Abstract
Fermented brown rice by Aspergillus oryzae (FBRA) is known to have the potential to prevent chemical carcinogenesis of the colon, liver, esophagus, urinary bladder, stomach and lungs in rodents. The present study examined the possible chemopreventive effects of FBRA on N-nitrosobis(2-oxopropyl)amine (BOP)-induced pancreatic tumorigenesis in hamsters. Five-week-old male Syrian golden hamsters were divided into seven groups. Groups 1–5 were subcutaneously injected with BOP (10 mg/kg body weight) four times during week 6 to induce pancreatic tumors, while groups 6 and 7 were injected with saline. Groups 2 and 3 were fed diets containing 5 and 10% FBRA, respectively, during the initiation phase. By contrast, groups 4 and 5 were fed diets containing 5 and 10% FBRA, respectively, during the post-initiation phase. Group 6 received a diet containing 10% FBRA throughout the experiment, and group 7 was kept on the basal diet alone and served as the untreated control. At the termination of the study (week 22), oral intake of 10% FBRA (group 5) during the post-initiation phase was identified to have significantly reduced the multiplicity (number of lesions/animal) of ductal adenocarcinoma [pancreatic intraepithelial neoplasia 3 (PanIN3); carcinoma in situ and invasive carcinoma] in comparison with group 1 control hamsters (0.24±0.44 vs. 0.71±0.72; P<0.05). Treatment with 10% FBRA in the post-initiation phase inhibited the progression of normal/precancerous lesions (PanIN1, mild hyperplastic lesions; and PanIN2, papillary hyperplasia) to ductal adenocarcinomas. Furthermore, dietary exposure to 10% FBRA during the initiation (group 3) and post-initiation phases (group 5) significantly reduced the multiplicity of PanIN2 (group 3, 0.55±0.69; group 5, 0.45±0.69; versus group 1, 1.26±1.24; P<0.05 and P<0.01, respectively). A significant reduction of Ki-67 positivity of PanIN2 in group 5 was also confirmed (group 5, 0.05±0.03; group 1, 0.22±0.12; P<0.01). Using terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling, augmentation of apoptosis by FBRA exposure in the non-lesional ductal epithelium and proliferative lesions was not evident. These findings indicate that FBRA exhibits inhibitory effects on BOP-induced pancreatic tumorigenesis in hamsters due to the reduced proliferation rate of tumor cells. Thus, FBRA may be a promising chemopreventive agent in human pancreatic cancer.
Keywords: fermented brown rice, rice bran, pancreatic cancer
Introduction
Pancreatic cancer is a highly lethal disease that affected ~38,000 individuals in the United States of America in 2013 (1). Early detection is difficult, and it is often too late to provide clinical help to the patient by the time the disease is identified. The poor prognosis of pancreatic cancer arises due to its aggressive local invasion, early metastasis and low responsiveness to conventional chemotherapies. Pancreatic intraepithelial neoplasia (PanIN), which is regarded as a cancer precursor, progresses slowly over a period of years and eventually develops into invasive carcinoma (2). Therefore, preventive strategies to delay the progression or cure this type of dormant pancreatic cancer are currently of interest.
Rice is one of the major cereals, and is the most consumed staple food worldwide, particularly in Asian countries. Rice seeds and rice germ contain fiber and various types of antioxidants, including phenolic acids, phytic acid, tocopherols and γ-oryzanol (3). Numerous cellular and preclinical studies support the hypothesis that antioxidants protect against cancer (4–6). Among them, α-tocopherol has been reported to prevent liver metastasis of chemically-induced pancreatic cancer in animal models (7). Fermented brown rice by Aspergillus oryzae (FBRA) is a processed food prepared by fermenting brown rice and rice bran with A. oryzae. It has been demonstrated that FBRA acts as a potent free-radical scavenger in vitro (8). FBRA has also been shown to exhibit chemopreventive effects against carcinogenesis in the colon (9,10), liver (11), esophagus (12), stomach (13) and urinary bladder (14) of rodents. As FBRA has no systemic toxicity in rodents, its potential chemopreventive effects against chemically-induced pancreatic carcinogenesis are attractive.
The animal model of Syrian golden hamsters injected with N-nitrosobis(2-oxopropyl)amine (BOP) provides pancreatic lesions with similarities to the major forms of pancreatic cancer in humans (15). PanIN lesions, such as mild hyperplastic lesions (PanIN1) and papillary hyperplasia (PanIN2), have been demonstrated to develop into carcinoma in situ (PanIN3) and invasive ductal cancer following the administration of BOP (16). For example, Syrian golden hamsters treated with BOP demonstrate a progression from mild hyperplasic lesions (PanIN1) to papillary hyperplasia (PanIN2) at 8 weeks, to carcinoma in situ (PanIN3) at 12 weeks and to invasive ductal adenocarcinoma at 24 weeks after the injection (17). Furthermore, point mutations in codon 12 of the K-ras gene frequently occur in this hamster pancreatic cancer model, as in human pancreatic tumors (17,18). Similarly, in mouse lung neoplasms induced by the tobacco-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), mutations of the K-ras gene are detected at a high frequency (19). Phutthaphadoong et al (20) revealed that FBRA treatement inhibited NNK-induced lung carcinogenesis in mice. In the present study, the chemopreventive activity of FBRA against BOP-induced pancreatic tumorigenesis was examined in male Syrian golden hamsters by evaluating the development of PanINs and invasive cancer.
