Abstract
Purpose of review
Exposure to traffic-related air pollutants (TRAP) has been implicated in asthma development, persistence, and exacerbation. This exposure is highly significant because increasingly large segments of the population worldwide reside in zones that have high levels of TRAP (1), including children since schools are often located in high traffic pollution exposure areas.
Recent findings
Recent findings include epidemiologic and mechanistic studies that shed new light on the impact of traffic pollution on allergic diseases and the biology underlying this impact. In addition, new innovative methods to assess and quantify traffic pollution have been developed to assess exposure and identify vulnerable populations and individuals.
Summary
This review will summarize the most recent findings in each of these areas. These findings will have substantial impact on clinical practice and research by development of novel methods to quantify exposure and identify at-risk individuals, as well as mechanistic studies that identify new targets for intervention for individuals most adversely affected by TRAP exposure.
Keywords: Asthma, air pollution, traffic pollution, allergy
Introduction
A recent comprehensive and systematic review of worldwide traffic emissions and health science by a Special Panel convened by the Health Effects Institute (HEI) found sufficient evidence that exposure to traffic-related air pollutants (TRAP) causes asthma exacerbation in children (1). Within the complex mixture of gaseous and particulate components of TRAP, diesel exhaust particles (DEP) are of particular concern with respect to health effects. DEP are estimated to contribute up to 90% of the particulate matter (PM) derived from traffic sources, are primarily ultrafine in size (< 100 nm), can be deposited in the nasal and peripheral airways, and have been shown to induce oxidative stress and airway hyper-responsiveness, enhance allergic responses and airway inflammation (2–4). This exposure is highly significant because in large cities in North America, up to 45% of the population resides in zones that are most impacted by TRAP (1) and over 30% of schools are located in high TRAP exposure areas (5). Evidence from our group and others suggests TRAP is also associated with reduced lung growth and the development of asthma, though recent studies have reported conflicting results (6–12). These inconsistent findings may be due to a lack of knowledge regarding the mechanistic basis of TRAP health effects and the characteristics of those most susceptibility to the harmful effects of TRAP exposure. Recent epidemiologic and mechanistic findings have started to fill gaps in knowledge regarding the health impact of TRAP exposure on allergic diseases, as well as the molecular mechanisms by which TRAP leads to adverse effects on allergic diseases such as asthma. Further, new methodologies for quantification of TRAP hold tremendous promise for rapid and reliable identification of individuals at-risk due to high exposure.
Epidemiology of the health impact of TRAP on allergic disease
The prevalence and incidence of allergic diseases have been increasing worldwide since the 1960’s (13, 14). More recent investigators suggest that the asthma prevalence has plateaued in developed countries, while in developing countries, where the prevalence was previously low, allergic diseases are on the rise (15). Environmental changes are suspected to be the major driver of this increasing trend (16), with air pollution identified as an important extrinsic agent (17). Motor vehicles produce a complex mixture of air pollutants including carbon monoxide, nitrogen oxides, particulate matter (PM) of varying size, polycyclic aromatic hydrocarbons (PAHs - e.g. benzo(a)pyrene), volatile organic compounds (VOCs – e.g. benzene, acetaldehyde) and other hazardous air pollutants (HAPs). Collectively referred to as traffic-related air pollutants (TRAP), these are the primary source of intraurban variability in air pollutant concentrations (1).
Asthma and Wheezing
There is sufficient evidence to suggest that TRAP can decrease lung function and trigger asthma exacerbation and hospitalizations (14, 18). Recent large studies on TRAP and respiratory outcomes substantiate these conclusions (Table 1). Findings from the Southern California Children’s Health Study, a cohort of 11,365 schoolchildren in 16 communities, indicate that exposure to higher local nitrogen dioxide (NO2) concentrations and close residential proximity to a freeway increase asthma prevalence (19). Asthmatic children in the cohort that lived in communities with higher levels of NO2, PM10 and PM2.5 had increased chronic lower respiratory symptoms, phlegm, production, bronchitis, wheeze and medication use (19). Living in areas with higher air pollution markers also affected lung function and growth. Children aged 10–18 living within 500 meters of a freeway had significant deficits in FEV1, FVC and maximal mid-expiratory flow rate compared to those living more than 1500 meters away (19). A recent study of 5,443 Korean children aged 6–14 found that children living within 200 meters of a main road that was ≥254 meters long had increased lifetime wheezing, lifetime asthma diagnosis and decreased lung function (20). A meta-analysis of six cohorts in the European Study of Cohorts for Air Pollution Effects (ESCAPE) that included 23,704 adults found that exposure to higher NO2 increased the incidence of adult-onset asthma, although the results did not reach significance (21). ESCAPE was also comprised of five birth cohort studies including 17,041 children. While these birth cohorts did not find any significant associations between six traffic-related pollution metrics and childhood asthma prevalence, the land-use regression (LUR) models used to estimate exposures were carried out as long as 15 years after the asthma outcomes were collected (10). During this time, campaigns to reduce air pollution could have reduced exposure levels compared to those present when the asthma outcomes were collected. We have also shown TRAP exposure levels at a child’s birth address to be associated with wheezing (22–24) and recurrent night cough (25) in the first three years of life.
Table 1.
Selected epidemiologic studies of TRAP associations with allergic disease.
| Outcome | Study Type | # of studies | Sample Size | Age Group | TRAP Markers | Main Findings | References |
|---|---|---|---|---|---|---|---|
| Asthma / Wheezing | Meta-analyses | 5 birth cohorts | 17,041 | Birth-10 | NO2, NOX, PM2.5, PM10, Coarse PM, traffic intensity | No significant associations between traffic-related pollution metrics and childhood asthma prevalence. | Molter et al. (10) |
| Review | 5 cohorts | 937 – 5,603 per cohort (11,365 total) | K - 10th grade | O3, PM2.5, PM10, NO2 | Higher local NO2 associated with increased asthma prevalence (OR 1.83, 1.04–3.22); asthmatics with higher NO2, PM10 and PM2.5 exposure had increased lower respiratory symptoms, bronchitis, phlegm production, wheeze and medication use; living within 500 meters of a freeway was associated with deficits in FEV1, FVC and MMEF. | Chen et al. (19) | |
| Case-control | 1 | 5,443 | 6–14 | Proximity | Living within 200 meters of a main road increased wheeze, asthma diagnosis and decreased lung function. | Jung et al. (20) | |
| Meta-analyses | 6 cohorts | 23,704 | Adults | NO2 | NO2 exposure modestly increased incidence of adult-onset asthma (OR 1.04, 0.99–1.21) | Jacquemin et al. (21) | |
| Case control | 1 birth cohort | 762 | Birth-3 | Proximity, land use regression model | Higher exposures to TRAP increase wheezing and night cough. | Ryan et al (22–24); Sucharew et al. (25) | |
| Meta-analyses | 5 birth cohorts | 272 – 37,401 | 1–12 | PM2.5, NO2, BC | Longitudinal childhood exposure to NO2 (OR 1.06, 1.01–1.12), PM2.5 (OR 1.05, 1.01–1.08) and BC (OR 1.19, 1.07–1.32) increased asthma incidence. Substantial heterogeneity between studies for NO2 and PM2.5. | Bowatte et al. (12) | |
| Eczema | Review | 6 | 400– 7,030 | Birth-13 | PM10, NO2, NOX, CO | Traffic pollutant markers act as risk factors for the development or aggravation of eczema. | Ahn et al. (26) |
| Case-control | 1 | 4,907 | 9–11 | Dispersion model | Eczema was significantly associated with PM10, NO2, NOX and CO. | Penard-Morand et al. (27) | |
| Case-control | 1 | 317,926 | 12–14 | SO2, NOX, O3, CO and PM10 | Flexural eczema is associated with NO2 and CO | Lee et al. (28) | |
| Allergic rhinitis / Sensitization | Case-control | 1 | 5,443 | 6–14 | Proximity | Living <75 meters from main road increased lifetime diagnoses and symptoms of AR. Distance to the main road and the length and proportion of the main road within 200 meters of the home were all associated with allergic sensitization to aeroallergen or food. | Jung et al. (20) |
