Abstract
Food reinforcement (RRVfood) is related to increased energy intake, cross-sectionally related to obesity, and prospectively related to weight gain. The fat mass and obesity-associated (FTO) gene is related to elevated body mass index and increased energy intake. The primary purpose of the current study was to determine whether any of 68 FTO single nucleotide polymorphisms (SNPs) or a FTO risk score moderate the association between food reinforcement and energy or macronutrient intake. Energy and macronutrient intake was measured using a laboratory ad libitum snack food consumption task in 237 adults of varying BMI. Controlling for BMI, the relative reinforcing value of reading (RRVreading) and proportion of African ancestry, RRVfood predicted 14.2% of the variance in energy intake, as well as predicted carbohydrate, fat, protein and sugar intake. In individual analyses, six FTO SNPs (rs12921970, rs9936768, rs12446047, rs7199716, rs8049933 and rs11076022, spanning approximately 251K bp) moderated the relationship between RRVfood and energy intake to predict an additional 4.9 - 7.4% of variance in energy intake. We created an FTO risk score based on 5 FTO SNPs (rs9939609, rs8050136, rs3751812, rs1421085, and rs1121980) that are related to BMI in multiple studies. The FTO risk score did not increase variance accounted for beyond individual FTO SNPs. Rs12921970 and rs12446047 served as moderators of the relationship between RRVfood and carbohydrate, fat, protein, and sugar intake. This study shows for the first time that the relationship between RRVfood and energy intake is moderated by FTO SNPs. Research is needed to understand how these processes interact to predict energy and macronutrient intake.
Keywords: Food Reinforcement, FTO, Energy Intake, Obesity
1. Introduction
Food reinforcement (RRVfood) refers to the motivation to eat, and is cross-sectionally related to increased energy intake in laboratory and usual intake situations [1, 2], body mass index (BMI) and obesity in children and adults [3, 4] and predicts body fat [5] and weight gain [5, 6] in children and adults. The fat mass and obesity-associated (FTO) gene has been related to elevated BMI [7-9] and increased energy intake [10-13] in adults and children [14, 15]. Animal models suggest that the FTO gene controls food intake through homeostatic mechanisms [16].
Both hedonic and homeostatic processes are involved in the control of food intake [17]. While these are often considered autonomous or independent processes, they may interact to predict food intake. For example, food deprivation, a process that is related to homeostatic mechanisms and the biological need for energy intake [18], also has strong effects on RRVfood and hedonic processes [19, 20]. Understanding the relationships between homeostatic and hedonic controls of food intake may help to explain differences in eating behavior. The primary purpose of the current study was to determine whether FTO single nucleotide polymorphisms (SNPs) interact with RRVfood to predict ad libitum energy intake in 237 adults of varying BMI. Since Frayling and colleagues’ [7] initial report of an FTO variant associated with obesity, many FTO SNPs have been identified which are associated with food intake and body weight [13, 21, 22], but none on how food reinforcement may be moderated by FTO SNPs to influence food intake. The secondary purpose was to determine whether FTO SNPs interact with RRVfood to predict macronutrient intake.
2. Methods
2.1 Participants
A sample of 237 participants (117 males, 120 females; 130 non-obese, 107 obese) from a study of genetic factors associated with food reinforcement was examined for single marker genetic associations. Details of the sample recruitment, inclusion/exclusion criteria [1] and findings [1, 2, 6, 23-25] have been previously published. Participants were excluded from the study if they were taking medications associated with loss of appetite, were smokers, had diabetes, had previously been diagnosed with an eating disorder or psychiatric disorder (e.g. anxiety, depression, attention deficit hyperactivity disorder), were allergic to the ingredients in the study foods, were currently dieting, or did not rate at least a moderate liking (≥4 on a 9 point Likert type scale) for five out of the six study foods. Participants received a $50 gift certificate to local stores for completing the study. The study was approved by the University at Buffalo Health Sciences Institutional Review Board. Participant characteristics are shown in Table 1.
Table 1.
