Abstract
BACKGROUND:
High bronchodilator reversibility in adult asthma is associated with distinct clinical characteristics. This analysis compares lung function, biomarker profiles, and disease control in patients with high reversibility (HR) and low reversibility (LR) asthma.
METHODS:
A retrospective analysis was performed with data from two completed clinical trials of similar design. Patients were divided into HR and LR subgroups based on their response to bronchodilators (HR = ΔFEV1 postbronchodilator ≥ 20%). Blood eosinophil count, serum IgE level, and fraction of exhaled nitric oxide concentration, biomarkers commonly used to stratify patients into T-helper (Th)-2-high vs Th2-low phenotypes, were measured in patients with not well controlled (1.5 ≤ Asthma Control Questionnaire [ACQ] ≤ 2.143) and very poorly controlled (ACQ > 2.143) disease.
RESULTS:
The majority of patients in the HR and LR subgroups displayed Th2-low biomarker profiles and very poor disease control. HR was more frequently associated with Th2-high biomarker profiles (40.1% vs 29.4%, P = .006), lower lung function (FEV1, 63.5 ± 7.7% predicted vs 67.9 ± 8.4% predicted; P < .001), and atopy (93.7% vs 86.5%, P = .005).
CONCLUSIONS:
HR is a physiologic indicator of reduced lung function and is more often associated with elevations in Th2 biomarkers than LR in moderate to severe asthma. However, the majority of patients with HR and LR asthma in this analysis had a Th2-low biomarker profile. Moreover, a Th2-high biomarker profile was not associated with worse disease control.
Data from large patient registries have identified clusters of patients with asthma and severe airflow limitation and disease that is difficult to control with conventional therapy.1‐4 Characteristics differentiating these patients from the general asthma population include longer duration of disease, high reversibility (HR) of the airway following bronchodilator administration, sputum neutrophilia, increased oral corticosteroid use, and increased health-care resource utilization.1,4 Indeed, HR is a distinctive physiologic characteristic of cluster 4 and 5 patients in the Severe Asthma Research Program (SARP), the two cohorts displaying the worst disease control and highest frequency of clinic and ED visits and hospitalizations. Serum and BAL fluid biomarkers have been analyzed to characterize the immune processes modulating disease severity.2,5 However, limited information exists regarding how serum biomarkers define endotypes across the asthma population, correlate with disease control, and relate to distinctive physiologic features such as HR. The present study uses combined baseline datasets from two randomized controlled trials involving patients with moderate to severe asthma to examine the relationship between immune pathway biomarkers and disease control in those with HR and low reversibility (LR).
Materials and Methods
A retrospective exploratory analysis was performed using data from two phase 2 clinical trials involving patients with partly or poorly controlled moderate to severe asthma (GINA [Global Initiative for Asthma] steps 3 and 4). The first trial was initiated on December 21, 2009, to assess the efficacy of a dual inhibitor of the prostaglandin D2 and chemoattractant receptor homologous molecule expressed on T-helper (Th) 2 cells.6 Full baseline datasets were available for 358 of 396 enrolled patients. The second trial was initiated October 4, 2010, to assess the efficacy of a monoclonal antibody against the IL-17 receptor A subunit in a similar cohort.7 Full baseline datasets were available for 272 of 302 enrolled patients. Although both trials were interventional, treatment responses were not considered in this analysis. Data collected beyond the baseline time point were used solely to assess stability of phenotypic characteristics in patients who did not receive experimental therapy.
Inclusion and exclusion criteria for the two trials were identical: (1) age 18 to 65 years, (2) disease requiring inhaled corticosteroids with and without a long-acting β-agonist, (3) baseline Asthma Control Questionnaire (ACQ) score ≥ 1.5, (4) FEV1 % predicted between 50% and 80%, (5) reversibility in FEV1 ≥ 12% (and at least 200 mL) following administration of a short-acting β-agonist, (6) no evidence of active infection, and (7) no medical conditions associated with immune suppression. Patients were excluded from study participation if they had COPD or asthma-COPD overlap syndrome,8 were receiving maintenance oral corticosteroids or IgE antibody therapy, were current or recent smokers, had a history of intubation within 3 years of enrollment, or had OSA.
