Abstract
The aim of this study was to investigate the effects of small interfering RNA (siRNA) targeting human growth hormone receptor (hGHR) combined with 5-fluorouracil (5-FU) on the hepatic metastasis of colon cancer. The animal model of liver metastases using human SW480 colon cancer cells was established on BALB/c mice and the siRNA interfering plasmid targeting hGHR gene was constructed. The tumor-bearing mice were randomly divided into the saline control, plasmid, growth hormone (GH), 5-FU, 5-FU+plasmid and 5-FU+plasmid+GH groups. The liver metastasis in each group was observed. All the animals showed liver metastases and using siRNA-interfering plasmid treatment the incidence of liver metastases was significantly reduced in the tumor groups compared to the saline or GH group. The combined treatment of interfering plasmid and 5-FU slightly decreased the incidence of liver metastases in the tumor groups compared to the plasmid alone or 5-FU alone treatment, although the findings were not statistically significant. On the basis of the combination of interfering plasmid and 5-FU, the additional GH did not increase the incidence of liver metastases (P>0.05), but improved the weight loss of the mice (P<0.05) induced by the inhibition of GHR and toxicity of 5-FU. The present results showed that siRNA targeting hGHR is able to reduce the incidence of liver metastases of human SW480 colon cancer cells in mice. Thus, GHR may be important in tumor metastasis.
Keywords: colonic neoplasms, liver metastasis, BALB/c mice, small interfering RNA
Introduction
Colon cancer cells, which invade the blood circulation, readily form metastases in the liver (1). The current available treatments for the hepatic metastasis of colonic carcinoma mainly focus on surgical removal of the metastasis; however, such treatments do not yield satisfactory results (2,3). Hepatic metastasis is considered the primary reason for the failure of colon cancer treatment in clinic, affecting patient prognosis and long-term survival (survival rate 10–20%) (4). The growth hormone receptor (GHR) genes are located on the fifth chromosome and GHR is widely distributed in human organs and tissues. Findings of a preliminary study showed that GHR is highly expressed in human colorectal carcinoma (5,6). Growth hormone (GH) induces cell differentiation and maturation by combining its receptor GHR with 191 amino acid monomeric peptides that are secreted from the eosinocyte anterior pituitary, initiating the anabolism inside the cells and promoting cell proliferation (7,8). GH/GHR plays an essential role in the occurrence of colon cancer and the development of hepatic metastases. By utilizing the technology of macromolecule interfering, we constructed a plasmid of small interfering RNA (siRNA) to interfere with GHR expression under diseased conditions involving hepatic metastases in colon cancer.
Materials and methods
Animals and cell lines
Thirty-six 8-week-old BALB/c mice with a body mass of 20–22 g were randomly selected from the Vital River Lab Animal Technology Co., Ltd. (Beijing, China). The mice were fed in a specific pathogen-free environment. The SW480 colorectal cancer cell line was purchased from the Cell Resource Center of the Shanghai Institutes for Biological Sciences at the Chinese Academy of Sciences (Shanghai, China). We have obtained the approval of the study by the Ethics Committee of Xiangyang Hospital Affiliated to Hubei University of Medicine and the humane treatment of the mice was ensured.
Cell suspension preparation
The human SW480 colonic cancer cells were cultivated in RPMI-1640 nutrient solution (Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), penicillin (100×103 U/l) and streptomycin (100 mg/l), and then placed in an incubator at 37°C, containing 5% CO2. The cells were collected at the exponential growth stage with 0.25% trypsinase and subsequently mechanically isolated to obtain cell suspension at a centrifugation of 200 × g for 5 min. The supernatant was discarded and moderate normal saline (NS) was added to adjust the cell concentration to 1×107/ml. The cell viability was measured using trypan blue 95% (Chongqing Chemicals Co., Chongqing, China).
Introduction of cancer cells via animal surgery
Mice were weighed prior to anesthesia. Confirmation that animals were anesthetized was indicated by a decrease in limb tension, corneal reflex showing no response and disappearance of skin pain. Aseptically, an oblique incision was performed in the left rear of the animals of 0.5–1.0 cm under the juncture of the left posterior axillary line and costal margin. The abdominal cavity was subsequently exposed and obliquely punctured using a size five needle along the length of the spleen into the membranes below. By forwarding the needle approximately 0.5 cm under the membrane, human SW480 colonic cancer cells were injected into the spleen. Each mouse was injected for 3 min with 0.1 ml cell suspension (or 1×106/mouse). When the spleen membranes swelled and became white, the needle was withdrawn and pressure was applied to the site using cotton for disinfection, avoiding the exposure of cancer cells and bleeding. The spleen was then returned and the abdominal cavity was secured. After the mice recovered, the animals were fed a regular diet.
