Abstract
Introduction
Despite successful preservation of low-frequency hearing in patients undergoing cochlear implantation (CI) with shorter electrode lengths, there is still controversy regarding which electrodes maximize hearing preservation (HP). The thin straight electrode array (TSEA) has been suggested as a full cochlear coverage option for HP. However, very little is known regarding its HP potential.
Methods
A retrospective review was performed at two tertiary academic medical centers, reviewing the electronic records for 52 patients (mean, 58.2 yr; range, 11–85 yr) implanted with the Cochlear Nucleus CI422 Slim Straight (Centennial, CO, USA) electrode array, referred to herein as the thin straight electrode array or TSEA. All patients had a preoperative low-frequency pure-tone average (LFPTA) of 85 dB HL or less. Hearing thresholds were measured at initial activation (t1) and 6 months after activation (t2). HP was assessed by evaluating functional HP using a cutoff level of 85 dB HL PTA.
Results
At t1, 54% of the subjects had functional hearing; 33% of these subjects had an LFPTA between 71 and 85 dB HL, and 17% had an LFPTA between 56 and 70 dB HL. At t2, 47% of the patients had functional hearing, with 31% having an LFPTA between 71 and 85 dB HL.
Discussion
Preliminary research suggests that the TSEA has the potential to preserve functional hearing in 54% of patients at t1. However, 22% (n = 6) of the patients who had functional hearing at t1 (n = 28) lost their hearing between t1 and t2. Further studies are needed to evaluate factors that influence HP with the TSEA electrode and determine the speech perception benefits using electric and acoustic hearing over electric alone.
Keywords: Atraumatic technique, Cochlear implantation, Electroacoustic stimulation, Hearing preservation, Soft technique
Cochlear implantation (CI) has revolutionized the management of patients presenting with severe to profound sensorineural hearing loss. Electrical stimulation can provide postlingually deafened adults with significant improvement in speech understanding; however, it does have limitations. Patients may report a “raspy” or “mechanical” quality to this electrical sound (1). This is because electrical speech processing does not provide the temporal fine structure that is necessary for music and melody representation (2) and improved hearing in noise (3). Historically, CI surgery was associated with unavoidable trauma resulting in loss of residual hearing. However, in 1993, Lehnhardt (4) from Hanover, Germany, theorized that, by using soft surgical techniques during CI, the surgeon might be able to minimize cochlear trauma. This spurred tremendous interest in identifying atraumatic surgical techniques as surgeons sought to preserve residual hearing in this subset of patients and improve the hearing experience by using both acoustic and electric hearing, or electroacoustic stimulation (EAS) (5,6). As hoped, studies evaluating EAS in CI have shown that patients with preserved low-frequency (LF) hearing demonstrate improved speech understanding in noise (5), better music appreciation (7), and improved localization of sound via preservation of binaural timing cues in complex listening environments (2,5–8). The question now is how to maximize hearing preservation (HP) without compromising electrical stimulation, and one of the key factors is the electrode array used.
The thin straight electrode array (TSEA) is publicized as the thinnest flexible straight electrode array in the global market. The 22 active intracochlear electrodes are spaced over 20 mm and allow for variable insertion depths of 20 to 25 mm. The diameter of the array is 0.3 mm at the apical end, tapering to 0.6 mm at the basal end. However, little is known about its potential for HP in CI. It has been hypothesized that full insertion of this array using standard soft surgical techniques would result in minimal trauma and allow for HP in patients with functional LFSH (defined as ≤85-dB 2 pure-tone average [PTA] of 250 and 500 Hz). Recent analysis of HP with varying lengths of electrode arrays has shown that the longer the electrode array, the poorer the HP. The purpose of this article is to obtain preliminary HP results for pa tients implanted with the TSEA who had a preoperative low-frequency PTA (LFPTA) of 85 dB HL or less.
MATERIALS AND METHODS
After institutional review board (IRB) approval at Mayo Clinic, Rochester, MN (IRB 12-001533), and the University of Iowa, Iowa City, IA (IRB 201006734 Iowa Cochlear Implant Clinical Research Registry), a retrospective chart review was performed on patients implanted from June 2012 through September 2013 using the electronic medical record. Patients were included if they met the following criteria: 1) postlingual deafness; 2) preoperative hearing thresholds considered functional, defined here as an LFPTA at 250 and 500 Hz no poorer than 85 dB HL; 3) typical cochlear anatomy; 4) no previous CI in the ear under consideration; and 5) implantation with the Cochlear Nucleus CI422 Slim Straight (Centennial, CO, USA) electrode array, referred to in this article as the TSEA.