Characteristics and predictions of characteristics with body mass index and energy intake (N = 237).
| Characteristic | BMI (r) | Energy Intake (r) | |
|---|---|---|---|
| Age (years) | 34.5 ± 10.6 | 0.142* | −0.189** |
| BMI | 30.1 ± 7.6 | . | 0.125 |
| Restraint | 7.7 ± 4.7 | −0.006 | −0.263*** |
| Disinhibition | 6.3 ± 3.4 | 0.315*** | 0.099 |
| Hunger | 5.3 ± 3.2 | 0.054 | 0.168** |
| Sex (M/F) | 117/120 | −0.152* | −0.350*** |
| Race/ethnicity | |||
| Non-minority/minority | (176/61) | 0.039 | −0.088 |
| Laboratory intake (kcal) | |||
| Energy intake | 601.2 ± 319.3 | 0.125 | . |
| Fat intake | 275.6 ± 147.2 | 0.121 | 0.997*** |
| Carbohydrate intake | 312.6 ± 164.6 | 0.122 | 0.998*** |
| Protein intake | 134.0 ± 68.1 | 0.192** | 0.908*** |
| Sugar intake | 167.4 ± 91.7 | 0.148** | 0.932*** |
| Food reinforcement task | |||
| RRVfood | 61.8 ± 132.4 | 0.141* | 0.419*** |
| RRVreading | 82.3 ± 114.6 | −0.005 | 0.011 |
| Liking of favorite food | 8.3 ± 0.9 | −0.031 | −0.003 |
Mean ± SD
r = correlation coefficient, RRVfood = Highest fixed ratio schedule completed for food, RRVreading = Highest fixed ratio schedule completed for reading time, liking ratings on a 9 point Likert type scale.
p<0.05.
p<0.01.
p<0.001
2.2 Procedures
Participants visited the laboratory for two sessions, an ad libitum snack-eating session, and a food reinforcement session. Both experimental sessions were scheduled between the hours of 2PM and 5PM, during a normal period that individuals would consume additional energy outside of meal time. Participants were asked to refrain from eating or drinking, with the exclusion of water, for at least 3 h prior to the test session and to refrain from consuming the experimental foods in the 24 h prior to the test session. Upon initial arrival to the laboratory, participants read and signed consent forms, completed a same day and 24 h food recall, hunger questionnaires and were asked to provide a saliva DNA sample. Participants were asked to rinse their mouth with water and then spit into a plastic vial. Prior to the start of each session participants were provided with a preload of a Luna Sunrise Blueberry Bliss, Strawberry Crumble or Vanilla Almond Breakfast bar (Clif Bar & Company; Berkeley, CA, 42g, 150kcal, 4g fat, 23g carbohydrates, 7g protein) to minimize the effects of hunger on energy intake and food reinforcement. Demographic information, height and weight measurements and three dietary habit questionnaires were administered.
2.3 Measurement
2.3.1 Height and weight
The participant's weight and height were measured using a digital scale (TANITA Corporation of America Inc., Arlington Heights, IL) and a digital stadiometer (Measurement Concepts & Quick Medical, North Bend, WA).
2.3.2 Ad libitum eating task
The ad libitum snack food consumption task was presented as a taste test. Participants were provided 210–305 kcal (42–60 g) servings of six palatable, high-energy-dense snack foods (amount of food presented (g) and energy density (kcal/g) shown in parentheses): Wavy Lay's Potato Chips (57 g, 5.4); Cooler Ranch Doritos (56 g, 5.4); M&M's (60 g, 5.0);Twix (48 g, 5.0); Kit Kat (42 g, 5.0); and Butterfinger (57 g, 4.5). Water was provided ad libitum. Participants were told that they could consume as much or as little of the food that they wanted as long as they tasted each food. Participants rated each food on a number of different characteristics including pleasurability, sweetness, blandness, flavorfulness, and bitterness using 9-point Likert-type scales. Food from the taste test was left in the room and participants were told that the food would be discarded after the session and they could continue eating if they choose to do so. When participants indicated that they were finished, they were asked to identify their favorite food from among the six available.
2.3.3 Food reinforcement task
The reinforcing value of food was measured based on responding participants made for food or food alternatives on progressive ratio schedules of reinforcement. The experimental environment included two computer stations that participants could go back and forth between. At one station, participants could earn points toward food and at the other station they could earn points for time to spend reading Time and Newsweek magazines. This alternative activity was provided to reduce the likelihood that participants would engage in responding out of boredom. Participants were instructed on how to use the computer task and given a practice session.