Construction of siRNA synthesis and eukaryotic expression vector
The mRNA sequence of the human GHR gene was obtained from the GenBank database, and the full-length genomic sequence was 4,414 bp (access no.: X06562, GI: 31737). According to the design principle of siRNA (http://bioinfo.clontech.com/rnaidesigner), the siRNA was designed to the genetic locus of hGHR online which is 1602–1622 bp: TGGTCTCACTCTGCCAAGAAA. The restriction sites for BamHI and HindIII were inserted in the siRNA with enzyme digestion into the eukaryotic expression vector pcDNATM6.2- GW/EmGFPmiRmiRNA, which was designated as pcDNATM6.2-GW/EmGFPmiRmiRNA-GHR-4 (G4).
Interfering particle
The bacterial solution containing the interfering particle was added into the lysozyme nutrient solution and centrifuged at 220 × g overnight at 37°C. An effective and efficient plasmid with high purity was extracted using Plasmid Midiprep kit (high-quality; Sigma) to prepare the plasmid according to the manufacturer's instructions.
Experiment reagents and treatment
TRIzol reagent was purchased from Invitrogen Life Technologies (Carlsbad, CA, USA), and the one-step RT-PCR kit and Qiagen plasmid mini kit were purchased from Qiagen (Hilden, Germany). BamHI and HindIII were produced by Promega Corp. (Madison, WI, USA), 5-fluorouracil (5-FU) was produced in the Hubei Yuancheng Pharmaceutical Co., Ltd. (Wuhan, China) and recombinant human growth hormone (rhGH) was produced by Merck Serono [(Schweiz) AG (Zug, Switzerland)].
Inoculation of animals
The mice were injected on the first day of inoculation with tumor cells. Based on the delivery and dose of treatment, the animals were divided as follows: i) NS, each mouse was injected with 10 µl NS in the abdominal cavity. ii) Plasmid G4, where animals were injected subcutaneously with eukaryotic expression plasmid as 10 µg/mouse. iii) GH, each mouse was injected subcutaneously with rhGH 2 IU/kg. iv) 5-FU, the intraperitoneal injection was applied at a dose of 20 mg of 5-FU/kg. v) FU+G4, intraperitoneal injection at a dose of 20 mg 5-FU/kg and 10 µg G4/mouse. vi) FU+G4+GH, intraperitoneal injection performed as 20 mg of 5-FU/kg, 10 µg of G4/mouse and rhGH 2 IU/kg. The mice in the abovementioned groups were injected once every 3 days and subsequently continually injected 10-fold.
The observation index
Body mass, volume of drinking water, food intake, mental and activity condition were observed on the 1st, 5th, 10th, 15th, 20th, 25th and 30th day piror to and following inoculation. On the 30th day of inoculation, the animals were sacrificed to collect liver and spleen and fixed in 4% of formaldehyde solution. Sections (4 µm) were obtained and paraffin-embedded, followed by hematoxylin and eosin (H&E) staining for histology. The animals were sacrificed by cervical dislocation.
Statistical analysis
Data were shown as mean ± standard deviation, and one-way analysis of variance was used for multi-groups. For comparison between two groups, the q-value was used for analysis. P<0.05 was considered to indicate a statistically significant result.
Results
Animal weight
BALB/c mice inoculated with human SW480 colon cancer cells survived. Following surgery, the mice were weighed every 5 days (Table I). On the first day, the body mass of mice in each group appeared to decrease, with a lack of activeness and a reduction in the consumption of water and food. This lack of activity may be explained by the anesthesia and subsequent surgical procedure. By the 15th day, the mice of the GH group regained their body mass (21.87±0.74) to that prior to the operation (21.93±0.58). By the 30th day, the body mass of the mice (22.00±0.46) increased slightly as compared to that prior to the operation. In the FU+GH group, the weight of the mice was recovered at the 4th week. For the remaining four groups, the body mass of the mice was decreased as compared to that prior to the operation (NS, 21.69±0.37 vs. 20.18±0.35; G4, 21.78±0.6 vs. 20.01±0.49; FU, 21.98±0.53 vs. 19.35±0.42; FU+G4, 21.47±0.68 vs. 19.27±0.57), particularly the FU+G4 group. The decrease for these groups was statistically significant (Table I).