Surgical Procedure
All patients underwent CI via a standard post–auricular in cision and a limited mastoidectomy and facial recess approach. All but one patient underwent round window insertion. The general principles of soft surgical technique were followed with some variability between institutions and from surgeon to surgeon. A standard surgical protocol was not used. Factors associated with soft surgical technique were recorded. The general surgical technique has previously been described (9). Intravenous dexamethasone was administered during the procedure. At the University of Iowa, 10 mg was the standard dose. A variable dosage was administered at the Mayo Clinic (0, 4, 8, or 10 mg).
Threshold Assessment
Hearing thresholds were measured before implantation (t0), at implant activation (t1), and 6 months after activation (t2). Behavioral air-conduction hearing thresholds were measured in double-walled sound booths, ANSI S3.1-1999 (10), using a Hugheson-Westlake threshold determination approach, ANSI S3.21-2004 (11), using calibrated audiometers, ANSI S3.6-2004 (12). At each time point, measurements were recorded at 0.25k, 0.5k, 1K, 2K, and 4K Hz. The outcome variable of interest was hearing threshold in units of decibel hearing loss.
Functional HP was defined as an LFPTA at 0.25k and 0.5k dB HL no poorer than 85 dB HL. This was selected because frequency regions with thresholds greater than 85 dB are unlikely to benefit from acoustic amplification (13).The amount of HP, using the LFPTA, was then defined based on a modified version of Clark’s hearing loss classification (14): 1) 25 dB HL or less, 2) 26 to 40 dB HL, 3) 41 to 55 dB HL, 4) 56 to 70 dB HL, 5) 71 to 85 dB HL, and 6) more than 85 dB HL.
Data analyses were completed using SAS version 9.3. Hearing preservation was modeled using general linear mixed models that accounts for repeated measurements across time and fre quencies. Threshold in decibels was set as the dependent variable with time interval (t0, t1, t2) and frequency (250, 500, 1K, 2K, and 4K HZ) as the within-subject factors using an unstructured covariance matrix. Duration of deafness and age at implant, both in years, were used as covariates.
RESULTS
Patient and Surgical Data
Fifty-two patients met inclusion criteria, and their demographics are detailed in Table 1. All patients successfully underwent CI using the TSEA. A single patient underwent a cochleostomy for implant insertion. This was a 71-year-old with a 12-year history of right-sided deafness and a preoperative PTA at 250 and 500 Hz of 77.5 dB, which was considered nonfunctional. The PTA shifted to 105 dB at 3 months and 98 dB at 6 months after surgery. The remainder underwent round window insertion.One patient had an accidental removal of the stimulating electrode during closure and underwent immediate reimplantation. No other intraoperative complications were encountered. Surgical details are reported in Table 2.
TABLE 1.
Demographic data for 52 patients undergoing CI with the TSEA
| Mean (range) | |
|---|---|
| Age at implantation (yr) | 58.2 (11–85) |
| Duration of deafness (yr) | 9.8 (0–50) |
|
| |
| n (%) | |
|
| |
| Sex | |
| Male | 20 (38.5) |
| Female | 32 (61.5) |
| Laterality | |
| Right | 24 (46.2) |
| Left | 28 (53.8) |
| Site of implantation | |
| Mayo Clinic | 26 (50) |
| University of Iowa | 26 (50) |
TABLE 2.
Surgical data for 53 patients undergoing CI with the TSEA
| Surgical variables | Mean (range) |
|---|---|
| Dose of IV steroid (mg) | 7.7 (0–10) |
|
| |
| n (%) | |
|
| |
| Intraoperative IV steroid | |
| Yes | 49 (94.2) |
| No | 3 (5.8) |
| 0 mg | 3 (5.8) |
| 4 mg | 15 (28.8) |
| 8 mg | 8 (15.4) |
| 10 mg | 26 (50.0) |
| Healona | |
| Yes | 13 (25.0) |
| No | 39 (75.0) |
| Site of insertion | |
| Round window | 51 (98.1) |
| Cochleostomy | 1 (1.9) |
| Depth of electrode insertion | |
| First white line (20 mm) | 32 (61.5) |
| Between first and second lines (20–25 mm) | 14 (26.9) |
| Second white line (25 mm) | 6 (11.5) |
Surgical lubricant made of sodium hyaluronate.
Threshold Shift
Average thresholds for each frequency at each test interval are shown in Table 3. The PTA at 250 and 500 Hz shifted by 30 dB HL or less in 76.9% (40 of 52) of patients from t0 to t1 and 67.4% (29 of 43) of patients from t0 to t2. Results showed a significant overall main effect of time interval F2,47 = 45.99, p < 0.0001, frequency F4,47 = 37.47, p < 0.0001, and interaction F8,47 = 5.43, p < 0.0001. Duration of deafness was also statistically significant, F1,47 = 5.84, p = 0.0196, whereas age at implant was not statistically significant F1,47 = 0.98, p = 0.3274. The estimated slope for duration of deafness was 0.27, indicating that a longer duration of deafness is related to higher threshold levels.