The reinforcement task is similar to a slot machine with shapes that rotate on the screen and a point is earned each time the three shapes match in shape and color. For every five points earned, the subject was able to receive a 70-101 kcal (14 - 20 g) portion of his or her preferred snack food or 2 min of time to spend reading depending on which reward they were working for. The programmed reinforcement schedules for food and reading were progressive fixed ratio schedules with response requirements of 4, 8, 16, 32, 64, 128, 256, 512, 1,024, 2,048 and so forth for each point. Participants were instructed to perform one activity at a time (i.e. play the computer game, eat or read), and that the session would end when they no longer wished to earn points for access to food or time to spend reading. Water was provided ad libitum.
RRVfood was defined as the highest fixed ratio schedule completed for food also known as the breakpoint or Pmax [1]. The RRVreading was used as a covariate and was defined as the highest fixed ratio schedule completed for reading time.
2.3.4 Eating questionnaire
Participants completed the Three Factor Eating Questionnaire (TFEQ) [26], a validated instrument [27] with subscales that assess dietary restraint, hunger and disinhibition.
2.3.5 Food liking, hunger
Subjective ratings of hunger and food hedonics were collected pre- and post intake of the pre-load and after both test sessions. For hunger, 1 indicated not at all hungry/not at all full and 10 indicated extremely hungry/extremely full, while for hedonics 1 indicated not liking at all and 9 indicated liking very much.
2.4 Genotyping
DNA was collected from saliva samples using a commercially available genomic DNA quick preparation kit (Oragene, DNA Genotek, Ottawa, Canada). DNA was extracted from the samples yielding 20μL of DNA at a concentration of 100-130ng/μL. After DNA purification, each sample was stored at −20°C for later analysis.
2.4.1 Genotyping and population stratification
384 SNPs were genotyped on an Illumina Golden gate platform (Illumina, San Diego, CA). The SNPs included 110 markers used to estimate individual (continental) ancestry. The FTO gene was chosen based on the association between FTO and obesity [7-9]. Sixty-eight FTO SNPs were chosen in two ways; as representatives for regions of high linkage disequilibrium (tag SNPs) using the software program TAGGER [28] and SNPs that were especially interesting due to previous associations or functional effects on gene transcription or performance [7-9]. All subsequent genetic analyses were performed using PLINK [29].
2.4.2 Ancestry informative markers
Population stratification controls for individual differences that are correlated with the gene distribution of a subpopulation [30, 31]. We genotyped a panel of 110 SNPs to estimate each individual's genetic proportion of European, Asian and African ancestry using the program STRUCTURE v2 [32]. Proportion African ancestry was used as a covariate in the current study.
2.4.3 FTO risk score
Since the function of individual SNPs may interact with other SNPs to influence how FTO genes may interact with food reinforcement to predict energy or macronutrient intake, we created an FTO risk score based on 5 FTO SNPs (rs9939609, rs8050136, rs3751812, rs1421085, and rs1121980) that are related to BMI in multiple studies and provide data on which allele is the risk allele. The rs9939609 risk allele (A) is related to increased body weight [9, 14], BMI [7, 9, 14, 33-36], waist circumference [34], hip circumference [9], body fat [34], and energy intake [10, 13-15]. The rs8050136 risk allele (A) is related to increased BMI [34-36], waist circumference [34], body fat [34, 37], and energy intake [37]. The rs3751812 risk allele (T) is related to increased BMI [34] and body fat [34]. The rs1421085 risk allele (C) is related to increased BMI [8, 34], waist circumference [34], and body fat [34]. The rs1121980 risk allele (T) is related to increased BMI [33, 34], waist circumference [34], and body fat [34]. Each FTO risk allele was treated equally in calculating the FTO score. For example using 5 FTO SNPs, if an individual was homozygous for all 5 risk alleles they would have a FTO risk score of 10 or if an individual was homozygous for all 11 non-risk alleles they would have a FTO risk score of 0. The individual FTO risk scores ranged from 0 to 10. The mean FTO risk score was 4.2 ± 3.3.