Table I.
The variation of mice body mass in each group of liver metastases.
| Mean body mass for mice from each group (mean ± standard deviation) | ||||||
|---|---|---|---|---|---|---|
| Time | NS | G4 | GH | FU | FU+G4 | FU+G4+GH |
| Prior to surgery | 21.69±0.37 | 21.78±0.6 | 21.93±0.58 | 21.98±0.53 | 21.47±0.68 | 21.75±0.38 |
| 1 day after surgery | 20.15±0.56 | 20.33±0.42 | 20.89±0.67 | 20.43±0.31 | 20.52±0.59 | 20.41±0.48 |
| 5 days after surgery | 20.02±0.45 | 20.14±0.78 | 21.14±0.70a | 20.10±0.56 | 19.69±0.64a | 20.75±1.63 |
| 10 days after surgery | 19.96±0.68 | 19.87±0.68 | 21.22±0.92a | 19.58±0.66 | 19.78±0.98 | 20.98±0.62a,b |
| 15 days after surgery | 19.81±0.38 | 19.85±0.74 | 21.87±0.74a | 19.71±0.45 | 18.87±0.57a | 21.13±0.72a,b |
| 20 days after surgery | 20.01±0.65 | 19.78±0.62a | 21.62±0.73a | 19.65±0.61a | 18.99±0.78a | 21.21±0.63a,b |
| 25 days after surgery | 19.91±0.44 | 19.92±0.23 | 21.80±0.55a | 19.38±0.69 | 19.14±0.65 | 21.13±0.65a,b |
| 30 days after surgery | 20.18±0.35 | 20.01±0.49 | 22.00±0.46a | 19.35±0.42a | 19.27±0.57a | 21.29±0.37a,b |
In comparison with group NS, P<0.05.
In comparison with group G4, P<0.05. NS, normal saline; GH, growth hormone; FU, fluorouracil.
Morphology of liver metastases
Metastatic tumors were identified on the surface of the liver of the BALB/c mice, indicating a 100% increase of th eliver metastatic rate. Liver volume became smaller and the texture appeared as crisp and hard. The metastasis tumor foci of the liver surface were mainly identified in the lobe margin and lobe visceral surface, especially the right lobe. The liver micrometastases for 26 animals showed diffused distribution with hoary appearance. Some tumor surface ulceration was observed, while the hepatic tissue was destroyed (Table II). Different numbers of liver metastases for mice in each group were observed under the microscope. The numbers of liver metastases in the NS group (10.17±1.94) and GH group (10.50±1.38) were significantly higher than those in the G4 group (2.67±137), FU group (3.17±0.98), G4+FU group (2.33±1.03) and G4+GH+FU group (2.17±0.75) (P<0.05). There were no significant differences between the NS and GH groups (10.17±1.94 vs. 10.50±1.38; P>0.5). A low number of liver metastasis was evident in the G4+GH+FU group (2.17±0.75). There were significant differences compared to those of the G4 group (2.67±137), FU group (3.17±0.98) and G4+FU group (2.33±1.03) (P<0.5).
Table II.
Comparison of number of liver metastases for mice in each group.
| Number of liver metastases | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Mean ± SD |
| NS | 9 | 8 | 10 | 9 | 12 | 13 | 10.17±1.94 |
| G4 | 3 | 2 | 2 | 3 | 5 | 1 | 2.67±1.37a |
| GH | 10 | 9 | 11 | 12 | 9 | 12 | 10.50±1.38b |
| FU | 3 | 3 | 3 | 2 | 5 | 3 | 3.17±0.98a |
| FU+G4 | 2 | 2 | 4 | 1 | 3 | 2 | 2.33±1.03a |
| FU+G4+GH | 3 | 1 | 2 | 2 | 3 | 2 | 2.17±0.75a |
In comparison with group NS, P<0.05.
In comparison with group NS, P>0.05. NS, normal saline; GH, growth hormone; FU, fluorouracil; SD, standard deviation.
