky D.A., Jiang J., Murphy M., Epperly M., Li S., Greenberger J., Kagan V., Bayir H. Design and synthesis of a mitochondria-targeted mimic of glutathione peroxidase, MitoEbselen-2, as a radiation mitigator. ACS Med. Chem. Lett. 2014;5:1304–1307. doi: 10.1021/ml5003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberger J.S., Kagan V.E., Pearce L., Boriseniao G., Tyurina Y., Epperly M.W. Modulation of redox signal transduction pathways in the treatment of cancer. Antioxid. Redox Signal. 2001;3:347–359. doi: 10.1089/15230860152409004. [DOI] [PubMed] [Google Scholar]
- 31.Pearce L.L., Epperly M.W., Greenberger J.S., Pitt B., Peterson J. Identification of respiratory complexes I and III as mitochondrial sites of damage following exposure to ionizing radiation and nitric oxide. Nitric Oxide: Biol. Chem. 2001;5:128–136. doi: 10.1006/niox.2001.0338. [DOI] [PubMed] [Google Scholar]
- 32.Epperly M.W., Bernarding M., Gretton J., Jefferson M., Nie S., Greenberger J.S. Overexpression of the transgene for manganese Superoxide dismutase (MnSOD) in 32D cl 3 cells prevents apoptosis induction by TNF-α, IL-3 withdrawal and ionizing irradiation. Exp. Hematol. 2003;31:465–474. doi: 10.1016/S0301-472X(03)00041-9. [DOI] [PubMed] [Google Scholar]
- 33.Epperly M.W., Rugo R., Cao S., Wang H., Franicola D., Goff J.P., Shen H., Zhang X., Wiktor-Brown D., Engelward B., et al. Investigation of the effects of aging on homologous recombination in long-term bone marrow cultures. In Vivo. 2009;23:669–678. [PMC free article] [PubMed] [Google Scholar]
- 34.Epperly M.W., Kagan V.E., Sikora C.A., Gretton J.E., Defilippi S.J., Bar-Sagi D., Greenberger J.S. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated irradiation. Int. J. Cancer. 2001;96:221–233. doi: 10.1002/ijc.1023. [DOI] [PubMed] [Google Scholar]
- 35.Epperly M.W., Sikora C.A., DeFilippi S.J., Gretton J.E., Bar-Sagi D., Carlos T., Guo H.L., Greenberger J.S. Pulmonary irradiation-induced expression of VCAM-1 and ICAM-1 is decreased by MnSOD-PL gene therapy. Biol. Blood Marrow Transplant. 2002;8:175–187. doi: 10.1053/bbmt.2002.v8.pm12014807. [DOI] [PubMed] [Google Scholar]
- 36.Epperly M.W., Sikora C., Defilippi S., Gretton J., Zhan Q., Kufe D.W., Greenberger J.S. MnSOD inhibits irradiation-induced apoptosis by stabilization of the mitochondrial membrane against the effects of SAP kinases p38 and Jnk1 translocation. Radiat. Res. 2002;157:568–577. doi: 10.1667/0033-7587(2002)157[0568:MSDSIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Kanai A.J., Zeidel M.L., Lavelle J.P., Greenberger J.S., Birder L.A., de Groat W.C., Apodaca G.L., Meyers S.A., Ramage R., van Bibber M.M., et al. Manganese superoxide dismutase gene therapy protects against irradiation-induced cystitis. Am. J. Physiol. 2002;44:1152–1160. doi: 10.1152/ajprenal.00228.2002. [DOI] [PubMed] [Google Scholar]
- 38.Epperly M.W., Defilippi S., Sikora C., Gretton J., Greenberger J.S. Radioprotection of lung and esophagus by overexpression of the human manganese superoxide dismutase transgene. Mil. Med. 2002;167:71–73. [PubMed] [Google Scholar]
- 39.Epperly M.W., Guo H.L., Jefferson M., Wong S., Gretton J., Bernarding M., Bar-Sagi D., Greenberger J.S. Cell phenotype specific duration of expression of epitope-tagged HA-MnSOD in cells of the murine lung following intratracheal plasmid liposome gene therapy. Gene Ther. 2003;10:163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 40.Guo H.L., Seixas-Silva J.A., Epperly M.W., Gretton J.E., Shin D.M., Greenberger J.S. Prevention of irradiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat. Res. 2003;159:361–370. doi: 10.1667/0033-7587(2003)159[0361:PORIOC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Guo H., Epperly M.W., Bernarding M., Nie S., Gretton J., Jefferson M., Greenberger J.S. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) intratracheal gene therapy reduction of irradiation-induced inflammatory cytokines does not protect orthotopic lewis lung carcinomas. In Vivo. 2003;17:13–22. [PubMed] [Google Scholar]