Subjects were classified into (1) an HR group of those with high airway reversibility defined as a ≥ 20% increase in FEV1 following administration of a short-acting bronchodilator during screening and baseline pulmonary function testing and (2) an LR group of those with reversibility below this level.1,4 Subgroups were further divided into subjects with not well controlled (NWC) (ACQ score ≥ 1.5 and ≤ 2.143) or very poorly controlled (VPC) (ACQ > 2.143) disease. The ACQ cut point defining VPC disease was derived from a linear extrapolation of ACQ scores of well controlled (five total ACQ points corresponding to an ACQ cut point of 0.75) and NWC (10 total ACQ points corresponding to an ACQ cut point of 1.5) disease.9 VPC disease was defined as corresponding to an additional five-point increment in total ACQ score beyond the NWC cut point (ie, ACQ > 2.143). Disease control was summarized in terms of both ACQ6 and ACQ7 values. Biomarkers reflecting Th2 immune activation were assessed in each patient and included serum IgE level, circulating eosinophil count, and fraction of exhaled nitric oxide (Feno) concentration. For each biomarker, cut points used to define a high level were as follows: IgE ≥ 100 IU/mL, eosinophil count ≥ 300/μL, and Feno ≥ 30 parts per billion.10,11 Patients were classified as having either a positive or a negative Th2 Immune Profile Index (IPI) (positive IPI indicates elevation in two or more Th2 biomarkers; negative IPI indicates elevation in one or no Th2 biomarkers).
Results are reported using descriptive statistics as mean ± SD, median, or quartiles as appropriate. Comparisons of baseline data between the two trials6,7 and between the HR and LR subgroups were performed by either Student t or Wilcoxon rank sum test. Correlations between baseline and week 12 biomarker assessments were performed using the Pearson product-moment correlation method. Assessments of the significance of relationships between Th2 biomarker elevations and disease control were performed by χ2 test (applying Yates correction as appropriate). A classification and regression tree (CART) analysis was included in the sensitivity analysis of cut points defining Th2 biomarker status. Statistically significant differences between groups were defined by P < .05.
Results
Demographics, biometrics, medical history, pulmonary function values, and medication use for all patients (N = 698) in both studies are summarized in Table 1. Patients studied in the first clinical trial6 were more likely to be atopic (93% vs 83%, P < .001) and had a higher FEV1 % predicted (66.9% ± 8.24% vs 65.2% ± 8.53%, P = .0081) than those in the second trial.7 However, these differences were small and not clinically significant or substantial enough to affect interpretation of results when considered as a single combined cohort.
TABLE 1 ] .
Comparison of Patients in the Clinical Trials Used to Define HR and LR Subgroups for the Present Analysis
| Variable | Study NCT010185506 (N = 396) | Study NCT011992897 (N = 302) | P Valuea |
| Baseline ACQ7 score | 2.5 ± 0.60 | 2.5 ± 0.65 | .4552 |
| Baseline ACQ6 score | 2.3 ± 0.67 | 2.3 ± 0.74 | .8223 |
| Age, y | 44.8 ± 11.37 | 45.7 ± 11.40 | .3088 |
| BMI, kg/m2 | 31.2 ± 7.08 | 30.2 ± 7.12 | .0917 |
| Baseline eosinophil count, cells/μL | 199.5 (122, 292) | 191.5 (130.5, 294.5) | .8815 |
| Baseline Feno, parts/billion | 23.5 (15.0, 36.3) | 23.5 (14.8, 35.8) | .6111 |
| Baseline FEV1, L | 2.22 ± 0.54 | 2.16 ± 0.57 | .1945 |
| Baseline FEV1 % predicted | 66.9 ± 8.24 | 65.2 ± 8.53 | .0081 |
| Baseline IgE, IU/mL | 176.2 (58.6, 423.3) | 132.2 (51.7, 319.7) | .0572 |
| Airway reversibility, % change | 17.9 ± 12.13 | 19.4 ± 13.29 | .1168 |
| Female sex | 60 | 59 | .8777 |
| Positive atopy status | 93 | 83 | < .0001 |
| Elevated eosinophil count | 24 | 24 | .9746 |
| High-dose ICS | 47 | 53 | .1312 |
| High-dose ICS + LABA | 40 | 41 | .6811 |
Data are presented as mean ± SD, median (quartile 1, quartile 2), or %. ACQ = Asthma Control Questionnaire; Feno = fraction of exhaled nitric oxide; HR = high reversibility; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LR = low reversibility.
P value from two-sample t test for means and from Wilcoxon rank sum test for medians.
