Abstract
BACKGROUND:
Oxygen saturation as measured by pulse oximetry/Fio2 (SF) ratio is highly correlated with the Pao2/Fio2 (PF) ratio in patients with ARDS. However, it remains uncertain whether SF ratio can be substituted for PF ratio for diagnosis of ARDS and whether SF ratio might identify patients who are systemically different from patients diagnosed by PF ratio.
METHODS:
We conducted a secondary analysis of a large observational prospective cohort study. Patients were eligible if they were admitted to the medical ICU and fulfilled the Berlin definition of ARDS with hypoxemia criteria using either the standard PF threshold (PF ratio ≤ 300) or a previously published SF threshold (SF ratio ≤ 315).
RESULTS:
Of 362 patients with ARDS, 238 (66%) received a diagnosis by PF ratio and 124 (34%) by SF ratio. In a small group of patients who received diagnoses of ARDS by SF ratio who had arterial blood gas measurements on the same day (n = 10), the PF ratio did not meet ARDS criteria. There were no major differences in clinical characteristics or comorbidities between groups with the exception of APACHE (Acute Physiology and Chronic Health Evaluation) II scores, which were higher in the group diagnosed by PF ratio. However, this difference was no longer apparent when arterial blood gas-dependent variables (pH, Pao2) were removed from the APACHE II score. There were also no differences in clinical outcomes including duration of mechanical ventilation (mean, 7 days in both groups; P = .25), duration of ICU stay (mean, 10 days vs 9 days in PF ratio vs SF ratio; P = .26), or hospital mortality (36% in both groups, P = .9).
CONCLUSIONS:
Patients with ARDS diagnosed by SF ratio have very similar clinical characteristics and outcomes compared with patients diagnosed by PF ratio. These findings suggest that SF ratio could be considered as a diagnostic tool for early enrollment into clinical trials.
ARDS is a catastrophic syndrome of pulmonary inflammation and injury that carries a high risk of morbidity and mortality.1,2 Although short-term mortality has been improved with lung-protective mechanical ventilation, failure to diagnose ARDS is common3 and potentially contributes to undertreatment. The clinical syndrome of ARDS is diagnosed by consensus definitions. The longstanding American-European Consensus Conference definition4 has been replaced by the Berlin definition.5 Like the American-European Consensus Conference definition, the Berlin definition requires arterial blood gas analysis to assess the severity of hypoxemia. However, the widespread adoption of continuous pulse oximetry, concerns about excessive blood draws and high costs, and reductions in placement of arterial lines have reduced the use of arterial blood gas sampling in critical illness.6‐8 Arterial blood gas sampling also may not be readily available in practice settings with limited resources. Thus, lack of arterial blood gas measurements in many patients may contribute to underdiagnosis of ARDS.
To determine if pulse oximetric measurement of the oxygen saturation as measured by pulse oximetry/Fio2 (SF) ratio could be substituted for Pao2/Fio2 (PF) ratio for diagnosis of ARDS, Rice et al9 analyzed corresponding measurements of oxygen saturation as measured by pulse oximetry (Spo2) (values < 97%) and Pao2 from patients enrolled in one of the ARDS Network clinical trials and found that SF ratios were highly correlated with PF ratios. The SF ratio has also been used to identify children with ARDS,10 substituted for PF ratio in the Lung Injury Score of ARDS severity,11 and substituted for the PF ratio in the Sequential Organ Failure Assessment scores.12 However, in a single-center study of 102 patients in the ICU, the SF ratio was not always concordant with PF ratio,13,14 and there continues to be uncertainty as to whether diagnosis of ARDS using the SF ratio might lead to identification of a less severely ill group of patients. To address this uncertainty, we performed a cross-sectional analysis of a prospective observational cohort of critically ill patients that includes patients who received diagnoses of ARDS by PF ratio and by the SF cutoffs of Rice et al.9 The aims of this study were to determine (1) whether patients with ARDS diagnosed by SF ratio have similar clinical characteristics and outcomes with those diagnosed by PF ratio and (2) to determine whether ARDS severity could be reliably stratified by the SF ratio compared with the PF ratio.