- 42.Fink M.P., Macias C.A., Xiao J., Tyurina Y.Y., Delude R.L., Greenberger J.S., Kagan V.E., Wipf P. Hemigramicidin-TEMPO conjugates: Novel mitochondria-targeted anti-oxidants. Biochem. Pharmacol. 2007;74:801–809. doi: 10.1016/j.bcp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Jiang J., Maeda A., Ji J., Baty C.J., Watkins S.C., Greenberger J.S., Kagan V.E. Are mitochondrial reactive oxygen species required for autophagy? Biochem. Biophys. Res. Commun. 2011;412:55–60. doi: 10.1016/j.bbrc.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glowacki J., Mizuno S., Kung J., Goff J., Epperly M., Dixon T., Wang H., Greenberger J.S. Effects of mouse genotype on bone wound healing and irradiation-induced delay. In Vivo. 2014;28:189–196. [PMC free article] [PubMed] [Google Scholar]
- 45.Greenberger J.S., Epperly M.W., Gretton J., Jefferson M., Nie S., Bernarding M., Kagan V., Guo H.L. Radioprotective gene therapy. Curr. Gene Ther. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 46.Guo H.L., Wolfe D., Epperly M.W., Huang S., Liu K., Glorioso J.C., Greenberger J., Blumberg D. Gene transfer of human manganese superoxide dismutase protects small intestinal villi from radiation injury. J. Gastrointest. Surg. 2003;7:229–236. doi: 10.1016/S1091-255X(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 47.Epperly M.W., Guo H.L., Bernarding M., Gretton J., Jefferson M., Greenberger J.S. Delayed intratracheal injection of manganese superoxide dismutase (MnSOD)-plasmid/liposomes provides suboptimal protection against irradiation-induced pulmonary injury compared to treatment before irradiation. Gene Ther. Mol. Biol. 2003;7:61–68. [Google Scholar]
- 48.Epperly M.W., Sikora C.A., Defilippi S., Gretton J.E., Greenberger J.S. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am. J. Respir. Mol. Cell Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 49.Epperly M.W., Gretton J.E., Bernarding M., Nie S., Rasul B., Greenberger J.S. Mitochondrial localization of copper/zinc superoxide dismutase (Cu/ZnSOD) confers radioprotective functions in vitro and in vivo. Radiat. Res. 2003;160:568–578. doi: 10.1667/RR3081. [DOI] [PubMed] [Google Scholar]
- 50.Kanai A., Epperly M.W., Pearce L., Birder L., Zeidel M., Meyers S., Greenberger J., de Groat W., Apodaca G., Peterson J. Differing roles of mitochondrial nitric oxide synthase in cardiomyocytes and urothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H13–H21. doi: 10.1152/ajpheart.00737.2003. [DOI] [PubMed] [Google Scholar]
- 51.Epperly M.W., Osipov A.N., Martin I., Kawai K., Borisenko G.G., Jefferson M., Bernarding M., Greenberger J.S., Kagan V.E. Ascorbate as a “redox-sensor” and protector against irradiation-induced oxidative stress in 32D cl 3 hematopoietic cells and subclones overexpressing human manganese Superoxide Dismutase. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:851–861. doi: 10.1016/j.ijrobp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 52.Epperly M.W., Hongliang G., Shields D., Zhang X., Flanders K., Lambert P., Greenberger J.S. Correlation of ionizing irradiation-induced late pulmonary fibrosis with long-term bone marrow culture fibroblast progenitor cell biology in mice homozygous deletion recombinant negative for endothelial cell adhesion molecules. In Vivo. 2004;18:1–14. [PubMed] [Google Scholar]