TABLE 3.
Average hearing threshold levels and standard deviations (in parentheses) for octave frequencies preoperatively, initial activation, 6 months after activation
| Frequency (Hz) |
|||||
|---|---|---|---|---|---|
| 250 | 500 | 1,000 | 2,000 | 4,000 | |
| t0 | 57.55 (16.51) | 69.62 (15.31) | 86.04 (14.82) | 102.83 (20.84) | 108.96 (23.15) |
| t1 | 81.60 (25.77) | 91.98 (20.41) | 105.57 (15.74) | 112.83 (16.42) | 119.91 (17.44) |
| t2 | 83.30 (29.07) | 94.77 (24.01) | 105.45 (17.78) | 115.45 (15.69) | 122.59 (15.33) |
Because the interaction term was statistically signifi cant, we examine pairwise comparisons on the interaction rather than the main effects. Significant drops in hearing threshold between preoperative and initial activation (t1) were found at all frequency levels (p < 0.0001). However, hearing only changed significantly from t1 to t2 at 500 Hz (p = 0.0314) but not at 250 Hz (p = 0.1069), 1K Hz (p = 0.2534), 2K Hz (p = 0.0755), and 4K Hz (p = 0.0844). Note that if one adjusts the p values for multiple comparisons, all of the t0 to t1 changes remain significant whereas none of the t1 to t2 comparisons remain significant.
Functional Hearing
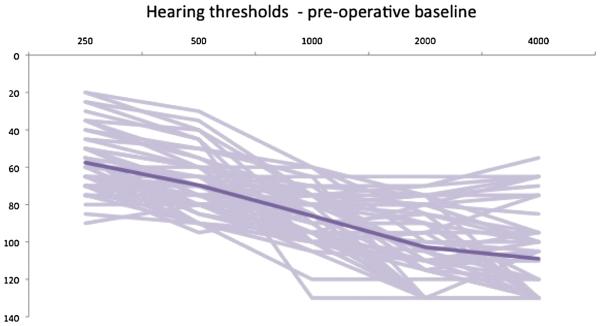
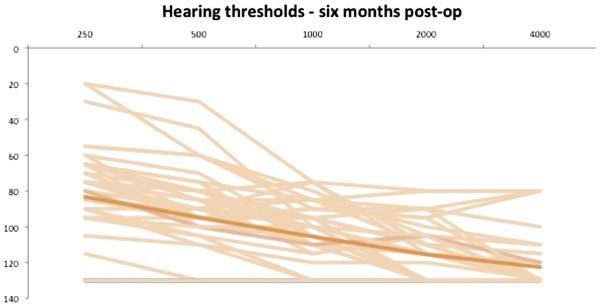
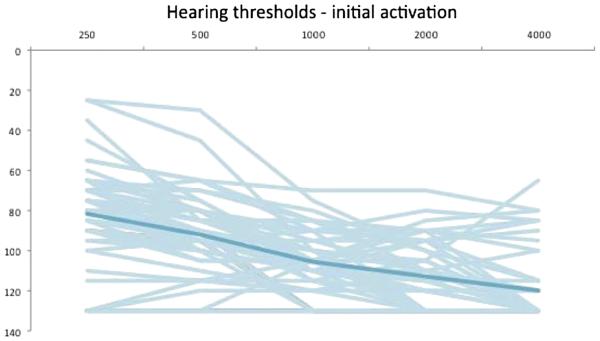
Individual and average threshold data are shown in Figures 1 to 3. The overall group LFPTA was 63.1 dB HL at t0, 86.0 dB HL at t1, and 88.1 dB HL at t2. Percentage and number of patients were classified according to their LFPTA at t0, t1, and t2. This is shown in detail in Table 4. At t2, one subject had not yet reached the 6-month data point. In addition, eight subjects were not retested at t2 because they experienced profound hearing loss at t1. Their PTA from t1 was carried forward to t2. The majority of subjects at t0 had an LFPTA between 56 and 70 dB HL (40%) and 33% had an LFPTA between 71 and 85 dB HL. At t1, functional HP was accomplished in 54% of patients in this study, with the majority of those (33%) having an LFPTA between 71 and 85 dB HL. At t2, functional HP was present in 47% of all patients in the study. Of the 27 patients with functional hearing at t1, 22% (n = 6) of those lost their hearing between initial activation and 6 months after activation.