Materials and Methods
Study Design
We studied patients who were prospectively enrolled from January 23, 2006, to July 14, 2013, in the Validating Acute Lung Injury Markers for Diagnosis (VALID) study. The VALID study was designed to identify and validate plasma biomarkers for diagnosis and prognosis of ARDS, and all subjects in the study are carefully phenotyped for ARDS. The Vanderbilt University Institutional Review Board approved the study protocol (IRB #051065) with a waiver of informed consent. However, written informed consent was obtained from the patient or their surrogate whenever possible.
Patients eligible for the VALID study were those aged ≥ 18 years admitted to the medical, surgical, cardiovascular, and trauma ICUs who remained in the ICU for at least 2 days and were evaluated for enrollment the morning after ICU admission. Detailed inclusion and exclusion criteria for patients in VALID were described previously.15 Clinical data, including demographics, prehospital medications, medical history, code status, and admission diagnoses were collected on enrollment; clinical data such as hemodynamics, ventilator variables, laboratory values, and in-hospital medications were collected daily for the first 4 days in the ICU. The lowest PF and SF ratios for each study day were recorded. For the SF ratio, only Spo2 values < 97% were used for calculation.9 The diagnoses of sepsis, organ dysfunction, and ARDS were determined daily for the first 4 ICU days according to published consensus definitions.4,16,17 The primary outcome was any ARDS during the first 4 days in the ICU as defined by consensus of two physician investigators using the American European Consensus Conference ALI and ARDS definitions; this outcome is subsequently referred to as ARDS herein, in keeping with the Berlin definition of ARDS.5 Throughout the VALID study, the diagnosis of ARDS was made if either the SF ratio or the PF ratio met criteria for ARDS; a previously established SF ratio cutoff of < 315 was used.9 Outcome data, including duration of mechanical ventilation, duration of ICU stay, duration of hospital stay, and hospital mortality, were collected.
Study Population
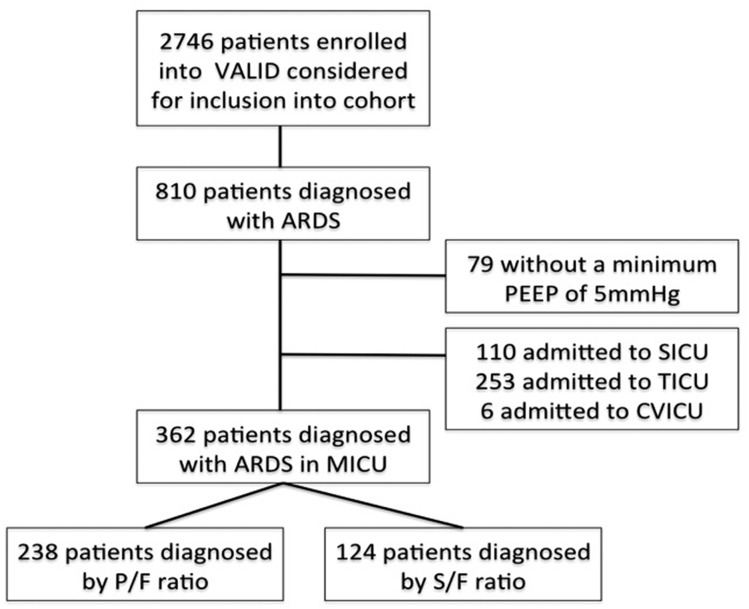
During the 7-year study period, 2,746 patients were enrolled into the VALID study. Among these, 810 patients received a diagnosis of ARDS during the first 4 days of ICU admission and were considered for inclusion (Fig 1). Based on the Berlin definition,5 we excluded patients who did not have a minimum CPAP or positive end-expiratory pressure of 5 cm H2O on the day of ARDS diagnosis. We also excluded patients admitted to the trauma, surgical, and cardiovascular ICUs. Arterial blood gas sampling was much more common in these ICUs, and only a small minority of patients (5%-10%) received a diagnosis of ARDS using the SF ratio compared with 34% in the medical ICU.
Figure 1 –
Study flow diagram. CVICU = cardiovascular ICU; MICU = medical ICU; PEEP = positive end-expiratory pressure; P/F = Pao2/Fio2; S/F = oxygen saturation as measured by pulse oximetry/Fio2; SICU = surgical ICU; TICU = trauma ICU; VALID = Validating Acute Lung Injury Markers for Diagnosis.
