Abstract
Breast cancer encompasses a heterogeneous group of diseases at the molecular level. It is known that chemo-sensitivity of breast cancer depends on its molecular subtype. We investigated the growth inhibitory effect of valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, and the mechanism of this inhibition on four breast cancer cell lines with different molecular subtypes. The growth inhibitory effect of VPA in the four different breast cancer cell lines was investigated. The alteration of levels of p21 WAF1, cleaved caspase-3, acetylated Heat shock protein (Hsp) 90, acetylated Hsp70, and acetylated α-tubulin by VPA was examined in VPA-sensitive, human epidermal receptor 2 (HER2)-overexpressing SKBR3 cells. The cell growth inhibition of breast cancer cell lines was dependent on the dose and exposure time of VPA. The cell growth of HER2-overexpressing SKBR3 cell line was inhibited by VPA to a much greater degree than other cell lines studied. In SKBR3 cell line, VPA upregulated expression of p21 WAF1 and cleaved caspase-3 in the early phase. VPA markedly increased Hsp70 acetylation in a time-dependent manner but did not increase Hsp90 acetylation. Our data demonstrated that VPA inhibited cell proliferation and induced cell cycle arrest and apoptosis of HER2-overexpressing breast cancer cells. This anti-proliferation effect might be the direct function of VPA as an HDAC inhibitor. We propose an alternative mechanism whereby acetylation of Hsp70 disrupts the function of Hsp90 and leads to downregulation of its client proteins, including HER2 that might be the indirect function of VPA, in the sense that non-histone proteins are acetylated.
Keywords: valproic acid, HDAC inhibitor, Hsp70, Hsp90, breast cancer, HER2
Introduction
Breast cancer is one of the most common cancers in women. In recent years, major advances in breast cancer therapy have been achieved. Nevertheless, because cancer cells may present a number of resistance mechanisms reducing the effectiveness of chemotherapeutic drugs, new and more effective strategies for treatment and prevention are still much needed. Breast cancer encompasses a heterogeneous group of diseases at the molecular level and can be classified into at least five distinct subtypes by gene-expression profiling: luminal A, luminal B, normal breast-like, human epidermal receptor 2 (HER2), and basal-like. Recent studies based on neoadjuvant clinical trials for breast cancer suggested that chemosensitivity depends on its molecular subtype (1).
There is increasing evidence that epigenetic alterations, such as histone acetylation and promoter methylation, play important roles in regulation of gene expression associated with the cell cycle and apoptosis (2). Chromatin remodeling regulates gene transcription and is in turn regulated by two enzymes, histone acetyltransferase (HAT) and histone deacetylase (HDAC), that control post-translational modifications of histones. Acetylation of lysine residues on the histones weakens their binding to DNA and induces a change in DNA conformation and allows transcription factors to bind to the promoter regions of target genes (3,4).
In mammalian cells, there are 18 different HDACs, which can be further divided into four types. HDAC 1, 2, 3, and 8 are class I deacetylases that localize almost exclusively to the nucleus and are ubiquitously expressed in various human cell lines and tissues. HDAC 4, 5, 6, 7, 9, and 10 are class II deacetylases, which shuttle between the nucleus and cytoplasm with certain cellular signals. Class III comprises the conserved nicotinamide adenine dinucleotide-dependent Sir2 family of deacetylases. HDAC11 is the only member of the class IV HDACs (5). Aberrant levels of HDAC activity have been found in a variety of human malignancies and result in repression of tumor-suppressor genes and promotion of tumorigenesis (6).
Valproic acid (VPA), which has long been used clinically for treatment of epilepsy and bipolar disorder without significant toxicity, causes hyperacetylation of the N-terminal tails of histones H3 and H4 in vitro and in vivo and inhibits HDAC activity, probably by binding to the catalytic center and, thereby, blocking substrate access (7,8). VPA inhibits both class I and II HDACs, with high potency for class I HDACs (9). Earlier studies indicated that p21 WAF1, one of the target genes induced by VPA, affects differentiation and decreases tumor cell growth (10,11). Another report focused on the apoptotic activity of VPA (12). However, the detailed mechanism of apoptosis induced by VPA has not been elucidated. In addition, recent evidence suggests that HDAC inhibitors also enhance the acetylation of non-histone proteins, such as p53, c-Jun, and α-tubulin (13–15).
Although VPA has been shown to reduce cancer proliferation to some extent, there is insufficient amount of data on its effect in breast cancer cells. Studies on the specificity of VPA against breast cancer subtypes have often yielded contrasting results and conflicting conclusions (16–18).
Several studies have found that inhibition of HDAC increases acetylation levels of the core histones as well as some non-histone proteins (13,19), raising the possibility that transcription-independent effects of HDAC inhibitors are also important for their anticancer activity (5). It has recently been reported that pan-HDAC inhibitors such as LAQ824, LBH589, and SAHA exert their antitumor activity by inhibition of HDAC6, a deacetylase of α-tubulin and heat shock protein (Hsp) 90 (19–21). The inhibition of HDAC6 results in acetylation of Hsp90 and disruption of its chaperone function (21–23).
Heat shock proteins (Hsps) are a group of highly conserved molecular chaperones which were originally identified by their induction in response to cellular stresses. Hsps are classified according to their molecular weight and in mammals five distinct families have been defined: Hsp100, Hsp90, Hsp70, Hsp60 and the small Hsps (24). As Hsp90 controls the intra-cellular trafficking and folding of diverse cellular proteins, disruption of Hsp90 chaperone function will lead to the destabilization and eventual degradation of Hsp90 client proteins and induces apoptosis (25).
HDAC6 is an unusual histone deacetylase, which harbors two functional catalytic domains and is localized in the cytoplasm (26). Some recent reports have demonstrated that HDAC6 is responsible for the deacetylation of acetyl-α-tubulin and acetyl-Hsp90 (23,27). It has been reported that the HDAC inhibitor FR901228 disrupts the chaperone function of Hsp90 and induces apoptosis in human non-small cell lung cancer cells (13). However, as a class I HDAC inhibitor, VPA has only a weak effect on inhibition of HDAC6 (14,28).
