Abstract
The aim of the present study was to analyze the clinical significance of the dynamic monitoring of blood lactic acid levels, the oxygenation index and C-reactive protein (CRP) levels in patients with severe pneumonia. The clinical data of 34 cases with severe pneumonia were collected. According to the clinical outcome, the patients were divided into a survival group (n=26) and a fatality group (n=8). Various factors, including the blood lactic acid level, oxygenation index, CRP level and acute physiology and chronic health evaluation II (APACHE II) score, were retrospectively analyzed in order to investigate whether these values had clinical significance for the prognosis of the patients. No statistically significant differences with regard to age, gender, initial concentrations of blood lactic acid and CRP, and APACHE II scores were observed between the two groups at admission to the Intensive Care Unit. However, the blood lactic acid levels were found to decrease to a normal level within 12–24 h after treatment in the survival group, while the levels were maintained at a higher concentration in the fatality group, even at 72 h after treatment (P<0.05). Furthermore, the oxygenation index in the survival group was significantly higher when compared with that in the fatality group. The oxygenation index was maintained at a normal level in the survival group, while the oxygenation index levels were below normal and continued to decline in the fatality group. A positive correlation was observed between the blood lactic acid level and the APACHE II scores (r=0.656, P<0.05). Therefore, the present study demonstrated that dynamic monitoring of blood lactic acid, oxygenation index and CRP levels in patients with severe pneumonia can be used to evaluate the therapeutic efficiency, in addition to serving as a prognosis indicator, for patients with severe pneumonia.
Keywords: severe pneumonia, systemic inflammation response syndrome, blood lactic acid, oxygenation index, C-reactive protein
Introduction
Severe pneumonia complicated with pulmonary infection in clinical practice (1,2) is accompanied by multiple organ dysfunction syndrome characterized by a physiological and pathological foundation, primarily hypoxia and hypoperfusion (3,4). Patients with severe pneumonia are critically ill, and if the disease is not treated in time, proneto water, electrolyte, acid-base balance disorders may occur, causing multiple organ dysfunction and even the emergence of septic shock. Early treatment with antibiotics and supportive therapy is an important measure to improve the prognosis of patients. In addition, timely access to a patients response to treatment, in order to evaluate the prognosis, has important significance for the prognosis of patients in Intensive Care Units (ICUs) and the adjustment of a treatment plan.
Blood lactate levels, oxygen metabolism and tissue perfusion can be used to monitor and assess the systemic index. The level of lactate in the blood reflects the increase in anaerobic metabolism, while the oxygenation index (PaO2/FiO2), moving average for mixed venous oxygen saturation from the whole body perfusion vascular bed values, reflects the balance between the oxygen supply and the oxygen required, which can be used to determine the tissue oxygenation status. Normal values vary between 400 and 500 mmHg (5,6). C-reactive protein (CRP) is the most sensitive acute phase protein. In the process of inflammation, a rapid increase in the blood lactic acid concentration is observed (7–9). PaO2, CRP three combined test, and dynamic observation of, can from different aspects to reflect the association between the oxygen supply and demand and inflammation.
Therefore, it was hypothesized that blood test results may correlate with the severity of pneumonia, and certain parameters may be used as an indicator to identify patients who require intensive care, and subsequently improve the treatment efficacy. Thus, the primary objective of the present study was to assess the correlations between the blood lactic acid level, oxygenation index and CRP level with the mortality risk scores, focusing on the prognosis of patients with acute physiology, age and chronic health evaluation II (APACHE II) score.
Materials and methods
Patient population and sample preparation
In total, 34 patients with severe pneumonia, hospitalized at the ICU of the First Hospital of Jilin University (Changchun, China) between July 1, 2012 and December 31, 2013, were recruited as research subjects. The study was conducted in accordance with the Declaration of Helsinki and with approval from the Ethics Committee of Jilin University. Written informed consent was obtained from all the participants.
