Abstract
Purpose
To measure expression of COX-2 and CD34 in pre-treatment tumor biopsies from patients on the RTOG C0128 phase II study, and to correlate expression of these biomarkers, using quantitative immunohistochemistry (IHC), with clinical outcome parameters.
Methods and Materials
Pre-treatment biopsies were placed into tissue microarrays. COX-2 and CD34 expression were measured using automated quantitative IHC (AQUA®). Cox regression models and Fisher's exact test were used to explore associations between expression of the biomarkers and clinical endpoints.
Results
84 patients were accrued between 2001 and 2004; 78 were eligible and analyzable. Pathology specimen submission was optional; COX-2 expression was determined for 37 (47%) of patients, and CD34 scoring was determined for 34 (44%) of patients. Median follow-up was 44.5 months. In tumors where COX-2 data was available, 6 of 37 (16%) patients had local-regional failure; 4 of these patients had tumors with COX-2 scores below the AQUA® score median [HR=0.39; 95%CI= (0.07, 2.16); p=0.28]. Of the 8 patients with DFS failures, 5 had tumors with COX-2 levels below the median [HR=0.49; 95%CI= (0.12, 2.04); p=0.32]. The 4 patients who died all had COX-2 levels below the median value. COX-2 levels below the median were associated with worse 2-year survival (Fisher's p=0.046). There was no statistically significant association between CD34 status and clinical outcome.
Conclusions
Low COX-2 expression measured by AQUA® was associated with worse overall survival in this subset of patients available for analysis from RTOG C0128. Application of AQUA® technology, in a larger study, will be required to definitively evaluate the association COX-2 with clinical outcome in cervical cancer.
Keywords: cervical cancer, RTOG C0128, COX-2, CD34, predictive markers
Introduction
Cisplatin-based chemoradiotherapy (CRT) has been shown to improve overall survival in women with advanced cervical cancer (1). However, one-third of these patients will fail conventional therapy within two years. The ability to screen for adverse molecular features of tumors, which may predict patient outcome, could allow for tailoring of therapy and better clinical results.
Cyclooxygenase-2 (COX-2) is an inducible cytokine which has been shown to contribute to neovascularization, leading to solid tumor growth and increased metastatic potential (2,3). Increased tumoral expression of COX-2 in patients with cervical cancer has been shown to be associated with poor outcome (4-6). In addition, high COX-2 expression is a marker of resistance to platinum-based chemotherapy in cervical cancer (7). Inhibition of COX-2 decreases tumor growth by promoting apoptosis, inhibiting angiogenesis, and sensitizing cells to radiation (8,9). The measurement of tumor microvessel density reflects the extent of tumor angiogenesis, and has been shown to positively correlate with COX-2 expression (10). CD34 is a transmembrane glycoprotein constitutively expressed on vascular endothelial cells, and is a commonly used method to calculate vessel density (11-12).
COX-2 inhibition, in combination with CRT has been has been investigated in clinical cancer studies, including cervical cancer trials (13,14). The RTOG C0128 trial was a phase I/II study investigating the efficacy, toxicity, and patterns of failure in patients with locally advanced cervical cancer treated with celecoxib, cisplatin, 5-FU, and pelvic radiotherapy (RT). At 2 years, the estimated disease-free survival (DFS) and overall survival (OS) rate for patients on the study was 69% and 83%, respectively. Local-regional recurrence was the most significant site of failure (13).
All existing published trials evaluating COX-2 inhibition and CRT have not pre-selected patients for treatment using pre-treatment tumor COX-2 evaluation. Few of these studies have demonstrated significant clinical benefit with the addition of a COX-2 inhibitor to CRT (15). Although there are no published data evaluating COX-2 expression in patients treated with CRT and a COX-2 inhibitor, such data exists in patients treated with chemotherapy and celecoxib. Although results are mixed regarding the predictive value of COX-2 expression and outcome, there is evidence of a significant association between high tumor expression of COX-2 and probability of response to celecoxib and chemotherapy (16; 17-19). Pre-treatment COX-2 tumor measurement could serve as an important predictor of clinical response to anti-COX-2 agents, and treating the appropriate subset of patients with an anti-COX-2 agent may improve clinical outcome.