Materials and methods
Animals, diet and chemicals
Male 4-week-old Syrian golden hamsters (n=172) were purchased from Japan SLC, Inc. (Hamamatsu, Japan). After a 1-week quarantine, the hamsters were housed between 3–5 animals per cage on pulp-chip bedding in an air-conditioned animal room at 23±2°C and 50±10% humidity. All hamsters were maintained under specific pathogen-free conditions with a 12-h light/dark cycle, with free access to water, and were maintained on a basal diet of MF (Oriental Yeast Co., Ltd. Tokyo, Japan) until they reached 5 weeks of age. The MF diet was used as the basal diet throughout the study. The experimental diets were prepared by mixing 5 and 10% FBRA, which were previously reported as effective concentrations (9–14,20), with the MF diet. FBRA was supplied by Genmai Koso Co., Ltd. (Sapporo, Japan). Briefly, the manufacturing process for FBRA included producing a fermentation base by steaming brown rice and rice bran. A. oryzae was then seeded into the fermentation base and the fermentation process was continued for 18–24 h. Subsequently, a second fermentation was continued for an additional 12–24 h for aging purposes. The fermented product was then dried and powdered. BOP was obtained from Toronto Research Chemicals, Inc. (Ontario, Canada).
Experimental procedure
Five-week-old male Syrian golden hamsters were randomly divided into seven groups (group 1, n=34; groups 2 and 4, n=30; groups 3 and 5, n=29; groups 6 and 7, n=10). The uneven group distribution is due to one hamster in groups 1, 3 and 5 succumbing during the early stages of the experiment. When they were 6 weeks old, the hamsters in groups 1–5 received subcutaneous injections of BOP 4 times (on days 1, 3, 5 and 7) at a dose of 10 mg/kg body weight, whereas the 20 hamsters in groups 6 and 7 were treated with saline as a vehicle control. Group 1 received a basal diet during the experimental period, while the hamsters in groups 2 and 3 were administered with 5 and 10% FBRA in the diet, respectively, during the initiation stage (during and 1 week after BOP treatment) and then were changed to the control diet until they were 27 weeks of age. The hamsters in groups 4 and 5, which were maintained on the basal diet during BOP treatment, received 5% and 10% FBRA in the diet, respectively, during the post-initiation stage (1 week after BOP treatment until 27 weeks of age). The hamsters in group 6 received 10% FBRA in their diet throughout the experimental period. The animals in group 7 served as non-treatment controls. All animals were weighed weekly using a dial scale (DH-3100N; Shinko Denshi Co., Ltd., Tokyo, Japan). At the end of the experiment when the hamsters were 27 weeks old, all surviving animals were sacrificed under deep anesthesia by deep inhalation of isoflurane and blood samples were collected from the aorta after overnight fasting. Serum was obtained from the blood samples by centrifugation at 1,000 × g for 30 min at 4°C and stored at −80°C prior to analysis. The serum levels of triglyceride, total cholesterol, high-density lipoprotein cholesterol and amylase, a pancreatic exocrine enzyme, were measured by SRL, Inc. (Tokyo, Japan). The effects of FBRA on a serum lipid profile possibly associated with chemically-induced pancreatic cancer development in rodents, as well as on amylase, were determined (21).
The animal husbandry and experimental protocols were conducted in accordance with the guidelines for the use and care of experimental animals, and were approved by the Committee for Animal Research and Welfare of Gifu University (Gifu, Japan; project code no. 25-1; approved on April 4, 2013).
Histopathological examination
At necropsy, the pancreas, kidneys, liver and bile duct were carefully examined macroscopically. Four anatomical sections of the pancreas (the gastric and splenic sections, duodenal lobes, and the head portion) were fixed in 10% phosphate-buffered formalin. All tissues were routinely processed, embedded in paraffin, serially sectioned to a thickness of 4 µm, and stained with hematoxylin and eosin (H&E) to assess the histopathological features. Two independent investigators blinded to the sample identity evaluated the sections of pancreas and scored them according to the PanIN criteria within the following categories: PanIN1, PanIN2, PanIN3 and invasive ductal adenocarcinoma (17,22). The tumor incidence (percentage of hamsters with pancreatic lesions) and multiplicity (number of pancreatic lesions per hamster) were calculated on the basis of these scores. In addition, the histopathological features of liver tumors stained with H&E were assessed to distinguish hepatocellular and cholangiocellular tumors, according to previously established criteria (23). Histologically, hepatocellular adenoma presents as single nodules, which compress the surrounding parenchyma. By contrast to normal hepatocytes, those within adenomas usually form solid aggregates or irregular sheets, which vary in cellular thickness. Cholangiocellular adenoma is usually composed of multilocular cystic structures lined by flattened epithelium. Furthermore, cholangiocellular carcinoma, defined as a malignant tumor arising from the intrahepatic ductular epithelial cells, which range from columnar to cuboidal in shape, forms distorted ductal structures.
