Abstract
Background
High osteoprotegerin (OPG) has been reported in association with insulin resistance and type 2 diabetes. We aimed to evaluate the association of serum OPG with impaired glucose regulation (IGR) and microalbuminuria among middle-aged and older Chinese.
Methods
Serum OPG was measured in 599 individuals with normal glucose regulation, 730 with impaired glucose regulation and 327 newly diagnosed patients with diabetes. Serum OPG was measured using ELISA methods and urine albumin/creatinine ratio was used to determine the urinary albumin excretion.
Results
Serum OPG levels were significantly higher in subjects with isolated impaired fasting glucose, isolated impaired glucose tolerance, combined impaired fasting glucose/impaired glucose tolerance and diabetes than in those with normal glucose regulation, whereas serum OPG levels were not different in the four groups with dysregulation of glucose metabolism. OPG was associated with a higher risk for IGR (OR 1.108 for each 0.1 μg/l increase in OPG, 95 % CI 1.009–1.117, p = 0.01) after adjustment for gender, age, BMI, current smoking and alcohol intake, family history of diabetes, homeostasis model assessment of insulin resistance (HOMA-IR), lipid profile; the corresponding OR of combined impaired glucose regulation and type 2 diabetes was 1.121 (95 % CI 1.101–1.141, p = 0.0005). OPG was associated with the risk of microalbuminuria (OR 1.025, 95 % CI 1.006–1.044, p = 0.02) after adjustment for gender, age, current smoking, current alcohol intake, family history of diabetes, BMI, waist/hip ratio, HOMA-IR, eGFR and lipid profile.
Conclusions
Serum OPG level is closely and independently associated with IGR and is an independent risk factor for microalbuminuria.
Keywords: Impaired glucose regulation, Osteoprotegerin, Microalbuminuria
What’s new?
In this study, we found a strong association between serum OPG levels and the risk of impaired glucose regulation in a large Chinese cohort aged 40 years or older.
Serum OPG was also associated with a higher risk of microalbuminuria independent of various confounding factors.
To our knowledge, this is the first study to evaluate the relationship between OPG levels and impaired glucose regulation in a large-scale population.
Background
Osteoprotegerin (OPG), a soluble member of the tumor necrosis factor (TNF) receptor superfamily is a decoy receptor for the receptor activator of nucleus factor-κB ligand (RANKL) and TNF-related apoptosis-inducing ligand (TRAIL) [1, 2], has been identified as having an effect on systemic insulin sensitivity and glucose homeostasis [3–5]. The association of serum OPG with insulin resistance has been most commonly studied; however, the results from these studies are not consistent. While serum OPG levels were increased in certain metabolic disorders, such as obesity, type 2 diabetes, and polycystic ovarian syndrome, that were closely related to insulin resistance [3–11], some other studies reported that serum OPG levels was not related to insulin resistance or even negative related to insulin resistance [12, 13].
Insulin resistance is a conspicuous characteristic of prediabetic states [14]. Impaired glucose regulation (IGR), also termed as prediabetes consisting of impaired fasting glucose and/or impaired glucose tolerance (IFG and/or IGT) is definitely a risk factor for type 2 diabetes and cardiovascular disease [15]. Recently, epidemiological studies showed that the prediabetes is abruptly increased in the world, as well as in China [16]. Elevated serum OPG levels have been reported in connection with IGT in study with small sample sizes [17]; however, the association of OPG with isolated IFG, isolated IGT and combined IFG and IGT has rarely been studied. Thus, it is indispensable to evaluate the association of serum OPG with type 2 diabetes, as well as with prediabetes. Microalbuminuria, which is an early indicator of renal dysfunction, represents a risk factor for atherosclerosis and cardiovascular disease independent of conventional risk factors [18]. Previous studies demonstrated that microalbuminuria was associated with insulin resistance in patients with type 2 diabetes as well as in patients without diabetes [19]. Studies have also shown an association of serum OPG with albumin excretion in type 2 diabetes, although this relationship was not fully elucidated [7].
In the present study, we evaluated the association of serum OPG with states of glucose metabolism and urinary albumin excretion in a cross-sectional population study of middle-aged and older Chinese subjects.