Severity of ARDS
For analysis of ARDS severity, patients who had a PF ratio available on the day of ARDS diagnosis were stratified into mild, moderate, and severe using the lowest PF ratio for that day according to the Berlin definition.5 Patients who only had an SF ratio at ARDS diagnosis were stratified into mild, moderate, and severe by applying cutoffs derived from the relationship between Spo2/Fio2 and Pao2/Fio2 by Rice et al,9 (cutoffs of 315, 235, and 144 for mild, moderate, and severe ARDS, respectively) to the lowest SF ratio for that day.
Statistical Analysis
Continuous variables are expressed as mean value with SD. Categorical variables are expressed as counts and percentages. Comparison of two groups with continuous variables was conducted using Wilcoxon rank-sum test. Comparison of categorical variables between two groups was performed with a χ2 test or Fisher exact test. SPSS Statistics, version 21.0 (IBM Corporation) was used for statistical analysis; a two-sided significance level of 0.05 was used for statistical inference.
Results
Characteristics of Patients Who Received a Diagnosis of ARDS by PF Ratio Compared With SF Ratio
A total of 362 patients with ARDS met the inclusion and exclusion criteria and were included in the current study (Fig 1). Of these, 238 patients with ARDS (66%) received a diagnosis by PF ratio, and 124 patients (34%) received a diagnosis by SF ratio. Table 1 includes a comparison of baseline characteristics and risk factors for ARDS between patients who received a diagnosis by PF and SF ratios. Overall, patients with ARDS diagnosed by PF ratio had similar characteristics to patients who received a diagnosis by SF ratio except for a significantly higher severity of illness as measured by the APACHE (Acute Physiology and Chronic Health Evaluation) II score (31 ± 8 vs 26 ± 8, P < .001). Because two variables used to calculate the APACHE II score require arterial blood gas analysis (pH and Pao2), and arterial blood gas analysis was not available in the majority of patients diagnosed by SF ratio, we calculated a modified APACHE II score for all patients that did not include points for pH or Pao2. This modified APACHE II score did not differ between the two groups (P = .205) (Table 1). The most common risk factors for ARDS were nonpulmonary sepsis and pneumonia. Risk factors did not differ between patients who received a diagnosis by PF ratio or SF ratio.
TABLE 1 ] .
Comparison of Demographic Data and Risk Factors Between Patients With ARDS Diagnosed by PF Ratio and by SF Ratio
| Characteristic | Diagnosed by PF Ratio (n = 238) | Diagnosed by SF Ratio (n = 124) | P Value |
| Age, y | 51 ± 18 | 52 ± 17 | .626 |
| APACHE II | 31 ± 8 | 26 ± 8 | < .001 |
| Modified APACHE II without pH, Pao2 | 27 ± 7 | 26 ± 8 | .205 |
| Male | 115 (48) | 65 (52) | .507 |
| White | 210 (88) | 101 (82) | .082 |
| Current smoker | 86 (36) | 36 (29) | .198 |
| Diabetes | 69 (29) | 32 (26) | .540 |
| Alcohol | 36 (15) | 26 (21) | .186 |
| Chronic liver disease | 27 (11) | 18 (15) | .404 |
| Chronic kidney disease | 48 (20) | 23 (19) | .781 |
| On dialysis | 5 (2.1) | 8 (6.5) | .069 |
| ARDS risk factor | .300 | ||
| Sepsis | 82 (35) | 54 (44) | |
| Pneumonia | 87 (37) | 31 (25) | |
| Aspiration | 55 (23) | 28 (23) | |
| Other | 10 (4) | 7 (6) |
Data presented as mean ± SD or No. (%). APACHE = Acute Physiology and Chronic Health Evaluation; PF = Pao2/Fio2; SF = oxygen saturation as measured by pulse oximetry/Fio2.
Concordance Between PF and SF Ratios in the Same Patients
The overall rate of discordance between PF and SF ratios for ARDS diagnosis was 8.2% (n = 30 of 362). All patients (n = 238) diagnosed with ARDS by PF ratio had at least one SF ratio recorded on the same day. For 20 of the 238 patients (8.4%) who received a diagnosis of ARDS by PF ratio, the lowest SF ratio on the day of ARDS diagnosis was > 315, indicating diagnostic discordance. Among 124 patients with ARDS that was diagnosed by SF, only 10 patients (8.0%) underwent arterial blood gas tests on the same day. For these 10 patients, the lowest PF ratio on the day of ARDS diagnosis was > 300, indicating diagnostic discordance. In this group, reliance on the PF alone would have missed the diagnosis of ARDS.