Ki-67 labeling index of pancreatic proliferative lesions
To assess cell proliferation, the avidin-biotin peroxidase complex technique was used. The paraffin-embedded sections (4 µm) were deparaffinized and rehydrated in phosphate-buffered saline (PBS; pH 7.4, 0.01 mol/l; Wako Pure Chemical Industries, Ltd., Osaka, Japan). The sections were then placed in 10 mmol/l citrate buffer (pH 6.0) and heated in a Pascal pressure cooker (S2800; Dako Japan KK, Tokyo, Japan) programmed for 1 min at 120°C for pretreatment. Endogenous peroxidase activity was blocked by incubation with 3% H2O2 solution for 20 min. After washing three times in PBS (pH 7.4; 0.01 mol/l), the sections were pre-incubated with 2% bovine serum albumin (Wako Pure Chemical Industries, Ltd.) for 40 min at room temperature to block general protein. Subsequently, the sections were incubated with a rabbit anti-hamster monoclonal Ki-67 antibody (cat no. ab16667; 1:100 dilution; Abcam, Cambridge, MA, USA) overnight at 4°C. Sequential incubation with a biotinylated anti-rabbit secondary antibody for 30 min and avidin-coupled peroxidase for 30 min (Vectastain Elite ABC kit, cat no. PK-6101; Vector Laboratory, Burlingame, CA, USA) was performed, according to the manufacturer's instructions. Binding was then visualized with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO, USA). Sections were counterstained with hematoxylin for microscopic examination (BX40; Olympus Corporation, Tokyo, Japan). The proliferating cells were quantified by counting the Ki-67-positive cells at a magnification of ×400. The Ki-67 labeling index of the pancreatic proliferative lesions was determined using Ki-67-stained immunohistochemical sections of between 4–7 hamsters per group, as certain PanINs were too small to evaluate and not all hamsters developed adenocarcinoma. The Ki-67 status was determined in >400 cells in each lesion and non-lesional epithelial sample, and the Ki-67 labeling index was expressed as the percentage of cells stained with the Ki-67 antibody.
Analysis of apoptosis
Apoptotic cells were detected by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay using an in situ Apoptosis Detection kit (Takara Bio Inc., Otsu, Japan). The apoptosis rate was calculated as the number of TUNEL-positive tumor cells as a proportion of the total cell number. Cells were counted at a magnification of ×400 and the mean TUNEL-positive rate was calculated for three or four randomly selected areas. At least 400 cells were counted per lesion for evaluation, following counterstaining with hematoxylin, using a BX40 microscope (Olympus Corporation).
Statistical analysis
The data are expressed as the mean ± standard deviation, and significant differences among the 5 BOP-treated groups were determined using one-way analysis of variance with the Tukey-Kramer post-hoc test. The pathological grade distribution was analyzed using the Kruskal-Wallis test with Dunn's post-hoc test. All statistical analyses were performed using GraphPad Prism software (version 6; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Final body weights, and weights of the liver and kidneys
All animals remained healthy throughout the experimental period. The mean body, liver and kidney weights in all groups at the end of the study are listed in Table I. The mean body and liver weights of the hamsters in groups 4 (BOP→5% FBRA; P<0.0001 and P<0.0001, respectively) and 5 (BOP→10% FBRA; P<0.0001 and P<0.0001, respectively) were significantly higher compared with those of the hamsters in group 1 (BOP alone). Furthermore, the mean relative liver weight of group 4 (P=0.0024) was significantly greater than that of group 1. No significant differences in kidney weight were observed among the groups (P>0.05). There were no clear signs of toxicity in the liver or kidneys of any of the hamsters as examined by histological analysis.
Table I.
Body, liver and kidney weightsa of the hamsters (age, 27 weeks).
| Group | Treatment (no. of animals examined) | Body weight, g | Liver weight, g | Relative liver weight, g/100 g body weight | Kidney weight, g |
|---|---|---|---|---|---|
| 1 | BOP (34) | 166.2±16.2 | 7.3±1.2 | 4.4±0.7 | 1.2±0.2 |
| 2 | BOP+5% FBRA (30) | 174.5±14.2 | 7.8±0.9 | 4.5±0.4 | 1.2±0.3 |
| 3 | BOP+10% FBRA (29) | 167.6±18.2 | 7.5±1.3 | 4.5±0.5 | 1.2±0.2 |
| 4 | BOP→5% FBRA (30) | 186.0±19.1b | 9.4±2.0b | 5.0±0.7b | 1.2±0.2 |
| 5 | BOP→10% FBRA (29) | 186.9±13.2b | 9.1±1.7b | 4.8±0.6 | 1.2±0.1 |
| 6 | 10% FBRA (10) | 191.0±20.3 | 8.7±1.6 | 4.5±0.6 | 1.3±0.2 |
| 7 | Control diet (10) | 189.3±18.4b | 8.2±1.4 | 4.3±0.6 | 1.3±0.1 |
Mean ± standard deviation.
P<0.01 vs. group 1. BOP, N-nitrosobis(2-oxopropyl)amine; FBRA, fermented brown rice by Aspergillus oryzae.
Serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDLC) and amylase levels
The TG, TC, HDLC and amylase levels of the 27-week-old hamsters are listed in Table II. A post-initiation diet of FBRA at a dose of 10% significantly increased the TG level compared with group 1 (P=0.0343). The concentration of amylase in group 4 was significantly higher compared with in group 1 (P=0.0209). However, differences in TG and amylase levels between groups 6 and 7 were not significant (P=0.3134 and P=0.9994, respectively). There were no statistical differences in TC and HDLC levels (P>0.05).
Table II.