2.5 Analytic plan
The genetic dataset was cleaned by removing participants who were not successfully genotyped for at least 90% of the SNPs. Due to the diversity of our sample, both minor allele frequency (MAF) and Hardy–Weinberg equilibrium (HWE) proportions were examined within and across populations (European American, African American and other based on self-identified ethnicity). Screening within populations removed SNPs if HWE proportions p<0.001. A MAF difference between populations greater than or equal to 30% was considered ancestry informative and was excluded from association analysis of the full population due to potential for false positive associations. Screening within populations removed SNPs if MAF<0.05.
Zero order correlations were used to examine the relationship between BMI, energy intake and participant characteristics. Regression analyses were used to examine the interaction of FTO SNPs and the FTO risk score with RRVfood to predict food intake (total energy intake, and carbohydrate, protein, fat intake, or sugar intake). Covariates to control for population stratification (proportion African ancestry as estimated using STRUCTURE), the RRVreading and BMI were included in all models. False discovery rate was used to control the family wise error rate for all 68 SNP association analyses [38].
Simple slopes were used to examine the moderating association of the models by calculating the slopes of the variants of the FTO SNPs at RRVfood ± 1 standard deviation (SD) from the mean. The regression was graphed using the constant and the coefficients in the regression model to explore the features of the interaction between rs12921970 and RRVfood. Data were analyzed using SYSTAT 11 (Systat Software, 2004).
3.0 Results
Characteristics of the sample are presented in Table 1. The final dataset included 64 of 68 FTO SNPs; 4 were removed based on minor allele frequency racial differences ≥30%. The remaining 64 SNPs had a minor allele frequency>0.05 and did not have any violations of Hardy–Weinberg equilibrium (<0.05).
RRVfood explained 14.2% of the variance in total energy intake (β = 0.371, p < 0.001). Additionally, 6 FTO SNPs (rs9936768, rs8049933, rs7199716, rs12921970, rs12446047, and rs11076022) moderated the relationship between RRVfood and energy intake to predict an additional 4.9 - 7.4% of variance of energy intake, for a total of 19.1 - 21.6% of the variance of energy intake (Table 2). The standardized regression coefficients for the individual SNPs (from each individual analysis) are also presented in Table 2 and the direction of the standardized regression coefficient of the individual SNPs are informative about whether the minor allele of each SNP would likely be considered a ‘risk’ allele (positive standardized regression coefficient) or a ‘protective’ allele (negative standardized regression coefficient).
Table 2.
Standardized regression coefficients (β) and R2 from hierarchical regression models for individual FTO SNPs predicting total energy intake, carbohydrate intake, fat intake, protein intake and sugar intake.a
|
rs9936768 52457064 |
rs8049933 52651631 |
rs7199716 52590749 |
rs12921970 52422727 |
rs12446047 52554803 |
rs11076022 52708479 |
|
|---|---|---|---|---|---|---|
| Total Energy Intake (kcal) | ||||||
| RRVfoodb | 0.371* | 0.371* | 0.371* | 0.371* | 0.371* | 0.371* |
| SNP | −0.111 | 0.024 | 0.129 | 0.096 | −0.039 | 0.063 |
| RRVfood x SNP | −0.323** | −0.295** | 0.318** | 0.294** | 0.344** | 0.306** |
| Total R2 | 0.206 | 0.191 | 0.216 | 0.205 | 0.211 | 0.203 |
| Carbohydrate Intake (kcal) | ||||||
| RRVfood | 0.367* | 0.367* | 0.367* | 0.367* | 0.367* | 0.367* |
| SNP | −0.109 | 0.018 | 0.127 | 0.098 | −0.032 | 0.062 |
| RRVfood x SNP | −0.311** | −0.284** | 0.312** | 0.295** | 0.335** | 0.297** |
| Total R2 | 0.201 | 0.186 | 0.213 | 0.205 | 0.206 | 0.199 |
| Fat Intake (kcal) | ||||||
| RRVfood | 0.375* | 0.375* | 0.375* | 0.375* | 0.375* | 0.375* |
| SNP | −0.114 | 0.029 | 0.132 | 0.094 | −0.038 | 0.064 |
| RRVfood x SNP | −0.332** | −0.305** | 0.318** | 0.293** | 0.349** | 0.311** |
| Total R2 | 0.208 | 0.193 | 0.216 | 0.204 | 0.212 | 0.203 |
| Protein Intake (kcal) | ||||||
| RRVfood | 0.289* | 0.289* | 0.289* | 0.289* | 0.289* | 0.289* |
| SNP | −0.117 | 0.012 | 0.133 | 0.122 | −0.052 | 0.033 |
| RRVfood x SNP | −0.228 | −0.231 | 0.294** | 0.303** | 0.287** | 0.223 |
| Total R2 | 0.172 | 0.164 | 0.203 | 0.206 | 0.183 | 0.165 |
| Sugar Intake (kcal) | ||||||
| RRVfood | 0.364* | 0.364* | 0.364* | 0.364* | 0.364* | 0.364* |
| SNP | −0.114 | −0.012 | 0.133 | 0.105 | −0.060 | 0.090 |
| RRVfood x SNP | −0.253 | −0.246 | 0.216 | 0.276** | 0.264** | 0.270** |
| Total R2 | 0.199 | 0.189 | 0.202 | 0.214 | 0.199 | 0.207 |
p < 0.001
p < 0.000735 (corrected using false discovery estimates for the 68 FTO SNPs studied) using PLINK.