| Case-control | 1 birth cohort | 762 | Birth-4 | Land use regression model | High TRAP exposure modestly increased the risk of aeroallergen sensitization (OR 1.4, 0.97–2.0) | Codispoti et al. (29) | |
| Case-control | 1 birth cohort | 2,545 | Birth-8 | Land use regression model | TRAP exposure in first year of life increased risk of pollen sensitization (OR 1.83, 1.02–3.28); TRAP increased risk of food sensitization at age 8 in those not sensitized at age 4 (OR 2.3, 1.1–4.82). | Gruzieva et al. (30) | |
| Meta-analyses | 5 birth cohorts | 272– 37,401 | 1–12 | PM2.5, NO2, BC | Higher PM associated with increased sensitization to outdoor allergens (pollen and grass). No association with TRAP measures and sensitization to indoor allergens (cat dog, or mold). | Bowatte et al. (12) |
Allergic Sensitization and Eczema
Studies also support an increase of allergic sensitization and eczema with TRAP exposures (Table 1). A 2014 review of epidemiologic data on the role of air pollution in eczema concluded that a variety of air pollutants, including those related to TRAP such as PM and nitrogen oxides, act as risk factors for the development or aggravation of eczema (26). A study of 4,907 children aged 9–11 showed that lifetime eczema was significantly associated with 3-year averaged concentrations of PM10, NO2, NOX and CO (27). A Taiwanese study of 317,926 middle school students demonstrated that flexural eczema was associated with levels of CO and NOX (28). In the Southern California Children’s Health Study, children living less than 75 meters from the main road were significantly more likely to have lifetime diagnoses and symptoms of allergic rhinitis (AR) (20). The distance to the main road and the length and proportion of the main road within 200 meters of the home were all associated with allergic sensitization, defined as positive skin prick test (SPT) to an aeroallergen or food (20). Our data and others have also shown high TRAP exposure in the first year of life to increase the risk of aeroallergen sensitization by the age of four by 40–83% (29, 30). Children exposed to high TRAP levels before age 1 also have an increased risk of developing food allergy by age eight, particularly those that are not sensitized at age four (30).
Insights from Birth Cohort Studies
To evaluate the impact of TRAP on asthma and allergy development, birth cohort studies are needed (Table 1). A recent meta-analysis conducted a systematic review of birth cohort studies to understand the association between early childhood TRAP and subsequent allergies, asthma and allergic sensitization (12). While modest associations were observed between asthma incidence/prevalence and wheeze and nitrogen oxides, PM, black carbon (BC), and road proximity, there was substantial heterogeneity observed (likely due to differences in study design, participants and exposure and outcome definitions) between the studies (12). The meta-analyses showed no association with TRAP measures and sensitization to indoor allergens (cat dog, or mold). While there was a significant association between PM and sensitization to outdoor allergens (pollen and grass), there was again high heterogeneity between studies (12). The associations between the markers of air pollution (NO2, BC, and road proximity) and hay fever and eczema were inconsistent. With respect to timing of exposure and disease development, this review suggests that TRAP exposure is associated with new onset asthma and may have an ongoing effect with a lag time of about 3 years (12). We recently reported that a child’s risk for persistent wheeze and asthma development varies depending on the timing and duration of TRAP exposure (31). Children exposed to high levels of TRAP at time of birth were nearly twice as likely to experience persistent wheezing at age seven; however, a longer duration of exposure to high levels of TRAP (beginning early in life and continuing through age seven) was the only time period of exposure related to asthma development (31).
Collectively, there is considerable evidence that TRAP plays a role in the development, and/or symptoms of allergic disease. However, heterogeneity in both the definitions of TRAP exposure and outcomes and unmeasured confounding limit the ability to draw firm conclusions from the data. As discussed in the Bowatte et al. meta-analyses, there is substantial variability in the exposure measurements across TRAP-related studies. Land use regression (LUR) models are among the most common methods to assess TRAP exposures (12). Other methods include passive samplers, central monitoring stations and proximity to major roads. The most frequent markers of pollutants include PM, oxides of nitrogen, carbon monoxide and ozone. PM may be further reported as BC, PM10, or PM2.5. While this vast variation in the definition of TRAP exposure limit the ability to conduct sound meta-analyses, it highlights the importance of appropriate exposure assessment, as discussed below.
Assessment of TRAP exposure
Given the increasingly evident health impact of TRAP, methodologies to accurately assess exposure are needed. While TRAP affects air quality on urban and regional scales, their impact is greatest on a local scale, particularly near roadways, as their concentrations are significantly elevated within approximately 300 to 500 m of their source (32). Further influencing individuals’ TRAP exposure is its temporal variability combined with complex and variable personal behavior including time spent indoors/outdoors (33). In order to meet the intrinsic challenge of accurately assessing TRAP exposure for epidemiologic studies both modeling and personal measurement approaches have been proposed. Herein, we briefly review approaches to TRAP exposure assessment for epidemiologic studies with an emphasis on recent methodologic advances. An overview of each approach and their strengths and limitations is also provided in Table 2.
Table 2.
Summary of TRAP exposure assessment approaches
| Category | Approach | Overview | Strengths | Limitations |
|---|---|---|---|---|
| Modeling | Proximity | Distance to nearby roads serves as surrogate of exposure | Straightforward, minimal data requirements, potential to incorporate traffic volume/mix | Crude estimates of exposure may result in misclassification |
| Dispersion | Emission, atmospheric, meteorological, and topographic data are used to mathematically model pollutant concentrations from traffic sources | Consideration of emissions, meteorology, source specific, high-resolution | Computationally intensive, requires assumptions regarding pollutant transport, extensive data including traffic characteristics, source emissions, fleet mix, meteorology, and topography often not available in study areas | |
| Land-use regression | Geographic variables measured in buffer regions surrounding multiple air sampling sites are used to estimate measured air pollutant variability, resulting regression equation used to predict pollutant concentrations at unsampled locations | Straightforward approach, able to capture high spatial variability of TRAP, can be applied to varying measured air pollutants, potential to incorporate temporal information | Requires air sampling at sufficient density to capture TRAP variability and geographic predictors, limited transferability of developed model | |
| Hybrid | Combination of multiple approaches, i.e. LUR with additional spatial interpolation models or personal monitoring | Improved exposure estimates | Requires data / expertise for multiple approaches | |
| Personal | Personal monitoring | Individuals’ exposure to air pollutants is measured using wearable sampling devices | Direct measure of exposure | Requires wearable devices capable of accurately measuring exposure over long time periods, participant burden, cost of measurement devices, requires study personnel time |
| Biomarkers | Concentration of internal biomarker, frequently in blood or urine, measured as marker of exposure or effect | Individual exposure assessment, potential use as measure of effect | Lack of specific markers, difficulty differentiating markers of effect from exposure, cost |
Modeling Approaches
While regulatory air monitoring provide valuable data to link regional and temporal variability of air pollutants to population-level health outcomes including increased cardiopulmonary morbidity and mortality, (34–37) these networks are unable to capture the high spatial variability of TRAP concentrations within an urban area. Measuring proximity (i.e. distance) to major roadways is a straightforward approach to estimate TRAP exposure, though this method does not account for traffic density and other geographic and land-use characteristics which impact TRAP concentrations. (38) An alternative approach, dispersion modeling, has not been extensively utilized in epidemiologic studies due to the required data (e.g. meteorology, traffic volume and makeup) and expertise required for its use (33).