Serum levelsa of triglycerides, total cholesterol, HDLC and amylase in hamsters (age, 27 weeks).
| Group | Treatment (no. of animals examined) | Triglycerides, mg/dl | Total cholesterol, mg/dl | HDLC, mg/dl | Amylase, U/l |
|---|---|---|---|---|---|
| 1 | BOP (5) | 216.4±82.9 | 199.4±20.1 | 102.0±6.4 | 1080.4±124.8 |
| 2 | BOP+5% FBRA (5) | 267.2±75.3 | 203.4±31.8 | 98.0±19.2 | 1087.0±183.8 |
| 3 | BOP+10% FBRA (5) | 225.8±65.2 | 188.0±25.9 | 95.5±16.7 | 965.5±167.1 |
| 4 | BOP→5% FBRA (5) | 319.8±44.8 | 195.2±12.4 | 103.2±10.5 | 1502.0±198.9b |
| 5 | BOP→10% FBRA (5) | 392.0±65.6b | 212.6±8.6 | 112.8±7.0 | 1417.0±245.2 |
| 6 | 10% FBRA (5) | 245.4±111.9 | 188.2±25.4 | 103.3±21.5 | 1369.0±189.2 |
| 7 | Control diet (5) | 362.6±113.2 | 190.2±17.6 | 91.8±27.1 | 1317.0±176.0 |
Mean ± standard deviation.
P<0.05 vs. group 1. HDLC, high density lipoprotein cholesterol; BOP, N-nitrosobis(2-oxopropyl)amine; FBRA, fermented brown rice by Aspergillus oryzae.
Incidence and multiplicity of pancreatic proliferative lesions
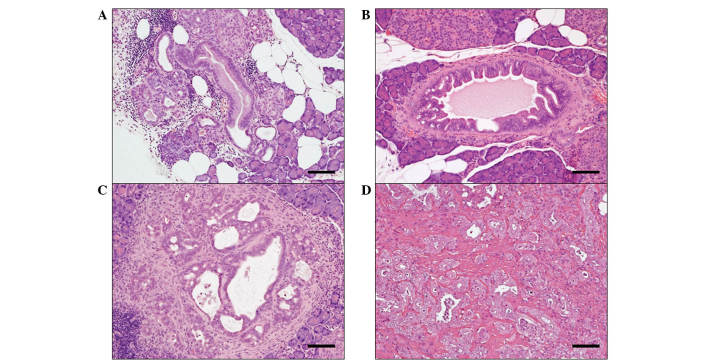
The administration of BOP induced pancreatic proliferative lesions (Fig. 1A and D) that were histopathologically diagnosed as PanIN1 (Fig. 1A), PanIN2 (Fig. 1B), PanIN3 (Fig. 1C) or invasive ductal adenocarcinoma (Fig. 1D). PanIN1s (mild hyperplastic lesions) are flat epithelial lesions composed of tall columnar cells with basally located nuclei or a papillary pseudostratified architecture (24). PanIN2s (papillary hyperplasia) are flat or papillary mucinous lesions with nuclear abnormalities, which may include loss of polarity, nuclear crowding, enlarged nuclei and pseudostratification. PanIN3s (carcinoma in situ) are identified as papillary or micropapillary structures characterized with high-grade dysplasia and loss of nuclear polarity, indicating the development of pancreatic cancer. On the basis of the above criteria and on the histopathological examination of H&E-stained pancreatic tissue, the number and type of PanINs in the pancreatic tissue samples were recorded.
Figure 1.
Histopathological features of representative pancreatic proliferative lesions, including (A) pancreatic intraepithelial neoplasia (PanIN) 1 (mild hyperplastic lesions), (B) PanIN2 (papillary hyperplasia), (C) PanIN3 (carcinoma in situ) and (D) invasive ductal adenocarcinoma (hematoxylin and eosin staining; scale bar, 100 µm).
The incidence of pancreatic proliferative lesions is summarized in Table III. PanINs and invasive ductal adenocarcinomas appeared in groups 1–5. There was no evidence of these lesions in groups 6 and 7. The majority of the animals in group 1 developed ductal adenocarcinomas (56%; including 32% PanIN3 and 24% invasive carcinoma), 41% had precancerous lesions (12% PanIN1 and 29% PanIN2) and one hamster exhibited a normal pancreas with no lesions (3%). Among the animals administered 10% FBRA during the post-initiation phase, 3 hamsters did not develop lesions in the pancreas (10%), 66% had precancerous lesions (42% PanIN1 and 24% PanIN2), and 10 and 14% of the hamsters exhibited PanIN3 and invasive carcinoma, respectively. Thus, treatment with 10% FBRA was associated with a shift in the pathological grade distribution toward normal/precancerous lesions, and significantly inhibited the progression to pancreatic cancer (P=0.0259).
Table III.
Effects of FBRA on the incidence and distribution of pathological lesions in the pancreas.
| Incidence of highest-grade lesions in affected animals, n (%) | ||||||
|---|---|---|---|---|---|---|
| Group | Treatment (no. of animals examined) | Animals with no lesions, n (%) | PanIN1 | PanIN2 | PanIN3 | Invasive ca. |
| 1 | BOP (34) | 1 (3) | 4 (12) | 10 (29) | 11 (32) | 8 (24) |
| 2 | BOP+5% FBRA (30) | 2 (7) | 3 (10) | 13 (43) | 5 (17) | 7 (23) |
| 3 | BOP+10% FBRA (29) | 1 (4) | 9 (31) | 11 (38) | 3 (10) | 5 (17) |
| 4 | BOP→5% FBRA (30) | 0 (0) | 2 (7) | 13 (43) | 7 (23) | 8 (27) |
| 5 | BOP→10% FBRA (29) | 3 (10) | 12 (42) | 7 (24) | 3 (10) | 4 (14) |
| 6 | 10% FBRA (10) | 10 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 7 | Control diet (10) | 10 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Kruskal-Wallis test with Dunn's post-hoc test showed significant differences between groups 1 and 5 (P<0.05). PanIN, pancreatic intraepithelial neoplasia; ca., adenocarcinoma; BOP, N-nitrosobis(2-oxopropyl)amine; FBRA, fermented brown rice by Aspergillus oryzae.