Standard of diagnosis for severe pneumonia
Recruited subjects were diagnosed with severe pneumonia according to the diagnostic criteria of severe community-acquired pneumonia outlined by the American Thoracic Society (2007) (10). The main criteria were as follows: i) Respiratory failure requiring mechanical ventilation of the lungs; ii) invasion expansion of >50% within 48 h; iii) an infection or requiring the use of vocative drugs >4 h after the shock; and iv) acute renal failure, as demonstrated by urine levels of <80 ml/4 h or non-chronic renal insufficiency patients with serum creatinine levels of >200 µg/dl. The minor criteria were as follows: i) 7 breaths/>30 owed; ii) PaO2/FiO2 of <250; iii) bilateral or multi leaf inflammation; and iv) systolic and diastolic blood pressure of <90 and <60 mmHg, respectively. The patients exhibited all the major criteria and a minimum of two minor criteria. In total, 26/34 patients survived who comprised the survival group, while there were 16 cases of mortality (fatality group). The demographics of the two groups are shown in Table I.
Table I.
Patient demographics.
| Parameter | Survival group (n=26) | Fatality group (n=8) |
|---|---|---|
| Age, years | 63.24±18.76 | 62.6±18.34 |
| Gender, male/female, n | 14/12 | 5/3 |
| Blood lactic acid, mmol/l | 4.45±1.27 | 4.62±1.43 |
| Oxygenation index, mmHg | 200±50 | 205±45 |
| C-reactive protein, mg/l | 52±10 | 52±8 |
| APACHE II score | 22.84±5.12 | 23.75±3.89 |
APACHE II, acute physiology and chronic health evaluation II.
Exclusion criteria
Patients were excluded from the study if they had not received antibiotic treatment for their clinical symptoms or had lung lesions with marked absorption. Furthermore, previous history of a serious heart, brain, kidney or vascular disease, or cancer, was used as exclusion criteria. In addition, patients were excluded if they suffered from an autoimmune disease, or were suffering from an additional infection. If the patients had undergone ~1 month of a special treatment history, including radiation therapy, chemotherapy, biological treatment and surgical immunosuppressive therapy, they were excluded. Pregnant women were also excluded from the study. The study was approved by the Ethics Committee of the First Hospital of Jilin University, and all subjects signed the informed consent.
Patients and methods
In order to monitor the oxygenation index (PaO2/FiO2) and CRP levels, blood gas analysis and biochemical examination were carried out. In addition, various etiological parameters were recorded in the ICU, as well as the length of stay in the ICU and the number of days of mechanical ventilation, which were used to assess the prognosis of the patients. At 6, 24 and 72 h following treatment, all the patients underwent a review, in which the arterial blood lactate concentration, oxygenation index (PaO2/FiO2), and the 6-h lactic acid clearance rate were calculated (11). Furthermore, the changes in the CRP levels were assessed at 24 and 72 h, and the APACHE II score was calculated at 72 h. Patients were administered bundle treatment following diagnosis, including prior to the routine use of antibiotics and the collection of blood, urine, sputum and any other relevant specimens for bacterial culture, in the hospital l day according to the results of bacterial culture and antibiotics in the treatment of hypotension or experience; blood lactic acid levels of >4 mmol/l, immediate fluid resuscitation, a dose of 20 ml/kg, such as low blood pressure cannot be corrected, while the use of vocative drugs was applied to maintain the mean arterial pressure at >65 mmHg. Sustained hypotension or blood lactic acid levels of >4 mmol/l, for fluid resuscitation, the central venous pressure (CVP) >8 mmHg, SaO2 >90%, urine level of >0.5/ml (kg/h). If the liquid recovery following CVP was 8–12 mmHg, or dobutamine infusion. According to the adjustment of mechanical ventilation parameters of blood gas, liquid amount of vocative drugs, treatment measures. All patients received treatment for 6 h to achieve these goals. Through monitoring and adjusting the treatment protocol with mechanical ventilation and the application of glucocorticoids, hemodynamic stability was maintained and the blood sugar levels were actively controlled, according to the requirements of continuous intravenous infusion of insulin to control the blood sugar level at <8.3 mmol/l.
Statistical analysis
Categorical variables are expressed as a number and percentage, while continuous variables are expressed as the mean ± standard deviation or as the median and interquartile range (IQR), as appropriate. The blood test findings are expressed as the median and IQR. Correlation analysis was performed using Spearmans rank correlation, while the 2-test was used to compare categorical variables. P<0.05 was considered to indicate a statistically significant difference. The commercial statistical software package used was SPSS 15.0 (SPSS, Inc., Chicago, IL, USA). Samples were collected from 34 patients after retro.