Two hundred thirty-seven patients (38%) in the combined study cohort met the HR criterion. Relative to those with LR (n = 385), patients with HR asthma tended to be younger; were more often atopic; and had a significantly lower BMI, lower baseline pulmonary function, higher mean Feno and IgE values, and higher ACQ7 scores (Table 2). One hundred thirty-six patients (63.6%) with HR asthma met the criterion for VPC disease defined as ACQ6 > 2.143. Compared with patients with HR and NWC asthma (ACQ, 1.5-2.143), the subgroup with VPC asthma tended to be older (44.1 ± 11.0 years vs 42.0 ± 12.3 years, P = .184), had lower baseline FEV1 values (2.06 ± 0.51 L vs 2.19 ± 0.59 L, P = .1038), and had a lower FEV1 % predicted (62.8% ± 7.50% vs 64.9% ± 8.04%, P = .062) (Table 3). Patients with HR and NWC disease did not differ from those reporting VPC disease with respect to sex distribution, BMI, medication use, or degree of airway reversibility. A parallel analysis using ACQ7 as the measure of disease control applying the same cut point showed similar findings. Patients with LR and NWC disease did not differ significantly from those with VPC disease with respect to demographics, baseline biomarker profiles, lung function, bronchodilator reversibility, or medication use (Table 4).
TABLE 2 ] .
Comparison of HR (n = 237; ΔFEV1 ≥ 20%) vs LR (n = 385; ΔFEV1 < 20%) Subgroups From the Clinical Trials6,7 Used in the Present Analysis
| Variable | HR (n = 237) | LR (n = 385) | P Valuea |
| Baseline ACQ7 score | 2.6 ± 0.62 | 2.4 ± 0.63 | .0247 |
| Baseline ACQ6 score | 2.3 ± 0.70 | 2.2 ± 0.71 | .2799 |
| Age, y | 43.1 ± 11.63 | 46.4 ± 11.14 | .0004 |
| BMI, kg/m2 | 29.9 ± 6.71 | 31.3 ± 7.03 | .0154 |
| Baseline eosinophil count, cells/μL | 214.0 (136.0, 303.0) | 191.0 (126.0, 288.0) | .173 |
| Baseline Feno, parts/billion | 25.7 (16.0, 40.0) | 22.0 (14.3, 31.7) | .004 |
| Baseline FEV1, L | 2.1 ± 0.54 | 2.2 ± 0.56 | .006 |
| Baseline FEV1 % predicted | 63.5 ± 7.72 | 67.9 ± 8.35 | < .0001 |
| Baseline IgE, IU/mL | 192.9 (71.7, 400.3) | 127.5 (50.3, 357.6) | .0111 |
| Airway reversibility, %Δ | 31.2 ± 11.39 | 11.2 ± 5.76 | < .0001 |
| Female sex | 149 (62.9) | 223 (57.9) | .2217 |
| Positive atopy status | 222 (93.7) | 333 (86.5) | .0050 |
Data are presented as mean ± SD, median (quartile 1, quartile 2), or No. (%). See Table 1 legend for expansion of abbreviations.
P value from two-sample t test for means and from Wilcoxon rank sum test for medians.
TABLE 3 ] .
Comparison of Patients With HR Based on Disease Control Assessed Through ACQ6
| Variable | Very Poorly Controlled (n = 136) | Not Well Controlled (n = 78) | P Valuea |
| Baseline ACQ7 score | 3.0 ± 0.45 | 2.1 ± 0.18 | < .0001 |
| Baseline ACQ6 score | 2.8 ± 0.51 | 1.8 ± 0.17 | < .0001 |
| Age, y | 44.1 ± 11.04 | 42.0 ± 12.28 | .1841 |
| BMI, kg/m2 | 30.0 ± 6.44 | 29.6 ± 6.73 | .6767 |
| Disease duration at study enrollment, y | 25.3 ± 13.44 | 23.4 ± 12.66 | .3081 |
| Baseline eosinophil count, cells/μL | 219.5 (146.5, 311.0) | 188.0 (129.0, 298.0) | .2486 |
| Baseline Feno, parts/billion | 25.3 (15.5, 40.1) | 26.0 (15.0, 42.0) | .7655 |
| Baseline FEV1, L | 2.06 ± 0.51 | 2.19 ± 0.59 | .1038 |
| Baseline FEV1 % predicted | 62.8 ± 7.50 | 64.9 ± 8.04 | .0620 |
| Baseline IgE, IU/mL | 197.7 (61.1, 396.2) | 192.8 (91.6, 430.7) | .4567 |
| Age at disease onset, y | 18.9 ± 16.79 | 18.6 ± 16.35 | .9000 |
| Airway reversibility, %Δ | 31.8 ± 12.39 | 30.3 ± 10.06 | .3663 |
| Female sex | 67 | 60 | .2443 |
| Positive atopy status | 91 | 99 | .0262 |
| High-dose ICS | 48 | 49 | .8271 |
| ICS + LABA | 39 | 39 | .9989 |
Data are presented as mean ± SD, median (quartile 1, quartile 2), or %. See Table 1 legend for expansion of abbreviations.