Methods
Study population
In 2011 China a national survey of Risk Evaluation of cancers in Chinese diabetic Individuals: a longitudinal (REACTION) study, which was conducted among 259,657 adults, aged 40 years and older in 25 communities across mainland China, from 2011 to 2012 [20]. The data presented in this article are based on the baseline survey of subsamples from Shanghai in eastern China. All studied individuals came from the Chongming District in Shanghai, China. Informed consent was obtained from all participants, and approval was given by the Institutional Review Board of Xinhua Hospital affiliated with Shanghai Jiaotong University School of Medicine. The study population, design and protocols of this cohort study have been described previously [21]. Briefly, a two-stage stratified sampling method was used. First, 12 residential communities or streets were randomly selected from the Chongming District. Of these, 8 urban communities and 4 rural communities were chosen to represent people with high to low socioeconomic status. Secondly, Within each community/street, all eligible individuals were sampled, with the exception that in households with more than one eligible individual, one individual was randomly selected. During the recruiting phase, inhabitants aged ≥40 years in these 12 communities were invited by telephone or door-to-door visit to participate in this study.
A total of 9,930 subjects were recruited and agreed to participate in the first step of our survey. After the exclusion of 990 individuals with self-reported diabetes, a random subcohort of 1800 individuals was selected from all participants of the study population. Although we considered missing anthropometry as exclusion criteria, none of the subcohort members qualified for exclusion here. Furthermore, we excluded 123 participants for whom not all biomarkers were available and 21 individuals with either unexplainable low plasma glucose concentrations (<2.8 mmol/l). Altogether, 1,656 participants were included in the random subcohort for analyses. The process of randomly selecting a subcohort, together with the appropriate statistics for this type of research design, renders the results generalizable without measurements of biomarkers in the entire cohort. [22] A comparison of the randomly selected subcohort, before and after exclusions, and the full cohort was performed. There were no significant differences in age, BMI, or sex distribution between full and subcohort before exclusions, suggesting that the subcohort was representative for the full cohort.
Data collection
A standardized questionnaire was used by trained physicians to collect information such as age, gender, smoking (yes/no), alcohol drinking (yes/no). Physical activity level was classified as low, moderate, or high according to the International Physical Activity Questionnaire scoring protocol. According to participants’ responses to the corresponding questions, family history of diabetes was classified as yes or no.
The details of anthropometric measurements including height, weight, waist circumference, hip circumference were carried by trained physicians. Blood pressure was measured at the right arm with an automated electronic device (OMRON Model1 Plus; Omron Company, Kyoto, Japan) three times consecutively with 1 min intervals after at least 5 min rest in the seated position; the three readings were averaged for analysis. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
All subjects were assessed after overnight fasting for at least 10 h. Overnight fasting and 2 h OGTT blood samples were collected in tubes containing EDTA and were centrifuged at 4 °C and stored at −80 °C until analysis. The fasting glucose, glucose 2 h after oral glucose tolerance test, total cholesterol (TC), triglycerides, low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol were measured on an automatic analyzer (Hitachi 7080; Tokyo, Japan). Fasting insulin was determined by RIA (Linco Research, St. Charles, MO). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the equation described by Matthews et al. [23] The abbreviated Modification of Diet in Renal Disease formula recalibrated for Chinese was used to estimate glomerular filtration rate expressed in milliliters per minute per 1.73 m2: estimated glomerular filtration rate (eGFR) = 186 × [serum creatinine × 0.011]-1.154 × [age]-0.203 × [0.742 if female] × 1.233, where serum creatinine is expressed as micromoles per liter and 1.233 is the adjusting coefficient for Chinese [24].
Urinary albumin and creatinine concentrations were determined using the first-void sterile urine sample in the early morning by rate nephelometry (Beckman Coulter, Fullerton, CA, USA) and alkaline nitroxanthic acid method, respectively. The sensitivity of the urinary albumin assay was 2.0 g/l. The albumin/creatinine ratio (ACR) was calculated as milligrams of urinary albumin excretion per gram of urinary creatinine and used for the diagnosis of normal, micro- and macroalbuminuria, defined as an ACR <3.4, 3.4–34, and >34 mg/mmol, respectively.
Measurements of serum osteoprotegerin
The serum OPG was determined in duplicate by ELISA with Duoset kit (DY805; R&D Systems, Minneapolis, MN) as recommended by the manufacturer. The ELISA system had an intraassay coefficient of variation of 3–9 % and an interassay coefficient of variation of 3–10 %, respectively.