FIG. 1.
Individual and average (dark line) hearing thresholds at baseline (N = 52).
FIG. 3.
Individual and average (dark line) hearing thresholds 6 months postoperatively (n = 44).
TABLE 4.
Percent of functional hearing for patients undergoing CI with the TSEA device preoperatively (t0), initial activation (t1), and 6 months after activation (t2) using an LFPTA at 0.25k and 0.5k Hz (based on a modified version of Clark’s hearing loss classification) (14)
| PTA (dB HL) Classification (0.25k and 0.5k Hz) |
t0 (%) | t1 (%) | t2 (%) |
|---|---|---|---|
| <25 | 2 | 0 | 2 |
| 26–40 | 10 | 4 | 4 |
| 41–55 | 15 | 0 | 0 |
| 56–70 | 40 | 17 | 10 |
| 71–85 | 33 | 33 | 31 |
| >85 | 0 | 46 | 53 |
| Total N | 52 | 52 | 51 |
The relationships between HP and other surgical factors were also examined. Depth of insertion was assigned values (1 = first band or 20 mm, 2 = between first and second bands or 20 to 25 mm, 3 = second white band or 25 mm). Analysis of variance was used to test for LFPTA differences between the depth levels, but no significant differences were found. Note that all patients from the University of Iowa had a depth = 1, and there were no significant differences both including and excluding the Iowa patients in the analysis. The patient who had accidental removal of the electrode intraoperatively went from an LFPTA of 62.5 dB HL preoperatively to 130 dB HL at t1 and 108 dB HL at t2.
DISCUSSION
Despite successful preservation of LFSH in patients undergoing CI, there remains controversy over which devices should be used to maximize HP and EAS. Although shorter electrodes may minimize trauma to the apical cochlea, they may fail to electrically stimulate the distal cochlear neurons in some with a longer duration of high-frequency hearing loss. This may result in poor performance in the event that LFSH is not preserved after surgery or preserved hearing is lost across time (15). It is important to note that, even when using the shortest electrodes, 100% HP has not been feasible (15). Therefore, investigators are actively searching for an electrode that maximizes acoustic potential without compromising electric potential. The TSEA introduces a straight, medium-length (20–25 mm), thin (0.3–0.6 mm), and flexible option that has the potential to provide broader electrical coverage than the traditional short hybrid arrays and less intracochlear trauma than both the traditional length and advance-off stylet arrays.
The goal of this study was therefore to investigate functional HP for the TSEA in patients who had a pre operative PTA no poorer than 85 dB HL. Thus, all subjects were considered to have functional hearing at the time of CI. Our preliminary results suggest that functional HP is possible using the TSEA, with nearly half of the subjects at initial activation considered to still have aidable hearing. However, at 6 months after activation, an additional 22% of the subjects who had functional hearing after initial activation lost their hearing for a total LFHP rate of 47% at 6 months of cochlear implant experience. Some of the hearing loss at initial activation might be explained by a deeper insertion depth between 20 and 24 mm. However, the mechanism related to the cause of hearing loss after cochlear implant experience is yet to be clarified.
Mick et al. (15) recently investigated HP among 35 adult patients using a 33.5-mm long device with a di ameter of 0.36 mm. Using the old classification system (16), at 1 year after implantation, they found a nearcomplete HP rate of 22% (8 of 36) and partial HP rate of 47% (17 of 36). The shorter TSEA array may result in less intracochlear trauma, thus explaining the higher HP rate, although it is difficult to make comparisons between classification systems, and both the old classification system used by Mick et al. as well as the classification system suggested by Skarzynski et al. (16) include patients who do not have functional HP in their classification system.
As expected, despite the success of the TSEA in preserving LFSH in a significant number of patients, previously de scribed, the rate of HP is less than for patients being implanted with shorter hybrid devices (17–19). However, HP was better for the TSEA compared with perimodiolar electrode array that uses the stiffer “advance-off stylet” technique in a study by Fraysse et al. (20), in which only 26% of patients retained hearing within 20 dB of preoperative levels, suggesting that the flexible array of the TSEA may result in less trauma. Again, variations in definitions make direct comparison challenging.
Finally, although the aims of this study were to focus primarily on the preservation of functional hearing in patients implanted with the TSEA, we were also interested in additional factors that might influence HP outcomes. Surgical variables that have been shown to impact HP include the presence of blood or bone dust at the in sertion site, round window insertion, surgical lubricant such as sodium hyaluronate, administration of a topical steroid, avoidance of perilymph leakage, and suctioning in addition to slow insertion speed (9,21). We did not find an association between age at implantation, duration of deafness, and depth of insertion on hearing outcomes (p > 0.05). Further study will be needed to specifically evaluate each of these factors as they related to HP with the TSEA device.