Clinical Course and Outcomes of Patients Who Received a Diagnosis by PF Ratio vs SF Ratio
A total of 329 patients with ARDS (91%) received invasive mechanical ventilation during the first 4 study days. There was no difference between the PF ratio and SF ratio diagnosis group (92% vs 88%, P = .179) (Table 2). There was also no significant difference between the PF ratio and SF ratio diagnosis groups in the need for invasive mechanical ventilation on the day of ARDS diagnosis (91% vs 86%, P = .109). There was a trend toward more noninvasive ventilation in the SF ratio diagnosis group with 18 patients (14%) receiving noninvasive ventilation on the day of ARDS diagnosis compared with 9% of the PF ratio diagnosis group; three of these patients required invasive mechanical ventilation before the fourth day of study enrollment.
TABLE 2 ] .
Comparison of Clinical Course and Outcomes Between Patients With ARDS Diagnosed by PF Ratio and by SF Ratio
| Clinical Course or Outcome | Diagnosed by PF Ratio (n = 238) | Diagnosed by SF Ratio (n = 124) | P Value |
| Ventilation mode on the day of ARDS diagnosis | .109 | ||
| Mechanical ventilator | 217 (91) | 106 (86) | |
| NIPPV | 21 (9) | 18 (14) | |
| Ventilation mode on the fourth day of study | .179 | ||
| Mechanical ventilator | 220 (92) | 109 (88) | |
| NIPPV | 18 (7.6) | 15 (12) | |
| No. of study days with ARDS | .002 | ||
| 1 | 13 (5.5) | 16 (13) | |
| 2 | 34 (14) | 26 (21) | |
| 3 | 34 (14) | 25 (20) | |
| 4 | 157 (66) | 57 (46) | |
| Timing of diagnosis of ARDS | < .001 | ||
| First day | 229 (96) | 94 (76) | |
| Second day | 5 (2.1) | 15 (12) | |
| Third day | 2 (0.8) | 8 (6.5) | |
| Fourth day | 2 (0.8) | 7 (5.6) | |
| Length of ICU stay | 10 ± 8 | 9 ± 8 | .263 |
| Length of ventilation | 7 ± 7 | 7 ± 8 | .251 |
| Ventilator-free days | 13 ± 10 | 15 ± 10 | .169 |
| Length of hospital stay, d | 17 ± 13 | 17 ± 13 | .710 |
| Hospital mortality | 85 (36) | 45 (36) | .909 |
Data presented as mean ± SD or No. (%). NIPPV = noninvasive positive pressure ventilation. See Table 1 legend for expansion of other abbreviations.
Patients who received a diagnosis by PF ratio were more likely to be diagnosed with ARDS on the first day of ICU admission compared with those diagnosed by SF ratio (96% vs 76%, P < .001), as shown in Table 2. There were no significant differences between the two groups in length of ICU stay, length of mechanical ventilation, ventilator-free days, length of hospital stay, or hospital mortality (Table 2).
ARDS Severity Assessment by PF Ratio vs SF Ratio
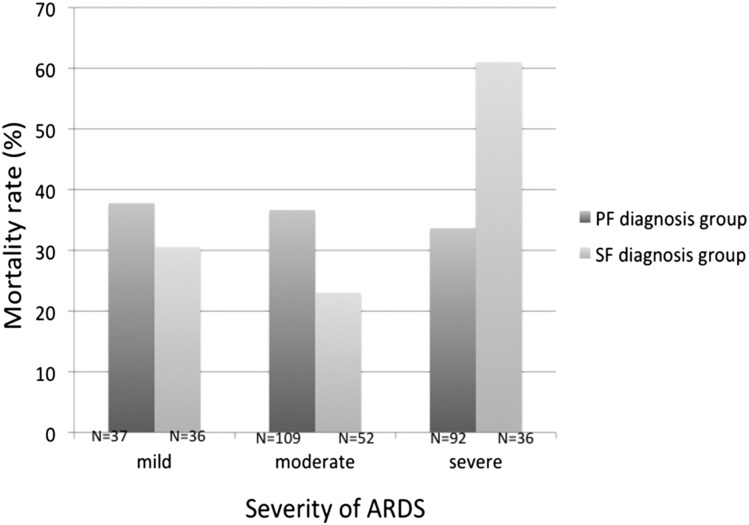
In the PF diagnosis group, the mortality rates of mild, moderate, and severe ARDS as defined by Berlin criteria were 37.8%, 36.7%, and 33.7%, respectively (P = .86). Using the SF ratio to assess severity, the mortality rates of mild, moderate, and severe ARDS were 30.6%, 23.1%, and 61.1%, respectively (P < .001) (Fig 2). Clinical characteristics and outcomes in patients who did not have shock on the day of ARDS diagnosis were also analyzed (e-Appendix 1 (92.8KB, pdf) , e-Tables 1, 2 (92.8KB, pdf) ).