Results
Blood test results
Blood lactic acid levels, the APACHE II score and the change in CRP concentration were assessed. As shown in Fig. 1A, the blood lactate levels were significantly lower in the survival group when compared with the fatality group. During the 12–24 h hospital treatment period, the blood lactic acid levels rapidly decreased to normal levels in the survival group. However, in the fatality group, during the observation period, almost no changes were observed in the blood lactate level, with a sustained high state. Fig. 1B indicates that the oxygenation index (PaO2/FiO2) in the survival group was significantly higher when compared with that of the fatality group, and was maintained at a normal level. However, in the fatality group, the oxygenation index (PaO2/FiO2) was lower than the normal level and was shown to continually decline. Furthermore, Fig. 1C shows that the CRP levels in the fatality group at 6, 24 and 72 h were significantly higher when compared with the survival group, and the difference was statistically significant (P<0.05). No statistically significant difference was observed in the CRP level of the fatality group at 24 and 72 h (P=0.17).
Figure 1.
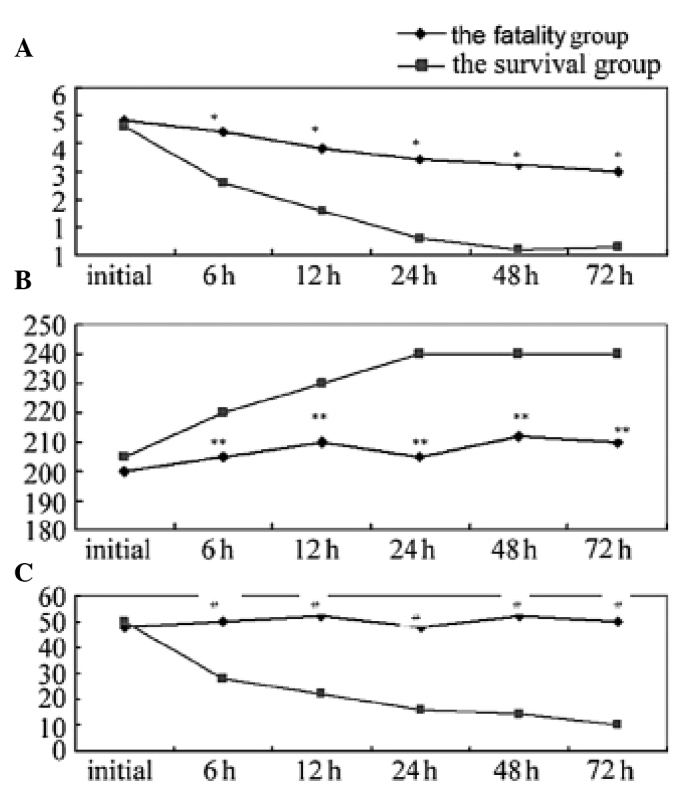
Results from the blood test. (A) Blood lactate levels (mmol/l); (B) Oxygenation index (PaO2/FiO2); (C) Dynamic testing C-reactive protein levels. *P<0.05, **P<0.05 and #P<0.05 vs. the survival group.
Severity of the pneumonia prognosis
Table II shows the lactate clearance rate and the incidence of sepsis in the two groups. The results revealed that the 6-h lactate clearance rate in the survival group was significantly higher when compared with the fatality group, while the incidence of sepsis was significantly lower in the survival group when compared with the fatality group; the differences were statistically significant (P<0.05). In the fatality group, the APACHE II scores were shown to increase in accordance with the arterial blood lactate concentration, and the APACHE score and blood lactate concentration were found to correlate (r=0.656, P<0.05).
Table II.
Lactate clearance rates and the incidence of sepsis in the two groups.
| Group | Initial lactate level, mmol/l | 6-h lactate clearance rate, % | APACHE II score | Sepsis incidence, % (n) |
|---|---|---|---|---|
| Survival | 4.45±1.27 | 29.43±9.71 | 14.6±1.43 | 30.77 (16/52) |
| Fatality | 4.62±1.43 | 11.27±2.61 | 23.2±3.9 | 81.25 (13/16) |
| P-value | 0.43 | 0.01 | <0.001 | <0.001 |
APACHE II, acute physiology and chronic health evaluation II.