- 53.Kalash R., Berhane H., Goff J., Houghton F., Epperly M.W., Dixon T., Zhang X., Sprachman M.M., Wipf P., Franicola D., et al. Thoracic irradiation effects on pulmonary endothelial compared to alveolar type II cells in fibrosis prone C57BL/6NTac mice. In Vivo. 2013;27:291–298. [PMC free article] [PubMed] [Google Scholar]
- 54.Kalash R., Epperly M.W., Goff J., Dixon T., Sprachman M.M., Zhang X., Shields D., Cao S., Wipf P., Franicola D., et al. Amelioration of irradiation pulmonary fibrosis by a water-soluble bi-functional sulfoxide radiation mitigator (MMS350) Radiat. Res. 2013;180:474–490. doi: 10.1667/RR3233.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalash R., Berhane H., Au J., Rhieu B.H., Epperly M.W., Goff J., Dixon T., Wang H., Zhang X., Franicola D., et al. Differences in irradiated lung gene transcription between fibrosis-prone C57BL/6NHsd and fibrosis-resistant C3H/HeNHsd mice. In Vivo. 2014;28:147–172. [PMC free article] [PubMed] [Google Scholar]
- 56.Epperly M.W., Franicola D., Shields D., Rwigema J.-C., Stone B., Zhang X., McBride W., Georges G., Wipf P., Greenberger J.S. Screening for in vitro radiation protection and mitigation capacity of antimicrobial agents including those used in supportive care regimens for bone marrow transplant recipients. In Vivo. 2010;24:9–20. [PMC free article] [PubMed] [Google Scholar]
- 57.Greenberger J.S., Epperly M.W. Antioxidant therapeutic approaches toward amelioration of the pulmonary pathophysiological damaging effects of ionizing irradiation. Curr. Respir. Med. Rev. 2007;3:29–37. doi: 10.2174/157339807779941767. [DOI] [Google Scholar]
- 58.Greenberger J.S. Gene therapy approaches for stem cell protection. Gene Ther. 2008;15:100–108. doi: 10.1038/sj.gt.3303004. [DOI] [PubMed] [Google Scholar]
- 59.Greenberger J.S. Radioprotection. In Vivo. 2009;23:323–336. [PMC free article] [PubMed] [Google Scholar]
- 60.Koide K., Osman S., Garner A.L., Song F., Dixon T., Greenberger J.S., Epperly M.W. The use of 3,5,4′-Tri-O-acetylresveratrol as a potential prodrug for Resveratrol protects mice from γ-irradiation-induced death. ACS Med. Chem. Lett. 2011;2:270–274. doi: 10.1021/ml100159p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pearce L.L., Martinez-Bosch S., Khlangwiset P., Zhang X., Epperly M.W., Fink M.P., Greenberger J.S., Peterson J. l-argrinine is a protector but not a mitigator of the effects of ionizing radiation on hematopoietic progenitor cells. Radiat. Res. 2012;177:792–803. doi: 10.1667/RR1281.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miao W., Feng R.X., Park M.-R., Gu H., Hu L., Kang J., Wook M.S., Liang P.H., Li Y., Cheng H., et al. Hematopoietic stem cell regeneration enhanced by ectopic expression of ROS-detoxifying enzymes in transplant mice. Mol. Ther. 2013;21:423–432. doi: 10.1038/mt.2012.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenberger J.S., Epperly M.W. Radioprotective antioxidant gene therapy: Potential mechanisms of action. Gene Ther. Mol. Biol. 2004;8:31–44. [Google Scholar]
- 64.Greenberger J.S., Epperly M.W. Pleiotrophic stem cell and tissue effects of ionizing irradiation protection by MnSOD-plasmid liposome gene therapy. In: Columbus F., editor. Progress in Gene Therapy. Nova Science Publications; Hauppauge, NY, USA: 2005. pp. 110–118. [Google Scholar]
- 65.Epperly M.W., Goff J.P., Sikora C.A., Shields D.S., Greenberger J.S. Bone marrow origin of cells with capacity for homing and differentiation to esophageal squamous epithelium. Radiat. Res. 2004;162:233–240. doi: 10.1667/RR3224. [DOI] [PubMed] [Google Scholar]
- 66.Epperly M.W., Carpenter M., Agarwal A., Mitra P., Nie S., Greenberger J.S. Intra-oral manganese superoxide dismutase plasmid liposome radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo. 2004;18:401–410. [PubMed] [Google Scholar]
- 67.Epperly M.W., Chaillet J.R., Kalash R., Shaffer B., Goff J., Shields D., Dixon T., Wang H., Berhane H., Kim J.-H., et al. Conditional radioresistance of Tet-inducible manganese superoxide dismutase bone marrow stromal cells. Radiat. Res. 2013;180:189–204. doi: 10.1667/RR3177.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rwigema J.-C.M., Beck B., Wang W., Doemling A., Epperly M.W., Shields D., Franicola D., Dixon T., Frantz M.-C., Wipf P., et al. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:860–868. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang J., Stoyanovsky D., Belikova N.A., Tyurina Y.Y., Zhao Q., Tungekar M.A., Kapralova V., Huang Z., Mintz A., Greenberger J.S., et al. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat. Res. 2009;172:706–714. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajagopalan M.S., Gupta K., Epperly M.W., Franicola D., Zhang X., Wang H., Zhao H., Tyurin V.A., Kagan V.E., Wipf P., et al. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo. 2009;23:717–726. [PMC free article] [PubMed] [Google Scholar]
- 71.Samhan-Arias A.K., Ji J., Demidova O.M., Sparvero L.J., Feng W., Tyurin V., Tyurina Y.Y., Epperly M.W., Shvedova A.A., Greenberger J.S., et al. Oxidized phospholipids as biomarkers of tissue and cell damage with a focus on cardiolipin. Biochim. Biophys. Acta. 2012;1818:2413–2423. doi: 10.1016/j.bbamem.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenberger J.S., Berhane H., Shinde A., Rhieu B.H., Bernard M., Wipf P., Skoda E.M., Epperly M.W. Can radiosensitivity associated with defects in DNA repair be overcome by mitochondrial-targeted antioxidant radioprotectors? Front. Radiat. Oncol. 2014;4:1. doi: 10.3389/fonc.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shinde A., Epperly M.W., Franicola D., Cao S., Goff J., Shields D., Franicola D., Wipf P., Wang H., Greenberger J.S. Increased longevity of GS-nitroxide (JP4-039) treated mouse long-term bone marrow cultures and radioresistance of derived bone marrow stromal cell lines. In Vivo. 2014;28:699–708. [PMC free article] [PubMed] [Google Scholar]
- 74.Shinde A., Epperly M.W., Cao S., Franicola D., Shields D., Wang H., Greenberger J.S. Effects of the radiation mitigator bifunctional sulfoxide MMS350 on hematopoiesis and long-term bone marrow cultures. In Vivo. 2014;28:457–466. [PMC free article] [PubMed] [Google Scholar]
- 75.Berhane H., Epperly M.W., Goff J., Kalash R., Cao S., Franicola D., Zhang X., Shields D., Houghton F., Wang H., et al. Radiobiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi Anemia (Fancd2−/−) mice. Radiat. Res. 2014;181:76–89. doi: 10.1667/RR13405.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berhane H., Shinde A., Kalash R., Xu K., Epperly M.W., Goff J., Franicola D., Zhang X., Dixon T., Shields D., et al. Amelioration of irradiation induced oral cavity mucositis and distant bone marrow suppression in Fancd2−/− (FVB/N) mice by intraoral JP4-039/F15. Radiat. Res. 2014;182:35–49. doi: 10.1667/RR13633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kagan V.E., Tyurina Y.Y., Bayir H., Chu C.T., Kapralov A.A., Vlasova B.N.A., II, Tyurin V.A., Amoscato A., Epperly M., Greenberger J., et al. The “pro-apoptotic genies” get out of mitochondria: Oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem. Biol. Interact. 