It is known that Hsp70 is required for the assembly of the signaling protein-Hsp90 heterocomplex. Hsp90 is involved in two multi-chaperone complexes and promotes correct folding or degradation of client proteins, depending on its conformation. When adenosine triphosphate (ATP) is bound to the amino-terminal nucleotide-binding pocket, Hsp90 is associated with co-chaperone proteins p23 and p50Cdc37 and directly binds to the client protein to stabilize the interactions. When adenosine diphosphate (ADP) is bound, Hsp90 is assembled into the complex with co-chaperone proteins Hsp70 and p60Hop. Within the complex, Hsp70 directly interacts with the client protein to promote ubiquitination and degradation (25,29). Therefore, the function of Hsp70 may indirectly affect the chaperone function of Hsp90. However, it is unknown whether VPA can influence the chaperone function of Hsp70 and the Hsp90 in breast cancer cells. We speculate that VPA could enhance the acetylation of Hsp70 and Hsp90 through its inhibitory effect on HDAC6.
In this study, we investigated both inhibitory and pro-apoptotic effects of VPA on breast cancer cell lines with various molecular subtypes. In addition, we explored whether VPA enhanced the acetylation of Hsp70 and Hsp90 and its contribution to tumor growth inhibition in the VPA-sensitive cell line.
Materials and methods
Materials
VPA and trichostatin A (TSA) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Cell lines and cell culture
Two cancer cell lines derived from human breast cancer, estrogen receptor (ER)-positive and HER2-negative MCF7 cells and ER-negative and HER2-overexpressing SKBR3 cells were provided by the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University. The other two cell lines derived from human breast cancer, ER-negative and HER2-negative MDA-MB-231 cells and ER-positive and HER2-overexpressing BT474 cells, were purchased from the American Type Culture Collection (Rockville, MD, USA). These breast cancer cells were seeded in 75-cm2 dishes (Becton-Dickinson, Franklin Lakes, NJ, USA) and cultured in 10 ml of medium at 37ºC in a humidified atmosphere of 5% CO2. These cells were grown in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Nichirei Bioscience Inc., Japan), 100 IU/ml penicillin, 100 mg/ml streptomycin (Invitrogen), 2 mM glutamine (Nissui Pharmaceutical Co., Ltd., Japan), and 0.5 mM sodium pyruvate. Cells were grown to confluence and harvested by trypsinization with 0.25 mg/ml trypsin/EDTA solution (Invitrogen) and suspended in culture medium before use.
Cell growth assay
The viability of breast cancer cells treated with VPA was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2H-tetrazolium (MTS) assay. Breast cancer cells were seeded at 5×103 cells per well in 96-well plates and incubated overnight at 37ºC. After incubation, the supernatant was discarded and replaced with fresh serum-free culture medium. VPA was dissolved in phosphate-buffered saline (PBS) and added to the cell culture medium at various concentrations (0–10 mM). At 24, 72, and 120 h after exposure to VPA, the supernatant was discarded and MTS solution (CellTiter 96 AQuous One Solution Reagent, Promega, Fitchburg, WI, USA) was added to each well and incubated at 37ºC for 2 h. Then, the absorbance of the solution was read at a wavelength of 490 nm using a microplate reader (Bio-Rad, Fitchburg, WI, USA). The percentage inhibition was determined by comparing the cell density of the drug-treated cells with that of untreated controls. All experiments were repeated at least three times. The median growth inhibition (GI50) corresponding to the concentration of the compound that inhibits 50% net cell growth was calculated for each cell line.
Western blotting
The mechanism of growth inhibition by VPA was analyzed using SKBR3 cell line, which we found to be the most sensitive to VPA among the four cell lines studied. The effects of VPA on acetylation of histone H3 and cell cycle regulatory and apoptosis-related proteins were analyzed by western blotting. Breast cancer cells were seeded at a density of 1×106 cells per 75-cm2 dish and cultured in 10 ml of medium overnight. Lysates were obtained from the cells harvested at 0, 3, 6, 12, 24, and 48 h after incubation with 1 mM VPA, which corresponded approximately to the maximum level obtained by administering a clinical dose of VPA. Whole-cell lysates were prepared in denaturing SDS sample buffer and resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a PVDF membrane (ATTO Co. Ltd., Japan). As primary antibodies, rabbit polyclonal acetyl-histone H3 (Lys9) antibody (1:5,000) (Cell Signaling Technology, Beverly, MA, USA), rabbit polyclonal histone H3 antibody (1:1,000) (Cell Signaling Technology), and mouse monoclonal β-actin antibody (1:5,000) (Sigma-Aidrich) were used. A mouse monoclonal p21 WAF1 (1:1,000) (Pharmingen, San Diego, CA, USA) was used against cell cycle regulatory proteins. As antibodies against apoptosis-related proteins, a rabbit polyclonal cleaved caspase-3 (Asp175) antibody (1:1,000) (Cell Signaling Technology) was used. The immunoblots were visualized using an ECL Plus (GE Healthcare UK, Ltd., Buckinghamshire, UK). Immuno-complex was detected by an ECL detection system (GE Healthcare). Chemiluminescent signal was detected by the Light-Capture system (ATTO), and the intensity of protein bands were quantified using the CS analyzer program (ATTO).
Immunoprecipitation analyses
Lysates were obtained from the SKBR3 cells harvested at 0, 6, 12, 24, and 48 h after incubation with 1 mM VPA. Cellular extracts from ~1×106 cells were prepared in radio-immunoprecipitation (RIPA) buffer, and ~500 μg of total proteins was incubated with 2 μg of primary antibody at room tempreture for 60 min on a rotator. As primary antibodies, rabbit polyclonal Hsp70 antibody (Upstate Biotechnology, Lake Placid, NY, USA) and mouse monoclonal Hsp90 (Upstate) antibody were used. Then 20 μl of protein A/G-Plus-Agarose (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to the mixture and incubated at 4ºC for 36 h. Agarose-antibody-protein complexes were washed three times with lysis buffer. After discarding the supernatant from the final wash, the immune-complexes were resuspended in 50 μl of 1× electrophoresis sample buffer and boiled at 95ºC for 3 min. The immunoprecipitated proteins were separated by SDS-PAGE, and detected by using an ECL Plus (GE Healthcare). As primary antibodies, mouse monoclonal acetyl-Lysine antibody (1:1,000) (Upstate), Hsp70 antibody (1:1,000) and Hsp90 antibody (1:1,000) were used. The antibody-antigen complex was detected by western blotting.
Immunohistochemical examination and TUNEL assay
SKBR3 cells were seeded in 10-cm2 dishes and incubated overnight at 37ºC. After incubation, the supernatant was discarded and replaced the cell culture medium including 1 mM VPA. At 48 h after exposure to VPA, the supernatant was discarded, and cells were fixed in 10% neutral buffered formalin and embedded in paraffin. The sections were stained with H&E and immunostained with a rabbit polyclonal cleaved caspase-3 antibody (1:200) (Cell Signaling Technology) at 4ºC overnight. The sections were reacted with EnVision reagent (Dako Co., Japan) for visualization. For quantitative analysis, the stained cells were counted under ×400 magnification in 6 randomly chosen fields representing ≥1,000 cells. The degree of apoptosis was evaluated using the TdT-mediated dUTP nick-end labeling (TUNEL) method (Apoptosis In Situ Detection kit; Wako, Osaka, Japan).
Results
Effects of VPA on the growth of breast cancer cell lines in vitro
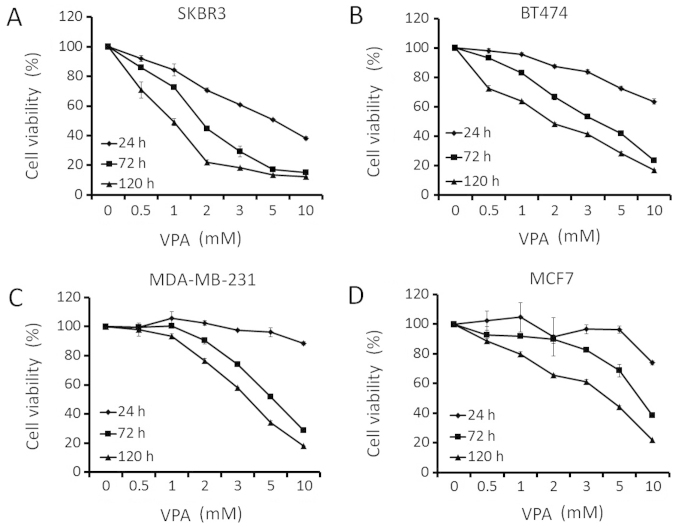
To explore whether VPA might be a potential therapeutic agent against breast cancer, we investigated its effects of growth inhibition in human breast cancer cell lines (SKBR3, BT474, MDA-MB-231, and MCF7) that differed in their ER- or HER2-expression status. Breast cancer cells were treated with increasing doses of VPA for ≤5 days. The inhibition of cell growth in breast cancer cell lines was dependent on the dose and incubation time of VPA (Fig. 1). GI50 values for VPA (mM) in SKBR3, BT474, MDA-MB-231, and MCF7 at 72 h were 1.8, 3.6, 5.4 and 8.1 mM, respectively (Table I). SKBR3 cells, which overexpressed HER2 and were ER-negative, exhibited the most sensitivity towards VPA in growth inhibition. SKBR3 cell growth was inhibited by VPA at clinically achievable doses.
Figure 1.
Effect of VPA on the growth of breast cancer cell lines. Cell viability was assessed by MTS assay. Breast cancer cell lines [SKBR3 (A), BT474 (B), MDA-MB-231 (C) and MCF7 (D)] were treated with the indicated doses of VPA (0–10 mM) for 24, 48 and 120 h in serum-free medium.
Table I.
Subtype and the median growth inhibition (GI50) for VPA of breast cancer cell lines.
| SKBR3 | BT474 | MDA-MB-231 | MCF7 | |
|---|---|---|---|---|
| ER | − | + | − | + |
| HER2 | ++ | + | − | − |
| GI50 (mM) | 1.8 | 3.6 | 5.4 | 8.1 |
Effects of VPA on histone H3 acetylation
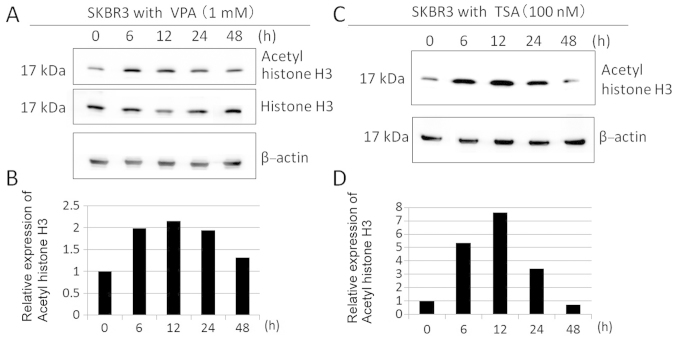
We evaluated the effects of HDAC inhibition by VPA in SKBR3 cells. The acetylation status of histone H3 in SKBR3 cells was determined during 48-h incubation with 1 mM VPA, using an antibody that specifically recognizes hyper-acetylated forms of histone H3. VPA markedly increased acetyl-histone H3 expression by 2-fold with maximal induction at 12 h of incubation with VPA (Fig. 2A and B) This time frame of the maximal acetylation of histone H3 was consistent with that of the maximal acetylation of histone H3 treated with 100 nM TSA, a known, strong HDAC inhibitor (Fig. 2C and D).
Figure 2.
Effect of VPA on the acetylation of histone H3 in SKBR3 cells. (A) Time courses of changes in acetyl-histone H3 and histone H3. SKBR3 cells were treated with 1 mM VPA, and cell lysates were harvested after time courses of ≤48 h. Western blotting was performed using a series of primary antibodies. (C) Time courses of changes in acetyl-histone H3 of SKBR3 cells treated with 100 nM TSA after ≤48 h. (A and C) The amount of β-actin in each sample was used as the loading control. (B and D) The expression levels of acetyl-histone H3 were analyzed by the Light-Capture system and then quantified using the CS analyzer program and reported in the histogram after normalization against β-actin, relative to the values obtained in the absence of VPA (ratio).
Effects of VPA on cell cycle arrest
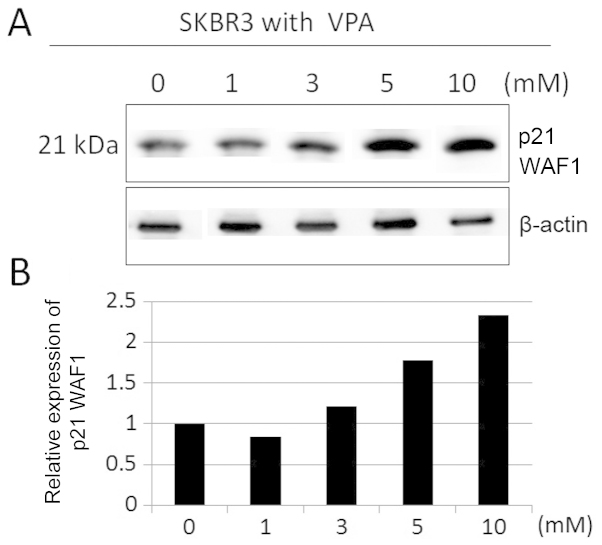
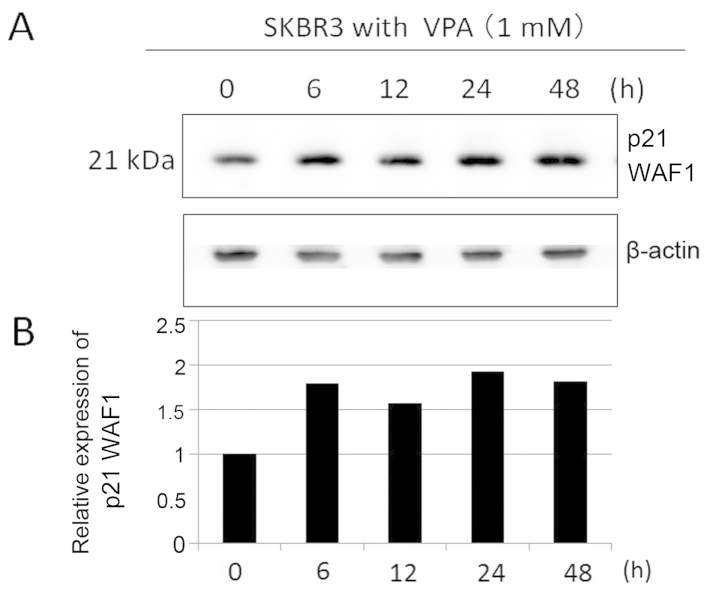
We studied differentiation effects of VPA by evaluating p21 WAF1 expression in SKBR3 cells by immunoblotting. Incubation for 48 h in VPA markedly increased p21 WAF1 expression in SKBR3 cells in a dose-dependent manner (Fig. 3). SKBR3 cells were treated with 1 mM VPA ≤48 h (Fig. 4). The expression levels of p21 WAF1 increased by ~2–3-fold during the 6–24-h incubation time in the presence of VPA.
Figure 3.
Effect of VPA on the expression of p21 WAF1 in SKBR3 cells with the indicated doses. (A) The expression levels of p21 WAF1 were detected by immunoblotting on protein extracts prepared from SKBR3 cells treated with the indicated doses of VPA for 48 h. (B) The expression levels of p21 WAF1 were reported in the histogram after normalization against β-actin, relative to the value obtained in the absence of VPA (ratio).
Figure 4.
Effect of VPA on the expression of p21 WAF1 in SKBR3 cells with the indicated incubation times. (A) The expression levels of p21 WAF1 were detected by immunoblotting on protein extracts prepared from SKBR3 cells treated with 1 mM VPA for ≤48 h. The amount of β-actin in each sample was used as the loading control. (B) The expression levels of p21 WAF1 were reported in the histogram after normalization against β-actin, relative to the value obtained in the absence of VPA (ratio).
Effects of VPA on apoptosis induction
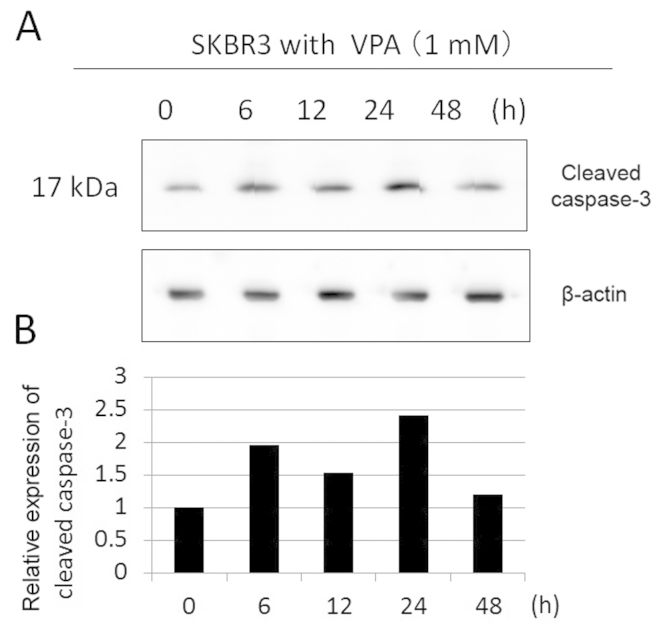
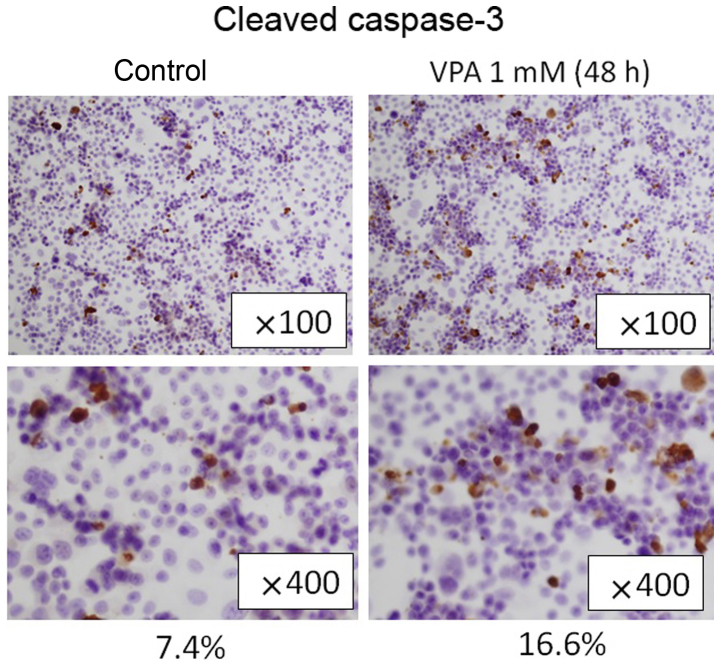
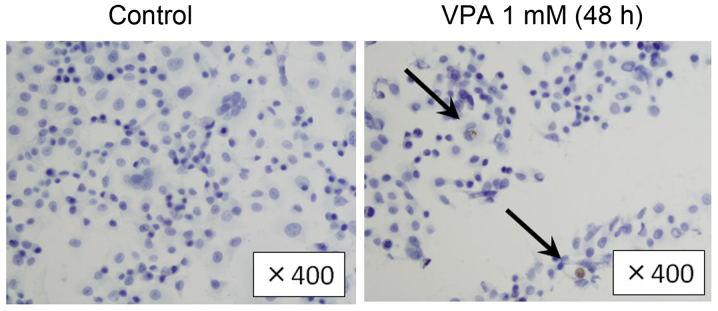
To examine the apoptosis induction effects of VPA, we evaluated cleaved caspase-3 expression in SKBR3 cells by immunoblotting. SKBR3 cells were treated with 1 mM VPA for ≤48 h (Fig. 5). The expression levels of cleaved caspase-3 increased by ~2-fold during the incubation times of 6–24 h in VPA. Immunohistochemical examination showed that the population of cleaved caspase-3-positive SKBR3 cells was higher in VPA-treated group in comparison to the control group (16.6 vs. 7.4%, Fig. 6). Consistently, in TUNEL assay, the population of TUNEL-positive cells was higher in VPA-treated SKBR3 cells than in the control group (Fig. 7).
Figure 5.
Effect of VPA on the expression of cleaved caspase-3 in SKBR3 cells with the indicated incubation times. (A) The expression levels of cleaved caspase-3 were detected by immunoblotting on protein extracts prepared from SKBR3 cells treated with 1 mM VPA for ≤48 h. The amount of β-actin in each sample was used as the loading control. (B) The expression levels of cleaved caspase-3 were reported in the histogram after normalization against β-actin, relative to the value obtained in the absence of VPA (ratio).
Figure 6.
Effects of VPA on the expression of cleaved caspase-3 in SKBR3 cells. Immunohistochemical examination showed that cleaved caspase-3-positive SKBR3 cells treated with VPA for 48 h were increased compared with the control group (16.6% to 7.4%).
Figure 7.
Effects of VPA on apoptosis in SKBR3 cells. Shrunken tumor cells showed positive reactivity in the TUNEL assay. Positive cells (arrow) in SKBR3 cells treated with VPA for 48 h were increased compared with the control group.
Effects of VPA on acetylation of Hsp70 and Hsp90
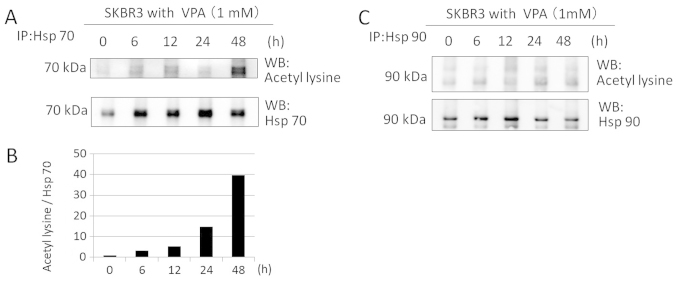
We investigated whether VPA acetylates Hsp70 and Hsp90 in SKBR3 cells by immunoprecipitation and immunoblotting. SKBR3 cells were treated with 1 mM VPA for ≤48 h. Western blotting with anti-aceyl-lysine, anti-Hsp70, and anti-Hsp90 antibodies after immunoprecipitation revealed that VPA markedly increased Hsp70 acetylation of SKBR3 cells at 1 mM for ≤48 h in a time-dependent manner (40-fold increase at 48 h) (Fig. 8A and B). However, VPA did not induce Hsp90 acetylation at 1 mM within the time frame of the study (Fig. 8C).
Figure 8.
The effects of VPA on Hsp70 and Hsp90 acetylation in SKBR3 cells. VPA increased Hsp70, but not Hsp90, acetylation. (A and C) The RIPA lysates from SKBR3 cells treated with VPA for the times indicated were immunoprecipitated with anti-Hsp90 or anti-Hsp70 antibodies and immunoblotted with anti-acetyl-lysine antibody and anti-Hsp70 or anti-Hsp90 antibodies. The levels of Hsp70 and Hsp90 in the immunoprecipitated pellet were used as the loading controls. (B) The expression levels of acetylated Hsp70 were reported in the histogram after normalization against Hsp70 in the absence of VPA (ratio).