Unfortunately, there is no standardized laboratory methodology to measure COX-2 expression in tumors, and techniques used in studies evaluating COX-2 and CD34 are heterogenous. Automated quantitative immunohistochemisty (IHC) enables less subjective protein expression scoring than traditional pathologist-read techniques, and is convenient for the measurement of multiple samples on a tissue microarray. AQUA® is one such technique, and unlike other quantitative techniques, can measure the protein of interest in a specific compartment within the cell. However, there are currently no published studies evaluating COX-2 and CD34 using this quantitative technology.
The objectives of this study were to measure expression of COX-2 and CD34, using AQUA®, in pre-treatment tumor biopsies in patients with locally advanced cervical cancer enrolled on the RTOG C0128 phase I/II trial, and to explore potential associations between pre-treatment COX-2 and CD34 expression on toxicity, local-regional failure, DFS and OS. This is the first study to evaluate COX-2 expression in patients with cervical cancer treated with radical CRT and a COX-2 inhibitor.
Methods and Materials
Patient and tumor characteristics
Patients were eligible for inclusion on this study if they were ≥ 18 years of age, had histological diagnosis of carcinoma of the uterine cervix (squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma), and had FIGO stage IIB-IVA or FIGO Stage IB-IIA disease with biopsy-proven pelvic lymph node metastases, or tumor size ≥ 5 cm. Additional inclusion factors are provided in the reference (13). Patients had the option to participate in a tissue collection portion of the protocol. All patients gave written informed consent for participation on this study.
Treatment
Radiotherapy
RT was delivered to the whole pelvis to a dose of 45 Gy in 25 fractions over 5 weeks. Low dose rate (LDR) or high dose rate (HDR) brachytherapy (BT) was permitted: with LDR, two insertions were performed, at 20 Gy per fraction; with HDR, five insertions were performed, at 6 Gy per fraction.
Chemotherapy
Cisplatin was administered at 75 mg/m2 to a maximum of 150 mg, on days 1, 22, and 43. 5-Fluorouracil was administered at 1g/m2/d for 4 days, bolus or continuous infusion, on days 2-5, 23-26, and 44-47. Celecoxib was given at 400 mg po twice daily, starting day 1 of RT, and continuing for twelve months.
Biosampling and analysis
Pre-treatment biopsies were placed into tissue microarrays (TMAs). COX-2 protein expression and CD34 vessel density were evaluated using fluorescent IHC and automated quantitative image analysis (AQUA®). The following antibodies were used: COX-2 (mouse monoclonal, Cayman Chemical, 1:300 dilution), CD34 Clone QBEnd/10 (mouse monoclonal, Vector Laboratories, 1:500 dilution). All slides were treated using Target Retrieval Solution from Dako at 121 °C for 8 minutes using a Biocare Medical tissue processor. Slides were developed using an anti-mouse EnVision + system HRP (DAB) development kit from Dakocytomation. An incubation time of 60 minutes was used for both primary and secondary antibodies. Four micron thick TMA sections were rehydrated and stained with the above antibodies using the EnVision+ HRP kit and the target antibodies were fluorescently labeled for 10 minutes with a TSA-Plus CY5 Tyramide Signal Amplification kit from PerkinElmer. TMA sections were also co-stained with a rabbit anti-pan-cytokeratin antibody (Wide-spectrum-species Anti-Pan-Cytokeratin, Dakocytomation, 1:200 dilution) visualized using a goat anti-rabbit Alexa 555 SFX kit from Invitrogen. After staining, slides were coverslipped using a DAPI-containing mounting media to visualize the nuclei (Vector Laboratories, Inc.). Acquisition of slide images was performed using a HistoRx PM-2000 imaging system and image analysis performed using the AQUA® quantitation software (AQUAnalysis Ver 1.2) as previously described (20,21). Cytoplasmic intensity of COX-2 fluorescent signal was collected within the cytokeratin mask (enabling measurement of COX-2 specifically within tumor cells); percentage area (% area) was collected for CD34 within the stromal compartment. This calculation was performed by measuring the total number of CD34 pixels above a designated threshold and dividing by total pixel area of the TMA core. This measurement was chosen as an automated evaluation of vessel density.