Figure 2 –
Mortality rate classified by severity of ARDS according to PF (n = 238, P = .869) or SF ratio (n = 124, P = .001) by χ2 test. See Figure 1 legend for expansion of abbreviations.
Discussion
Because of continued uncertainty regarding the usefulness of the SF ratio for diagnosis of ARDS, we sought to investigate whether there are systematic differences between patients who received a diagnosis of ARDS by PF ratio compared with patients with ARDS diagnosed by SF ratio. In a large cohort of 362 carefully phenotyped patients with ARDS in the medical ICU, we found no differences in demographics, comorbidities, or severity of illness between patients with ARDS diagnosed by PF ratio compared with SF ratio. The only difference in clinical presentation between the two groups was the timing of diagnosis of ARDS. Patients who received a diagnosis by PF ratio were more likely to be diagnosed on the first ICU day compared with patients who received a diagnosis by SF ratio. However, the length of hospital stay, length of mechanical ventilation, and hospital mortality rates were similar between the two groups.
Since a protective lung ventilator strategy significantly improves the outcomes of patients with ARDS, early identification of ARDS and early application of lower tidal volume ventilation are crucial. Levitt et al18 defined a clinical diagnosis of early acute lung injury at hospital admission if there were bilateral opacities on chest radiograph and an initial oxygen requirement of > 2 L/min. This definition showed a high sensitivity and specificity for progression to acute lung injury.18 Moreover, Festic et al19 showed that SF ratio, measured within the first 6 h after hospital admission, is an independent indicator of ARDS development among patients at risk.19 In the current study, diagnosis of ARDS using SF ratio rather than PF ratio identified patients with similar outcomes. Since SF is continuously available, its use may facilitate earlier diagnosis and application of protective ventilation.
In addition to comparing clinical characteristics between patients who received a diagnosis ARDS by PF vs SF ratio, we also sought to determine if PF or SF ratio was superior for stratifying severity of ARDS into mild, moderate, and severe categories by comparing hospital mortality rates by severity of hypoxemia. Using this approach, we found no association between the severity of ARDS diagnosed by PF ratios and hospital mortality. These findings are concordant with several studies that have shown that the PF ratio measured at the onset of ARDS is not an independent predictor of mortality.20‐25 One possible explanation for the lack of the power of the PF ratio to discriminate adverse outcome in ARDS is that the PF ratio is highly variable depending on the ventilator strategy chosen. In one study, standardization of ventilator settings greatly improved risk stratification of ARDS by PF ratio.26 In the current study, use of the SF ratio to classify severity performed somewhat better than the PF ratio; patients with severe ARDS by SF ratio had a significantly higher mortality rate compared with those with moderate and mild ARDS.
Continuous pulse oximetry has been incorporated into standard monitoring in the intensive care unit for decades. Use of the pulse oximetry to monitor the SF ratio for hypoxemia in critically ill patients has a number of theoretical advantages over arterial blood gas monitoring. First, the noninvasive nature of pulse oximetry avoids excessive arterial blood draws, which may lead to anemia, hemorrhage, vascular injury, and procedure-associated complications.27 Second, pulse oximetry allows continuous monitoring of the oxygen saturation, which may increase the likelihood of early detection of ARDS or recognition of patients who may have ARDS but have not undergone arterial blood gas sampling. As such, use of pulse oximetry to screen for ARDS may facilitate early enrollment into clinical trials and early diagnosis and treatment in clinical practice.9 These theoretical advantages, coupled with data from the current study suggesting that ARDS diagnosed by SF ratio is not clinically different from ARDS diagnosed by PF ratio, support a broader role for the SF ratio for ARDS diagnosis in both clinical and research settings. Although there have been some concerns surrounding accuracy of pulse oximetry in dark-skinned individuals, previous reports indicated that the arterial oxygen saturation was slightly different (3.56% ± 2.45%) from Spo2 only at very low oxygen saturations (60%-70% arterial oxygen saturation).28 In our study, none of the patients with ARDS diagnosed by SF ratio was at below 80% Spo2, making it unlikely that any differences in skin color between groups confounded the results.