This study is limited by the retrospective nature of data collection, relatively short follow-up, and the lack of a strict soft technique surgical protocol for CI.
CONCLUSION
Further investigation into the EAS and electrical stimulation–only outcomes associated with the TSEA will aid in assessing the performance of the TSEA in the HP arena and are forthcoming. In the present study, functional low-frequency HP was attained in only 47% of our subjects. In addition, implementing strict soft surgical technique protocols may allow investigators to further identify specific factors that most significantly impact HP outcomes.
FIG. 2.
Individual and average (dark line) hearing thresholds at initial activation (N = 52).
Acknowledgments
Internal departmental funding was used without commercial sponsorship or support.
This study was presented as a podium presentation during the AOS section of the COSM meeting from May 16 to 17, 2014, in Las Vegas, NV.
Footnotes
Colin L. W. Driscoll is a consultant for Cochlear Corporation, Med El Corporation, and Advanced Bionics.
REFERENCES
- 1.Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- 2.Dunn CC, Perreau A, Gantz B, Tyler RS. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol. 2010;21:44–51. doi: 10.3766/jaaa.21.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorman MF, Loizou PC, Fitzke J. The identification of speech in noise by cochlear implant patients and normal-hearing listeners using 6-channel signal processors. Ear Hear. 1998;19:481–4. doi: 10.1097/00003446-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41:356–9. [PubMed] [Google Scholar]
- 5.Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113:1726–30. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol. 2004;124:344–7. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- 7.Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol. 2006;11(Suppl 1):12–15. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- 8.Gifford RH, Dorman MF, Skarzynski H, et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear. 2013;34:413–25. doi: 10.1097/AUD.0b013e31827e8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson ML, Driscoll CLW, Gifford RH, et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol. 2011;32:962–8. doi: 10.1097/MAO.0b013e3182204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American National Standards Institute . Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. (ANSI S3.1- 1999) American National Standards Institute; New York, NY: [Google Scholar]
- 11.American National Standards Institute . Methods for Manual Pure-Tone Threshold Audiometry (ANSI S3.21-2004) American National Standards Institute; New York, NY: [Google Scholar]
- 12.American National Standards Institute . Specifications for Audiometers (ANSI S3.6-2004) American National Standards Institute; New York, NY: [Google Scholar]
- 13.Hornsby BWY, Ricketts TA. The effects of hearing loss on the contribution of high- and low-frequency speech information to speech understanding. II. Sloping hearing loss. J Acoust Soc Am. 2006;119:1752–63. doi: 10.1121/1.2161432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23:493–500. [PubMed] [Google Scholar]
- 15.Mick P, Amoodi H, Shipp D, et al. Hearing preservation with full insertion of the FLEXsoft electrode. Otol Neurotol. 2014;35:e40–4. doi: 10.1097/MAO.0b013e318291c66d. [DOI] [PubMed] [Google Scholar]
- 16.Skarzynski H, van de Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol Suppl. 2013:3–13. doi: 10.3109/00016489.2013.869059. [DOI] [PubMed] [Google Scholar]
- 17.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial. Audiol Neurotol. 2009;14:32–8. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenarz T, Stöver T, Buechner A, Lesinski-Schiedat A, Patrick J, Pesch J. Hearing conservation surgery using the Hybrid-L electrode. Results from the first clinical trial at the Medical University of Hannover. Audiol Neurotol. 2009;1(14 Suppl):22–31. doi: 10.1159/000206492. [DOI] [PubMed] [Google Scholar]
- 19.Skarzynski H, Lorens A, Matusiak M, Porowski M, Skarzynski PH, James CJ. Cochlear implantation with the nucleus thin straight electrode in subjects with residual low-frequency hearing. Ear Hear. 2014;35:e33–43. doi: 10.1097/01.aud.0000444781.15858.f1. [DOI] [PubMed] [Google Scholar]
- 20.Fraysse B, Macías AR, Sterkers O, et al. Residual hearing conservation and electroacoustic stimulation with the nucleus 24 contour advance cochlear implant. Otol Neurotol. 2006;27:624–33. doi: 10.1097/01.mao.0000226289.04048.0f. [DOI] [PubMed] [Google Scholar]
- 21.Friedland DR, Runge-Samuelson C. Soft cochlear implantation: rationale for the surgical approach. Trends Amplif. 2009;13:124–38. doi: 10.1177/1084713809336422. [DOI] [PMC free article] [PubMed] [Google Scholar]