Discussion
Severe pneumonia is defined as respiratory insufficiency, which is caused by tissue hypoxia and metabolic dysfunction (12,13). Sequential failure, severe pneumonia in children, the elderly, the frail, immunocompromised patients, children's basic physiology and pathology of special schools, elderly patients prior to the onset of the majority of existing heart, lung disease, mainly in chronic obstructive pulmonary disease, diabetes, coronary heart disease, low immunity, tumor etc. The pathological basis of severe pneumonia infection is the triggering of a series of inflammatory events, including inflammatory cytokine release, which results in hemodynamic changes. For example, elevated endotoxin levels in the blood and an uncontrolled systemic inflammatory response leads to multiple organ failure. Changes to the in vivo microenvironment ##and normal metabolic disorder caused by cells due to a lack of oxygen are the main reasons for the formation of multiple organ dysfunction syndrome (14). CRP is a product of fibrin dissolution, and is increased in inflammatory diseases. Therefore, the protein can be used as an important index to evaluate systemic inflammation. In patients with severe pneumonia, tissue organ effective blood volume reduction, which further exacerbates tissue hypoxia and increases anaerobic metabolism. Blood lactic acid is a product of the anaerobic glycolysis of glucose, and can directly reflect the tissue hypoperfusion and hypoxia conditions. Lactic acidosis is an important index of shock, hypoxia and oxygen metabolism, and quantitative detection and monitoring of the lactic acid levels in patients undergoing recovery from severe pneumonia is an important indicator (6,15,16), with significant value for assessment of the disease. The oxygenation index (PaO2/FiO2) is an indicator of the oxygen supply levels reflect the extracellular, is the average mixed venous oxygen saturation from the whole body perfusion vascular beds value. The index reflects the state of tissue oxygenation and perfusion, tissue oxygen supply and oxygen requirements (17–19). In addition, the oxygenation index (PaO2/FiO2) is an important predictor of the prognosis of patients in a critical condition. Through dynamic monitoring of the oxygenation index (PaO2/FiO2), treatment protocols can be effectively guided, while evaluating the prognosis. Strengthen the monitoring of oxygen metabolism in patients with early, early detection and correct body anoxic condition may improve the survival rate of the patients, the Nguy -en and so on the oxygenation index (PaO2/FiO2) <300 mmHg as septic shock early goal-directed therapy can improve prognosis. The results of the present study demonstrated that the early course of oxygenation index (PaO2/FiO2) for 2 times less than 250 of patients, the prognosis is not good. Therefore, monitoring the oxygenation index (PaO2/FiO2) level, particularly continuous detection for condition assessment, has important clinical significance. In patients with persistent pneumonia, the oxygenation index (PaO2/FiO2) was shown to decrease, indicating that the body was in severe hypoxia. In ICU, positive measures can be applied to increase the oxygen intake, improve microcirculation and reduce oxygen consumption and other measures to correct the severe hypoxia. However, the oxygenation index (PaO2/FiO2) only reflects the systemic oxygen supply and consumption balance; thus, is unable to evaluate the local situation. Therefore, certain studies have attempted to assess the local oxygen saturation and oxygen load using additional methods, with the aim to further understand the tissue oxygen supply for disease detection and the guidance of treatment (20,21).