The multiplicity of pancreatic proliferative lesions are indicated in Table IV. The multiplicity of total ductal adenocarcinomas in group 5 (10% FBRA during the post-initiation phase) were significantly reduced compared with group 1 that received BOP alone (0.24±0.44 vs. 0.71±0.72; P=0.0401). The multiplicity of PanIN2 in group 5 was also significantly smaller than that of group 1 (0.45±0.69 vs. 1.26±1.24; P=0.0021). Conversely, the value of PanIN1 in group 5 was greater than the control group. Treatment with 10% FBRA during the initiation phase (group 3) also significantly reduced the multiplicity of PanIN2 compared with group 1 (P=0.0105).
Table IV.
Multiplicity of pancreatic proliferative lesions.
| Lesions/animala, n | ||||||
|---|---|---|---|---|---|---|
| Ductal adenocarcinoma | ||||||
| Group | Treatment (no. of animals examined) | PanIN1 | PanIN2 | PanIN3 | Invasive ca. | Total |
| 1 | BOP (34) | 0.68±0.84 | 1.26±1.24 | 0.44±0.61 | 0.26±0.51 | 0.71±0.72 |
| 2 | BOP+5% FBRA (30) | 0.70±0.79 | 0.87±0.82 | 0.23±0.50 | 0.23±0.43 | 0.47±0.63 |
| 3 | BOP+10% FBRA (29) | 1.00±1.03 | 0.55±0.69b | 0.14±0.35 | 0.21±0.49 | 0.34±0.61 |
| 4 | BOP→5% FBRA (30) | 1.13±0.94 | 0.97±0.61 | 0.37±0.56 | 0.27±0.45 | 0.63±0.76 |
| 5 | BOP→10% FBRA (29) | 1.21±1.01 | 0.45±0.69c | 0.10±0.31 | 0.14±0.35 | 0.24±0.44b |
Mean ± standard deviation.
P<0.05 vs. group 1;
P<0.01 vs. group 1. PanIN, pancreatic intraepithelial neoplasia; ca, adenocarcinoma; BOP, N-nitrosobis(2-oxopropyl)amine; FBRA, fermented brown rice by Aspergillus oryzae.
Cell proliferation and apoptosis in pancreatic proliferative lesions
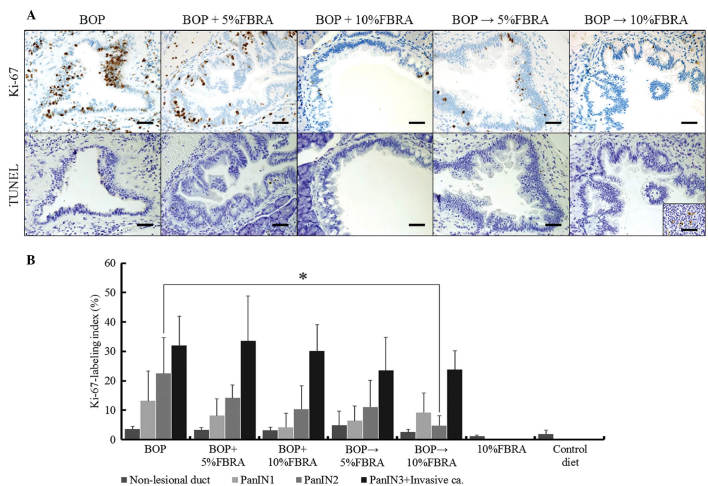
The proliferative potential of pancreatic proliferative lesions were investigated, and the results of the Ki-67-labeling index in the lesions of the hamsters in each group are indicated in Fig. 2A and B. Notably, the Ki-67-labeling index of the PanIN2 in group 5 was significantly lower than that of group 1 (P=0.0054). Groups 6 and 7 were also analyzed; however PanINs and adenocarcinoma were not observed due to a lack of BOP treatment. Furthermore, there was no significant difference in the Ki-67 labeling index of non-lesional ductal epithelia between the two groups (data not shown).
Figure 2.
Ki-67 labeling index and immunohistochemistry of BOP-induced pancreatic proliferative lesions from hamsters treated with or without FBRA. (A) Representative features of Ki-67 immunohistochemical staining and the TUNEL assay in the PanIN2 lesions of hamsters treated with BOP (groups 1–5) (scale bars, 50 µm). (B) Dietary exposure to FBRA significantly reduced the Ki-67 labeling index of the PanIN2 lesions in group 5 compared with those in the hamsters treated with BOP alone (group 1) (*P<0.01). BOP, N-nitrosobis(2-oxopropyl)amine; FBRA, fermented brown rice by Aspergillus oryzae; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; PanIN, pancreatic intraepithelial neoplasia; ca, carcinoma.
The apoptosis of pancreatic proliferative lesions was also investigated. Expression of cells positive for the TUNEL assay were frequently detected in the control section (lymph node; Fig. 2A; inset of group 5). By contrast, positive cells were rarely identified in BOP-induced pancreatic tumors from the different groups, indicating that cellular apoptosis rarely occurs in such tumors (data not shown).