Among the most frequently used method to estimate TRAP exposure in epidemiologic studies is land use regression (LUR) modeling (38–41). In the most straightforward LUR approach a single pollutant from the TRAP mixture is measured at multiple stationary sites within a defined study region and characteristics of the area surrounding each sampling site (e.g. elevation, nearby roads, traffic) are used as predictors of the measured concentrations in a linear model. The resultant LUR model is then applied to estimate pollutant concentrations at non-sampled locations including schools and homes where significant geographic predictor variables can be determined. First described by Briggs et al, (42) LUR models are now among the most commonly used techniques in epidemiologic studies of respiratory health (10, 11, 38, 40, 43–49). While initial LUR models were most often developed for NO2 and, less commonly, PM, more recent models have been developed for additional traffic pollutants including elemental components of PM, ultrafine particles, VOCs, PAHs, and black carbon (50–54). The temporal variability of TRAP concentrations have also been incorporated into LUR models through the addition of mobile or continuous monitoring allowing for short-term and daily estimates of TRAP exposure for study participants (44, 55–58). New data inputs for LUR models, including satellite-derived pollutant measurements (59, 60) and the development of hybrid models combining LUR with Bayesian Maximum Entropy and other statistical approaches have also improved the accuracy of TRAP exposure assessment (61, 62) In studies with available participant-reported time spent in locations outside the home, LUR models have been used to derive time-weighted estimates of exposure based on location (40) More recent application of this time-weighted approach have utilized smartphones and GPS-derived location data to improve estimates of TRAP exposure by combining LUR or other modeled TRAP estimates with individuals’ location through space and time (63)
Personal Approaches
Despite advances in modeling TRAP and the incorporation of GPS to improve estimates of individual-level exposure, personal monitoring remains the ‘gold-standard’ for TRAP exposure assessment. While there have been limited applications of personal monitoring in air pollution epidemiology, usually in the context of short-term panel studies, (64–66) assessing long-term exposure by personal sampling is not routinely conducted due to a current lack of wearable devices and the associated cost, time, and participant burden of personal monitoring (67). However, new technological advances and the miniaturization of personal sensor technology for PM and black carbon will lead to increased applications of personal monitoring technology in epidemiologic studies (68–70). Another potential approach to assessing personal exposure to TRAP is the use of a measured biomarker of exposure in a biological specimen (e.g. urine or blood). While measuring PAH metabolites as biomarkers of TRAP exposure has been conducted primarily in occupational settings, more recent studies have identified PAH metabolites specific to environmental traffic exposure making this approach more feasible for applications in environmental epidemiology (71–73).
Mechanistic insights into TRAP effects on the pathogenesis of allergic disease
Although there is strong evidence that TRAP exposure contributes to childhood asthma (1, 6, 7, 24) the mechanistic basis of TRAP effects on asthma has been elusive. The molecular and cellular pathways triggered by exposure to TRAP and their impact on allergen-induced immune responses have been studied in human studies as well as in reductionist models in vitro and in animal models in vivo.
DEP induces epithelial stress responses
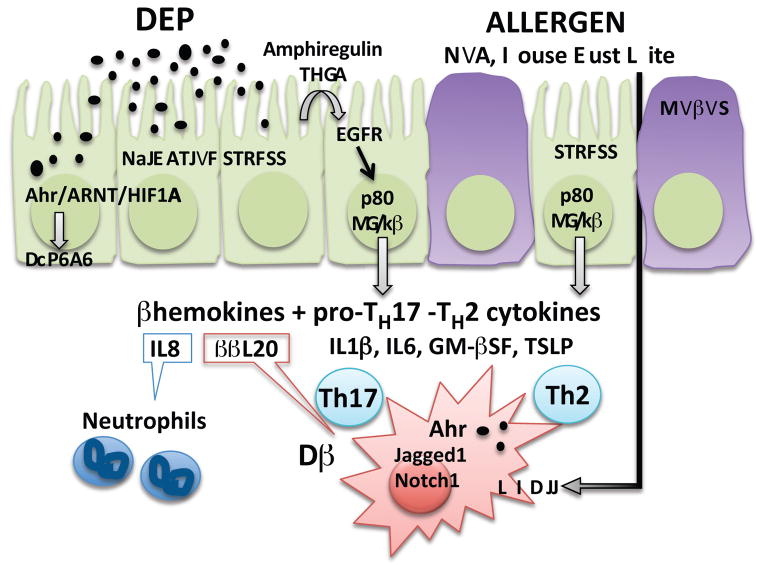
As the main barrier against airway pathogens and pollutants, lung epithelial cells are commonly used to study the toxicity of different traffic related pollutants and their components and have been reviewed elsewhere (74, 75). The inability of epithelial cells to effectively detoxify DEP results in release of pro-inflammatory cytokines including cytokines involved in TH17 differentiation (IL1β, IL6) and neutrophil chemokines, such as IL8 (CXCL8). DEP-mediated induction of IL8 is dependent on p38 MAP kinase and NF-kB signaling (76). Upstream, IL8 secretion has been shown to be dependent on the EGFR pathway, as DEP induces secretion of endogenous EGFR ligands, and neutralizing antibodies against TGFα, heparin-binding EGF or amphiregulin decrease IL8 secretion by primary bronchial epithelial cells following DEP exposure (77). Since TGFα neutralizing antibodies or TNFα-converting enzyme (TACE; ADAM-17) inhibitors significantly decrease IL8 generation following DEP exposure, a model by which cleavage of pro-TGFα by TACE leads to EGFR signaling has been proposed (78, 79). A similar cascade of events has been demonstrated for allergen (house dust mite)-mediated induction of GM-CSF by human bronchial epithelial cells, underscoring that these pathways are part of a broad epithelial stress response (Figure 1).
Figure 1. Mechanistic insights into DEP effects on asthma pathogenesis.
Lung epithelial cells recognize polycyclic aromatic hydrocarbons present in diesel exhaust particles (DEP) via the aryl hydrocarbon receptor (AhR), promoting cytochrome P450 family 1 A1 (CYP1A1) mediated detoxification. Failure to detoxify results in oxidative stress and release of repair cytokines (amphiregulin, TGFα), which signal through the epidermal growth factor receptor (EGFR), p38 mitogen-activated protein kinase and NF-κB to induce secretion of chemokines, as well as cytokines involved in TH17 and TH2 differentiation (TSLP). DEP promotes allergic airway inflammation by upregulating the expression of the Jagged1/Notch1 pathway in dendritic cells (DC) in an AhR dependent manner in concert with allergens.