CRP is an acute phase protein that is an important index of inflammation. Thus, the CRP concentration in patients with infectious diseases, particularly infectious shock and severe infection, increases significantly. The underlying mechanism is hypothesized to primarily involve endotoxin or antigen antibody complexes functioning as activators to directly activate the release of proinflammatory cytokines. Inflammation can be caused by the release of a variety of inflammatory factors, which subsequently leads to hypoxia, acidosis-induced pulmonary microvascular contraction and a slow blood supply. In the present study, the CRP level in the survival group was lower when compared with the fatality group, which exhibited significantly increased levels. If a decrease in the CRP concentration is not evident, or the levels continue to increase, the disease prognosis is not good. Following treatment, Lin Zhejiang (22) detected significantly increased levels of CRP in 48 elderly patients with severe pneumonia, as compared with the healthy group, indicating that body in elderly patients with severe pneumonia in inflammatory activity anomaly detection. Therefore, dynamic monitoring of the CRP level may aid the understanding of the in vivo inflammatory medium water in patients with severe pneumonia, purification for guiding clinical use of anti-inflammatory medium drugs or continuous blood (CRRT) provide the basis, thus reducing the mortality rate. A single blood lactic acid, is the reflection of the oxygen supply and the anaerobic metabolism state, the rain during the ICU treatment, doctors would like to grasp the improving hypoxia in patients after treatment. Therefore, the dynamic determination of the blood lactic acid level may be more meaningful. Nguyen et al (11) demonstrated that in patients with septic shock resuscitation, a 6-h lactic acid clearance rate of ≥10% resulted in the dosage of vocative drugs being significantly lower when compared with the patients who had a low clearance rate, and the mortality rate was also significantly reduced. Therefore, determination of the lactate clearance rate may better reflect the patient prognosis compared with the pure lactic acid concentration. Treatment to effectively reduce the blood lactic acid level is limited; thus, improving the tissue perfusion and oxygenation status is challenging. Subsequently, the progression of the disease may deteriorate and the condition may easily develop into septic shock, with an increased mortality rate. Retrospective analysis of the present study revealed that patients in the survival group had a higher lactate clearance rate when compared with the fatality group, and the decreasing speed, survival group blood lactate within 24 h were normal, while the fatality group until 72 h remained significantly higher compared with the survival group. The APACHE II score is an indicator of vital signs in the patient response, and is an important index in ICU management. In addition, the APACHE II score was shown to be closely associated with the disease state; the higher the score, the more severe the prognosis.
The present study had a number of limitations. Firstly, as the study was retrospective, there was a lack of strict control with regard to case inclusion and exclusion criteria. Secondly, the detection methods have evidently progressed in modern ICU treatment; thus, the majority of patients had a good prognosis, subsequently resulting in the number of cases in the survival and fatality groups being unequal. Therefore, there may be bias in the data statistics. The pathological basis of severe pneumonia is known to be a series of responses to infection, and the present study observed three downstream indicators of the disease, namely a balanced supply of blood oxygen, cell anaerobic metabolism and inflammation. However, the present study only assessed the disease in terms of the developing reaction, and in order to fully understand the prognosis and outcome of severe pneumonia (25,26), other indicators should be considered. For example, numerous factors, including the age of the patient, the basic disease, lung inflammation and treatment measures, may affect the prognosis of patients. Therefore, further study is required in this research direction.
In summary, dynamic monitoring of the levels of blood lactic acid, the oxygenation index and CRP in patients with severe pneumonia can be used to evaluate the therapeutic efficiency, in addition to functioning as a prognosis indicator for patients with severe pneumonia. Although the number of patients included in the present study was low, the results provide a preliminary basis, and are useful and important data that require confirmation in further studies.
Acknowledgements
The authors thank Liping Peng for her assistance with the specimen preparation and processing.