P value from two-sample t test for means and from Wilcoxon rank sum test for medians.
TABLE 4 ] .
Comparison of Patients With LR Based on Disease Control Assessed Through ACQ6
| Variable | Very Poorly Controlled (n = 202) | Not Well Controlled (n = 149) | P Valuea |
| Baseline ACQ7 score | 2.9 ± 0.52 | 2.0 ± 0.19 | < .0001 |
| Baseline ACQ6 score | 2.8 ± 0.57 | 1.8 ± 0.17 | < .0001 |
| Age, y | 47.3 ± 11.19 | 45.8 ± 10.97 | .2110 |
| BMI, kg/m2 | 32.0 ± 7.48 | 30.7 ± 6.46 | .1092 |
| Disease duration at study enrollment | 26.6 ± 14.30 | 27.9 ± 14.72 | .4023 |
| Baseline eosinophil count, cells/μL | 188.0 (123.0, 288.0) | 205.0 (140.0, 290.0) | .2499 |
| Baseline Feno, parts/billion | 21.3 (14.0, 30.5) | 24.5 (14.7, 34.3) | .1074 |
| Baseline FEV1, L | 2.17 ± 0.58 | 2.33 ± 0.54 | .0087 |
| Baseline FEV1 % predicted | 67.4 ± 8.58 | 69.0 ± 8.05 | .0805 |
| Baseline IgE, International Units/mL | 112.9 (40.7, 311.0) | 143.2 (64.7, 356.3) | .0808 |
| Age at disease onset, y | 20.7 ± 16.25 | 18.0 ± 15.01 | .1021 |
| Airway reversibility, %Δ | 11.2 ± 5.66 | 11.0 ± 6.09 | .7280 |
| Female sex | 62 | 54 | .1574 |
| Positive atopy status | 87 | 88 | .7217 |
| High-dose ICS | 53 | 48 | .4141 |
| ICS + LABA | 43 | 40 | .5720 |
Data are presented as mean ± SD, median (quartile 1, quartile 2), or %. See Table 1 legend for expansion of abbreviations.
P value from two-sample t test for means and from Wilcoxon rank sum test for medians.
To assess the consistency of Th2 biomarker values as indicators of inflammatory status in this cohort, we compared blood eosinophil counts, serum IgE levels, and Feno concentrations at baseline with those at week 12 in subjects assigned to placebo treatment. The results are summarized in Figures 1A‐C and show significant correlations between baseline and week 12 for all three Th2 biomarkers. We also examined within-patient temporal consistency of bronchodilator reversibility measurements and ACQ assessments using a similar approach. The results summarized in Figures 1D and 1E confirm that these were also quite stable over the 12-week assessment interval.
Figure 1 –
A-E, Correlations between baseline and wk 12 blood eosinophil counts (cells/μL3) (A), serum IgE levels (IU/mL) (B), FeNO concentrations (parts per billion) (C), disease control assessed in terms of ACQ6 (U) (D), and bronchodilator reversibility (E) are summarized for patients who did not receive active therapy in the clinical trials studied.6,7 The results confirm the stability of these measures out to 12 wk. ACQ = Asthma Control Questionnaire; EOS = eosinophil count; FeNO = fraction of expired nitric oxide.
We next examined whether within-patient changes from baseline to week 12 in individual biomarkers correlated among one another or with changes in either bronchodilator reversibility or ACQ over this same interval. No correlations of significance were found between baseline to week 12 changes among Th2 biomarkers or between biomarker changes and changes in bronchodilator reversibility or ACQ.