Hepatocellular and cholangiocellular tumors
Liver tumors of hepatocellular or cholangiocellular origin were sporadically observed, but no significant intergroup variations were noted [P=0.5520, 0.5993, 0.5339 and 0.4724, for hepatocellular adenoma, cholangiocellular adenoma, cholangiocellular carcinoma, and cholangiocellular tumors (adenoma + adenocarcinoma), respectively; Table V].
Table V.
Incidences of liver and bile duct tumors induced by BOP.
| Animals with tumors, n (%) | |||||
|---|---|---|---|---|---|
| Cholangiocellular tumors | |||||
| Group | Treatment (no. of animals examined) | Hepatocellular adenoma | Adenoma | Adenocarcinoma | Total |
| 1 | BOP (34) | 2 (6) | 1 (3) | 0 (0) | 1 (3) |
| 2 | BOP+5% FBRA (30) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| 3 | BOP+10% FBRA (29) | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
| 4 | BOP→5% FBRA (30) | 1 (3) | 1 (3) | 1 (3) | 2 (7) |
| 5 | BOP→10% FBRA (29) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
BOP, N-nitrosobis(2-oxopropyl)amine; FBRA, fermented brown rice by Aspergillus oryzae.
Discussion
In the present study, dietary exposure to 10% FBRA during the post-initiation phase of BOP-induced pancreatic carcinogenesis significantly reduced the development of pancreatic ductal adenocarcinoma (PanIN3 and invasive carcinoma). Furthermore, post-initiation feeding of 10% FBRA also resulted in a significant shift in the pathological grade distribution from pancreatic cancerous lesions toward normal/precancerous lesions compared with the predominance of cancerous lesions in group 1, where the animals were treated with BOP alone. Thus, it appears that 10% FBRA was effective for suppressing the progression of precancerous lesions to pancreatic cancers. In BOP-induced pancreatic carcinogenesis, FBRA appears to inhibit the occurrence of the various proliferative lesions throughout tumorigenesis. Accordingly, it is suggested that the suppressive effect of FBRA on pancreatic carcinogenesis is associated with an arrest of the onset of carcinogenesis, and FBRA blocks the transition from PanIN1 to PanIN2 and from PanIN2 to PanIN3.
FBRA has previously been proven to inhibit chemically-induced carcinogenesis in different organs, such as the colon (9,10), liver (11), esophagus (12), stomach (13), urinary bladder (14) and lung (20) of rodents. Taken together, these results and the results of the present study indicate that FBRA is a candidate chemopreventive agent that may protect against carcinogenesis in multiple organs, including the pancreas. Various mechanisms by which chemopreventive agents exert their inhibitory effects on tumorigenesis have been considered. For example, cell proliferation is important in multistage carcinogenesis and involves multiple genetic alterations (25). In the present study, FBRA inhibited the cell proliferation of BOP-induced pancreatic intraductal neoplasia. However, an association between FBRA intake and the induction of apoptosis in the pancreatic lesions was not evident. These results are in line with our previous studies using different models of carcinogenesis (9–14,20). In general, controlling cell proliferation has been proposed to be relevant to carcinogenesis in various organs (26). Indeed, a high level of Ki-67 immunoreactivity is an indicator of poor prognosis of human pancreatic ductal adenocarcinomas (27). In the present study, it was demonstrated that dietary exposure to FBRA during the post-initiation phase reduced tumor development in the pancreas. Furthermore, the Ki-67 scores in PanIN2 lesions were reduced by post-initiation treatment with FBRA. Similar results for FBRA were previously obtained in precancerous lesions in the lungs of mice (20). Thus, inhibiting the proliferative activity in tumor cells may be one of the important mechanisms underlying the chemopreventive effects of FBRA administered at the post-initiation phase.
In the present study, simultaneous exposure to FBRA and BOP decreased the multiplicity of PanIN2. These results are consistent with our previous report, which identified that FBRA suppresses NNK-induced lung carcinogenesis in A/J mice when administered in the initiation phase (20). It has previously been proposed that the chemopreventive effects of FBRA on lung carcinogenesis are predominantly associated with inhibition of the metabolic activation of carcinogens by cytochrome P450s (phase I enzymes). Similarly, the intake of phenethyl isothiocyanate (PEITC) during the initiation phase was reported to inhibit pulmonary and pancreatic tumorigenesis in BOP-treated hamsters (28). Previous studies using mice also demonstrated preventive effects of PEITC against NNK-induced lung carcinogenesis, possibly through the inhibition of cytochrome P450s (29,30). It may be important that BOP and NNK share a metabolic pathway leading to active carcinogenic forms (31). Accordingly, it is possible that the mechanism underlying the anti-initiation effect of FBRA on BOP-induced pancreatic carcinogenesis in hamsters is associated with the inhibition of phase I enzymes.