Histology
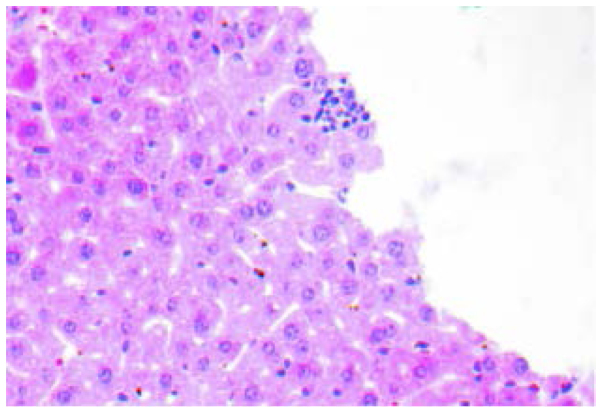
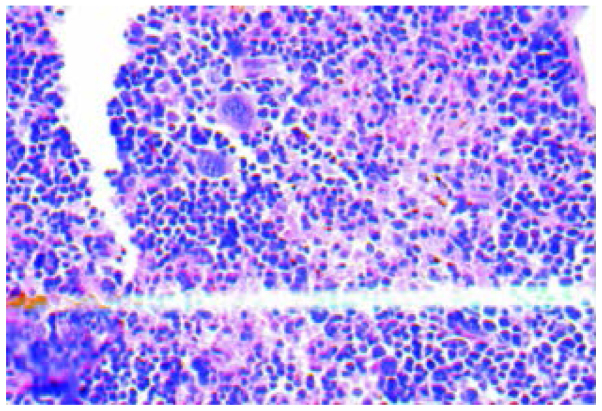
The H&E staining of tissues showed that inner liver metastatic tumors were clustered. The normal liver lobule structure was eradicated, cell volume was reduced, the cancer cell differentiation was poor with obvious atypia and the cytoplasm, karyopyknosis, karyorrhexis, dissolution and mitosis increased (Fig. 1). Various hoary nodes, with a diameter of 0.2–3 mm, were evident in part of the inoculated spleen. The tumor formation rate of orthotopic inoculation was 100%. The cancer cells of the spleen orthotopic inoculation were mainly distributed near the splenic sinusoids with obvious atypia, concentrated in the nucleus and cytoplasm, with the chromosomal becoming hard, and more intense staining (Fig. 2). The morphological structure of the liver metastases was similar to that of the tumor nodules of the spleen orthotopic inoculation, conforming to the structural features of colon low-differentiated adenocarcinoma.
Figure 1.
The liver metastases (hematoxylin and eosin; magnification, ×400). The image is from a representative mouse.
Figure 2.
The inoculated tumor of the spleen in situ (hematoxylin and eosin; magnification, ×400). The image is from a representative mouse.
Discussion
Hepatic metastases occur in almost 500,000 colon cancer patients during disease progression annually (9). The liver is the primary target organ of hematogenous gastric and colorectal metastasis (10), which constitutes the highest hepatic metastatic rate of colorectal cancer in alimentary cancer. Currently, the treatment for hepatic metastasis of colon cancer is mainly focused on hepatectomy while combining the adjuvant therapy of chemo- and radiotherapy. However, surgery may not be a viable treatment option for advanced stage patients. Additionally, post-surgery tumor cells are not sensitive to chemotherapy leading to treatment failure. The patient survival rate following surgery is between 50 and 70% (11–13). Previous studies have focused on the molecular mechanism of hepatic metastases of colorectal cancer to select appropriate genes associated with tumor as therapeutic target in order to identify the relevant therapy (1). Previous findings have shown that organizing the specificity of 5-fluorocytosine/cytosine deaminase to identify a thermochemotherapy system can effectively obtain the targeting and inhibitory effect of hepatic metastases in colon cancer of nude mice (14).
RNAi technology is associated with double-stranded RNA, which is complementary with endogenous mRNA in cells, leading to the specific degradation of mRNA and resulting in mRNA encoding genes not expressing the result of gene silencing. The emergence of RNAi has been beneficial in the study of the function of genes and identification of the target of gene therapy (15). The rhGH is widely used in the surgical field of opsonizing the metabolism, enhancing the immune system, relieving postoperative fatigue, promoting wound healing, maintaining the intestinal mucosal barrier, and reducing bacterial translocation. The rhGH binds to its receptor (GHR) on the cell surface and via the GH-GHR-insulin-like growth factors (IGFs) axis triggers a series of biological effects (16). In recent years, investigators have identified that the GH-GHR-IGFs axis markedly contributes to the occurrence, development and metastasis of maligant tumors (17,18). The expression of colon cancer tissues in GHR is high and rhGH can promote the proliferation, differentiation, and metastasis of tumor cells of postoperative residuals. Previous findings have suggested that rhGH should be employed with care in patients with a high expression of colon cancer (19–25). Considering the role of GHR in tumor metastasis, the aim of the present study was to investigate the GHR response by constructing GHR siRNA. GRH is stimulated following colon surgery, in which the cancer cells flow backward into the vein and then to the liver with blood dissemination leading to hepatic metastasis.