Statistical Considerations
Failure for the OS endpoint was death due to any cause and was measured from the date of registration to the date of death or last follow-up for patients who had not failed. Local-failure was persistence of local disease or local recurrence. Regional failure was persistence, appearance or recurrence of regional nodal disease. Patients with persistent disease were considered as having failures, defined at one day post registration. Patients were considered as having failures for local-regional disease if they had a local and/or regional failure, measured from the date of registration to the date of failure or last follow-up for patients who have not failed. Patients were considered as having failures for the DFS endpoint if they failed any of the above mentioned efficacy endpoints. DFS was measured from the date of registration to the date of the site of first failure, death (with no other failures) or last follow-up for patients who did not fail. OS and DFS were estimated using the Kaplan-Meier method. Time to local-regional failure was estimated by the cumulative incidence method (22).
Protein expression data was recorded as a continuous variable. Marker expression was analyzed at the median value. Cox regression models were used to explore associations between expression of the protein markers and clinical endpoints (local-regional failure, DFS, OS). Fisher's exact test was used to determine association of COX-2 expression with both 2-year DFS and 2-year OS.
Results
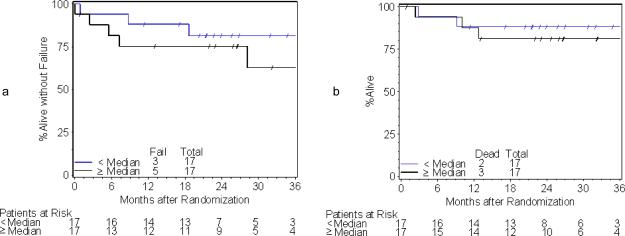
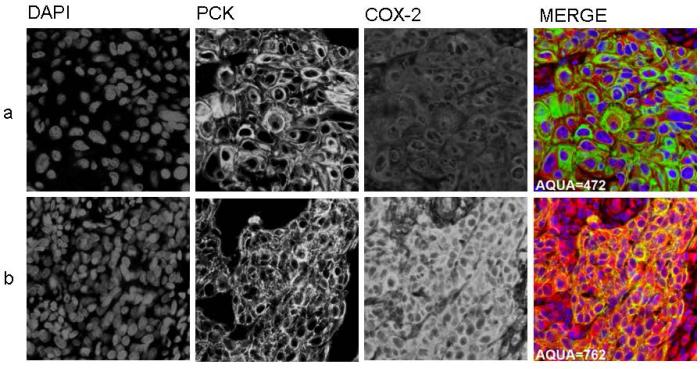
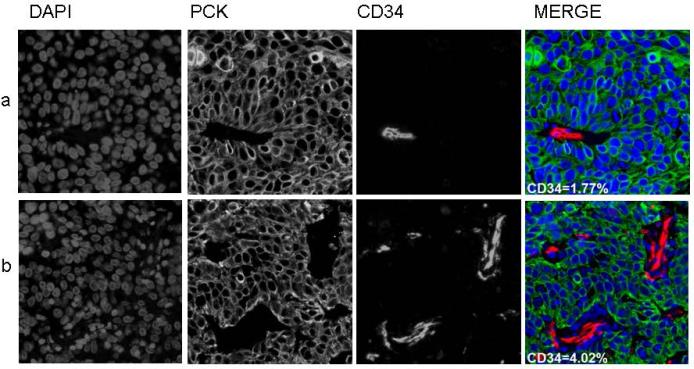
A total of 84 patients were accrued to RTOG C0128 between August 2001 and March 2004, of whom 78 were eligible and analyzable. COX-2 expression by AQUA® and was determined for 37 (47%) of patients. CD34 scoring by AQUA® was determined for 34 (44%) of patients. Loss of data is due to unavailability of pre-treatment biopsy specimens, or loss of cores due to sectioning from the TMA. Pre-treatment patient and tumor characteristics for COX-2 and CD34 status are shown in Tables 1 and 2, respectively. There were no statistically significant differences in pre-treatment patient and tumor characteristics between the missing versus determined biomarker groups. Median COX-2 score was 694.2 (range 472.0-1223.5). Median CD34 scores (% area) was 3.9 (range 0.3-32.5). A tumor with low (below median) expression of COX-2 is shown in Figure 1a; high (above median) expression is shown in Figure 1b. A tumor with low percentage area CD34 within the stroma is shown in Figure 2a; high percentage area CD34 in Figure 2b. Median follow-up for patients with COX-2 AQUA® data was 44.5 