Definitions
Impaired glucose regulation was defined as impaired fasting glucose (fasting plasma glucose level ≥6.1 and <7.0 mmol/l) and/or impaired glucose tolerance (IGT, 2 h OGTT plasma glucose level ≥7.8 and <11.1 mmol/l). Isolated IFG: fasting plasma glucose ≥6.1 mmol/l and <7.0 mmol/l and 2 h OGTT plasma glucose <7.8 mmol/l. Isolated IGT: 2 h OGTT plasma glucose ≥7.8 mmol/l and <11.1 mmol/l and fasting glucose <6.1 mmol/l. IFG/IGT: fasting plasma glucose 6.1 mmol/l to 6.9 mmol/l and 2 h OGTT plasma glucose 7.8 mmol/l to 11.0 mmol/l. Type 2 diabetes was diagnosed according to the 1999 World Health Organization criteria (fasting plasma glucose level ≥7.0 mmol/l and/or 2 h OGTT plasma glucose level ≥11.1 mmol/l). A fasting glucose level lower than 6.1 mmol/l and 2 h OGTT plasma glucose level below 7.8 mmol/l were defined as normal glucose regulation (NGR).
Statistical analysis
Normally distributed data were expressed as means ± SD, whereas variables with a skewed distribution were reported as median (interquartile range) and log transformed to approximate normality before analysis. Comparisons of means and proportions were performed with the standard normal z and χ2 tests, respectively. Homogeneity of groups was determined when the means showed significant differences. Means of these groups were compared by the Student–Newman–Keuls method.
To allow for covariates and confounders, we performed analysis of covariance and multiple linear and logistic regressions. Univariate and multivariable stepwise logistic regression analysis were used to investigate the association of serum OPG with clinical and biochemical characteristics. The forward regression procedure was used to obtain determinants of serum OPG, and we considered as potential covariates age, sex, body mass index, waist circumference, fasting plasma glucose, 2 h OGTT plasma glucose, log10 fasting serum insulin, log10 HOMA-IR index, serum concentrations of triacylglycerols, total cholesterol, HDL- and LDL-cholesterol, systolic and diastolic blood pressure. To study the association of impaired glucose regulation with OPG, we defined participants with normal glucose regulation as 0 (n = 599) and impaired glucose regulation as 1 (n = 730), and excluded patients with type 2 diabetes from the logistic regression analyses. For the association of combined impaired glucose regulation and type 2 diabetes with OPG, we defined participants with normal glucose regulation as 0 (n = 599) and combined impaired glucose regulation and type 2 diabetes as 1 (n = 1,057) in the logistic regression analyses. To investigate the association of microalbuminuria with OPG, we defined participants with normal urinary albumin excretion as 0 (n = 1,539) and microalbuminuria as 1 (n = 103), excluding macroalbuminuria (n = 14) from the logistic regression analyses. For association of combined micro- and macroalbuminuria with OPG, we defined participants with normal urinary albumin excretion as 0 (n = 1,539) and combined micro- and macroalbuminuria as 1 (n = 117). Data management and statistical analysis were performed with the SPSS Statistical Package (version 13.0; SPSS Inc., Chicago, IL).P values < 0.05 were considered statistically significant.
Results
Characteristics of the participants
The study involved 599 participants with NGR, 730 with IGR and 327 with newly diagnosed type 2 diabetes (Table 1). Among the participants with IGR, 230 (13.9 %) had isolated IFG, 286 (17.3 %) had isolated IGT, and 214 (12.9 %) had combined IFG/IGT. There was no significant difference in current smoking and alcohol intake between these groups (both p > 0.05). The NGR and diabetes groups had, respectively, the most favourable and unfavourable metabolic profiles. There was no significant difference in age, BMI, waist/hip ratio, fasting serum insulin, HDL-cholesterol, white blood cell count, postmenopausal women between the groups with isolated IFG, isolated IGT and combined IFG/IGT. The isolated IGT and combined IFG/IGT groups had higher 2 h OGTT plasma glucose than the isolated IFG and NGR groups. The isolated IGT and combined IFG/IGT groups had higher serum fasting insulin concentration and urinary ACR than the isolated IFG and NGR groups. The metabolic profile of the combined IFG/IGT group showed a trend towards similarity to the type 2 diabetes group.
Table 1.
