Abstract
The present study aimed to visualize human motor neuronal activation in the brain using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET), and to develop an FDG-PET procedure for imaging neuronal activation. A male volunteer underwent 20 min periods of rest and motor activation, whilst being assessed using FDG-PET on two consecutive days. The motor task, which involved repetitively grasping and releasing the right hand, was performed during the initial 5 min of the activation period. Subtraction of the rest period signal from the activation PET images was performed using the subtraction ictal single-photon emission computed tomography co-registered to magnetic resonance imaging method. The subtracted image detected activation of the contralateral (left) primary motor cortex, supplementary motor area, and ipsilateral (right) cerebellum. In the present study, FDG-PET detected significantly increased motor-associated activation of the brain in a subject performing a motor task.
Keywords: motor cortex, motor cortex activation, motor image, neuroimaging, fluorodeoxyglucose positron emission tomography
Introduction
The performance of voluntary motor tasks is associated with the primary motor cortex of the brain (1). Furthermore, cerebral blood flow and glucose metabolism have been demonstrated to positively correlate with neuronal processes during neuronal transmission in a healthy brain (2).
Numerous methods, including computed tomography (CT), magnetic resonance imaging (MRI), and functional MRI have previously been used to image the motor cortex; however, limitations of these approaches are associated with their inability to fully capture a functional view of the motor cortex, instead only capturing the anatomical structure or blood flow (3,4).
Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET) detects FDG accumulation in tissue that is proportional to the amount of glucose metabolism, and has been in use for >35 years (5) in the study of neurology, oncology and cardiology (6). FDG-PET is regarded as an effective imaging method for visualizing glucose metabolism during cerebral blood flow (7) and is currently the most accurate method for the evaluation of human brain metabolism (8); however, studies that have investigated the use of FDG-PET for imaging motor cortex activation are limited.
The present study aimed to demonstrate human motor-associated activation of the brain using FDG-PET, and to develop an FDG-PET protocol for imaging neuronal activation.
Materials and methods
Patient
A healthy 30-year-old right-handed male volunteered to participate in the present study at Korea University Anam Hospital (Seoul, Republic of Korea). Written informed consent was obtained from the patient. This study was approval by the ethics committee of the Korea University Anam Hospital.
Imaging time and motor task
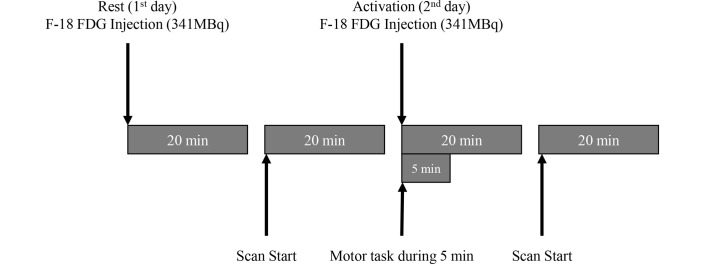
On day 1, following a 20-min preparatory rest period, the patient underwent FDG-PET analysis. On day 2, after performing 20 min of motor activation, a second session of FDG-PET was conducted (Fig. 1). The motor task, which involved repetitively grasping and releasing the right hand, was performed during the initial 5 min of the motor activation period.
Figure 1.
Schematic description of the 2-day F-18 FDG-positron emission tomography procedure. F-18 FDG, fluorine-18 fluorodeoxyglucose.
Image acquisition
Images were obtained using a PET-CT scanner (Gemini TF; Philips Healthcare, Cleveland, OH, USA). The volunteer fasted for ≥6 h, and serum glucose levels were <180 mg/dl, prior to scanning. Image acquisition (20 min) was initiated 20 min following intravenous injection of 341 MBq fluorine-18 FDG. CT scans were obtained followed by PET emission scans for 1 min per scan. The PET unit had an axial field of view of 18 cm and a spatial resolution of 4.4 mm. A low-dose CT scan was obtained for attenuation correction and localization, with a 16-slice multidetector helical CT unit, using the following parameters: 120 kVp; 50 mA; 0.75 sec rotation time; 0.75 mm slice collimation; 2 mm scan reconstruction with a reconstruction index of 2 mm; 60 cm field of view; and a 512×512 matrix. PET data were reconstructed iteratively using the 3 dimensional Row Action Maximum Likelihood Algorithm (9), with low-dose CT data-sets for attenuation correction. Maximum intensity projection, cross sectional views, and fusion images were generated and reviewed.
Image processing
Images were transformed and quantified using statistical probabilistic anatomical mapping, which is a well-established method for displaying functional brain images (10–12). Images were spatially normalized using the Statistical Parametric Mapping (SPM) 2 software (http://www.fil.ion.ucl.ac.uk/spm/), according to the Korean standard templates provided by the Korean Structural Statistical Probabilistic Anatomical Map, implemented in Matlab 6.5 (13), which is based on the Korean standard brain atlas (13). Normalized images were smoothed by convolution using a Gaussian filter with a 16 mm full width at half maximum. The pixel count of PET images was normalized to the mean pixel count of grey matter in each PET image (rest and activation), which was measured using a gray matter probability map (provided by SPM), according to the following equation:
Where Ii,j,k and Gi,j,k are the pixel counts of the PET images and the probability map of gray matter at the (Ii,j,k)th pixel, respectively. The Digital Imaging and Communications in Medicine (.dcm) format was used for all of the data files in the present study, and was converted to the Analyze format using MRIcro software (www.mricro.com; Chris Rorden, Columbia, SC, USA).
Image analysis
The subtraction method was used to analyze the images (11,14). Subtraction of the rest period signal from the activation PET images was performed using the Subtraction ictal single-photon emission computed tomography co-registered to MRI method (14). A metabolic change map was calculated using the following equation:
Where Iic and Iin are the normalized rest and activated PET images, respectively. Metabolic changes >20% were considered significant. Changes in the metabolic map containing significant pixels were superimposed onto the T1 MRI template (15,16).
Results and Discussion
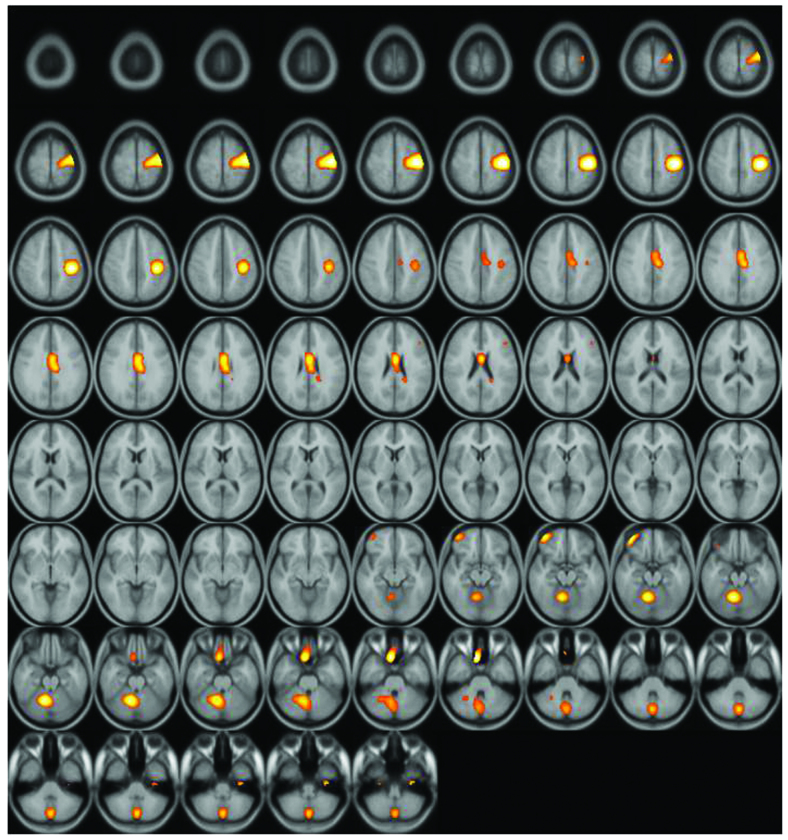
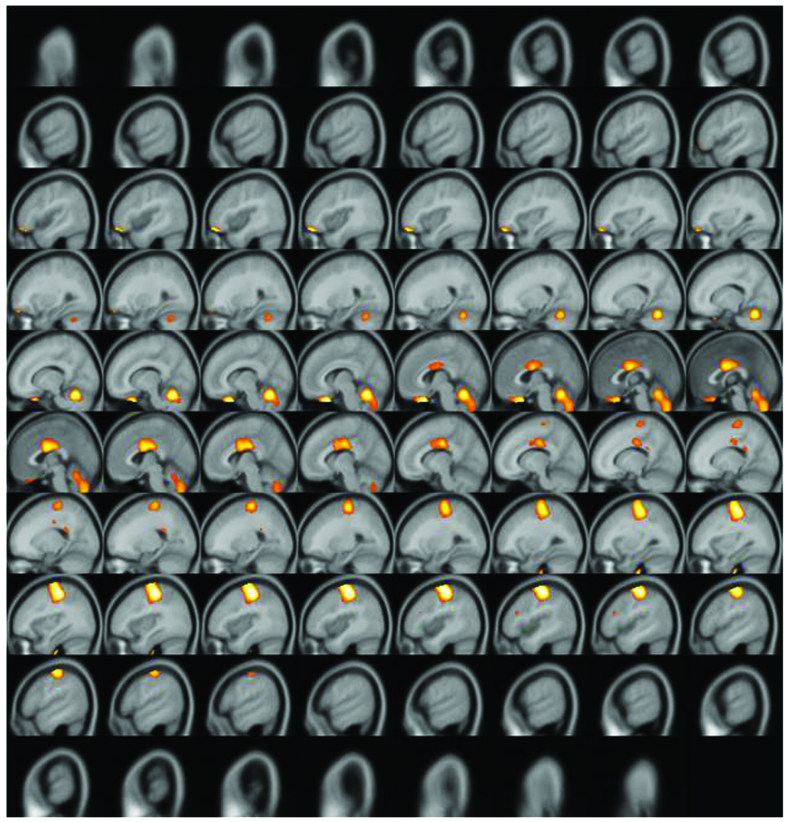
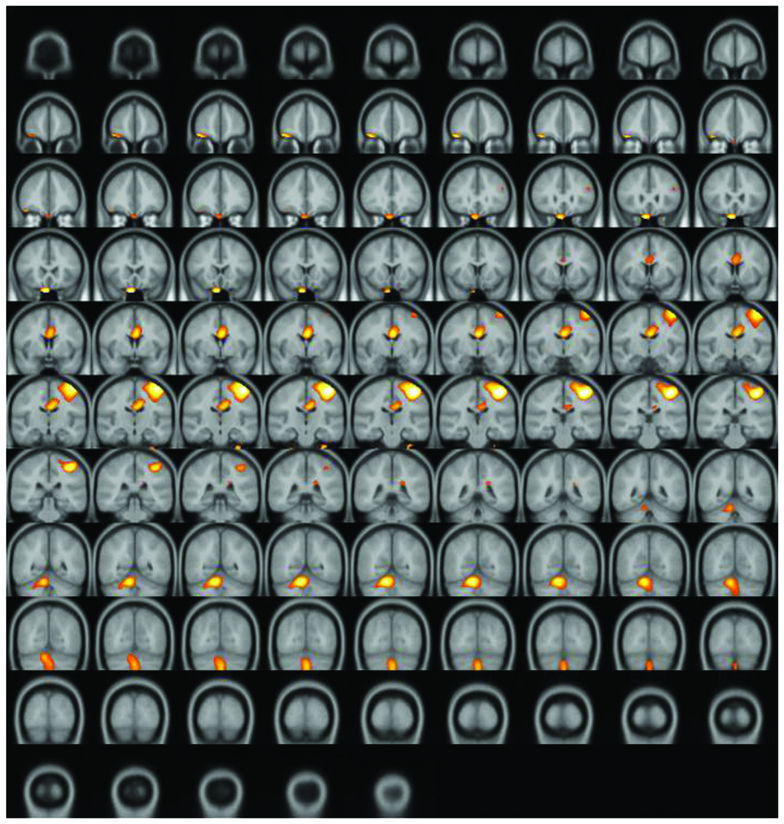
A significantly increased uptake of F-18 FDG was observed in the contralateral (left) primary motor cortex (M1), supplementary motor area (SMA), and ipsilateral (right) cerebellum (Figs. 2–4). Increased glucose uptake (metabolic connectivity) indicates increased neuronal activity (17). Therefore, the results shown in Figs. 2–4 indicated increased neuronal activity in the contralateral (left) primary motor cortex (M1), supplementary motor area (SMA) and ipsilateral (right) cerebellum. An increased number of regional blood flow connections between the M1, SMA and cerebellum during motor tasks has previously been demonstrated using MRI (18,19).
Figure 2.
Subtraction positron emission tomography image: Axial view. Enhanced uptake of fluorine-18 fluorodeoxyglucose was observed in the supplementary motor area and ipsilateral cerebellum.
Figure 4.
Subtraction positron emission tomography image: Sagittal view. Enhanced uptake of fluorine-18 fluorodeoxyglucose was observed in the supplementary motor area and ipsilateral cerebellum.
The results of the present study align with results from previous MRI studies (18,19), and demonstrated increased glucose metabolism in the contralateral M1, SMA, and ipsilateral cerebellum, using FDG-PET. In the process of neuronal activation, regional metabolic connections precede regional blood flow connections, which are typically measured by fMRI (20). The present results show that it is possible to detect metabolic connectivity using FDG-PET. Furthermore, the present study established a simple imaging procedure for FDG-PET, thus suggesting that FDG-PET may be considered a reliable neuronal imaging method for analyzing glucose metabolism in the brain.
In summary, the present study used PET to demonstrate increased human motor-associated activation of the brain following administration of FDG, in a subject performing a motor task. In addition, a two-day protocol was established for acquiring detailed images of neuronal activation. FDG-PET-based metabolic connectivity studies may be useful for investigating brain neuronal activation, particularly in motor neuronal activation.
Figure 3.
Subtraction positron emission tomography image: Coronal view. Enhanced uptake of fluorine-18 fluorodeoxyglucose was observed in the supplementary motor area and ipsilateral cerebellum.
Acknowledgements
This study was supported by a grant from Korea University (no. K1507831).