FBRA is a processed food prepared by the solid-state fermentation of brown rice and rice bran with A. oryzae. Rice antioxidants are particularly abundant in the bran (3). The nutritional and sanitary advantages of fermentation have long been recognized, although the details are not well known. Solid-state fermentation of rice bran has been employed to provide a higher bioavailable content of functional compounds when compared with unfermented bran. Schmidt et al (32) reported that the phenolic compound content in rice bran is increased by fermentation with Rhizopus oryzae. Solid-state fermentation of rice bran can be applied for producing phospholipids, as well as for decreasing the total fat and saturated fatty acids, with an increase of unsaturated fatty acids (33). A previous study reported that a diet high in certain unsaturated fatty acids is associated with a reduced risk of developing pancreatic cancer, whereas a diet high in saturated fatty acids may be associated with an increased risk of human pancreatic cancer (34). Although Takeuchi et al (21) reported that hyperlipidemia may be important for the development of pancreatic cancers in hamsters, the association was not clarified in the current study, which indicated that the inhibitory effects of FBRA on pancreatic tumorigenesis were not associated with serum lipid levels. Considering the aforementioned discussion and the absence of studies in humans, the detailed mechanisms underlying the inhibitory effects of FBRA on pancreatic tumorigenesis require further investigation.
In conclusion, the present study clarified that the administration of FBRA during the initiation and post-initiation phases inhibited pancreatic tumorigenesis and progression induced by BOP in male hamsters. Thus, FBRA is proposed to be a promising agent for the prevention of human pancreatic cancer. In the future, intervention experiments, which involve calorie-regulated high-fat diet supplementation, should be performed to clarify the association between BOP treatment and serum lipid profile, and to investigate cancer chemoprevention by the intake of FBRA.
Acknowledgements
The authors would like to thank Miss Kyoko Takahashi and Mr. Masayoshi Shimizu (Tumor Pathology, Graduate School of Medicine, Gifu University), as well as Mr. Kouji Kato (Experimental Pathology and Tumor Biology, Nagoya City University) for their technical assistance and animal care.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goufo P, Trindade H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, y-oryzanol and phytic acid. Food Sci Nutr. 2014;2:75–104. doi: 10.1002/fsn3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 5.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM. c-Myc can induce DNA damage, increase reactive oxygen species and mitigate p53 function: A mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–1044. doi: 10.1016/S1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 7.Heukamp I, Kilian M, Gregor JI, Neumann A, Jacobi CA, Guski H, Schimke I, Walz MK, Wenger FA. Effects of the antioxidative vitamins A, C and E on liver metastasis and intrametastatic lipid peroxidation in BOP-induced pancreatic cancer in Syrian hamsters. Pancreatology. 2005;5:403–409. doi: 10.1159/000086541. [DOI] [PubMed] [Google Scholar]
- 8.Tazawa K, Fuketa N, Hirohide N. Superoxide scavenging effect of fermented brown rice determined by ESR spin-trapping method. Food Style. 1999;3:32–37. (In Japanese) [Google Scholar]
- 9.Katyama M, Yoshimi N, Yamada Y, Sakata K, Kuno T, Yoshida K, Qiao Z, Vihn PQ, Iwasaki T, Kobayashi H, et al. Preventive effect of fermented brown rice and rice bran against colon carcinogenesis in male F344 rats. Oncol Rep. 2002;9:817–822. [PubMed] [Google Scholar]
- 10.Phutthaphadoong S, Yamada Y, Hirata A, Tomita H, Hara A, Limtrakul P, Iwasaki T, Kobayashi H, Mori H. Chemopreventive effect of fermented brown rice and rice bran (FBRA) on the inflammation-related colorectal carcinogenesis in ApcMin/+mice. Oncol Rep. 2010;23:53–59. [PubMed] [Google Scholar]
- 11.Katayama M, Sugie S, Yoshimi N, Yamada Y, Sakata K, Qiao Z, Iwasaki T, Kobayashi H, Mori H. Preventive effect of fermented brown rice and rice bran on diethylnitrosoamine and phenobarbital-induced hepatocarcinogenesis in male F344 rats. Oncol Rep. 2003;10:875–880. [PubMed] [Google Scholar]
- 12.Kuno T, Hirose Y, Hata K, Kato K, Qiang SH, Kitaori N, Hara A, Iwasaki T, Yoshimura T, Wada K, et al. Preventive effect of fermented brown rice and rice bran on N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats. Int J Oncol. 2004;25:1809–1815. [PubMed] [Google Scholar]
- 13.Tomita H, Kuno T, Yamada Y, Oyama T, Asano N, Miyazaki Y, Baba S, Taguchi A, Hara A, Iwasaki T, et al. Preventive effect of fermented brown rice and rice bran on N-methyl-N'-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Oncol Rep. 2008;19:11–15. [PubMed] [Google Scholar]
- 14.Kuno T, Hirose Y, Yamada Y, Hata K, Qiang SH, Asano N, Oyama T, Zhi H, Iwasaki T, Kobayashi H, et al. Chemoprevention of mouse urinary bladder carcinogenesis by fermented brown rice and rice bran. Oncol Rep. 2006;15:533–538. [PubMed] [Google Scholar]