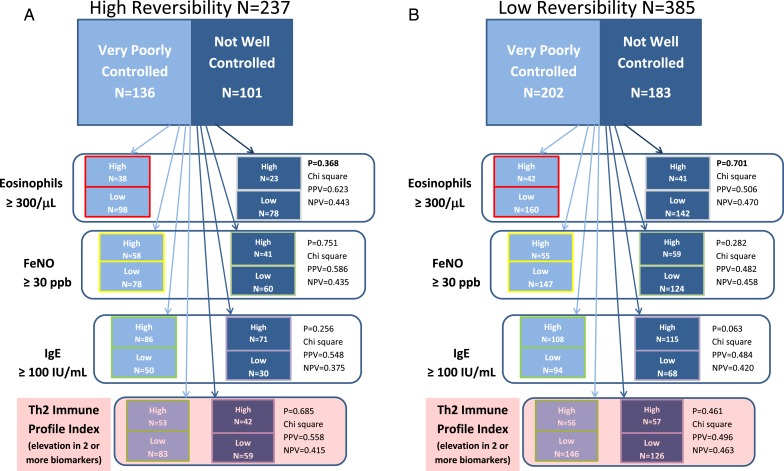
The relationships between biomarkers and disease control in the HR and LR subgroups are summarized in Figure 2. Among patients with HR asthma, an elevated blood eosinophil count was somewhat predictive (positive predictive value [PPV], 62%; P = .368 by χ2 test) of VPC disease. However, circulating eosinophil count was not a sensitive indicator (0.279) of disease control status because 98 of the 136 patients with VPC disease and HR had low eosinophil counts. Feno and serum IgE levels were also not associated with disease control in patients with HR. An elevated serum IgE level had moderate PPV (0.548) for identifying patients with VPC disease but lacked specificity (0.375). Likewise, an elevated Feno had a moderate PPV of VPC (0.586) but was neither sensitive (0.481) nor specific (0.435) as an indicator of level of disease control. The aggregate Th2 IPI also failed to reflect disease control status in the HR subgroup. A positive profile was indeed associated with poor disease control, but the majority of patients with VPC disease did not display a Th2-high profile.
Figure 2 –
A, Patients with very poorly controlled and not well controlled disease with high reversibility assessed via ACQ6. B, Patients with very poorly controlled and not well controlled disease with low reversibility assessed via ACQ6. Patients with high reversibility (A) and low reversibility (B) phenotypes are partitioned into those with poor and adequate disease control and then further subdivided based on individual Th2 biomarkers and the aggregate Th2 Immune Profile Index. The results of χ2 analysis assessing the statistical significance of the association of each biomarker with disease control status and PPV and NPV of each biomarker for predicting disease control status are shown. NPV = negative predictive value; PPV = positive predictive value; Th2 = T-helper 2. See Figure 1 legend for expansion of other abbreviations.
Similar poor associations between biomarker status (high vs low) and extent of disease control were observed in the LR subgroup (Fig 2B, Table 4). Th2 biomarkers alone and in aggregate when expressed as Th2 IPI failed to accurately reflect the level of disease control. This finding indicates that Th2 biomarkers in both the HR and the LR subgroups are not reliable indicators of disease control.
To assess whether application of various biomarker cut points might have rendered different findings, we performed a CART analysis. The CART analysis aimed to maximize differences between subgroups by exploring the continuous range of values for each Th2 biomarker to identify optimal cut point values to differentiate patients with NWC from those with VPC disease. CART results were unrevealing, however, because no cut point strategy, including > 25% of either the HR or the LR population, clearly separated NWC from VPC patients.
Discussion
To better understand endotype diversity in patients with asthma and identify profiles to guide therapy, patients have been grouped, or clustered, based on clinical data and biomarkers. Using this approach, SARP investigators identified subgroups differing with respect to age of disease onset, sex distribution, biometrics, health-care resource utilization, airway hyperresponsiveness, and bronchodilator reversibility.1,2 High airway bronchodilator reversibility has emerged as a reliable physiologic biomarker associated with severe airflow obstruction, increased health-care resource utilization, and poor disease control. By contrast, classic serum and blood biomarkers have not consistently correlated with disease activity.2,5 Indeed, several studies paradoxically suggest that higher levels of some cytokines may be associated with milder phenotypes.5
The present study attempts to better characterize this HR phenotype by comparing biomarker profiles and disease control status in patients with HR and LR asthma. The present analysis focused on three biomarkers (blood eosinophil counts, serum IgE levels, and Feno concentrations) and used ACQ to assess disease control status. Results presented in Figure 1 show that these three biomarkers are stable indicators of immune status. Patients not receiving active therapy in either clinical trial6,7 had 12-week biomarker values that highly correlated with baseline values. Although some variability was observed, 49% to 93% of the week 12 signal, as assessed in terms of r2 value, was accounted for by the baseline value, with Feno concentration showing the poorest correlation over time. A similar analysis involving ACQ and bronchodilator reversibility indicated that within-subject measures of disease control and airway reversibility also remained relatively stable over this time frame.