Characteristics of the study population
| Characteristic | NGR (0) | IGR | Type 2 diabetes (4) | p value | Homogeneity of groups | ||
|---|---|---|---|---|---|---|---|
| Isolated IFG (1) | Isolated IGT (2) | IFG and IGT (3) | |||||
| n | 599 | 230 | 286 | 214 | 327 | - | - |
| Male, n(%) | 200 (43.4) | 96 (41.7) | 84 (29.4) | 86 (40.2) | 129 (39.4) | 0.008 | - |
| Age (years) | 54.2 ± 8.3 | 56.3 ± 7.5 | 55.6 ± 8.1 | 56.9 ± 7.4 | 57.3 ± 7.7 | <0.0001 | (0) (1,2,3,4) |
| BMI (kg/m2) | 24.4 ± 3.7 | 25.1 ± 3.4 | 24.7 ± 3.4 | 25.1 ± 3.9 | 25.5 ± 3.6 | <0.0001 | (0,1,2,3) (1,3,4) |
| Waist circumference (cm) | 84.0 ± 10.3 | 86.8 ± 10.0 | 84.5 ± 9.7 | 85.3 ± 11.8 | 88.0 ± 10.8 | <0.0001 | (0,2,3) (1.3) (1,4) |
| Waist/hip ratio | 0.88 ± 0.08 | 0.90 ± 0.09 | 0.89 ± 0.07 | 0.89 ± 0.13 | 0.93 ± 0.16 | 0.012 | (0,1,2,3) (1,2,3,4) |
| Fasting plasma glucose (mmol/l) | 5.5 ± 0.3 | 6.4 ± 0.2 | 5.6 ± 0.3 | 6.4 ± 0.2 | 7.5 ± 2.3 | <0.0001 | (0,2) (1,3) (4) |
| 2 h OGTT plasma glucose (mmol/l) | 6.1 ± 1.1 | 6.3 ± 1.0 | 9.0 ± 0.9 | 9.1 ± 0.9 | 13.2 ± 5.0 | <0.0001 | (0,1) (2,3) (4) |
| Fasting serum insulin (mmol/l) | 5.50 (3.90–7.63) | 6.20 (4.30–8.78) | 6.25 (4.23–8.60) | 6.80 (4.50–9.90) | 7.20 (4.70–10.20) | <0.0001 | (0) (1,2,3) (4) |
| Hemoglobin A1c (%) | 5.4 ± 0.3 | 5.6 ± 0.4 | 5.5 ± 0.3 | 5.7 ± 0.4 | 6.3 ± 1.4 | <0.0001 | (0) (1,2) (1,3) (4) |
| HOMA-IR | 1.39 (0.95–1.87) | 1.79 (1.26–2.53) | 1.64 (1.15–2.31) | 1.93 (1.33–2.80) | 2.37 (1.47–3.39) | <0.0001 | (0) (1,2) (1,3) (4) |
| Systolic blood pressure (mmHg) | 130 ± 19 | 136 ± 20 | 132 ± 19 | 139 ± 17 | 141 ± 20 | <0.0001 | (0,2) (1) (3,4) |
| Diastolic blood pressure (mmHg) | 81 ± 11 | 82 ± 10 | 82 ± 10 | 84 ± 11 | 84 ± 11 | <0.0001 | (0,1,2) (1,3) (3,4) |
| Total cholesterol (mmol/l) | 4.81 ± 0.83 | 5.09 ± 0.84 | 4.92 ± 0.86 | 5.13 ± 0.91 | 5.23 ± 0.91 | <0.0001 | (0,2) (1,3,4) |
| Triacylglycerol (mmol/l) | 1.39 ± 0.85 | 1.75 ± 1.36 | 1.83 ± 1.11 | 2.20 ± 1.85 | 2.17 ± 1.55 | <0.0001 | (0) (1,2) (3,4) |
| LDL-cholesterol (mmol/l) | 2.67 ± 0.70 | 2.83 ± 0.68 | 2.68 ± 0.68 | 2.81 ± 0.68 | 2.87 ± 0.77 | <0.0001 | (0,2) (1,3,4) |
| HDL-cholesterol (mmol/l) | 1.37 ± 0.30 | 1.34 ± 0.30 | 1.32 ± 0.29 | 1.28 ± 0.35 | 1.32 ± 0.33 | 0.009 | (0,1,2,4) (1,2,3,4) |
| White blood cell count (×109) | 5.7 ± 1.3 | 6.2 ± 1.5 | 6.2 ± 1.5 | 6.4 ± 1.6 | 6.5 ± 1.7 | <0.0001 | (0) (1,2,3) (3,4) |
| ACR (mg/g) | 7 (4–11) | 8 (5–14) | 8 (4–13) | 8 (5–15) | 10 (6–20) | <0.0001 | (0) (1,2,3) (4) |
| Serum creatinine concentration (mmol/l) | 62.0 (56.4–69.1) | 66.1 (60.4–74.3) | 62.5 (57.4–69.4) | 65.6 (59.4–73.0) | 66.3 (60.2–73.9) | 0.55 | (0,1,2,3,4) |
| GFR (ml min−1 1.73 m−2) | 126.7 (112.7–142.7) | 120.6 (108.4–136.3) | 122.7 (110.3–138.9) | 120.4 (106.8–133.8) | 118.5 (105.6–134.9) | 0.029 | (0,1,2,3) (4) |
| Current smoking, n (%) | 147 (24.5) | 56 (24.3) | 59 (20.6) | 65 (30.4) | 78 (23.9) | 0.172 | - |