- 15.Pour P, Althoff J, Krüger FW, Mohr U. A potent pancreatic carcinogen in Syrian hamsters: N-nitrosobis (2-oxopropyl) amine. J Natl Cancer Inst. 1977;58:1449–1453. doi: 10.1093/jnci/58.5.1449. [DOI] [PubMed] [Google Scholar]
- 16.Grandhi BK, Thakkar A, Wang J, Prabhu S. A novel combinatorial nanotechnology-based oral chemopreventive regimen demonstrates significant suppression of pancreatic cancer neoplastic lesions. Cancer Pre Res V (Phila) 2013;6:1015–1025. doi: 10.1158/1940-6207.CAPR-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerny WL, Mangold KA, Scarpelli DG. K-ras mutation is an early event in pancreatic duct carcinogenesis in the Syrian golden hamster. Cancer Res. 1992;52:4507–4513. [PubMed] [Google Scholar]
- 18.Fujii H, Egami H, Chaney W, Pour P, Pelling J. Pancreatic ductal adenocarcinomas induced in Syrian hamsters by N-nitrosobis (2-oxopropyl) amine contain a c-Ki-ras oncogene with a point-mutated codon 12. Mol Carcinog. 1990;3:296–301. doi: 10.1002/mc.2940030510. [DOI] [PubMed] [Google Scholar]
- 19.Yamakawa K, Kuno T, Hashimoto N, Yokohira M, Suzuki S, Nakano Y, Saoo K, Imaida K. Molecular analysis of carcinogen-induced rodent lung tumors: Involvement of microRNA expression and Krαs or Egfr mutations. Mol Med Rep. 2010;3:141–147. doi: 10.3892/mmr_00000231. [DOI] [PubMed] [Google Scholar]
- 20.Phutthaphadoong S, Yamada Y, Hirata A, Tomita H, Taguchi A, Hara A, Limtrakul PN, Iwasaki T, Kobayashi H, Mori H. Chemopreventive effects of fermented brown rice and rice bran against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in female A/J mice. Oncol Rep. 2009;21:321–327. [PubMed] [Google Scholar]
- 21.Takeuchi Y, Takahashi M, Sakano K, Mutoh M, Niho N, Yamamoto M, Sato H, Sugimura T, Wakabayashi K. Suppression of N-nitrosobis(2-oxopropyl)amine-induced pancreatic carcinogenesis in hamsters by pioglitazone, a ligand of peroxisome proliferator-activated receptor gamma. Carcinogenesis. 2007;28:1692–1696. doi: 10.1093/carcin/bgm095. [DOI] [PubMed] [Google Scholar]
- 22.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, et al. Pancreatic intraepithelial neoplasia: A new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Mohr U, Turusov VS, editors. 2nd. III - Tumours of the Hamster. Lyon: IARC Press; 1996. International Agency for Research on Cancer: Pathology of Tumors in Laboratory Animals. [Google Scholar]
- 24.Löhr M, Klöppel G, Maisonneuve P, Lowenfels AB, Lüttges J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: A meta-analysis. Neoplasia. 2005;7:17–23. doi: 10.1593/neo.04445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Reboiras-Lopez MD, Rey Gandara JM, Garcia-Garcia A. Genetic and molecular alterations associated with oral squamous cell cancer (Review) Oncology Reports. 2009;22:1277–1282. doi: 10.3892/or_00000565. [DOI] [PubMed] [Google Scholar]
- 26.Mori H, Sugie S, Yoshimi N, Hara A, Tanaka T. Control of cell proliferation in cancer prevention. Mutat Res. 1999;428:291–298. doi: 10.1016/S1383-5742(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 27.Lundin J, Nordling S, von Boguslawsky K, Roberts PJ, Haglund C. Prognostic value of Ki-67 expression, ploidy and S-phase fraction in patients with pancreatic cancer. Anticancer Res. 1995;15:2659–2668. [PubMed] [Google Scholar]
- 28.Nishikawa A, Furukawa F, Uneyama C, Ikezaki S, Tanakamaru Z, Chung FL, Takahashi M, Hayashi Y. Chemopreventive effects of phenethyl isothiocyanate on lung and pancreatic tumorigenesis in N-nitrosobis (2-oxopropyl) amine-treated hamsters. Carcinogenesis. 1996;17:1381–1384. doi: 10.1093/carcin/17.6.1381. [DOI] [PubMed] [Google Scholar]
- 29.Morse MA, Amin SG, Hecht SS, Chung FL. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res. 1989;49:2894–2897. [PubMed] [Google Scholar]
- 30.Smith TJ, Guo Z, Li C, Ning SM, Thomas PE, Yang CS. Mechanisms of inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone bioactivation in mouse by dietary phenethyl isothiocyanate. Cancer Res. 1993;53:3276–3282. [PubMed] [Google Scholar]
- 31.Nishikawa A, Lee IS, Uneyama C, Furukawa F, Kim HC, Kasahara K, Huh N, Takahashi M. Mechanistic insights into chemopreventive effects of phenethyl isothiocyanate in N-nitrosobis (2-oxopropyl) amine-treated hamsters. Jpn J Cancer Res. 1997;88:1137–1142. doi: 10.1111/j.1349-7006.1997.tb00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt CG, Gonçalves LM, Prietto L, Hackbart HS, Furlong EB. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014;146:371–377. doi: 10.1016/j.foodchem.2013.09.101. [DOI] [PubMed] [Google Scholar]
- 33.Mdos Oliveira S, Feddern V, Kupski L, Cipolatti EP, Badiale-Furlong E, de Souza-Soares LA. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour Technol. 2011;102:8335–8338. doi: 10.1016/j.biortech.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Jansen RJ, Robinson DP, Frank RD, Anderson KE, Bamlet WR, Oberg AL, Rabe KG, Olson JE, Sinha R, Petersen GM, et al. Fatty acids found in dairy, protein and unsaturated fatty acids are associated with risk of pancreatic cancer in a case-control study. Int J Cancer. 2014;134:1935–1946. doi: 10.1002/ijc.28525. [DOI] [PMC free article] [PubMed] [Google Scholar]