Although baseline Th2 biomarker levels appear to be stable indicators of inflammation in a given patient, they were not useful indicators of HR vs LR or VPC vs NWC phenotypes. As shown in Figures 2A and 2B, the fraction of patients with a Th2-high biomarker profile in the HR subgroup was higher than in the LR subgroup (40.1% vs 29.3%, P = .006). However, the majority of patients in both subgroups displayed a Th2-low biomarker profile. Serum IgE level was elevated in a similar fraction of the HR (66.2%) and LR (57.9%) subgroups, consistent with the high incidence of atopy in both populations (93.7% and 86.5%, respectively). However, blood eosinophil counts and Feno concentrations were elevated in a minority of patients in both subgroups. This could reflect the effects of corticosteroid use on biomarker expression because this class of medication is known to affect circulating eosinophil counts and Feno levels and may influence cytokine expression.12,13 Thus, although the HR relative to the LR subgroup is enriched for patients with a Th2-high biomarker profile, the majority of patients with moderate to severe asthma in both studies6,7 had a Th2-low profile.
The present results further indicate that within the HR and LR subgroups, Th2 biomarker status does not align with disease control. Across both subgroups, the proportions of patients displaying Th2-high biomarker profiles with VPC and NWC asthma were similar (HR Th2-high, 39.0% vs 41.6%, respectively [P = .685]; LR Th2-high, 27.% vs 31.1%, respectively [P = .461]). To the extent that Th2 biomarkers levels accurately reflect inflammatory status, these findings do not support the hypothesis that disease activity in adult patients with poor asthma control is primarily driven by Th2 inflammation.
Several factors implicit in this analysis are relevant to interpreting these findings in the broader context of asthma research. The clinical trials6,7 excluded patients on oral corticosteroids and IgE immunotherapy (ie, GINA step 5), and study eligibility required that patients have a baseline FEV1 > 50% predicted and fewer than five exacerbations in the 12-month period before enrollment. Therefore, the findings apply specifically to a subset of patients in GINA steps 3 and 4. Extension of these findings to patients with different disease characteristics may not be valid. In addition, allocation of patients to Th2-high vs Th2-low biomarker profiles in this analysis is based on published IgE, eosinophil, and Feno cut points. Although the values used in this study are generally accepted and reasonable, results would vary if different cut points had been used. The CART analysis, however, failed to identify Th2 biomarker cut points that reliably separated patients with VPC and NWC asthma into either the HR or the LR subgroup. Finally, the ACQ-based cut points defining NWC and VPC are based on extrapolations from the literature, and although theoretically reasonable, these are specific to this analysis. A difference in cut point definition would affect comparisons of NWC vs VPC subgroups due to patient assignment. However, it would not likely change the overall conclusion that patients with HR asthma often display poor disease control along with Th2-low biomarker profiles.
In conclusion, the results indicate that conventional Th2 biomarker levels, including blood eosinophil counts, serum IgE levels, and Feno concentrations, are reasonably stable indicators of the inflammatory state of individuals with asthma receiving fixed medical therapy. Findings support prior results from SARP indicating that patients with HR asthma represent a distinct phenotype with worse pulmonary function and less-well-controlled disease. The data further suggest that the HR population, relative to the LR population, is enriched for patients displaying a Th2-high biomarker profile. However, the majority of patients in both the HR and the LR subgroups, although atopic, displayed Th2-low biomarker profiles. Finally, within the HR and LR subgroups with ACQ6 ≥ 1.5, a poor relationship exists between disease control and Th2 biomarker levels, suggesting that factors other than Th2 inflammation drive disease activity in a majority of patients with moderate to severe asthma.