Controlled for proportion African, the highest reinforcement schedule of reading and BMI.
RRVfood = the highest reinforcement schedule completed for access to food or alternatives in the RRV task.
The FTO risk score moderated the relationship between RRVfood and energy intake to predict additional 2.2% (β = 0.152, p = 0.016) of variance of energy intake, for a total of 16.4% of the variance of energy intake. The FTO risk score did not improve the proportion of variance accounted for compared to any of the individual SNPs.
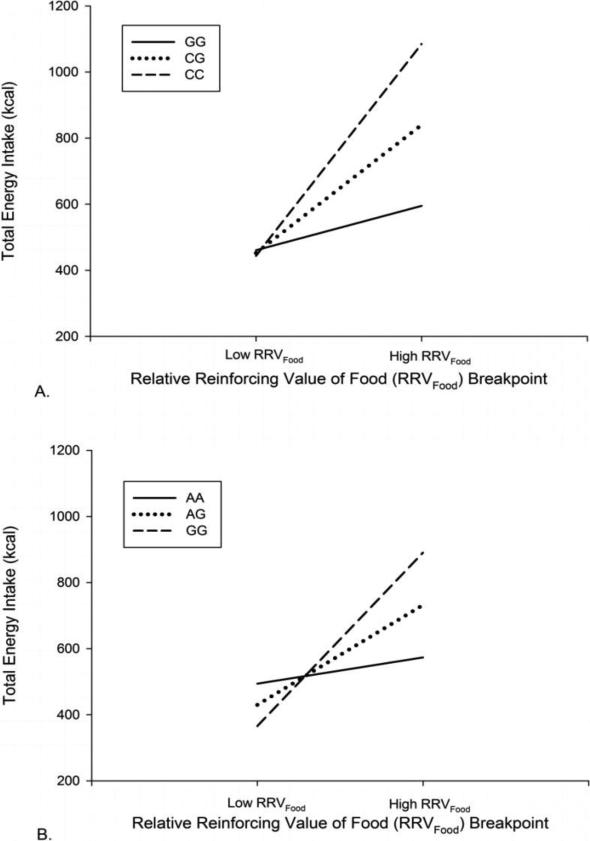
Table 2 demonstrates that in addition to energy intake, all of the FTO SNPs also moderated the relationship between RRVfood, fat intake, and carbohydrate intake (rs9936768, rs8049933, rs7199716, rs12921970, rs12446047, and rs11076022) and some of the FTO SNPs moderated the relationship between RRVfood and protein intake (rs7199716, rs12921970, rs11076022), and sugar intake (rs12921970, rs11076022, rs12446047). While all of the SNPs were significant moderators of RRVfood and energy intake at p < 0.000735 (corrected using false discovery estimates for the 68 FTO SNPs studied), only two SNPs (rs12921970 and rs12446047) moderated the relationship between RRVfood and energy intake plus all the macronutrients. For example, Figure 1 demonstrates that rs12921970 and rs12446047 moderated the relationship between RRVfood and total energy intake.
Figure 1.
The moderating relationship of the variants of the FTO SNPs (A) rs12921970 and (B) rs12446047 on food reinforcement (RRVfood) to predict energy intake. Low and high food reinforcement was defined by ± 1 SD from the mean RRVfood.
4.0 Discussion
High food reinforcement is related to increased energy intake and body weight [4, 39-41], and predicts weight gain [5, 6]. Rather than FTO having a main effect on energy intake as other investigators have shown [10, 13-15, 36], we have shown for the first time that the association of the RRVfood with energy intake is moderated by FTO SNPs and a FTO risk score, suggesting that people with a higher motivation to eat may be impacted more by selected FTO alleles compared to individuals with a lower motivation to eat.
The reinforcing value of food influences energy intake by hedonic mechanisms in addition to the homeostatic control of food intake [17]. In the current study, food reinforcement was a strong predictor of energy intake (14.2%) and accounted for more variance in energy intake than any of the individual FTO SNPs or the FTO risk score suggesting that low food reinforcement is protective against any increased energy intake caused by the FTO SNPs [42].
While food reinforcement is related to food intake in part by hedonic mechanisms, the FTO gene is thought to moderate food intake though homeostatic mechanisms [16]. Using a mouse model, Olszewski and colleagues [16] showed deprivation upregulated FTO mRNA, while consumption of palatable fat or sugar did not alter FTO mRNA, indicating that the expression of the FTO gene is related to homeostatic energy intake. Animal models also provide evidence that the FTO gene is expressed in the arcuate nucleus of the hypothalamus, an area of the brain important for energy homeostasis [16, 43].
While other investigators have demonstrated a relationship between FTO SNPs and energy intake [10-13], individual FTO SNPs may have relatively small genetic contributions to food intake and may be difficult to demonstrate in small populations. The FTO gene is only one of many genetic factors that play a role in energy balance and body weight. Many other human genes have been identified that are related to obesity, for example serotonin receptor, melanocortin 4 receptor, and opioid receptor genes and variants of these genes are related to increased BMI [7, 44-46]. Segal and colleagues [47] analyzed genetic components and environmental components of BMI in monozygotic twins, dizygotic twins and same-age unrelated siblings and estimated that genetic components contribute about 65% [47] to variations in BMI.
Six FTO SNPs (rs9936768, rs8049933, rs7199716, rs12921970, rs12446047, rs11076022) interacted with food reinforcement to predict energy intake, fat intake, and carbohydrate intake, and three FTO SNPs (rs12921970, rs11076022, rs12446047) interacted with food reinforcement to predict protein intake (rs7199716, rs12921970, rs12446047) and sugar intake (rs12921970, rs11076022, rs12446047). Since rs12921970 and rs12446047 interacted with food reinforcement to predict energy intake, fat intake, carbohydrate intake, protein intake and sugar intake, rs12921970 and rs12446047 appear to have a robust relationship with food intake and would be good candidates for future studies to examine the relationships between food intake and FTO genes. These genes would also be good candidates as moderators of other predictors of food intake, for example snacking or fast food intake.
In the current study energy intake was measured using ad libitum food consumption of snack foods. A major limitation to the current study is that the food intake procedure included only high-energy-dense snack foods (Wavy Lay's Potato Chips, Cooler Ranch Doritos, M&M's, Twix, Kit Kat and Butterfinger). Since snack foods are typically highly palatable and reinforcing, the current findings may only apply to highly palatable foods with a high reinforcing value.
In summary, this study is consistent with previous findings that food reinforcement is related to increased energy intake during an ad libitum snack food eating task [3]. For the first time we demonstrate that the association of food reinforcement with energy intake is moderated by FTO SNPs. The pattern of the interaction suggests that low food reinforcement may reduce the impact of FTO risk alleles on energy intake. Rather than provide the same obesity treatment to everyone, interventions need to be developed that target individual subgroups of obesity, for example a treatment could target people with FTO risk alleles. Treatments with the goal of decreasing the reinforcing value of food would be very beneficial to people with FTO risk alleles and high food reinforcement.
Highlights.
Food reinforcement (RRVfood) predicts increased energy intake.
FTO SNPs interacted with RRVfood to predict additional variance in energy intake.
FTO SNPs also moderated the association of RRVfood with macronutrient and sugar intake.