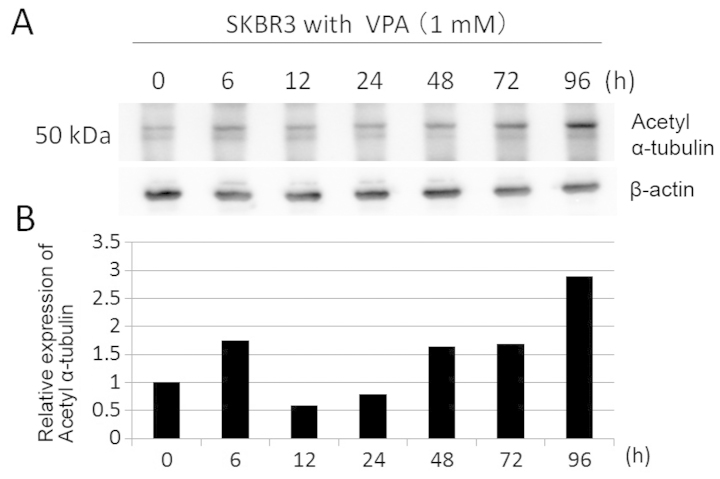
Effects of VPA on acetylation of α-tubulin
To confirm whether VPA has inhibitory effect on HDAC6 activity and leads to Hsp90 acetylation, we investigated acetylation of α-tubulin, which is deacetylated by HDAC6, in SKBR3 cells by immunoblotting. SKBR3 cells were treated with 1 mM VPA for ≤96 h. The expression levels of acetyl-α-tubulin were increased at later time (3-fold increase at 96 h) (Fig. 9). These data suggest that VPA has weak HDAC6 inhibitory activity.
Figure 9.
The effects of VPA on α-tubulin acetylation in SKBR3 cells. (A) The expression levels of acetyl-α-tubulin were detected by immunoblotting protein extracts prepared from SKBR3 cells treated with 1 mM VPA for ≤96 h. The amount of β-actin in each sample was used as the loading control. (B) The expression levels of acetyl α-tubulin were reported in the histogram after normalization against β-actin, relative to the value obtained in the absence of VPA (ratio).
Discussion
The present study reports that VPA has anti-proliferative activity in HER2-overexpressing breast cancer cells through cell cycle arrest and apoptosis induction at a clinically achievable dose of 1 mM. Proliferation-inhibiting effect of VPA in HER2-overexpressing breast cancer cells might be attributable to dysfunction of Hsp90, which affects its client protein HER2, indirectly through hyper-acetylation of Hsp70.
We suggested that the diverse cellular responses to VPA treatment largely depend on the intrinsic characteristics of breast cancer cells. It is controversial whether ER-expression status affects the growth-inhibitory effect of HDAC inhibitors. Fortunati et al (16) showed that growth of ER-positive breast cancer cells were suppressed by VPA in lower dose than ER-negative cells, while Travaglini et al (17) showed estrogen sensitivity had no relation with the extent of cell growth suppression by VPA. On the other hand, Giacinti et al (30) showed that ER-negative cells were more sensitive to HDAC inhibitor Scriptaid than ER-positive cells. Consistent with the results of Giacinti et al, ER-negative cell lines seem to be more sensitive to VPA in this study.
In our study, VPA exhibits a greater efficacy against HER2-overexpressing SKBR3 cells than HER2-negative cells, regardless of their ER-expression status. We focused on HER2-overexpressing SKBR3 cell line to investigate the mechanism of anti-proliferative activity of VPA.
Anti-proliferative activity of VPA has previously been reported in HER2-negative breast cancer cell lines (16–18,31–34) (Table II). Huang et al (35) showed HDAC inhibitor SNDX-275 induced strong apoptosis in HER2-overexpressing breast cancer cells compared with HER2-negative cells. Giacinti et al (30) also showed that HER2-overexpressing breast cancer cells were more sensitive to HDAC inhibitor Scriptaid than HER2-negative cells. To explore the differences among intrinsic subtypes of breast cancer cell lines, we studied the inhibition of cell growth in four different human breast cancer cell lines: ER-positive and HER2-negative MCF7 cells, ER-negative and HER2-overexpressing SKBR3 cells, ER-negative and HER2-negative MDA-MB-231 cells, and ER-positive and HER2-negative BT474 cells. We confirmed that HER2-overexpressing breast cancer cells were more sensitive to VPA treatment than HER2-negative ones. The results suggested that anti-proliferative mechanism of breast cancer cells by VPA is related to their HER2-expression status.
Table II.
The characteristics of breast cancer cell lines treated with VPA in past reports.
| Authors (Ref.) | Year | |
|---|---|---|
| Olsen et al (31) | 2004 | MCF7 (ER+, HER2−) |
| Chavez-Blanco et al (32) | 2006 | MCF7 (ER+, HER2−) |
| Hodges-Gallagher et al (33) | 2007 | MCF7 (ER+, HER2−) |
| Fortunati et al (16) | 2008 | MCF7 (ER+, HER2−) > ZR-75-1 (ER+, HER2+) > MDA-MB-231 (ER−, HER2−) > MDA-MB435 (ER−, HER2) |
| Travaglini et al (17) | 2009 | MCF7 (ER+, HER2−) = MDA-MB-231 (ER−, HER2−) |
| Li et al (34) | 2012 | MDA-MB-231 (ER−, HER2−) |
| Zhang et al (18) | 2012 | MDA-MB-231 (ER−, HER2−) |
| This study | 2015 | SKBR3 (ER+, HER2++) > BT474 (ER−, HER2+) > MDA-MB-231 (ER−, HER2−) > MCF7 (ER+, HER2−) |
The cyclin-dependent kinase inhibitor p21 WAF1, which is involved in both the G1-S and the G2-M transition, regulates cell cycle progression. Fortunati et al (16) showed the anti-proliferative effects of VPA were associated with upregulation of p21. In this investigation, we confirmed that VPA induced cell cycle arrest with upregulation of p21 at 1 mM, in concurrence with the times of histone H3 acetylation.