DEP exposure promotes TH17 responses including increased TH2/TH17 double-producing cells
Although asthma has long been characterized as a disease of dysregulated TH2 immune responses to environmental allergens, accumulating evidence suggests a role for TH17 cells, especially severe steroid resistant asthma (80, 81). Immunohistochemistry on bronchial biopsies from asthmatics reveals increased IL-17A+ cells in patients with severe asthma compared to mild asthma or controls (82). In both adults and children, serum IL-17A is significantly higher in severe asthmatics compared to mild asthmatics or controls (83–85). Recent evidence demonstrates that that DEP exposure promotes asthma by enhancing TH17 immune responses, and anti-IL17A treatment alleviates the negative effects of DEP on asthma in a mouse asthma model (4). Similarly, in children, TRAP exposure is associated with increased serum IL17A levels and increased asthma severity (4). In mice, DEP exposure results in accumulation and persistence of allergen specific TH2/TH17 cells in the lungs, potentiating secondary allergen recall responses (86). These cells rapidly produce both TH2 and TH17 cytokines upon re-exposure to antigen. The induction and persistence of these resident memory cells in the lungs following TRAP exposure may be responsible, in part, for the long lasting impact of early life TRAP exposure into later lifestages; and may contribute to persistence of asthma into adolescence. Recent studies have demonstrated that dual-positive TH2/TH17 cells and IL-17A were present at a higher frequency in the BALF from steroid resistant asthmatic patients (87). These TH2/TH17 cells were resistant to dexamethasone-induced cell death and the TH2/TH17 predominant subgroup of patients manifested the most severe form of asthma (87). Thus, these double-producing cells may be an excellent biomarker of severe, steroid resistant patients that would benefit from additional or alternate treatment. In a murine model, transfer of allergen-specific TH2/TH17 double-producing cells to naïve mice resulted in inflammation and exacerbated asthma (88).
Mice exposed to DEP alone develop airway neutrophilia, but do not present any of the hallmarks associated with asthma such as eosinophilia, TH2 cytokine release, mucus production or airway hyperresponsiveness (AHR) (2, 4, 89). However, chronic exposure to low doses of DEP over a 3-month period results in emphysema and accumulation of T cells (89). DEP exposure promotes accumulation of TREG, TH17 cells and neutrophils (2, 4). The observed increase in TReg and Th17 cells may result in part from the ability of DEP to promote TH17, TH22, and TReg differentiation through the aryl hydrocarbon receptor (AhR) (90, 91). Indeed, exposure to DEP and related polycyclic aromatic hydrocarbons (PAH) has been shown to increase TH22 cells in an Ahr-dependent manner (92, 93). Ahr signaling requires dimerization with the aryl hydrocarbon receptor nuclear translocator (ARNT), also known as hypoxia-inducible factor beta (HIF1β). Accordingly, a crosstalk between the hypoxia-inducible factor 1-alpha (HIF1α) and Ahr pathways has been proposed, as both might compete for ARNT binding (94). In T-cells, HIF1α driven glycolysis supports TH17 differentiation by promoting expression of RORγt, whereas in conditions supporting TREG differentiation HIF1α favors Foxp3 induction (95, 96). The respective contributions and interactions of DEP-induced Ahr signaling and oxidative stress related HIF1α signaling have not been explored.
DEP exposure exacerbates allergen-induced TH2 responses
Numerous studies have demonstrated that exposure to either particulate matter or DEP can exacerbate allergic airway responses (97). We recently demonstrated that co-exposure to HDM and DEP promotes a mixed TH2/TH17 response with increased TH2 and TH17 cells, as well as double-producing IL13+/IL17A+ TH cells (2, 4). Thus, DEP exposure exacerbates TH2 responses. Mechanisms by which DEP exposure increases allergen-induced Th2 differentiation have been reviewed elsewhere (98). A recent study using co-cultures of OVA transgenic CD4+ T cells and bone marrow derived dendritic cells (BMDC) pre-exposed to OVA with or without TRAP, demonstrated increased IFNγ, IL4, IL13, and IL17 levels in culture supernatants of OVA+TRAP exposed BMDC compared to BMDC exposed to OVA alone (99) supporting that TRAP exposure directly impacts dendritic cells. Further, TRAP upregulated TH cytokine levels, IgE production, and allergic airway inflammation in mice in a Jagged1- and Notch-dependent manner and TRAP-induced Jagged1 expression was mediated by the AhR, which bound to and activated AhR response elements in the Jag1 promoter (99). AhR blockade or its lineage-specific deletion in CD11c+ cells abrogated the augmentation of airway inflammation by TRAP. Thus, TRAP promotes allergic airway inflammation by upregulating the expression of components of the Notch pathway in human monocytes and murine DCs (99). Of note, TSLP released by epithelial cells upon DEP exposure, promotes Jagged1 and OX40 ligand expression by PBMC-derived DC, favoring TH2 differentiation (100).
The impact of early life TRAP exposure
The timing and duration of traffic-related air pollution (TRAP) exposure may be important for childhood wheezing and asthma development. This was examined in a recent article examining the relationship between TRAP exposure and longitudinal wheezing phenotypes and asthma at age seven in a birth cohort (31). High TRAP exposure at birth was significantly associated with both transient and persistent wheezing. In contrast, only children with high average TRAP exposure continuously from birth through age seven were at significantly increased risk for asthma (aOR = 1.71, 95% CI 1.01–2.88). Early-life exposure to TRAP is associated with increased risk for persistent wheezing, but only long-term exposure to high levels of TRAP throughout childhood was associated with asthma development. In mice, post-natal exposure to HDM and/or DEP followed by a secondary exposure to HDM 4 weeks later induced AHR only in mice that were co-exposed to HDM and DEP but not in mice that were only exposed to HDM (86). In another study where neonatal OT-II mice were repeatedly pre-exposed to TRAP before being co-exposed to OVA as adults, a secondary OVA recall response generated AHR only in mice previously co-exposed to TRAP and OVA as neonates (101). This was associated with the accumulation of CD4+ T-cells expressing IL4 or IL17A in the lungs. Collectively, these data strongly suggest that TRAP exposure promotes disease persistence. Indeed, in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) birth cohort, early TRAP exposure was associated with persistent wheeze while early and sustained exposure to TRAP was associated with asthma development (31). DEP exposure has been shown to enhance allergen specific memory, thereby potentiating secondary allergen recall responses and promoting the development of allergic asthma (86).
Prenatal TRAP exposure has been linked to asthma as well (102–105). Mothers who lived near highways during pregnancy are more likely to have children with asthma (103). Prenatal exposure to PAHs is associated with increased risk of allergic sensitization and early childhood wheeze (102, 105). A limited number of mechanistic studies have assessed the impact of in utero TRAP exposure on the development of allergic disorders. In one recent study, offspring of mice exposed to DEP were hypersensitive to OVA and developed increased OVA sensitization, airway inflammation, Th2/Th17 responses, and AHR compared to offspring with no prior in utero DEP exposure (106). Further, prenatal DEP exposure induced expression of genes downstream of AhR and this upregulation persisted 1 month after birth, even though mice were no longer exposed to DEP. Thus, in utero DEP exposure appears to result in a primed state where the neonate is hypersensitive to subsequent allergen exposure. Interestingly, the Th2 and Th17 cytokines were produced primarily by natural killer (NK) cells and other non-CD3+ T-cells. Repeated treatment with anti-NK1.1 prior to OVA challenge resulted in decreased airway inflammation (106). The importance of NK cells in allergic airway responses is supported by a recent study using NK-deficient mice (107). The respective contribution of NK cells and other innate cells to DEP exacerbation of adaptive immune responses offers a promising new avenue of research.