References
- 1.Lotze M, Montoya P, Erb M, Hülsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fMRI study. J Cogn Neurosci. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- 2.Schreckenberger M, Spetzger U, Sabri O, Meyer PT, Zeggel T, Zimny M, Gilsbach J, Buell U. Localisation of motor areas in brain tumour patients: A comparison of preoperative [18F]FDG-PET and intraoperative cortical electrostimulation. Eur J Nucl Med. 2001;28:1394–1403. doi: 10.1007/s002590100582. [DOI] [PubMed] [Google Scholar]
- 3.Rorden C, Karnath HO. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- 4.Lee DS, Kang H, Kim H, Park H, Oh JS, Lee JS, Lee MC. Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. Eur J Nucl Med Mol Imaging. 2008;35:1681–1691. doi: 10.1007/s00259-008-0808-z. [DOI] [PubMed] [Google Scholar]
- 5.Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, OShaughnessy J, Guyton KZ, Mankoff DA. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 6.Hoh CK. Clinical use of FDF PET. Nucl Med Biol. 2007;34:737–742. doi: 10.1016/j.nucmedbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: A critical review. Acta Psychiatr Scand. 2000;101:11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [DOI] [PubMed] [Google Scholar]
- 8.Varrone A, Asenbaum S, Van der Borght T, Booij J, Nobili F, Nagren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, Van Laere K. European Association of Nuclear Medicine Neuroimaging Committee: EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–2110. doi: 10.1007/s00259-009-1264-0. [DOI] [PubMed] [Google Scholar]
- 9.Daube-Witherspoon ME, Matej S, Karp JS, Lewitt RM. Application of the row action maximum likelihood algorithm with spherical basis functions to clinical PET imaging. IEEE Trans Nucl Sci. 2001;48:24–30. doi: 10.1109/23.910827. [DOI] [Google Scholar]
- 10.Lee DS, Lee JS, Kang KW, Jang MJ, Lee SK, Chung JK, Lee MC. Disparity of perfusion and glucose metabolism of epileptogenic zones in temporal lobe epilepsy demonstrated by SPM/SPAM analysis on 15O water PET, [18F]FDG-PET, and [99mTc]-HMPAO SPECT. Epilepsia. 2001;42:1515–1522. doi: 10.1046/j.1528-1157.2001.21801.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee JJ, Kang WJ, Lee DS, Lee JS, Hwang H, Kim KJ, Hwang YS, Chung JK, Lee MC. Diagnostic performance of 18F-FDG PET and ictal 99mTc-HMPAO SPET in pediatric temporal lobe epilepsy: Quantitative analysis by statistical parametric mapping, statistical probabilistic anatomical map, and subtraction ictal SPET. Seizure. 2005;14:213–220. doi: 10.1016/j.seizure.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Choi JY, Lee KH, Na DL, Byun HS, Lee SJ, Kim H, Kwon M, Lee KH, Kim BT. Subcortical aphasia after striatocapsular infarction: Quantitative analysis of brain perfusion SPECT using statistical parametric mapping and a statistical probabilistic anatomic map. J Nucl Med. 2007;48:194–200. [PubMed] [Google Scholar]
- 13.Lee JS, Lee DS. Analysis of functional brain images using population-based probabilistic atlas. Curr Med Imag Rev. 2005;1:81–87. doi: 10.2174/1573405052953056. [DOI] [Google Scholar]
- 14.Lee SK, Lee SY, Yun CH, Lee HY, Lee JS, Lee DS. Ictal SPECT in neocortical epilepsies: Clinical usefulness and factors affecting the pattern of hyperperfusion. Neuroradiology. 2006;48:678–684. doi: 10.1007/s00234-006-0106-z. [DOI] [PubMed] [Google Scholar]
- 15.Spanaki MV, Spencer SS, Corsi M, MacMullan J, Seibyl J, Zubal IG. Sensitivity and specificity of quantitative difference SPECT analysis in seizure localization. J Nucl Med. 1999;40:730–736. [PubMed] [Google Scholar]
- 16.Avery RA, Spencer SS, Studholme C, Stokking R, Morano G, Corsi M, Seibyl JP, Spencer DD, Zubal IG. Reproducibility of serial peri-ictal single-photon emission tomography difference images in epilepsy patients undergoing surgical resection. Eur J Nucl Med. 2000;27:50–55. doi: 10.1007/PL00006662. [DOI] [PubMed] [Google Scholar]
- 17.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai N, Lu MK, Ugawa Y, Ziemann U. Effective connectivity between human supplementary motor area and primary motor cortex: A paired-coil TMS study. Exp Brain Res. 2012;220:79–87. doi: 10.1007/s00221-012-3117-5. [DOI] [PubMed] [Google Scholar]
- 19.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DS, Kang H, Kim H, Park H, Oh JS, Lee JS, Lee MC. Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. Eur J Nucl Med Mol Imaging. 2008;35:1681–1691. doi: 10.1007/s00259-008-0808-z. [DOI] [PubMed] [Google Scholar]