The present study further showed that VPA induces apoptosis in SKBR3 cells in immunohistochemical examination and TUNEL assay. Caspase-3 is a critical executioner of apoptosis in its activated form of cleaved caspase-3. We showed that caspase-3 is activated in SKBR3 by VPA simultaneously as histone H3 acetylation. Thus VPA seemed to affect proliferation of breast cancer cells by cell cycle arrest and apoptosis induction.
Hsp90 is involved in two multi-chaperone complexes and promotes correct folding or degradation of client proteins, depending on its conformation (25). As class II deacetylase, HDAC6 is known as the deacetylase of Hsp90, and inhibiting the activity of HDAC6 leads to hyper-acetylation of Hsp90, disruption of its chaperone function, and cell apoptosis (21–23). VPA is known to have a weak inhibitory effect on HDAC6, and we could not directly observe VPA-induced acetylation of Hsp90 in SKBR3 cells. However, we showed that VPA had acetylation effect against α-tubulin. Considering that HDAC6 is the only known deacetylase of α-tubulin, the increased acetylation of α-tubulin indicates that VPA can partially inhibit HDAC6 activity. HDAC inhibition by VPA might be insufficient to acetylate Hsp90. On the other hand, we showed that VPA could acetylate Hsp70. It is known that Hsp70 is required for the assembly of the signaling protein-Hsp90 heterocomplex.
We thought VPA may disrupt the function of Hsp90 indirectly through hyper-acetylation of Hsp70. Wang et al (36) showed that FK228, a class I HDAC inhibitor, blocked Hsp90 chaperone function in K562 CML cells via hyper-acetylation of Hsp70. Fuino et al (37) showed that LAQ824, a hydroxamic acid analogue HDAC inhibitor, induced acetylation of Hsp90, and reduced its binding to ATP. In SKBR3 and BT474 cells, treatment with LAQ824 shifted the chaperone association of HER2 from Hsp90 to Hsp70, which efficiently ubiquitinates and downregulates HER2. Scott et al (38) showed that treatment with TSA, a strong HDAC inhibitor, in SKBR3 and BT474 cells produced the expected marked decline in their endogenous HER2 protein levels. Meng et al (39) showed that treatment with carbamazepine (CBZ), an anti-epileptic drug that acts as an HDAC inhibitor in SKBR3 cells, produced a marked decline in their HER2 protein levels. In the same study, CBZ was shown to synergize with trastuzumab inhibiting breast cancer cell proliferation more strongly than CBZ or trastuzumab alone. Therefore, VPA may synergize with trastuzumab to inhibit HER2-overexpressing breast cancer cell proliferation more strongly. The fact that HER2-overexpressing breast cancer cells were more sensitive to VPA than HER2-negative cells seems to be associated with the role of HER2 as a client protein of Hsp90. We speculate VPA may disrupt the function of Hsp90 indirectly through hyper-acetylation of Hsp70 and downregulation of HER2 expression, thus suppressing cell growth.
Our results showed that the class I and II HDAC inhibitor VPA preferably inhibited cell proliferation and induced cell cycle arrest and apoptosis of HER2-overexpressing breast cancer cells. This effect might be a direct function of VPA as an HDAC inhibitor. Moreover, we propose another mechanism of anti-proliferation that might be related to its non-histone acetylation effect by indirectly disrupting Hsp90 function via acetylation of Hsp70 and leading to downregulation of its client proteins, including HER2. We showed that, in addition to the direct function of VPA in histone acetylation that results in cell cycle arrest or apoptosis, its indirect function of acetylation of non-histone proteins could result in the anti-proliferative activity of VPA. To confirm this hypothesis, further study is required.
Acknowledgements
We thank Lynn Kimlicka for editing the manuscript.
Abbreviations
- VPA
valproic acid
- HDAC
histone deacetylase
- Hsp
heat shock protein
- HER2
human epidermal receptor 2
- HAT
histone acetyltransferase
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- TSA
trichostatin A
- ER
estrogen receptor
- RIPA
radio-immunoprecipitation
- TUNEL
TdT-mediated dUTP nick-end labeling
- CBZ
carbamazepine
References
- 1.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 2.Fang JY, Lu YY. Effects of histone acetylation and DNA methylation on p21(WAF1) regulation. World J Gastroenterol. 2002;8:400–405. doi: 10.3748/wjg.v8.i3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Davie JR, Moniwa M. Control of chromatin remodeling. Crit Rev Eukaryot Gene Expr. 2000;10:303–325. doi: 10.1615/CritRevEukarGeneExpr.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 5.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 6.Monneret C. Histone deacetylase inhibitors. Eur J Med Chem. 2005;40:1–13. doi: 10.1016/j.ejmech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Sami S, Höti N, Xu HM, Shen Z, Huang X. Valproic acid inhibits the growth of cervical cancer both in vitro and in vivo. J Biochem. 2008;144:357–362. doi: 10.1093/jb/mvn074. [DOI] [PubMed] [Google Scholar]
- 8.Krämer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, Brill B, Groner B, Bach I, Heinzel T, et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003;22:3411–3420. doi: 10.1093/emboj/cdg315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hrzenjak A, Moinfar F, Kremser ML, Strohmeier B, Staber PB, Zatloukal K, Denk H. Valproate inhibition of histone deacetylase 2 affects differentiation and decreases proliferation of endometrial stromal sarcoma cells. Mol Cancer Ther. 2006;5:2203–2210. doi: 10.1158/1535-7163.MCT-05-0480. [DOI] [PubMed] [Google Scholar]
- 11.Rocchi P, Tonelli R, Camerin C, Purgato S, Fronza R, Bianucci F, Guerra F, Pession A, Ferreri AM. p21Waf1/Cip1 is a common target induced by short-chain fatty acid HDAC inhibitors (valproic acid, tributyrin and sodium butyrate) in neuroblastoma cells. Oncol Rep. 2005;13:1139–1144. [PubMed] [Google Scholar]