References
- 1.Ethan AH, Alvin S. Management of community-acquired pneumonia. J Assoc Physicians India. 2013;61(Suppl):20–23. (In Chinese) [PubMed] [Google Scholar]
- 2.Di Pasquale M, Ferrer M, Esperatti M, et al. Assessment of severity of ICU-acquired pneumonia and association with etiology. Crit Care Med. 2014;42:303–312. doi: 10.1097/CCM.0b013e3182a272a2. [DOI] [PubMed] [Google Scholar]
- 3.Menter T, Giefing-Kroell C, Grubeck-Loebenstein B, Tzankov A. Characterization of the inflammatory infiltrate in Streptococcus pneumoniae pneumonia in young and elderly patients. Pathobiology. 2014;81:160–167. doi: 10.1159/000360165. [DOI] [PubMed] [Google Scholar]
- 4.Cameron MJ, Ran L, Xu L, et al. Canadian SARS Research Network: Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones AE, Shapiro NI, Trzeciak S, et al. Emergency Medicine Shock Research Network (EMShockNet) Investigators: Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechert RE, Park PK, Bartlett RH. Evaluation of the oxygenation index in adult respiratory failure. J Trauma Acute Care Surg. 2014;76:469–473. doi: 10.1097/TA.0b013e3182ab0d27. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, Takeda S. ECMO for the patients with severe respiratory failure associated with ARDS due to influenza. Masui. 2013;62:557–562. (In Japanese) [PubMed] [Google Scholar]
- 8.Wang T, Xia Y, Hao D, et al. The significance of lactic acid in early diagnosis and goal-directed therapy of septic shock patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:51–55. (In Chinese) [PubMed] [Google Scholar]
- 9.Marti C, Garin N, Grosgurin O, Poncet A, Combescure C, Carballo S, Perrier A. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141. doi: 10.1186/cc11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America; American Thoracic Society: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen HB, Rivers EP, Knoblieh BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. doi: 10.1097/01.CCM.0000132904.35713.A7. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign Management Guidelines Committee: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.CCM.0000117317.18092.E4. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001;29(Suppl):S99–S106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- 15.James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999;354:505–508. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]
- 16.Chalmers JD, Singanayagam A, Hill AT. Predicting the need for mechanical ventilation and/or inotropic support for young adults admitted to the hospital with community-acquired pneumonia. Clin Infect Dis. 2008;47:1571–1574. doi: 10.1086/593195. [DOI] [PubMed] [Google Scholar]
- 17.Miller MJ. Tissue oxygenation in clinical medicine: an historical review. Anesth Analg. 1982;61:527–535. doi: 10.1213/00000539-198206000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Journois D, Safran D. Continuous monitoring of mixed venous blood oxygen saturation. Ann Fr Anesth Reanim. 1993;12:393–408. doi: 10.1016/S0750-7658(05)80107-8. (In French) [DOI] [PubMed] [Google Scholar]
- 19.Krafft P, Steltzer H, Hiesmayr M, Klimscha W, Hammerle AF. Mixed venous oxygen saturation in critically ill septic shock patients. The role of defined events. Chest. 1993;103:900–906. doi: 10.1378/chest.103.3.900. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart K, Kuhn HJ, Hartog C, Bredle DL. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med. 2004;30:1572–1578. doi: 10.1007/s00134-004-2337-y. [DOI] [PubMed] [Google Scholar]
- 21.Kim KM, Ko JS, Gwak MS, Kim GS, Cho HS. Comparison of mixed venous oxygen saturation after in vitro calibration of pulmonary artery catheter with that of pulmonary arterial blood in patients undergoing living donor liver transplantation. Transplant Proc. 2013;45:1916–1919. doi: 10.1016/j.transproceed.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Krüger S, Ewig S, Giersdorf S, et al. German Competence Network for the Study of Community Acquired Pneumonia (CAPNETZ) Study Group: Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: results from the German Competence Network, CAPNETZ. Am J Respir Crit Care Med. 2010;182:1426–1434. doi: 10.1164/rccm.201003-0415OC. [DOI] [PubMed] [Google Scholar]
- 23.Borthwick HA, Brunt LK, Mitchem KL, Chaloner C. Does lactate measurement performed on admission predict clinical outcome on the intensive care unit? A concise systematic review. Ann Clin Biochem. 2012;49:391–394. doi: 10.1258/acb.2011.011227. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes A, Bennett ED. Early goal-directed therapy: An evidence-based review. Crit Care Med. 2004;32(Suppl):S448–S450. doi: 10.1097/01.CCM.0000145945.39002.8D. [DOI] [PubMed] [Google Scholar]
- 25.Dang TT, Eurich DT, Weir DL, Marrie TJ, Majumdar SR. Rates and risk factors for recurrent pneumonia in patients hospitalized with community-acquired pneumonia: population-based prospective cohort study with 5 years of follow-up. Clin Infect Dis. 2014;59:74–80. doi: 10.1093/cid/ciu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamuz J, Viasus D, Jiménez-Martínez E, Isla P, Garcia-Vidal C, Dorca J, Carratalà J. Incidence, timing and risk factors associated with 1-year mortality after hospitalization for community-acquired pneumonia. J Infect. 2014;68:534–541. doi: 10.1016/j.jinf.2014.02.006. [DOI] [PubMed] [Google Scholar]