Conclusion
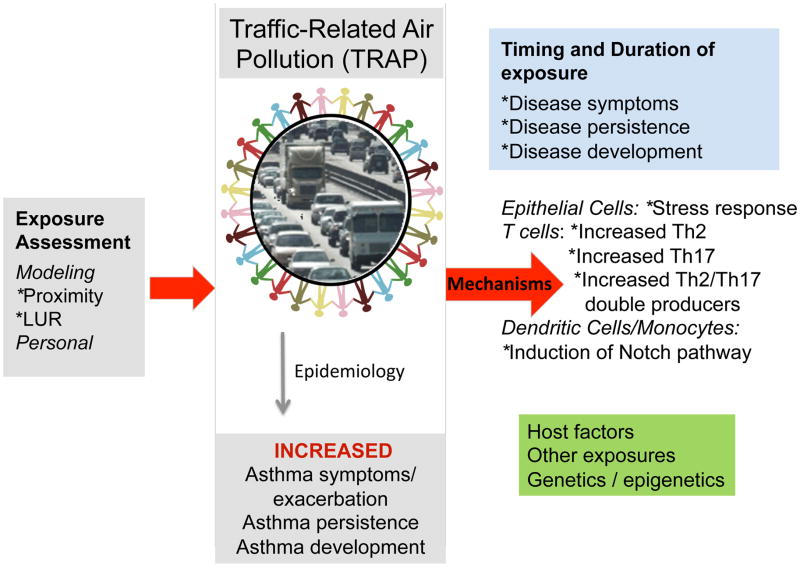
As discussed above, there is considerable evidence that exposure to TRAP is associated with childhood asthma symptoms and exacerbations (6, 7, 10, 24, 108, 109) and recent evidence suggests that TRAP is also associated with reduced lung growth and the development of asthma (31). Herein, we have reviewed the recent epidemiologic and mechanistic findings have started to fill gaps in knowledge regarding the health impact of TRAP exposure on allergic diseases, as well as new methodologies for quantification of TRAP that hold tremendous promise for rapid and reliable identification of individuals at-risk due to high exposure (Figure 2). These new methodologies will enable accurate assessment of exposure in real time such that interventions could be designed and implemented early in the course of exposure in vulnerable populations. The impact of TRAP exposure on allergic disease is complicated by the presence of additional host (genetic, obesity, co-morbidities, nutritional status) and environmental factors, which undoubtedly will affect the observed impact. Additional studies are needed to fill the remaining gaps including identification of the key host factors associated with enhanced susceptibility to TRAP exposure. These studies will have tremendous health impact and ultimately lead to design, testing, implementation, and dissemination of interventions to prevent the impact of TRAP exposure on asthma development, progression, and persistence.
Figure 2. Overview.
More accurate assessment of TRAP exposure will enable the design of epidemiologic and mechanistic studies aimed at discovery of biomarkers to identify the individuals most at-risk from the harmful effects of TRAP exposure and the design of novel targeted interventions.
Key Points.
Exposures to TRAP decrease lung function, trigger asthma exacerbation, and contribute to the development and aggravation of asthma, eczema, allergic rhinitis and sensitization.
Exposure models incorporating novel data and methodology and new technology, including smartphones and personal sensors, have led to improved exposure estimates in epidemiologic studies.
DEP can persist in the murine lungs for months after exposure, in association with chronic TH17 inflammation, without triggering immune features of asthma.
DEP exacerbates allergen-induced innate and adaptive TH2 responses and promotes disease persistence through increased accumulation of pathogenic memory TH2/TH17 cells.
Acknowledgments
Funding: 2U19AI70235 (GKKH, JBM), R01ES019890 (PHR)
We thank Cynthia Chappell for administrative assistance with this review.
Financial Support
This work was supported by the following grants: 2U19AI70235 (GKKH, JBM), R01ES019890 (PHR)
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
- 1.HEI Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. Boston, MA: Health Effects Institute; 2010. [Google Scholar]
- 2.Acciani TH, Brandt EB, Khurana Hershey GK, Le Cras TD. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin Exp Allergy. 2013;43(12):1406–18. doi: 10.1111/cea.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi G, Tanaka H, Wakahara K, Nasu R, Hashimoto M, Miyoshi K, et al. Effect of diesel exhaust particles on house dust mite-induced airway eosinophilic inflammation and remodeling in mice. Journal of pharmacological sciences. 2010;112(2):192–202. doi: 10.1254/jphs.09276fp. [DOI] [PubMed] [Google Scholar]
- 4.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. The Journal of allergy and clinical immunology. 2013;132(5):1194–204. e2. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appatova AS, Ryan PH, LeMasters GK, Grinshpun SA. Proximal exposure of public schools and students to major roadways: a nationwide US survey. J Environ Plann Man. 2008;51(5):631–46. [Google Scholar]
- 6.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environmental health perspectives. 2010;118(7):1021–6. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerrett M, Shankardass K, Berhane K, Gauderman WJ, Kunzli N, Avol E, et al. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116(10):1433–8. doi: 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369(9561):571–7. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 9.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. New England Journal of Medicine. 2004;351(11):1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- **10.Molter A, Simpson A, Berdel D, Brunekreef B, Custovic A, Cyrys J, et al. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J. 2014 doi: 10.1183/09031936.00083614. This study did not find an association between air pollution exposure and childhood asthma in 5 European cohorts. [DOI] [PubMed] [Google Scholar]
- 11.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environmental health perspectives. 2010;118(2):284–90. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70(3):245–56. doi: 10.1111/all.12561. This systematic review and meta-analyses of published birth cohort studies concludes that early childhood exposures to TRAP is associated with the development of asthma in childhood as well as sensitization to aero and food allergens. [DOI] [PubMed] [Google Scholar]
- 13.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 14.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 15.Hatzler L, Hofmaier S, Papadopoulos NG. Allergic airway diseases in childhood - marching from epidemiology to novel concepts of prevention. Pediatr Allergy Immunol. 2012;23(7):616–22. doi: 10.1111/pai.12022. [DOI] [PubMed] [Google Scholar]
- 16.Gehring U, Cyrys J, Sedlmeir G, Brunekreef B, Bellander T, Fischer P, et al. Traffic-related air pollution and respiratory health during the first 2 yrs of life. Eur Respir J. 2002;19(4):690–8. doi: 10.1183/09031936.02.01182001. [DOI] [PubMed] [Google Scholar]
- 17.Takizawa H. Impact of air pollution on allergic diseases. The Korean journal of internal medicine. 2011;26(3):262–73. doi: 10.3904/kjim.2011.26.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatum AJ, Shapiro GG. The effects of outdoor air pollution and tobacco smoke on asthma. Immunol Allergy Clin North Am. 2005;25(1):15–30. doi: 10.1016/j.iac.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Salam MT, Eckel SP, Breton CV, Gilliland FD. Chronic effects of air pollution on respiratory health in Southern California children: findings from the Southern California Children’s Health Study. Journal of thoracic disease. 2015;7(1):46–58. doi: 10.3978/j.issn.2072-1439.2014.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung DY, Leem JH, Kim HC, Kim JH, Hwang SS, Lee JY, et al. Effect of Traffic-Related Air Pollution on Allergic Disease: Results of the Children’s Health and Environmental Research. Allergy, asthma & immunology research. 2015;7(4):359–66. doi: 10.4168/aair.2015.7.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Jacquemin B, Siroux V, Sanchez M, Carsin AE, Schikowski T, Adam M, et al. Ambient Air Pollution and Adult Asthma Incidence in Six European Cohorts (ESCAPE) Environ Health Perspect. 2015;123(6):613–21. doi: 10.1289/ehp.1408206. This study highlights that exposures to ambient air pollution in adults can lead to the development of adult-onset asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S, et al. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am J Respir Crit Care Med. 2009;180(11):1068–75. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116(2):279–84. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Ryan PH, Levin L, Bernstein DI, Burkle J, Grinshpun SA, Lockey JE, et al. Early life exposure to traffic pollutants and wheezing throughout childhood: the Cincinnati Childhood Allergy and Air Pollutions Study (CCAAPS) Am J Respir Crit Care Med. 2011;183:A3748. [Google Scholar]