In this study, there were more patients who developed ARDS after the second day of enrollment among those who received a diagnosis by SF ratio compared with patients who received a diagnosis by PF ratio. One possible explanation for this finding is that the SF ratio is particularly well suited for diagnosis of delayed ARDS after ICU admission since the frequency of arterial blood gas measurement may diminish after the first ICU day. Although the difference did not reach significance, a higher percentage of patients in the SF diagnosis group were receiving noninvasive ventilation on the day of ARDS diagnosis. This finding suggests that the SF ratio may also be useful in diagnosis ARDS in those receiving noninvasive mechanical ventilation since this group may be less likely to have an arterial access catheter placed or to undergo arterial blood gas sampling.
This study has a number of strengths. First, the ARDS phenotyping was done prospectively by multiple expert physician investigators’ review of all chest radiographs, clinical history, and blood gas and pulse oximetry data. Second, the study includes a heterogeneous population of patients with ARDS in a large tertiary academic medical ICU with a broad spectrum of severity of illness and comorbidities, which should enhance generalizability. Third, all available blood gas and pulse oximetry data were recorded prospectively, for the explicit purpose of ARDS diagnosis. The study also has some limitations. First, it is a post hoc analysis, and we only studied patients admitted to the medical ICU, making the findings most applicable to this patient population. In the surgical and trauma ICUs, a very high frequency of arterial blood gas sampling led to few patients who had ARDS diagnosed by SF ratio. Second, the study was done in a medical ICU that generally has a low frequency of arterial blood gas analysis. The findings might not be generalizable to an ICU setting where arterial blood gas analysis is done multiple times per day in the majority of patients. Third, pulse oximetry is affected not only by Pao2 but by pH and venous oxygen saturation and oxygen extraction. For this reason, a low Spo2 could also signify local or global hypoperfusion and not arterial hypoxemia.29 Finally, since PF data were not available in the majority of patients who received a diagnosis of ARDS by SF ratio, it is not possible to determine whether those patients actually met criteria for diagnosis of ARDS by the current clinical definition of ARDS. However, the similar demographics, severity of illness, and clinical outcomes between the two diagnostic strategies suggest that the SF ratio does not select for a group of patients who are clinically different from those diagnosed by PF ratio.
In summary, in a large cohort of patients admitted to the medical ICU, patients with ARDS diagnosed by SF ratio have very similar clinical characteristics and outcomes to patients with ARDS diagnosed by PF ratio. These findings suggest that the SF ratio is a useful tool for assessment of hypoxemia in the diagnosis of ARDS. Since pulse oximetry is continuously available, use of the SF ratio could facilitate earlier diagnosis and treatment and improve overall rates of diagnosis and treatment of ARDS.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: L. B. W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. W. C. served as principal author. W. C. and L. B. W. contributed to the study concept design and writing of the manuscript; D. R. J., C. M. S., and J. A. B. contributed to data analysis and interpretation, study design, statistical analysis, and revision of the manuscript; and G. R. B. and L. B. W. contributed to review of the manuscript.