2006;163:15–28. doi: 10.1016/j.cbi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 78.Goff J., Shields D., Wang H., Skoda E., Sprachman M., Wipf P., Lazo J., Atkinson J., Kagan V., Epperly M., et al. Evaluation of ionizing irradiation protectors and mitigators using clonagenic survival of human umbilical cord blood hematopoietic progenitor cells. Exp. Hematol. 2013;41:957–966. doi: 10.1016/j.exphem.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Epperly M.W., Smith T., Wang H., Schlesselman J., Franicola D., Greenberger J.S. Modulation of total body irradiation induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy. Radiat. Res. 2008;170:437–444. doi: 10.1667/RR1286.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Epperly M.W., Smith T., Zhang X., Greenberger B., Komanduri P., Wang H., Greenberger J.S. Modulation of in utero total body irradiation induced newborn mouse growth retardation by maternal manganese superoxide dismutase-plamid liposome (MnSOD-PL) gene therapy. Gene Ther. 2010;18:579–583. doi: 10.1038/gt.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Epperly M.W., Wang H., Jones J., Dixon T., Montesinos C., Greenberger J.S. Antioxidant-chemoprevention diet ameliorates late effects of total body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat. Res. 2011;175:759–765. doi: 10.1667/RR2398.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajagopalan M.S., Stone B., Rwigema J.-C., Salimi U., Epperly M.W., Goff J., Franicola D., Dixon T., Cao S., Zhang X., et al. Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total body and thoracic irradiation sensitivity of homologous deletion recombinant negative nitric oxide synthase-1 (NOS1−/−) mice. Radiat. Res. 2010;174:297–312. doi: 10.1667/RR2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fink M.P., Macias C.A., Xiao J., Tyurina Y.Y., Delude R.L., Greenberger J.S., Kagan V.E., Wipf P. Hemigramicidin-TEMPO conjugates: Novel mitochondria-targeted antioxidants. Crit. Care Med. 2007;35:5461–5470. doi: 10.1097/01.CCM.0000279192.96303.E7. [DOI] [PubMed] [Google Scholar]
- 84.Epperly M.W., Rwigema J.-C.M., Li S., Gao X., Wipf P., Goff J., Wang H., Franicola D., Shen H., Kagan V., et al. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo. 2010;24:811–821. [PMC free article] [PubMed] [Google Scholar]
- 85.Jing J., Kline A.E., Amoscato A., Samhan-Arias A.K., Sparvero L.J., Tyurin V.A., Tyurina Y.Y., Fink B., Manoe M.D., Pucco A.M., et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 2012;15:1407–1413. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goff J.P., Epperly M.W., Shields D., Wipf P., Dixon T., Greenberger J.S. Radiobiologic effects of GS-nitroxide (JP4-039) in the hematopoietic syndrome. In Vivo. 2011;25:315–324. [PMC free article] [PubMed] [Google Scholar]
- 87.Greenberger J.S., Kagan V., Bayir H., Lazo J., Wipf P., Li S., Gao X., Clump D., Epperly M.W. Mitochondrial targeted small molecule radiation protectors and radiation mitigators. Front. Radiat. Oncol. 2012;1:1–12. doi: 10.3389/fonc.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Epperly M.W., Shen H., Jefferson M., Greenberger J.S. In vitro differentiation capacity of esophageal progenitor cells with capacity for homing and repopulation of the ionizing irradition damaged esophagus. In Vivo. 2004;18:675–685. [PubMed] [Google Scholar]
- 89.Stone H.B., Coleman C.N., Moulder J.E., Ang K.K., Anscher M.S., Barcellos-Hoff M.H., Dynan W.S., Fike J.R., Grdina D.J., Greenberger J.S., et al. Models for evaluating agents intended for the prophylaxis, mitigation, and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat. Res. 2004;162:711–718. doi: 10.1667/RR3276. [DOI] [PubMed] [Google Scholar]