months (range 30 – 66 months). The estimated two-year local-regional failure rate was 22% for patients with COX-2 scores below the median and 12% for patients with COX-2 scores ≥ median. In tumors where COX-2 AQUA® data was available, 6 out of 37 patients (16%) had local-regional failure; 4 of these patients had COX-2 scores below the median [HR=0.39; 95%CI=(0.07, 2.16); p=0.28]. The estimated two-year DFS rate was 72% for patients with COX-2 scores below the median and 88% for patients with COX-2 scores ≥ median (Figure 3a). Of the 8 DFS failures, 5 had COX-2 levels below the median [HR=0.49; 95%CI=(0.12, 2.04); p=0.32]. The 4 patients who died all had COX-2 levels below the median value. The estimated two-year OS rate was 78% for patients with COX-2 scores below the median and 100% for patients with COX-2 scores ≥ median (Figure 3b). Based on Fisher's exact test, there was a significant association between COX-2 AQUA® score and 2-year OS (p=0.046). There was no statistically significant correlation of CD34 status and clinical outcome (Figures 4a, 4b). In addition, there was no significant correlation with COX-2 or CD34 status and acute or late toxicity (data not shown). There was no statistically significant correlation between COX-2 expression and percentage area CD34 (data not shown).
Table 1.
Pre-treatment Characteristics, COX-2 measured by AQUA®
| Missing COX-2 (n=41) | Determined COX-2 (n=37) | COX-2 < Median (n=18) | COX-2 ≥ Median (n=19) | |
|---|---|---|---|---|
| Age | ||||
| Median | 44 | 47 | 42 | 47 |
| Range | 24 – 68 | 30 – 66 | 30 – 66 | 32 – 59 |
| n | % | n | % | n | % | n | % | |
|---|---|---|---|---|---|---|---|---|
| Zubrod | ||||||||
| 0 | 36 | 88 | 21 | 57 | 9 | 50 | 12 | 63 |
| 1 | 4 | 10 | 15 | 41 | 9 | 50 | 6 | 32 |
| 2 | 1 | 2 | 1 | 3 | 0 | 0 | 1 | 5 |
| FIGO Stage | ||||||||
| IB | 7 | 17 | 11 | 30 | 6 | 33 | 5 | 26 |
| IIA | 1 | 2 | 3 | 8 | 3 | 17 | 0 | 0 |
| IIB | 22 | 54 | 18 | 49 | 7 | 39 | 11 | 58 |
| IIIB | 9 | 22 | 4 | 11 | 2 | 11 | 2 | 11 |
| IVA | 2 | 5 | 1 | 3 | 0 | 0 | 1 | 5 |
| Pelvic Lymph Nodes | ||||||||
| Not Evaluable | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Negative | 32 | 78 | 32 | 86 | 14 | 78 | 18 | 95 |
| Positive | 7 | 17 | 3 | 8 | 2 | 11 | 1 | 5 |
| Equivocal | 0 | 0 | 2 | 5 | 2 | 11 | 0 | 0 |
| Histology | ||||||||
| Squamous cell Ccarcinoma | 36 | 88 | 32 | 86 | 17 | 94 | 15 | 79 |
| Adenocarcinoma | 3 | 7 | 5 | 14 | 1 | 6 | 4 | 21 |
| Adenosquamous | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydronephrosis | ||||||||
| None | 38 | 93 | 33 | 89 | 17 | 94 | 16 | 84 |
| Unilateral | 3 | 7 | 3 | 8 | 1 | 6 | 2 | 11 |
| Bilateral | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 5 |
Table 2.
Pre-treatment Characteristics, CD34 measured by AQUA®
| Missing CD34 (n=44) | Determined CD34 (n=34) | CD34 < Median (n=17) | CD34 ≥ Median (n=17) | |
|---|---|---|---|---|
| Age | ||||
| Median | 44 | 47 | 48 | 45 |
| Range | 24 – 68 | 30 – 66 | 30 – 66 | 31 – 59 |
| n | % | n | % | n | % | n | % | |
|---|---|---|---|---|---|---|---|---|
| Zubrod | ||||||||
| 0 | 37 | 84 | 20 | 59 | 10 | 59 | 10 | 59 |
| 1 | 6 | 14 | 13 | 38 | 7 | 41 | 6 | 35 |
| 2 | 1 | 2 | 1 | 3 | 0 | 0 | 1 | 6 |
| FIGO Stage | ||||||||
| IB | 8 | 18 | 10 | 29 | 6 | 35 | 4 | 24 |
| IIA | 1 | 2 | 3 | 9 | 3 | 18 | 0 | 0 |
| IIB | 24 | 55 | 16 | 47 | 7 | 41 | 9 | 53 |
| IIIB | 9 | 20 | 4 | 12 | 1 | 6 | 3 | 18 |
| IVA | 2 | 5 | 1 | 3 | 0 | 0 | 1 | 6 |
| Pelvic Lymph Nodes | ||||||||
| Not Evaluable | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Negative | 35 | 80 | 29 | 85 | 14 | 82 | 15 | 88 |
| Positive | 7 | 16 | 3 | 9 | 2 | 12 | 1 | 6 |
| Equivocal | 0 | 0 | 2 | 6 | 1 | 6 | 1 | 6 |
| Histology | ||||||||
| Squamous cell carcinoma | 39 | 89 | 29 | 85 | 15 | 88 | 14 | 82 |
| Adenocarcinoma | 3 | 7 | 5 | 15 | 2 | 12 | 3 | 18 |
| Adenosquamous | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydronephrosis | ||||||||
| None | 41 | 93 | 30 | 88 | 17 | 100 | 13 | 76 |
| Unilateral | 3 | 7 | 3 | 9 | 0 | 0 | 3 | 18 |
| Bilateral | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 6 |
Figure 1.
1a: Low tumoral expression of COX-2, AQUA®
1b: High tumoral expression of COX-2, AQUA®
Figure 2.
2a: Low tumoral percent area CD34, AQUA®
2b: High tumoral percent area CD34, AQUA®
Figure 3.
3a: Disease-free survival by COX-2 status
3b: Overall Survival by COX-2 status
Figure 4.
4a: Disease-free survival by CD34 status
4b: Overall survival by CD34 status
Discussion
This study demonstrates the ability to perform quantitative biomarker analyses using automated quantitative IHC analysis, in archival tumor specimens, as part of a cooperative group trial. It was observed that patients with pre-treatment tumors expressing relatively low COX-2 levels had worse OS. This adds support to previous studies identifying pre-treatment tumor level of COX-2 expression as a potential predictive marker of COX-2 inhibitor response. In patients with locally advanced lung cancer treated with celecoxib and chemotherapy, it has been shown that the level of tumoral COX-2 expression correlates with prognosis and benefit from celecoxib (17,18). In addition, it has been shown that patients with COX-2 over-expression who did not receive the drug demonstrated a markedly inferior outcome – the first time that this has been confirmed in a prospective trial, indicating that COX-2 could be a prognostic factor in lung cancer. Interestingly, a possible adverse effect was noted for those who received celecoxib and did not express COX-2 in their tumor, indicating that perhaps treatment is contraindicated in this subset of patients (17). This effect has also been seen in patients with esophageal and gastroesophageal junction cancer, treated with chemotherapy and celecoxib (23). It is possible that this finding may explain negative results in previous COX-2 trials, since any positive effect in the COX-2 expressing group may be obscured by the negative effects on patients whose tumors did not express COX-2. This has been demonstrated in other studies (19, 24), and may also explain the observations in our study. This finding has not been previously described in cervical cancer.