Acknowledgments
Funding: Funded in part by the National Institute of Drug Abuse (Grant No. R01DA024883).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Allison has, anticipates, or has had financial interests with the Frontiers Foundation; Federal Trade Commission; Vivus, Inc; Kraft Foods; University of Wisconsin; University of Arizona; Paul, Weiss, Wharton & Garrison LLP; and Sage Publications.
References
- 1.Epstein LH, Carr KA, Lin H, Fletcher KD. Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr. 2011;94:12–8. doi: 10.3945/ajcn.110.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein LH, Carr KA, Lin H, Fletcher KD, Roemmich JN. Usual energy intake mediates the relationship between food reinforcement and BMI. Obesity. 2012;20:1815–9. doi: 10.1038/oby.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008;87:1121–7. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121:877–86. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7--10-y-old children. Am J Clin Nutr. 2009;90:276–81. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- 6.Carr KA, Lin H, Fletcher KD, Temple J, Epstein LH. Food reinforcement, dietary disinhibition and weight gain in non-obese adults. Obesity. 2013;22:254–9. doi: 10.1002/oby.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougneres P, Kovacs P, Marre M, Balkau B, Cauchi S, Chevre JC, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–6. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 9.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity. 2008;16:1961–5. doi: 10.1038/oby.2008.318. [DOI] [PubMed] [Google Scholar]
- 11.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfalt E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90:1418–25. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- 12.Corella D, Arnett DK, Tucker KL, Kabagambe EK, Tsai M, Parnell LD, Lai CQ, Lee YC, Warodomwichit D, Hopkins PN, Ordovas JM. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr. 2011;141:2219–25. doi: 10.3945/jn.111.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timpson NJ, Emmett PM, Frayling TM, Rogers I, Hattersley AT, McCarthy MI, Davey Smith G. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88:971–8. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–66. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 15.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes. 2009;33:42–5. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- 16.Olszewski PK, Fredriksson R, Olszewska AM, Stephansson O, Alsio J, Radomska KJ, Levine AS, Schioth HB. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci. 2009;10:129. doi: 10.1186/1471-2202-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthoud HR. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity. 2006;14:197S–200S. doi: 10.1038/oby.2006.308. [DOI] [PubMed] [Google Scholar]
- 18.Lutter M, Nestler EJ. Homeostatic and Hedonic Signals Interact in the Regulation of Food Intake. J Nutr. 2009;139:629–32. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 20.Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav. 2003;78:221–7. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- 21.Wing MR, Ziegler J, Langefeld CD, Ng MC, Haffner SM, Norris JM, Goodarzi MO, Bowden DW. Analysis of FTO gene variants with measures of obesity and glucose homeostasis in the IRAS Family Study. Hum Genet. 2009;125:615–26. doi: 10.1007/s00439-009-0656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinney A, Nguyen TT, Scherag A, Friedel S, Bronner G, Muller TD, Grallert H, Illig T, Wichmann HE, Rief W, Schafer H, Hebebrand J. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS One. 2007;2:e1361. doi: 10.1371/journal.pone.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carr KA, Lin H, Fletcher KD, Sucheston L, Singh PK, Salis RJ, Erbe RW, Faith MS, Allison DB, Stice E, Epstein LH. Two functional serotonin polymorphisms moderate the effect of food reinforcement on BMI. Behav Neurosci. 2013;127:387–99. doi: 10.1037/a0032026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein LH, Lin H, Carr KA, Fletcher KD. Food reinforcement and obesity. Psychological moderators. Appetite. 2012;58:157–62. doi: 10.1016/j.appet.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin H, Carr KA, Fletcher KD, Epstein LH. Food Reinforcement Partially Mediates the Effect of Socioeconomic Status on Body Mass Index. Obesity. 2013;21:1307–12. doi: 10.1002/oby.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 27.Allison DB, Kalinsky LB, Gorman BS. A comparison of the psychometric properties of three measures of dietary restraint. Psychological Assessment. 1992;4:391–8. [Google Scholar]