- 90.Carpenter M., Epperly M.W., Agarwal A., Nie S., Hricisak L., Niu Y., Greenberger J.S. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) protects the murine lung from irradiation damage. Gene Ther. 2005;12:685–690. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 91.Epperly M.W., Zhang X., Nie S., Cao S., Kagan V., Tyurin V., Greenberger J.S. MnSOD-plasmid liposome gene therapy effects on ionizing irradiation induced lipid peroxidation of the esophagus. In Vivo. 2005;19:997–1004. [PubMed] [Google Scholar]
- 92.Epperly M.W., Shen H., Zhang X., Nie S., Cao S., Greenberger J.S. Protection of esophageal stem cells from ionizing irradiation by MnSOD-plasmid liposome gene therapy. In Vivo. 2005;19:965–974. [PubMed] [Google Scholar]
- 93.Lechpammer S., Epperly M.W., Zhou S., Nie S., Glowacki J., Greenberger J.S. Antioxidant pool regulated adipocyte differentiation Sod2−/− bone marrow stromal cells. Exp. Hematol. 2005;33:1201–1208. doi: 10.1016/j.exphem.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Bayir H., Fadeel B., Palladino M.J., Witasp E., Kurnikov I.V., Tyurina Y.Y., Tyurin V.A., Amoscato A.A., Jiang J., Kochanek P.M., et al. Apoptotic interactions of cytochrome c: Redox flirting with anionic phospholipids within and outside of mitochondria. Biochim. Biophys. Acta. 2006;1757:648–659. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 95.Epperly M.W., Franicola D., Zhang X., Nie S., Greenberger J.S. Effect of EGFR receptor antagonists gefitinib (Iressa) and C225 (Cetuximab) on MnSOD-plasmid liposome transgene radiosensitization of a murine squamous cell carcinoma cell line. In Vivo. 2006;20:791–796. [PubMed] [Google Scholar]
- 96.Epperly M.W., Wegner R., Kanai A.J., Kagan V., Greenberger E.E., Nie S., Greenberger J.S. Irradiated murine oral cavity orthotopic tumor antioxidant pool destabilization by MnSOD-plasmid liposome gene therapy mediates tumor radiosensitization. Radiat. Res. 2007;267:289–297. doi: 10.1667/RR0761.1. [DOI] [PubMed] [Google Scholar]
- 97.Jiang J., Belikova N.A., Xiao J., Zhao Q., Greenberger J.S., Wipf P., Kagan V.E. A mitochondria-targeted nitroxide/hemi-gramicidin S conjugate protects mouse embryonic cells against γ-irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tyurina Y.Y., Tyurin V.A., Epperly M.W., Greenberger J.S., Kagan V.E. Oxidative lipidomics of γ-irradiation induced intestinal injury. Free Radic. Biol. Med. 2008;44:299–314. doi: 10.1016/j.freeradbiomed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 99.Epperly M.W., Melendez A., Zhang X., Franicola D., Smith T., Greenberger B.A., Komanduri P., Greenberger J.S. Mitochondrial targeting of a catalase transgene product by plasmid liposomes increases radioresistance in vitro and in vivo. Radiat. Res. 2009;171:588–595. doi: 10.1667/RR1424.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atkinson J., Kapralov A.A., Yanamala N., Pearce L., Peterson J., Tyurina Y.Y., Epperly M.W., Huang Z., Jiang J., Maeda A., et al. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation induced death. Nat. Commun. 2011;2:497. doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tyurina Y.Y., Tungekar M.A., Jung M.-Y., Tyurin V.A., Greenberger J.S., Stoyanovsky D.A., Kagan V.E. Mitochondria targeting of non-peroxidizable triphenylphosphonium conjugated oleic acid protects mouse embryonic cells against apoptosis: Role of cardiolipin remodeling. FEBS Lett. 2012;586:235–241. doi: 10.1016/j.febslet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frantz M.-C., Skoda E.M., Davoren J.E., Wang Z., Epperly M.W., Stripay J.L., Tyurin V.A., Fink B., Kapralov A., Greenberger J.S., et al. Synthesis and biochemical analysis of mitochondria-targeted nitroxide conjugates based on gramicidin S. JACS. 2015 in press. [Google Scholar]