Acknowledgments
Author contributions: E. P. I. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. W. W. B. was a study investigator and contributed to the data analysis and writing and review of the manuscript; S. T. H. and S. S. W. were study investigators and contributed to the writing and review of the manuscript; P. K. was a sponsor medical director and contributed to the data analysis and review of the manuscript; Y. C. and J. F. contributed to the statistical analysis and table and figure preparation for the manuscript; E. P. I. was a sponsor medical director and contributed to the data analysis, table and figure preparation for the manuscript, and writing and review of the manuscript; and A. N. was the sponsor senior medical director and contributed to the data analysis and review of the manuscript.
Conflict of interest: W. W. B. has received personal fees from Novartis AG, Hoffmann-La Roche Ltd, AstraZeneca, Takeda Pharmaceutical Company Limited, Sanofi, Boston Scientific Corporation, and ICON plc. He also reports grants from the National Institute of Allergy and Infectious Diseases and National Heart, Lung, and Blood Institute, National Institutes of Health, outside the submitted work. S. T. H. has served as an occasional consultant to Amgen Inc. S. W. W. has been a principal investigator on multicenter industry-sponsored trials from Amgen Inc, AstraZeneca, sanofi aventis, Merck Sharp & Dohme Corp, and Pfizer Inc. She has received consulting fees from Amgen Inc, GlaxoSmithKline, AstraZeneca, Merck Sharp & Dohme Corp, Gilead, Novartis AG, and Boehringer Ingelheim GmbH. P. K., Y. C., J. F., and A. N. are employees of Amgen Inc. E. P. I. is a clinical medical director at Amgen Inc.
Role of sponsors: Amgen Inc funded the studies on which this manuscript is based. The data analysis, statistical assessments, and writing of the initial draft of the manuscript were performed by Amgen Inc employees.
Other contributions: The authors thank Maggie Wang, MS, for important contributions to the statistical analysis.
ABBREVIATIONS
- ACQ
Asthma Control Questionnaire
- CART
classification and regression tree
- Feno
fraction of exhaled nitric oxide
- GINA
Global Initiative for Asthma
- HR
high reversibility
- IPI
Immune Profile Index
- LR
low reversibility
- NWC
not well controlled
- PPV
positive predictive value
- SARP
Severe Asthma Research Program
- Th
T-helper
- VPC
very poorly controlled
Footnotes
FUNDING/SUPPORT: This study was supported by Amgen Inc, Thousand Oaks, CA.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Moore WC, Meyers DA, Wenzel SE, et al. ; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu W, Bleecker E, Moore W, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133(5):1280-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore WC, Hastie AT, Li X, et al. ; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133(6):1557-1563.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braiser AR, Victor S, Ju H, et al. Predicting intermediate phenotypes in asthma using bronchoalveolar lavage-derived cytokines. Clin Transl Sci. 2010;3(4):147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amgen Inc. AMG 853 phase 2 study in subjects with inadequately controlled asthma. NCT01018550. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2009; https://clinicaltrials.gov/ct2/show/NCT01018550. Updated May 19, 2011. [Google Scholar]
- 7.Amgen Inc. A study to evaluate the dosing of AMG 827 for subjects with inadequately controlled asthma. NCT01199289. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2010; https://clinicaltrials.gov/ct2/show/NCT01199289. Updated April 28, 2015. [Google Scholar]
- 8.Diagnosis of diseases of chronic airflow limitation: asthma, COPD and asthma-COPD overlap syndrome. Global Initiative for Chronic Obstructive Lung Disease website http://www.goldcopd.org/uploads/users/files/AsthmaCOPDOverlap.pdf. Accessed August 29, 2014.
- 9.National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma: full report 2007. National Heart, Lung, and Blood Institute website https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf. Accessed August 29, 2014.
- 10.Dweik RA, Boggs PB, Erzurum SC, et al. ; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran TN, Khatry DB, Ward CK, Gossage D. High blood eosinophil counts is associated with more frequent asthma attacks in asthma patients. Ann Allergy Asthma Immunol. 2014;113(1):19-24. doi:10.1016/janal.2014:04.001. [DOI] [PubMed] [Google Scholar]
- 12.de Jongste JC. Yes to NO: the first studies on exhaled nitric oxide-driven asthma treatment. Eur Respir J. 2005;26(3):379-381. [DOI] [PubMed] [Google Scholar]
- 13.Smits HH, Grünberg K, Derijk RH, Sterk PJ, Hiemstra PS. Cytokine release and its modulation by dexamethasone in whole blood following exercise. Clin Exp Immunol. 1998;111(2):463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]