- 25.Sucharew H, Ryan PH, Bernstein D, Succop P, Khurana Hershey GK, Lockey J, et al. Exposure to traffic exhaust and night cough during early childhood: the CCAAPS birth cohort. Pediatr Allergy Immunol. 2010;21(2 Pt 1):253–9. doi: 10.1111/j.1399-3038.2009.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134(5):993–9. doi: 10.1016/j.jaci.2014.09.023. discussion 1000. This review highlights recent epidemiologic and experimental data that support a role for air pollution in the development and aggravation of atopic dermatitis. [DOI] [PubMed] [Google Scholar]
- 27.Penard-Morand C, Raherison C, Charpin D, Kopferschmitt C, Lavaud F, Caillaud D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36(1):33–40. doi: 10.1183/09031936.00116109. [DOI] [PubMed] [Google Scholar]
- 28.Lee YL, Su HJ, Sheu HM, Yu HS, Guo YL. Traffic-related air pollution, climate, and prevalence of eczema in Taiwanese school children. J Invest Dermatol. 2008;128(10):2412–20. doi: 10.1038/jid.2008.110. [DOI] [PubMed] [Google Scholar]
- **29.Codispoti CD, LeMasters GK, Levin L, Reponen T, Ryan PH, Biagini Myers JM, et al. Traffic pollution is associated with early childhood aeroallergen sensitization. Ann Allergy Asthma Immunol. 2015;114(2):126–33. doi: 10.1016/j.anai.2014.10.020. This study supports that DEP exposure enhances the risk of aeroallergen senstitzation in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruzieva O, Bellander T, Eneroth K, Kull I, Melen E, Nordling E, et al. Traffic-related air pollution and development of allergic sensitization in children during the first 8 years of life. J Allergy Clin Immunol. 2012;129(1):240–6. doi: 10.1016/j.jaci.2011.11.001. [DOI] [PubMed] [Google Scholar]
- **31.Brunst KJ, Ryan PH, Brokamp C, Bernstein D, Reponen T, Lockey J, et al. Timing and Duration of Traffic-Related Air Pollution Exposure and the Risk for Childhood Wheeze and Asthma. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201407-1314OC. This study provides evidence that early-life exposure to high levels of TRAP is assoicated with wheeze symptoms during childhood and asthma developoment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karner AA, Eisinger DS, Niemeier DA. Near-roadway air quality: synthesizing the findings from real-world data. Environmental science & technology. 2010;44(14):5334–44. doi: 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- 33.Brauer M. How much, how long, what, and where: air pollution exposure assessment for epidemiologic studies of respiratory disease. Proceedings of the American Thoracic Society. 2010;7(2):111–5. doi: 10.1513/pats.200908-093RM. [DOI] [PubMed] [Google Scholar]
- 34.Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six US cities. New England journal of medicine. 1993;329(24):1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 35.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. American journal of respiratory and critical care medicine. 2009;179(12):1115–20. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA : the journal of the American Medical Association. 2006;295(10):1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng RD, Bell ML, Geyh AS, McDermott A, Zeger SL, Samet JM, et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environmental health perspectives. 2009;117(6):957–63. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan PH, Lemasters GK, Biswas P, Levin L, Hu S, Lindsey M, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect. 2007;115(2):278–84. doi: 10.1289/ehp.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan PH, LeMasters GK. A review of land-use regression models for characterizing intraurban air pollution exposure. Inhalation toxicology. 2007;19(Suppl 1):127–33. doi: 10.1080/08958370701495998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan PH, Lemasters GK, Levin L, Burkle J, Biswas P, Hu S, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ. 2008;404(1):139–47. doi: 10.1016/j.scitotenv.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 41.Hoek G, Beelen R, de Hoogh K, Vienneau D, Gulliver J, Fischer P, et al. A review of land-use regression models to assess spatial variation of outdoor air pollution. Atmos Environ. 2008;42(33):7561–78. [Google Scholar]
- 42.Briggs DJ, de Hoogh C, Gulliver J, Wills J, Elliott P, Kingham S, et al. A regression-based method for mapping traffic-related air pollution: application and testing in four contrasting urban environments. The Science of the total environment. 2000;253(1–3):151–67. doi: 10.1016/s0048-9697(00)00429-0. [DOI] [PubMed] [Google Scholar]
- 43.Clougherty JE, Wright RJ, Baxter LK, Levy JI. Land use regression modeling of intra-urban residential variability in multiple traffic-related air pollutants. Environmental health : a global access science source. 2008;7(1):17. doi: 10.1186/1476-069X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **44.Dons E, Van Poppel M, Int Panis L, De Prins S, Berghmans P, Koppen G, et al. Land use regression models as a tool for short, medium and long term exposure to traffic related air pollution. Science of The Total Environment. 2014;476:378–86. doi: 10.1016/j.scitotenv.2014.01.025. This study shows land-use regression to be a fast and accurate means for estimating long-term and daily BC and NO2 exposure. [DOI] [PubMed] [Google Scholar]
- 45.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. American journal of respiratory and critical care medicine. 2010;181(6):596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 46.Henderson SB, Beckerman B, Jerrett M, Brauer M. Application of land use regression to estimate long-term concentrations of traffic-related nitrogen oxides and fine particulate matter. Environmental science & technology. 2007;41(7):2422–8. doi: 10.1021/es0606780. [DOI] [PubMed] [Google Scholar]
- 47.Hoek G, Beelen R, Kos G, Dijkema M, Zee SCvd, Fischer PH, et al. Land Use Regression Model for Ultrafine Particles in Amsterdam. Environmental Science & Technology. 2011;45(2):622–8. doi: 10.1021/es1023042. [DOI] [PubMed] [Google Scholar]
- 48.Ross Z, English PB, Scalf R, Gunier R, Smorodinsky S, Wall S, et al. Nitrogen dioxide prediction in Southern California using land use regression modeling: potential for environmental health analyses. Journal of exposure science & environmental epidemiology. 2006;16(2):106–14. doi: 10.1038/sj.jea.7500442. [DOI] [PubMed] [Google Scholar]
- 49.Ross Z, Jerrett M, Ito K, Tempalski B, Thurston GD. A land use regression for predicting fine particulate matter concentrations in the New York City region. Atmos Environ. 2007;41(11):2255–69. [Google Scholar]
- 50.de Hoogh K, Wang M, Adam M, Badaloni C, Beelen R, Birk M, et al. Development of land use regression models for particle composition in twenty study areas in Europe. Environmental science & technology. 2013;47(11):5778–86. doi: 10.1021/es400156t. [DOI] [PubMed] [Google Scholar]
- *51.Patton AP, Collins C, Naumova, Zamore W, Brugge D, Durant JL. An Hourly Regression Model for Ultrafine Particles in a Near-Highway Urban Area. Environmental Science & Technology. 2014;48(6):3272–80. doi: 10.1021/es404838k. In this study, predictors of near-highway particle number concentrations were wind speed and direction, temperature, highway traffic and distance to the highway and major roads. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gale SL, Noth EM, Mann J, Balmes J, Hammond SK, Tager IB. Polycyclic aromatic hydrocarbon exposure and wheeze in a cohort of children with asthma in Fresno, CA. Journal of exposure science & environmental epidemiology. 2012;22(4):386–92. doi: 10.1038/jes.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *53.Jedynska A, Hoek G, Wang M, Eeftens M, Cyrys J, Keuken M, et al. Development of land use regression models for elemental, organic carbon, PAH, and hopanes/steranes in 10 ESCAPE/TRANSPHORM European study areas. Environmental science & technology. 2014;48(24):14435–44. doi: 10.1021/es502568z. This study developed LUR models for PAH, EC, OC and Σhophanes/steranes in 10 areas across Europe and found that predictions of the EC models correlated highly with the predictions of PM2.5. [DOI] [PubMed] [Google Scholar]