- 28.De Bakker PIW, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–23. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, De Bakker PIW, Daly MJ. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, Kittles R, Alarcon Riquelme ME, Gregersen PK, Belmont JW. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30:69–78. doi: 10.1002/humu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet. 2008;17:R143–50. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotta K, Nakata Y, Matsuo T, Kamohara S, Kotani K, Komatsu R, Itoh N, Mineo I, Wada J, Masuzaki H, Yoneda M, Nakajima A, Miyazaki S, Tokunaga K, Kawamoto M, Funahashi T, Hamaguchi K, Yamada K, Hanafusa T, Oikawa S, Yoshimatsu H, Nakao K, Sakata T, Matsuzawa Y, Tanaka K, Kamatani N, Nakamura Y. Variations in the FTO gene are associated with severe obesity in the Japanese. J Hum Genet. 2008;53:546–53. doi: 10.1007/s10038-008-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wing MR, Ziegler J, Langefeld CD, Ng MCY, Haffner SM, Norris JM, Goodarzi MO, Bowden DW. Analysis of FTO gene variants with measures of obesity and glucose homeostasis in the IRAS Family Study. Hum Genet. 2009;125:615–26. doi: 10.1007/s00439-009-0656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Liu Z, Song YQ, Zhou DZ, Zhang D, Zhao T, Chen Z, Yu L, Yang YF, Feng GY, Li J, Zhang J, Liu SM, Zhang ZF, He L, Xu H. Meta-analysis Added Power to Identify Variants in FTO Associated With Type 2 Diabetes and Obesity in the Asian Population. Obesity. 2010;18:1619–24. doi: 10.1038/oby.2009.469. [DOI] [PubMed] [Google Scholar]
- 36.Nock NL, Plummer SJ, Thompson CL, Casey G, Li L. FTO polymorphisms are associated with adult body mass index (BMI) and colorectal adenomas in African-Americans. Carcinogenesis. 2011;32:748–56. doi: 10.1093/carcin/bgr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haupt A, Thamer C, Staiger H, Tschritter O, Kirchhoff K, Machicao F, Haring HU, Stefan N, Fritsche A. Variation in the FTO Gene Influences Food Intake but not Energy Expenditure. Exp Clin Endocrinol Diabetes. 2009;117:194–7. doi: 10.1055/s-0028-1087176. [DOI] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995:289–300. [Google Scholar]
- 39.Epstein LH, Wright SM, Paluch RA, Leddy J, Hawk LW, Jr., Jaroni JL, Saad FG, Crystal-Mansour S, Lerman C. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol Behav. 2004;81:511–7. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Giesen JC, Havermans RC, Douven A, Tekelenburg M, Jansen A. Will work for snack food: the association of BMI and snack reinforcement. Obesity. 2010;18:966–70. doi: 10.1038/oby.2010.20. [DOI] [PubMed] [Google Scholar]
- 41.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 42.Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–5. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fredriksson R, Hagglund M, Olszewski PK, Stephansson O, Jacobsson JA, Olszewska AM, Levine AS, Lindblom J, Schioth HB. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149:2062–71. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Zhang F, Zhang DD, Chen XD, Lu M, Lin RY, Wen H, Jin L, Wang XF. OPRM1 Gene Is Associated With BMI in Uyghur Population. Obesity. 2009;17:121–5. doi: 10.1038/oby.2008.504. [DOI] [PubMed] [Google Scholar]
- 45.Loos RJF, Lindgren CM, Li SX, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney ASF, Elliott KS, Elliott P, Evans DM, Farooqi IS, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JDM, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O'Rahilly S, Owen KR, Palmer CNA, Papadakis K, Potter S, Pouta A, Qi L, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M, Vogel CIG, Wallace C, Waterworth DM, Weedon MN, Willer CJ, Wraight VL, Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Ovarian PLC, KORA, Study NH, Initiative DG, Study S, Consor WTCC, FUSION Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–75. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kring SII, Werge T, Holst C, Toubro S, Astrup A, Hansen T, Pedersen O, Sorensen TIA. Polymorphisms of Serotonin Receptor 2A and 2C Genes and COMT in Relation to Obesity and Type 2 Diabetes. PLoS One. 2009;4:e6696. doi: 10.1371/journal.pone.0006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal NL, Feng R, McGuire SA, Allison DB, Miller S. Genetic and environmental contributions to body mass index: comparative analysis of monozygotic twins, dizygotic twins and same-age unrelated siblings. Int J Obes. 2009;33:37–41. doi: 10.1038/ijo.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]