The majority of such protein biomarker studies, including those evaluating COX-2 expression, have used conventional, semi-quantitative scoring systems in which a pathologist interprets the staining and assigns a score or a descriptive coding. This type of histologic interpretation can be subjective, and difficult to reproduce in other laboratories. The advantages to automative quantitative IHC techniques include more objective and reproducible scoring, and the ability for high-throughput quantification of biomarkers. The specific subcellular quantification is not possible using the conventional, pathologist-read “eyeball” method.
This study has several limitations that should be stated. This is a relatively small study, and the small number of clinical endpoint events limits the power to detect significant associations between marker levels and efficacy endpoints. The use of tissue microarray cores of tumor may result in inadequate sampling if the marker of interest is heterogeneously expressed. This may be of particular importance in measuring vessel density as a surrogate for angiogenesis, as there is likely higher activity in the peripheral locations of the tumor (25). Also, CD34 does not distinguish neoangiogenic versus mature vessels; therefore, although a well-documented surrogate, it may not be the optimal marker for assessing tumoral neoangiogenesis. However, this is the first study to evaluate COX-2 and CD34 expression in cervical cancer patients treated with a COX-2 inhibitor. Application of AQUA® technology in a larger follow-up study will be required to definitively evaluate the association of these tumor markers with clinical outcome.
Targeted therapeutics (e.g. tamoxifen, imatinib, trastuzumab) in oncology have been shown to have the greatest clinical benefit when patients are pre-selected for treatment based on expression of the target molecule in the cancer tissue using robust laboratory assays. However, many trials investigating targeted therapeutics have not included tumoral testing of the marker or pathway of interest. The ultimate success of targeted agents in cancer therapy, such as COX-2 inhibitors, may hinge on the ability to pre-select patients most likely to respond, based on the biological features of their specific tumor. This knowledge and improved testing technology is required in the development of future targeted-agent clinical trials, to better facilitate patient selection and stratification, and to better demonstrate clinical benefit from these agents.
Acknowledgments
Supported by RTOG U10 CA21661 and CCOP U10 CA37422 grants from the NCI and the Pennsylvania Department of Health 2004 Formula Grant 4100026182. This manuscript's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vale C, Tierney JF, Stewart LA, et al. Chemoradiotherapy for cervical cancer meta-analysis collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masunaga R, Kohn H, Dhar D, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064–8. [PubMed] [Google Scholar]
- 3.Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 4.Gaffney D, Holden J, Davis M, et al. Increased cyclooxygenase-2 expression correlated with diminished survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:1213–17. doi: 10.1016/s0360-3016(00)01583-2. [DOI] [PubMed] [Google Scholar]
- 5.Chen HW, Wu-Chou S, Cheng-Yang C, et al. Increased expression of nitric oxide synthase and cyclooxygenase-2 is associated with poor survival in cervical cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:1093–1100. doi: 10.1016/j.ijrobp.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 6.Lindstrom AK, Stendahl U, Tot T, et al. Predicting the outcome of squamous cell carcinoma of the uterine cervix using combinations of individual tumor marker expressions. Anticanc Res. 2007;27:1609–1616. [PubMed] [Google Scholar]
- 7.Ferrandina G, Lauriola L, Distefano M, et al. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical patients. J Clin Oncol. 2002;20:973–81. doi: 10.1200/JCO.2002.20.4.973. [DOI] [PubMed] [Google Scholar]