- 54.Weichenthal S, Van Ryswyk K, Goldstein A, Shekarrizfard M, Hatzopoulou M. Characterizing the spatial distribution of ambient ultrafine particles in Toronto, Canada: A land use regression model. Environmental pollution. 2015 doi: 10.1016/j.envpol.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Dons E, Van Poppel M, Kochan B, Wets G, Int Panis L. Modeling temporal and spatial variability of traffic-related air pollution: Hourly land use regression models for black carbon. Atmos Environ. 2013;74:237–46. [Google Scholar]
- 56.Larson T, Henderson SB, Brauer M. Mobile Monitoring of Particle Light Absorption Coefficient in an Urban Area as a Basis for Land Use Regression. Environmental Science & Technology. 2009;43(13):4672–8. doi: 10.1021/es803068e. [DOI] [PubMed] [Google Scholar]
- 57.Smargiassi A, Brand A, Fournier M, Tessier F, Goudreau S, Rousseau J, et al. A spatiotemporal land-use regression model of winter fine particulate levels in residential neighbourhoods. Journal of exposure science & environmental epidemiology. 2012;22(4):331–8. doi: 10.1038/jes.2012.26. [DOI] [PubMed] [Google Scholar]
- 58.Saraswat A, Apte JS, Kandlikar M, Brauer M, Henderson SB, Marshall JD. Spatiotemporal land use regression models of fine, ultrafine, and black carbon particulate matter in New Delhi, India. Environmental science & technology. 2013;47(22):12903–11. doi: 10.1021/es401489h. [DOI] [PubMed] [Google Scholar]
- 59.Lee HJ, Koutrakis P. Daily Ambient NO2 Concentration Predictions Using Satellite Ozone Monitoring Instrument NO2 Data and Land Use Regression. Environmental science & technology. 2014;48(4):2305–11. doi: 10.1021/es404845f. [DOI] [PubMed] [Google Scholar]
- 60.Kloog I, Nordio F, Coull BA, Schwartz J. Incorporating local land use regression and satellite aerosol optical depth in a hybrid model of spatiotemporal PM2. 5 exposures in the Mid-Atlantic states. Environmental science & technology. 2012;46(21):11913–21. doi: 10.1021/es302673e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beckerman BS, Jerrett M, Serre M, Martin RV, Lee S-J, van Donkelaar A, et al. A hybrid approach to estimating national scale spatiotemporal variability of PM2. 5 in the contiguous United States. Environmental science & technology. 2013;47(13):7233–41. doi: 10.1021/es400039u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adam-Poupart A, Brand A, Fournier M, Jerrett M, Smargiassi A. Spatiotemporal modeling of ozone levels in Quebec (Canada): a comparison of kriging, land-use regression (LUR), and combined Bayesian maximum entropy-LUR approaches. Environmental health perspectives. 2014;122(9):970–6. doi: 10.1289/ehp.1306566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **63.Su JG, Jerrett M, Meng Y-Y, Pickett M, Ritz B. Integrating smart-phone based momentary location tracking with fixed site air quality monitoring for personal exposure assessment. Science of The Total Environment. 2015;506:518–26. doi: 10.1016/j.scitotenv.2014.11.022. This paper applied momentary location tracking services supplied by smart phones to identify an individual’s location in space-time over Wi-Fi and found that integrating these services with fixed site field monitoring, plus indoor-outdoor air exchange calibration, makes exposure assessment of a very large population over extended periods of time feasible. [DOI] [PubMed] [Google Scholar]
- 64.Delfino RJ, Quintana PJ, Floro J, Gastanaga VM, Samimi BS, Kleinman MT, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environmental health perspectives. 2004;112(8):932–41. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, et al. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect. 2006;114(11):1736–43. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dons E, Int Panis L, Van Poppel M, Theunis J, Wets G. Personal exposure to Black Carbon in transport microenvironments. Atmos Environ. 2012;55:392–8. [Google Scholar]
- 67.Zou B, Wilson JG, Zhan FB, Zeng Y. Air pollution exposure assessment methods utilized in epidemiological studies. Journal of Environmental Monitoring. 2009;11(3):475–90. doi: 10.1039/b813889c. [DOI] [PubMed] [Google Scholar]
- **68.Ryan PH, Son SY, Wolfe C, Lockey J, Brokamp C, LeMasters G. A field application of a personal sensor for ultrafine particle exposure in children. Science of The Total Environment. 2015;508:366–73. doi: 10.1016/j.scitotenv.2014.11.061. This study conducted a field test of a personal sensor for ultrafine particles and found that the sensor can measure near-real time exposures to UFP with high spaciotemporal resolution. [DOI] [PubMed] [Google Scholar]
- 69.Cai J, Yan B, Ross J, Zhang D, Kinney PL, Perzanowski MS, et al. Validation of MicroAeth® as a Black Carbon Monitor for Fixed-Site Measurement and Optimization for Personal Exposure Characterization. Aerosol and Air Quality Research. 2014;14(1):1–9. doi: 10.4209/aaqr.2013.03.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **70.Nieuwenhuijsen MJ, Donaire-Gonzalez D, Rivas I, de Castro M, Cirach M, Hoek G, et al. Variability in and agreement between modeled and personal continuously measured black carbon levels using novel smartphone and sensor technologies. Environmental science & technology. 2015;49(5):2977–82. doi: 10.1021/es505362x. This study used smartphones with tracking software and personal sensors to measure black carbon exposure in children. They found that personal black carbon levels vary substantially throughout the day and that the correlation of modeled and measured black carbon levels was generally good, except for commuting times. [DOI] [PubMed] [Google Scholar]
- 71.Neophytou AM, Hart JE, Chang Y, Zhang JJ, Smith TJ, Garshick E, et al. Short-term traffic related exposures and biomarkers of nitro-PAH exposure and oxidative DNA damage. Toxics. 2014;2(3):377–90. doi: 10.3390/toxics2030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nethery E, Wheeler AJ, Fisher M, Sjodin A, Li Z, Romanoff LC, et al. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. Journal of exposure science & environmental epidemiology. 2012;22(1):70–81. doi: 10.1038/jes.2011.32. [DOI] [PubMed] [Google Scholar]
- 73.Adetona O, Sjodin A, Zheng L, Romanoff LC, Aguilar-Villalobos M, Needham LL, et al. Personal exposure to PM(2.5) and urinary hydroxy-PAH levels in bus drivers exposed to traffic exhaust, in Trujillo, Peru. J Occup Environ Hyg. 2012;9(4):217–29. doi: 10.1080/15459624.2012.666142. [DOI] [PubMed] [Google Scholar]
- 74.Schwarze PE, Totlandsdal AI, Lag M, Refsnes M, Holme JA, Ovrevik J. Inflammation-related effects of diesel engine exhaust particles: studies on lung cells in vitro. BioMed research international. 2013;2013:685142. doi: 10.1155/2013/685142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li YJ, Kawada T, Azuma A. Nrf2 is a protective factor against oxidative stresses induced by diesel exhaust particle in allergic asthma. Oxidative medicine and cellular longevity. 2013;2013:323607. doi: 10.1155/2013/323607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Totlandsdal AI, Cassee FR, Schwarze P, Refsnes M, Lag M. Diesel exhaust particles induce CYP1A1 and pro-inflammatory responses via differential pathways in human bronchial epithelial cells. Part Fibre Toxicol. 2010;7:41. doi: 10.1186/1743-8977-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parnia S, Hamilton LM, Puddicombe SM, Holgate ST, Frew AJ, Davies DE. Autocrine ligands of the epithelial growth factor receptor mediate inflammatory responses to diesel exhaust particles. Respir Res. 2014;15:22. doi: 10.1186/1465-9921-15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ovrevik J, Refsnes M, Totlandsdal AI, Holme JA, Schwarze PE, Lag M. TACE/TGF-alpha/EGFR regulates CXCL8 in bronchial epithelial cells exposed to particulate matter components. Eur Respir J. 2011;38(5):1189–99. doi: 10.1183/09031936.00171110. [DOI] [PubMed] [Google Scholar]