In the present study, we developed a hepatic metastasis mouse model by injecting human SW480 colon cancer cells into the spleen of BALB/c mice. The animals were also treated with GHR siRNA-interfering plasmid and 5-FU was added. The formation of hepatic metastatic tumor in BALB/c mice was investigated. The experimental results showed that GHR siRNA is capable of inhibiting the hepatic metastasis of human SW480 colon cancer cells. In the joint group of FU+G4+GH, a comparison of the inhibition ratio of hepatic metastasis using 5-FU alone for the group FU+G4 yielded no statistically significant difference. However, in order to improve food intake in the body mass of mice, the latter two groups have an advantage over the group of FU+G4+GH. When GH binds with GHR-siRNA and 5-FU, the hepatic metastatic ratio of SW480 cells is not increased. Additionally, GHR-siRNA is capable of selectively inhibiting the metastasis of human SW480 colon cancer cells, confirming the significant role GHR plays in tumor metastasis (26–28). Therefore, results of the present study further enhance understanding for treating colon cancer through the combination therapy of 5-FU and siGHR.
References
- 1.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD): Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tropea A, Biondi A, Corsaro A, Donati M, Basile F, Gruttadauria S. Combined microwave thermal ablation and liver resection for single step treatment of otherwise unresectable colorectal liver metastases; a monoistitutional experiences. Eur Rev Med Pharmacol Sci. 2014;18(Suppl 2):6–10. [PubMed] [Google Scholar]
- 3.Giovinale M, Fonnesu C, Soriano A, Cerquaglia C, Curigliano V, Verrecchia E, De Socio G, Gasbarrini G, Manna R. Atypical sarcoidosis: Case reports and review of the literature. Eur Rev Med Pharmacol Sci. 2009;13(Suppl 1):37–44. [PubMed] [Google Scholar]
- 4.Otchy D, Hyman NH, Simmang C, et al. Standards Practice Task Force; American Society of Colon and Rectal Surgeons: Practice parameters for colon cancer. Dis Colon Rectum. 2004;47:1269–1284. doi: 10.1007/s10350-004-0598-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, Liang D, Zhang Y. The expression of human growth hormone receptor in colon cancer tissues and its clinical significance. China J Curr Adv Gen Surg. 2007;10:490–492. (In Chinese) [Google Scholar]
- 6.Liang DM, Chen JY, Zhang Y. The expression of human growth hormone receptor in rectal cancer tissues. China J Gen Surg. 2009;18:414–416. (In Chinese) [Google Scholar]
- 7.Shen XY, Holt RI, Miell JP, Justice S, Portmann B, Postel-Vinay MC, Ross RJ. Cirrhotic liver expresses low levels of the full-length and truncated growth hormone receptors. J Clin Endocrinol Metab. 1998;83:2532–2538. doi: 10.1210/jcem.83.7.4983. [DOI] [PubMed] [Google Scholar]
- 8.Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. New insights into growth hormone action. J Mol Endocrinol. 2006;36:1–7. doi: 10.1677/jme.1.01933. [DOI] [PubMed] [Google Scholar]
- 9.Wei C, Tan J, Xu L, Juan L, Zhang SW, Wang L, Wang Q. Differential diagnosis between hepatic metastases and benign focal lesions using DWI with parallel acquisition technique: A meta-analysis. Tumour Biol. 2015;36:983–990. doi: 10.1007/s13277-014-2663-9. [DOI] [PubMed] [Google Scholar]
- 10.Waisberg J, Ivankovics IG. Liver-first approach of colorectal cancer with synchronous hepatic metastases: A reverse strategy. World J Hepatol. 2015;7:1444–1449. doi: 10.4254/wjh.v7.i11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watzka FM, Fottner C, Miederer M, Schad A, Weber MM, Otto G, Lang H, Musholt TJ. Surgical therapy of neuroendocrine neoplasm with hepatic metastasis: Patient selection and prognosis. Langenbecks Arch Surg. 2015;400:349–358. doi: 10.1007/s00423-015-1277-z. erratum 359. [DOI] [PubMed] [Google Scholar]