- 8.Masferrer J, Leahy K, Koki A, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–11. [PubMed] [Google Scholar]
- 9.Kishi K, Petersen S, Petersen C, et al. Preferential enhancement of tumor radioresponse by a cycloocygenase-2 inhibitor. Cancer Res. 2000;60:1326–31. [PubMed] [Google Scholar]
- 10.Mao X, Wang X, Xu L. COX-2 expression in gastric cancer and its relationship with angiogenesis using tissue microarray. World J Gastroenterol. 2007;13(25):3466–3471. doi: 10.3748/wjg.v13.i25.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hlatky L, Hahnfeltd P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Nat Cancer Inst. 2002;94:883–93. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 12.Viera SC, Zeferino LC, Da Silva BB, et al. Quantification of angiogenesis in cervical cancer: a comparison among three endothelial cell markers. Gyne Onc. 2004;93:121–24. doi: 10.1016/j.ygyno.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Gaffney DK, Winter K, Dicker AP, et al. Efficacy and patterns of failure for locally advanced cancer of the cervix treated with Celebrex (celexoxib) and chemoradiotherapy in RTOG 0128. Int J Rad Onc Biol Phys. 2007;69(1):111–17. doi: 10.1016/j.ijrobp.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 14.Herrera F, Chan P, Doll C, et al. A prospective phase I/II trial of the cyclooxygenase-2 inhibitor celecoxib in patients with carcinoma of the cervix with biomaker assessment of the tumor microenvironment. Int J Radiat Oncol Biol Phys. 2007;67:97–103. doi: 10.1016/j.ijrobp.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadianpanah M, Razmjou-Ghalaei S, Shafizad A, et al. Efficacy and safety of concurrenct chemoradiation with weekly cisplatin +/_low-dose celecoxib in locally advanced undifferentiated nasopharyngeal carcinoma: a phase II/III clinical trial. J Can Res and Therapeut. 2011;7(4):442–447. doi: 10.4103/0973-1482.92013. [DOI] [PubMed] [Google Scholar]
- 16.Groen HJ, Sietsma H, Vincent A, et al. Randomized, placebo-controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase-2 expression as a biomarker for patients with advanced non-small-cell lung cancer: the NVALT-4 study. J Clin Oncol. 2011;29(32):4320–4326. doi: 10.1200/JCO.2011.35.5214. [DOI] [PubMed] [Google Scholar]
- 17.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy - Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 18.Fidler M, Argiris A, Patel, et al. The potential predictive value of cyclooxygenase-2 expression and increased risk of gastrointestinal hemorrhage in advanced non–small cell lung cancer patients treated with erlotinib and celecoxib. Clin Canc Res. 2008;14:2088–94. doi: 10.1158/1078-0432.CCR-07-4013. [DOI] [PubMed] [Google Scholar]
- 19.Rini BI, Weinberg V, Dunlap S, et al. Maximal COX-2 immunostaining and clinical response to celecoxib and interfereon alpha therapy in metastatic renal cell carcinoma. Cancer. 2006;106:566–75. doi: 10.1002/cncr.21661. [DOI] [PubMed] [Google Scholar]
- 20.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8(11):1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 21.Doll CM, Prystajecky M, Eliasziw M, et al. Low ERCC1 mRNA and protein expression are associated with worse survival in cervical cancer patients treated with radiation alone. Radiother Oncol. 2010;97(2):352–359. doi: 10.1016/j.radonc.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & sons; New York: 1980. [Google Scholar]
- 23.Altorki NK, Christos P, Port JL, et al. Preoperative taxane-based chemotherapy and celecoxib for carcinoma of the esophagus and gastroesophageal junction: results of a phase 2 trial. J Thorac Oncol. 2011;6(6):1121–1127. doi: 10.1097/JTO.0b013e31821529a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide and with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000 Feb;18(3):623–31. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 25.Ancuta C, Ancuta E, Zucon-Eloae FL, et al. Neoangiogenesis in cervical cancer: focus on CD34 assessment. Rom J Morph and Embryol. 2010;51(2):289–294. [PubMed] [Google Scholar]