- 103.Frantz M.-C., Skoda E.M., Sacher J.R., Epperly M.W., Goff J.P., Greenberger J.S., Wipf P. Synthesis of analogs of the radiation mitigator JP4-039 and visualization of BODIPY derivatives in mitochondria. Org. Biomol. Chem. 2013;11:4147–4153. doi: 10.1039/c3ob40489g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Epperly M.W., Cao S., Zhang X., Franicola D., Kanai A.J., Greenberger E.E., Greenberger J.S. Increased longevity of hematopoiesis in continuous bone marrow cultures derived from mtNOS−/− homozygous recombinant negative mice correlates with increased radioresistance of hematopoietic and bone marrow stromal cells. Exp. Hematol. 2007;35:137–145. doi: 10.1016/j.exphem.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Jiang J., Kurnikov I., Belikova N.A., Xiao J., Zhao Q., Vlasova I.L., Amoscato A.A., Braslau R., Studer A., Fink M.P., et al. Structural requirements for optimized delivery, inhibition of oxidative stress and anti-apoptotic activity of targeted nitroxides. J. Pharmacol. Exp. Ther. 2007;320:1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- 106.Epperly M.W., Epperly L.D., Zhang X., Franicola D., Greenberger J.S. Overexpression of MnSOD transgene product protects cryopreserved bone marrow hematopoietic progenitor cells from ionizing irradiation. Radiat. Res. 2007;168:560–566. doi: 10.1667/RR1071R.1. [DOI] [PubMed] [Google Scholar]
- 107.Niu Y., Epperly M.W., Shen H., Smith T., Lewis D., Gollin S., Greenberger J.S. Intraesophageal MnSOD-plasmid liposome administration enhances engraftment and self-renewal capacity of bone marrow derived progenitors of esophageal squamous epithelium. Gene Ther. 2008;15:347–356. doi: 10.1038/sj.gt.3303089. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X., Epperly M.W., Kay M.A., Chen Z.-Y., Smith T., Franicola D., Greenberger B.A., Komanduri P., Greenberger J.S. Radioprotection in vitro and in vivo by minicircle plasmid carrying the human manganese superoxide dismutase transgene. Hum. Gene Ther. 2008;19:820–826. doi: 10.1089/hum.2007.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niu Y., Wang H., Wiktor-Brown D., Rugo R., Shen H., Huq M.S., Engelward B., Epperly M., Greenberger J.S. Irradiated esophageal cells are protected from radiation-induced recombination by MnSOD gene therapy. Radiat. Res. 2010;173:453–461. doi: 10.1667/RR1763.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greenberger J.S., Epperly M. Bone marrow-derived stem cells and radiation response. Semin. Radiat. Oncol. 2009;19:133–139. doi: 10.1016/j.semradonc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Epperly M.W., Goff J.P., Franicola D., Wang H., Wipf P., Li S., Greenberger J.S. Esophageal radioprotection in thoracic irradiated mice with transgenic lung tumors by swallowed JP4-039/F15. In Vivo. 2014;28:435–440. [PMC free article] [PubMed] [Google Scholar]
- 112.Shinde A., Berhane H., Rhieu B.H., Kalash R., Xu K., Goff J., Epperly M.W., Franicola D., Zhang X., Dixon T., et al. Intraoral mitochondrial-targeted GS-Nitroxide, JP4-039, radioprotects normal tissue in tumor-bearing radiosensitive Fancd2−/− (C57BL/6) mice. Radiat. Res. 2015 doi: 10.1667/RR14035.1. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gokhale A.S., Epperly M., Glowacki J., Wang H., Wipf P., Pierce J.G., Dixon T., Patrene K., Greenberger J.S. Small molecule GS-nitroxide and MnSOD gene therapy ameliorate ionizing irradiation-induced delay in bone wound healing in a novel murine model. In Vivo. 2010;24:377–386. [PMC free article] [PubMed] [Google Scholar]
- 114.Bernard M.E., Kim H., Rwigema J.-C., Epperly M.W., Kelley E.E., Murdoch G.H., Dixon T., Wang H., Greenberger J.S. Role of the esophageal vagus neural pathway in ionizing irradiation-induced seizures in Nitric Oxide Synthase-1 homologous recombination negative NOS1−/− mice. In Vivo. 2011;25:861–870. [PMC free article] [PubMed] [Google Scholar]
- 115.Rhieu B.H., Epperly M.W., Cao S., Shields D., Franicola D., Wang H., Greenberger J.S. Improved longevity of long-term bone marrow cultures from Toll-Like Receptor-4 (TLR4) deletion recombinant negative mice. In Vivo. 2014;28:444–448. [PMC free article] [PubMed] [Google Scholar]
- 116.Epperly M.W., Goff J., Zhang X., Shields D., Wang H., Shen H., Franicola D., Bahnson A., Greenberger E.E., Greenberger J.S. Increased radioresistance, G2M checkpoint inhibition and impaired migratory capacity of bone marrow stromal cell lines derived from SMAD3−/− mice. Radiat. Res. 2006;165:671–677. doi: 10.1667/RR3572.1. [DOI] [PubMed] [Google Scholar]
- 117.Epperly M.W., Franicola D., Zhang X., Nie S., Wang H., Bahnson A., Shields D., Goff J., Greenberger J.S. Decreased irradiation pulmonary fibrosis in SMAD3−/− marrow chimeric mice correlates to reduced bone marrow stromal cell migration in vitro. In Vivo. 2006;20:573–582. [PubMed] [Google Scholar]
- 118.Ding B.-S., Cao Z., Lis R., Nolan D.J., Guo P., Simons M., Penfold M.E., Shido K., Rabbany S.Y., Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watson C.J., Collier P., Tea I., Neary R., Watson J.A., Robinson C., Phelan D., Ledwidge M.T., McDonald K.M., McCann A., et al. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum. Mol. Genet. 2014;23:2176–2188. doi: 10.1093/hmg/ddt614. [DOI] [PubMed] [Google Scholar]
- 120.Rhieu B.H., Epperly M.W., Cao S., Franicola D., Shields D., Goff J., Wang H., Greenberger J.S. Increased hematopoiesis in long-term bone marrow cultures derived from Fon Willebrand Factor homologous deletion recombinant mice (vWF−/−) In Vivo. 2014;28:449–456. [PMC free article] [PubMed] [Google Scholar]
- 121.Tarhini A.A., Belani C., Luketich J.D., Ramalingam S.S., Argiris A., Gooding W., Petro D., Kane K., Liggitt D., Championsmith T., et al. A phase I study of concurrent chemotherapy (Paclitaxel and Carboplatin) and thoracic radiotherapy with swallowed manganese superoxide dismutase (MnSOD) plasmid liposome (PL) protection in patients with locally advanced stage III non-small cell lung cancer. Hum. Gene Ther. 2011;22:336–343. doi: 10.1089/hum.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bernard M.E., Kim H., Berhane H., Epperly M.W., Franicola D., Zhang X., Houghton F., Shields D., Wang H., Bakkenist C.J., et al. GS-nitroxide (JP4-039) mediated radioprotection of human Fanconi Anemia cell lines. Radiat. Res. 2011;176:603–612. doi: 10.1667/RR2624.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim H., Bernard M.E., Epperly M.W., Shen H., Amoscato A., Dixon T.M., Doemling A.S., Li S., Gao X., Wipf P., et al. Amelioration of radiation esophagitis by orally administered p53/mdm2/mdm4 inhibitor (BEB55) or GS-Nitroxide. In Vivo. 2011;25:841–849. [PMC free article] [PubMed] [Google Scholar]
- 124.Stoyanovsky D.A., Huang Z., Jiang J., Belikova N.A., Tyurin V., Epperly M.W., Greenberger J.S., Bayir H., Kagan V.E. A manganese-porphyrin complex decomposes hydrogen peroxide, compartmentalizes into mitochondria, inhibits apoptosis, and acts as a radiation mitigator in vivo. JACS Med. Chem. Lett. 2011;362:21–34. doi: 10.1021/ml200142x. [DOI] [PMC free article] [PubMed] [Google Scholar]