- 79.Skuland T, Ovrevik J, Lag M, Schwarze P, Refsnes M. Silica nanoparticles induce cytokine responses in lung epithelial cells through activation of a p38/TACE/TGF-alpha/EGFR-pathway and NF-kappaBeta signalling. Toxicol Appl Pharmacol. 2014;279(1):76–86. doi: 10.1016/j.taap.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 80.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–97. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? American journal of respiratory and critical care medicine. 2014;190(10):1094–101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 82.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. The Journal of allergy and clinical immunology. 2009;123(5):1185–7. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 83.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104(8):1131–7. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy. 2013;43(9):1018–26. doi: 10.1111/cea.12119. [DOI] [PubMed] [Google Scholar]
- 85.Alyasin S, Karimi MH, Amin R, Babaei M, Darougar S. Interleukin-17 gene expression and serum levels in children with severe asthma. Iranian journal of immunology : IJI. 2013;10(3):177–85. [PubMed] [Google Scholar]
- **86.Brandt EB, Biagini Myers JM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. The Journal of allergy and clinical immunology. 2015 doi: 10.1016/j.jaci.2014.11.043. This study demonstrates that DEP persist in murine lungs for months with increased airway neutrophil and IL17A levels. DEP coexposure with HDM results in increased accumulation of allergen-specific TH2/TH17 cells in the lungs, potentiating HDM recall responses. Exposure to TRAP at birth is associated with earlier sensitization in young children and development of allergic asthma at age 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **87.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. The Journal of allergy and clinical immunology. 2014;134(5):1175–86. e7. doi: 10.1016/j.jaci.2014.05.038. This study highlights that an increased frequency of BALF TH2/TH17 cells is associated with more severe asthma and in vitro glucocorticoid resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. The Journal of experimental medicine. 2010;207(11):2479–91. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshizaki K, Brito JM, Moriya HT, Toledo AC, Ferzilan S, Ligeiro de Oliveira AP, et al. Chronic exposure of diesel exhaust particles induces alveolar enlargement in mice. Respir Res. 2015;16:18. doi: 10.1186/s12931-015-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 91.Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM, et al. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol. 2010;40(9):2450–9. doi: 10.1002/eji.201040461. [DOI] [PubMed] [Google Scholar]
- 92.Ple C, Fan Y, Ait Yahia S, Vorng H, Everaere L, Chenivesse C, et al. Polycyclic aromatic hydrocarbons reciprocally regulate IL-22 and IL-17 cytokines in peripheral blood mononuclear cells from both healthy and asthmatic subjects. PLoS One. 2015;10(4):e0122372. doi: 10.1371/journal.pone.0122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, et al. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8(12):e82545. doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vorrink SU, Domann FE. Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1alpha signaling node. Chemico-biological interactions. 2014;218:82–8. doi: 10.1016/j.cbi.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–84. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–28. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maes T, Provoost S, Lanckacker EA, Cataldo DD, Vanoirbeek JA, Nemery B, et al. Mouse models to unravel the role of inhaled pollutants on allergic sensitization and airway inflammation. Respir Res. 2010;11:7. doi: 10.1186/1465-9921-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li N, Buglak N. Convergence of air pollutant-induced redox-sensitive signals in the dendritic cells contributes to asthma pathogenesis. Toxicol Lett. 2015;237(1):55–60. doi: 10.1016/j.toxlet.2015.05.017. [DOI] [PubMed] [Google Scholar]
- **99.Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, et al. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.02.014. This study demonstrates that traffic related ambiant fine and ultrafine particles increase expression of Notch1 and Jagged1 in bone marrow derived dendritic cells in an Ahr dependent but TLR4, Nerf2 and Nlrp3 independent way. In mice, activation of this cascade exacerbated allergen-induced airway inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol. 2010;185(11):6636–45. doi: 10.4049/jimmunol.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *101.Saravia J, You D, Thevenot P, Lee GI, Shrestha B, Lomnicki S, et al. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal immunology. 2014;7(3):694–704. doi: 10.1038/mi.2013.88. This study demonstrates that exposure to traffic related PM during the first few weeks of life abrogates HDM-induced allergic airway responses. However, mice exposed to PM and OVA as neonates and rechallenged with OVA as adults display exacerbated allergic responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perzanowski MS, Chew GL, Divjan A, Jung KH, Ridder R, Tang D, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. The Journal of allergy and clinical immunology. 2013;131(3):886–93. doi: 10.1016/j.jaci.2012.12.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patel MM, Quinn JW, Jung KH, Hoepner L, Diaz D, Perzanowski M, et al. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environmental research. 2011;111(8):1222–9. doi: 10.1016/j.envres.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosa MJ, Jung KH, Perzanowski MS, Kelvin EA, Darling KW, Camann DE, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, environmental tobacco smoke and asthma. Respir Med. 2011;105(6):869–76. doi: 10.1016/j.rmed.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jedrychowski WA, Perera FP, Maugeri U, Mrozek-Budzyn D, Mroz E, Klimaszewska-Rembiasz M, et al. Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze. Prospective birth cohort study in 4-year olds. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2010;21(4 Pt 2):e723–32. doi: 10.1111/j.1399-3038.2010.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **106.Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol. 2014;134(1):63–72. doi: 10.1016/j.jaci.2013.10.047. In this study, mice exposed first to DEP in utero and then sensitized and challenged with OVA as neonates developped increased allergic airway responses mediated by NK cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mathias CB, Guernsey LA, Zammit D, Brammer C, Wu CA, Thrall RS, et al. Pro-inflammatory role of natural killer cells in the development of allergic airway disease. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014;44(4):589–601. doi: 10.1111/cea.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spira-Cohen A, Chen LC, Kendall M, Lall R, Thurston GD. Personal exposures to traffic-related air pollution and acute respiratory health among Bronx schoolchildren with asthma. Environ Health Perspect. 2011;119(4):559–65. doi: 10.1289/ehp.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **109.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–92. doi: 10.1016/S0140-6736(14)60617-6. This article is a great overall review of outdoor air pollution and its detrimental effect on asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]