- 12.Kemeny NE, Chou JF, Capanu M, Gewirtz AN, Cercek A, Kingham TP, Jarnagin WR, Fong YC, DeMatteo RP, Allen PJ, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer. 2014;120:3965–3971. doi: 10.1002/cncr.28954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang XY, Zhang XP, Huang JH, Luo RG, Miao BJ, Wang Y. Effects of intra-arterial infusion of 3-bromopyruvate on metastases and survival benefit of hepatic VX2 tumor in rabbits. Zhonghua Yi Xue Za Zhi. 2013;93:3139–3142. (In Chinese) [PubMed] [Google Scholar]
- 14.Cai C. Reflections on clinical problems of hepatic metastasis from gastric and colorectal cancer. China J Gen Surg. 2005;14:721–722. (In Chinese) [Google Scholar]
- 15.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschoep K, Kohlmann A, Schlemmer M, Haferlach T, Issels RD. Gene expression profiling in sarcomas. Crit Rev Oncol Hematol. 2007;63:111–124. doi: 10.1016/j.critrevonc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Zhang B, Wang L. The targeting effect of pro-drug thermochemotherapy on mice hepatic metastasis of gene transfection of colon cancer. China J Gen Surg. 2009;18:348–352. (In Chinese) [Google Scholar]
- 18.Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007;26:456–461. doi: 10.1038/sj.onc.1209796. [DOI] [PubMed] [Google Scholar]
- 19.Campbell GS. Growth-hormone signal transduction. J Pediatr. 1997;131:S42–S44. doi: 10.1016/S0022-3476(97)70010-6. [DOI] [PubMed] [Google Scholar]
- 20.Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257–2263. doi: 10.1016/S0959-8049(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Yang D. The research development on the relation between GH-IGFs and tumor. China Cancer. 2002;11:720–722. [Google Scholar]
- 22.Yang XD, Liu FK, Xu Z, Li JS. Growth hormone receptor expression in human colorectal cancer and its implication. Zhonghua Wei Chang Wai Ke Za Zhi. 2005;8:252–254. (In Chinese) [PubMed] [Google Scholar]
- 23.Lim C, Broqueres-You D, Brouland JP, Merkulova-Rainon T, Faussat AM, Hilal R, Rouquie D, Eveno C, Audollent R, Levy BI, et al. Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J Surg Res. 2013;184:888–897. doi: 10.1016/j.jss.2013.04.069. [DOI] [PubMed] [Google Scholar]
- 24.Paschos KA, Majeed AW, Bird NC. Natural history of hepatic metastases from colorectal cancer - pathobiological pathways with clinical significance. World J Gastroenterol. 2014;20:3719–3737. doi: 10.3748/wjg.v20.i14.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamao T, Hayashi H, Higashi T, Takeyama H, Kaida T, Nitta H, Hashimoto D, Chikamoto A, Beppu T, Baba H. Colon cancer metastasis mimicking intraductal papillary neoplasm of the extra-hepatic bile duct. Int J Surg Case Rep. 2015;10:91–93. doi: 10.1016/j.ijscr.2015.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Ning SL, Chen YX, Xu KS, Shou NH. Role of vascular cell adhesion molecule-1 in the mouse model of hepatic ischemia/reperfusion and the hematogenic metastasis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2014;36:426–431. doi: 10.3881/j.issn.1000-503X.2014.04.014. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 27.Barber EL, Schink JC, Lurain JR. Hepatic metastasis in gestational trophoblastic neoplasia: Patient characteristics, prognostic factors, and outcomes. J Reprod Med. 2014;59:199–203. [PubMed] [Google Scholar]
- 28.Padda RS, Gkouvatsos K, Guido M, Mui J, Vali H, Pantopoulos K. A high-fat diet modulates iron metabolism but does not promote liver fibrosis in hemochromatotic Hjv-/- mice. Am J Physiol Gastrointest Liver Physiol. 2015;308:G251–G261. doi: 10.1152/ajpgi.00137.2014. [DOI] [PubMed] [Google Scholar]