Conflict of interest: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- APACHE
Acute Physiology and Chronic Health Evaluation
- PF
Pao2/Fio2
- SF
oxygen saturation as measured by pulse oximetry/Fio2
- Spo2
oxygen saturation as measured by pulse oximetry
- VALID
Validating Acute Lung Injury Markers for Diagnosis
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health [Grants NIH HL103836, HL112656-02, T32 HL087738, and UL1 RR024975], an American Heart Association Clinical Research Award, and an American Heart Association Established Investigator Award.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334-1349. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685-1693. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228-2234. [DOI] [PubMed] [Google Scholar]
- 4.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818-824. [DOI] [PubMed] [Google Scholar]
- 5.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. [DOI] [PubMed] [Google Scholar]
- 6.Merlani P, Garnerin P, Diby M, Ferring M, Ricou B. Quality improvement report: Linking guideline to regular feedback to increase appropriate requests for clinical tests: blood gas analysis in intensive care. BMJ. 2001;323(7313):620-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilon CS, Leathley M, London R, et al. Practice guideline for arterial blood gas measurement in the intensive care unit decreases numbers and increases appropriateness of tests. Crit Care Med. 1997;25(8):1308-1313. [DOI] [PubMed] [Google Scholar]
- 8.Roberts D, Ostryzniuk P, Loewen E, et al. Control of blood gas measurements in intensive-care units. Lancet. 1991;337(8757):1580-1582. [DOI] [PubMed] [Google Scholar]
- 9.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB; National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410-417. [DOI] [PubMed] [Google Scholar]
- 10.Khemani RG, Patel NR, Bart RD, III, Newth CJ. Comparison of the pulse oximetric saturation/fraction of inspired oxygen ratio and the PaO2/fraction of inspired oxygen ratio in children. Chest. 2009;135(3):662-668. [DOI] [PubMed] [Google Scholar]
- 11.Khemani RG, Thomas NJ, Venkatachalam V, et al. ; Pediatric Acute Lung Injury and Sepsis Network Investigators (PALISI). Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med. 2012;40(4):1309-1316. [DOI] [PubMed] [Google Scholar]
- 12.Pandharipande PP, Shintani AK, Hagerman HE, et al. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med. 2009;37(4):1317-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573-1582. [DOI] [PubMed] [Google Scholar]
- 14.Van de Louw A, Cracco C, Cerf C, et al. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med. 2001;27(10):1606-1613. [DOI] [PubMed] [Google Scholar]
- 15.O’Neal HR, Jr, Koyama T, Koehler EA, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39(6):1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bone RC, Sprung CL, Sibbald WJ. Definitions for sepsis and organ failure. Crit Care Med. 1992;20(6):724-726. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707-710. [DOI] [PubMed] [Google Scholar]
- 18.Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Festic E, Bansal V, Kor DJ, Gajic O; US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG–LIPS). SpO2/FiO2 ratio on hospital admission is an indicator of early acute respiratory distress syndrome development among patients at risk. J Intensive Care Med. 2015;30(4):209-216. [DOI] [PubMed] [Google Scholar]
- 20.Bone RC, Maunder R, Slotman G, et al. An early test of survival in patients with the adult respiratory distress syndrome. The PaO2/FIo2 ratio and its differential response to conventional therapy. Prostaglandin E1 Study Group. Chest. 1989;96(4):849-851. [DOI] [PubMed] [Google Scholar]
- 21.Sloane PJ, Gee MH, Gottlieb JE, et al. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. Am Rev Respir Dis. 1992;146(2):419-426. [DOI] [PubMed] [Google Scholar]
- 22.Knaus WA, Sun X, Hakim RB, Wagner DP. Evaluation of definitions for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150(2):311-317. [DOI] [PubMed] [Google Scholar]
- 23.Luhr OR, Karlsson M, Thorsteinsson A, Rylander C, Frostell CG. The impact of respiratory variables on mortality in non-ARDS and ARDS patients requiring mechanical ventilation. Intensive Care Med. 2000;26(5):508-517. [DOI] [PubMed] [Google Scholar]
- 24.Bersten AD, Edibam C, Hunt T, Moran J; Australian and New Zealand Intensive Care Society Clinical Trials Group. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165(4):443-448. [DOI] [PubMed] [Google Scholar]
- 25.Brun-Buisson C, Minelli C, Bertolini G, et al. ; ALIVE Study Group. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30(1):51-61. [DOI] [PubMed] [Google Scholar]
- 26.Villar J, Pérez-Méndez L, Blanco J, et al. ; Spanish Initiative for Epidemiology, Stratification, and Therapies for ARDS (SIESTA) Network. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting–a prospective, multicenter validation study. Intensive Care Med. 2013;39(4):583-592. [DOI] [PubMed] [Google Scholar]
- 27.Baskın SB, Oray NC, Yanturalı S, Bayram B. The comparison of heparinized insulin syringes and safety-engineered blood gas syringes used in arterial blood gas sampling in the ED setting (randomized controlled study). Am J Emerg Med. 2014;32(5):432-437. [DOI] [PubMed] [Google Scholar]
- 28.Bickler PE, Feiner JR, Severinghaus JW. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology. 2005;102(4):715-719. [DOI] [PubMed] [Google Scholar]
- 29.Mardirossian G, Schneider RE. Limitations of pulse oximetry. Anesth Prog. 1992;39(6):194-196. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement