Abstract
In this communication the mismatch between simultaneous 18F-FDG-PET and a 13C-lactate imaging (hyperPET) in a biopsy verified squamous cell carcinoma in the right tonsil of a canine cancer patient is shown. The results demonstrate that 18F-FDG-PET may not always reflect the Warburg effect in all tumors.
Keywords: cancer, dynamic nuclear polarization, hyperpolarized, 13C-pyruvate, MR, 18F-FDG-PET, PET/MR, molecular imaging, hyperPET
Figure 1.
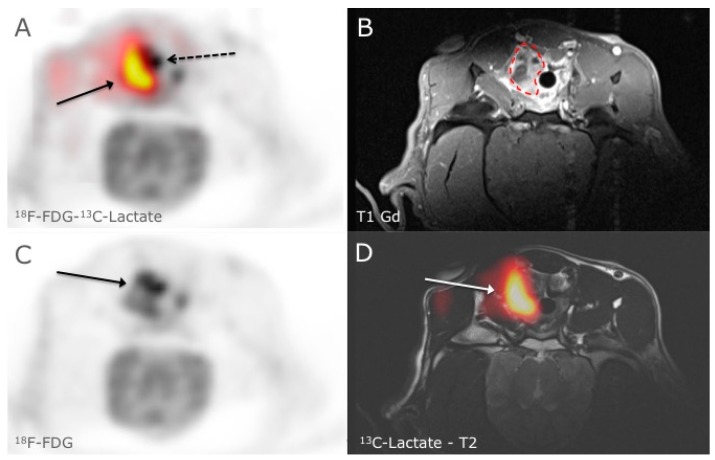
HyperPET is a new in vivo imaging modality that consists of combining a PET scan with magnetic resonance spectroscopic imaging (MRSI) of hyperpolarized 13C-pyruvate made possible by integrated hybrid PET/MRI systems [1]. The metabolism of cancer cells is characterized by a shift to glycolysis with production of lactate even in the presence of sufficient oxygen, this phenomenon is also known as the Warburg effect [2,3,4]. With the introduction of hyperpolarized 13C-pyruvate/13C-lactate MRSI it is probably now possible to directly study the metabolism of lactate and Warburg effect in real time. This is opposed to imaging with 18F-FDG PET scan alone, which demonstrates the Warburg effect only indirectly through increased glucose utilization and uptake. In Figure 1, 18F-FDG-PET and 13C-lactate MRSI in a spontaneous canine tumor is shown. A clear mismatch between 18F-FDG uptake and 13C-lactate production is seen. In an axial slice of the neck in a canine cancer patient with a biopsy verified squamous cell carcinoma in the right tonsil, we noticed in panel A clear discrepancy between the 18F-FDG-PET (18F-FDG activity is shown in grey scale and the dashed arrow points at the margin of tumor) and the 13C-lactate production (red to yellow color corresponds to the 13C-Lactate production and the arrow points to the margin of tumor) in a large heterogeneous tumor. 18F-FDG uptake in the tumor was variable in the tumor (panel C) and corresponded to the anatomical MR images in that high 18F-FDG levels paralleled the uptake of Gadolinium in the T1 sequence (panel B, dashed line outlines the contour of the tumor). However 13C-lactate did not correspond to the 18F-FDG uptake, especially in the more profound region of the tumor where we demonstrated a large production of 13C-lactate indicating higher degree of glycolysis (panel D). The Ethics and Administrative Committee, Department of Veterinary Clinical and Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen approved the study. Whereas 18F-FDG-PET has generally been accepted as an indicator of the Warburg effect the hyperPET imaging of a canine tumor demonstrates that this may not always be the case. Accordingly, the new technique of hyperPET that we recently introduced can expose such diversity in metabolism. We suggest that hyperPET may become a valuable tool for better phenotyping of tumors to be used for prognostication, treatment planning and response monitoring.
Acknowledgments
The financial support from the John and Birthe Meyer Foundation and the Capital Region of Denmark is gratefully acknowledged. Karin Stahr, Marianne Federspiel, Jakup Poulsen and Betina Senius Pedersen are acknowledged for invaluable technical assistance.
Author Contributions
Henrik Gutte wrote the initial draft of the manuscript, with all authors making substantial contributions to evaluation of data and critically reviewing its content. All authors have approved the final version of the manuscript prior to its submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gutte H., Hansen A.E., Henriksen S.T., Johannesen H.H., Ardenkjaer-Larsen J., Vignaud A., Hansen A.E., Børresen B., Klausen T.L., Wittekind A.M.N., et al. Simultaneous hyperpolarized 13C-pyruvate MRI and 18F-FDG-PET in cancer (hyperPET): Feasibility of a new imaging concept using a clinical PET/MRI scanner. Am. J. Nuclear Med. Mol. Imaging. 2015;5:38–45. [PMC free article] [PubMed] [Google Scholar]
- 2.Johnbeck C.B., Jensen M.M., Nielsen C.H., Hag A.M.F., Knigge U., Kjær A. 18F-FDG and 18F-FLT-PET Imaging for Monitoring Everolimus Effect on Tumor-Growth in Neuroendocrine Tumors: Studies in Human Tumor Xenografts in Mice. PLoS ONE. 2014;9:e91387. doi: 10.1371/journal.pone.0091387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen M.M., Erichsen K.D., Johnbeck C.B., Björkling F., Madsen J., Jensen P.B., Sehested M., Højgaard L., Kjær A. [18F]FDG and [18F]FLT positron emission tomography imaging following treatment with belinostat in human ovary cancer xenografts in mice. BMC Cancer. 2013;13:168. doi: 10.1186/1471-2407-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]