- 12.Takai N, Narahara H, Takai N, Narahara H. Human endometrial and ovarian cancer cells: Histone deacetylase inhibitors exhibit antiproliferative activity, potently induce cell cycle arrest, and stimulate apoptosis. Curr Med Chem. 2007;14:2548–2553. doi: 10.2174/092986707782023299. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 14.Blagosklonny MV, Robey R, Sackett DL, Du L, Traganos F, Darzynkiewicz Z, Fojo T, Bates SE. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther. 2002;1:937–941. [PubMed] [Google Scholar]
- 15.Catalano MG, Poli R, Pugliese M, Fortunati N, Boccuzzi G. Valproic acid enhances tubulin acetylation and apoptotic activity of paclitaxel on anaplastic thyroid cancer cell lines. Endocr Relat Cancer. 2007;14:839–845. doi: 10.1677/ERC-07-0096. [DOI] [PubMed] [Google Scholar]
- 16.Fortunati N, Bertino S, Costantino L, Bosco O, Vercellinatto I, Catalano MG, Boccuzzi G. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259:156–164. doi: 10.1016/j.canlet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Travaglini L, Vian L, Billi M, Grignani F, Nervi C. Epigenetic reprogramming of breast cancer cells by valproic acid occurs regardless of estrogen receptor status. Int J Biochem Cell Biol. 2009;41:225–234. doi: 10.1016/j.biocel.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Wang G, Wang L, Song C, Leng Y, Wang X, Kang J. VPA inhibits breast cancer cell migration by specifically targeting HDAC2 and down-regulating Survivin. Mol Cell Biochem. 2012;361:39–45. doi: 10.1007/s11010-011-1085-x. [DOI] [PubMed] [Google Scholar]
- 19.Nimmanapalli R, Fuino L, Bali P, Gasparetto M, Glozak M, Tao J, Moscinski L, Smith C, Wu J, Jove R, et al. Histone deacetylase inhibitor LAQ824 both lowers expression and promotes proteasomal degradation of Bcr-Abl and induces apoptosis of imatinib mesylate-sensitive or -refractory chronic myelogenous leukemia-blast crisis cells. Cancer Res. 2003;63:5126–5135. [PubMed] [Google Scholar]
- 20.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 21.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 22.Bali P, Pranpat M, Swaby R, et al. Activity of suberoylanilide hydroxamic Acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005;11:6382–6389. doi: 10.1158/1078-0432.CCR-05-0344. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of gluco-corticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Powers MV, Clarke PA, Workman P. Death by chaperone: HSP90, HSP70 or both? Cell Cycle. 2009;8:518–526. doi: 10.4161/cc.8.4.7583. [DOI] [PubMed] [Google Scholar]
- 25.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 26.Boyault C, Zhang Y, Fritah S, Caron C, Gilquin B, Kwon SH, Garrido C, Yao TP, Vourc'h C, Matthias P, et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21:2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22:1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furumai R, Matsuyama A, Kobashi N, Lee KH, Nishiyama M, Nakajima H, Tanaka A, Komatsu Y, Nishino N, Yoshida M, et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 29.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8(Suppl):S55–S61. doi: 10.1016/S1471-4914(02)02316-X. [DOI] [PubMed] [Google Scholar]
- 30.Giacinti L, Giacinti C, Gabellini C, Rizzuto E, Lopez M, Giordano A. Scriptaid effects on breast cancer cell lines. J Cell Physiol. 2012;227:3426–3433. doi: 10.1002/jcp.24043. [DOI] [PubMed] [Google Scholar]
- 31.Olsen CM, Meussen-Elholm ET, Røste LS, Taubøll E. Antiepileptic drugs inhibit cell growth in the human breast cancer cell line MCF7. Mol Cell Endocrinol. 2004;213:173–179. doi: 10.1016/j.mce.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 32.Chavez-Blanco A, Perez-Plasencia C, Perez-Cardenas E, Carrasco-Legleu C, Rangel-Lopez E, Segura-Pacheco B, Taja-Chayeb L, Trejo-Becerril C, Gonzalez-Fierro A, Candelaria M, et al. Antineoplastic effects of the DNA methylation inhibitor hydralazine and the histone deacetylase inhibitor valproic acid in cancer cell lines. Cancer Cell Int. 2006;6:2. doi: 10.1186/1475-2867-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodges-Gallagher L, Valentine CD, Bader SE, Kushner PJ. Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat. 2007;105:297–309. doi: 10.1007/s10549-006-9459-6. [DOI] [PubMed] [Google Scholar]
- 34.Li GF, Qian TL, Li GS, Yang CX, Qin M, Huang J, Sun M, Han YQ. Sodium valproate inhibits MDA-MB-231 breast cancer cell migration by upregulating NM23H1 expression. Genet Mol Res. 2012;11:77–86. doi: 10.4238/2012.January.13.1. [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Gao L, Wang S, Lee CK, Ordentlich P, Liu B. HDAC inhibitor SNDX-275 induces apoptosis in erbB2-overexpressing breast cancer cells via down-regulation of erbB3 expression. Cancer Res. 2009;69:8403–8411. doi: 10.1158/0008-5472.CAN-09-2146. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wang SY, Zhang XH, Zhao M, Hou CM, Xu YJ, Du ZY, Yu XD. FK228 inhibits Hsp90 chaperone function in K562 cells via hyperacetylation of Hsp70. Biochem Biophys Res Commun. 2007;356:998–1003. doi: 10.1016/j.bbrc.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 37.Fuino L, Bali P, Wittmann S, Donapaty S, Guo F, Yamaguchi H, Wang HG, Atadja P, Bhalla K. Histone deacetylase inhibitor LAQ824 down-regulates Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone B. Mol Cancer Ther. 2003;2:971–984. [PubMed] [Google Scholar]
- 38.Scott GK, Marden C, Xu F, Kirk L, Benz CC. Transcriptional repression of ErbB2 by histone deacetylase inhibitors detected by a genomically integrated ErbB2 promoter-reporting cell screen. Mol Cancer Ther. 2002;1:385–392. [PubMed] [Google Scholar]
- 39.Meng Q, Chen X, Sun L, Zhao C, Sui G, Cai L. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348:165–171. doi: 10.1007/s11010-010-0651-y. [DOI] [PubMed] [Google Scholar]