| Alcohol intake, n (%) | 148 (24.7) | 68 (30.0) | 72 (25.2) | 72 (33.6) | 93 (28.4) | 0.099 | - |
| Postmenopausal women, n (%) | 301 (50.3) | 102 (44.3) | 153 (53.5) | 101 (47.2) | 156 (47.7) | 0.149 | - |
| OPG (μg/l) | 2.63 ± 0.95 | 2.95 ± 1.01 | 2.98 ± 1.03 | 2.96 ± 1.00 | 3.02 ± 1.09 | <0.0001 | (0) (1,2,3,4) |
Values are mean ± SD or median (interquartile range) or number (proportion)
p values were for the ANOVA or χ2 analyses across the five groups
Serum OPG levels by sex and age
There was no significant difference in serum OPG levels between the men and women (p > 0.05). Age was positively associated with serum OPG levels (r = 0.377, p < 0.0001). OPG concentrations increased by 0.30 μg/ml with each 10 year increase in age (p < 0.0001).
Determinants of serum OPG levels
Age, 2 h OGTT plasma glucose, HbA1c, log10 HOMA-IR, triacylglycerol and log10-ACR were independent determinants for serum OPG concentrations in a stepwise linear regression analysis (Table 2). Furthermore, serum OPG levels were associated with white blood cell count (r = 0.225, p < 0.0001) in this sudy.
Table 2.
Univariate and stepwise regression analysis with serum OPG concentration as a dependent variable
| Co-variable | Univariate | Stepwise | ||
|---|---|---|---|---|
| r | p value | Beta | p value | |
| Age (years) | 0.377 | <0.0001 | 0.331 | <0.0001 |
| Sex (male = 1, female = 2) | –0.12 | 0.440 | - | - |
| Body mass index (kg/m2) | 0.068 | 0.193 | - | - |
| Waist/hip ratio | 0.023 | 0.262 | - | - |
| Fasting plasma glucose (mmol/l) | 0.191 | 0.007 | - | - |
| 2 h OGTT plasma glucose (mmol/l) | 0.266 | <0.0001 | 0.222 | 0.002 |
| Log10 fasting serum insulin (mmol/l) | –0.125 | 0.056 | –0.175 | 0.021 |
| Hemoglobin A1c (%) | 0.251 | 0.001 | - | - |
| Log10 HOMA-IR | 0. 29 | <0.0001 | 0.20 | 0.003 |
| Systolic blood pressure (mmHg) | 0.104 | 0.092 | - | - |
| Diastolic blood pressure (mmHg) | 0.003 | 0.483 | - | - |
| Total cholesterol (mmol/l) | 0.113 | 0.075 | - | - |
| Triacylglycerol (mmol/l) | 0.222 | 0.002 | 0.255 | 0.001 |
| LDL-cholesterol (mmol/l) | 0.045 | 0.282 | - | - |
| HDL-cholesterol (mmol/l) | –0.057 | 0.235 | - | - |
| Log10 ACR | 0.09 | 0.05 | 0.16 | 0.025 |
| Blood cell count | 0.225 | <0.0001 | - | - |
Beta, Regression coefficient; r, Pearson correlation coefficient
OPG in impaired glucose regulation and newly diagnosed type 2 diabetes
Serum OPG concentrations were significantly higher in subjects with isolated IFG, isolated IGT, combined IFG and IGT, and newly diagnosed type 2 diabetes compared with concentrations in participants with NGR (2.95, 2.98, 2.96, 3.02 μg/l vs 2.63 μg/l, all p < 0.01), after adjustment for sex, age, BMI, current smoking and current alcohol intake and the family history of diabetes. There was no statistical difference between these four groups with dysregulation of glucose metabolism (all p > 0.05) (Fig. 1).
Fig. 1.
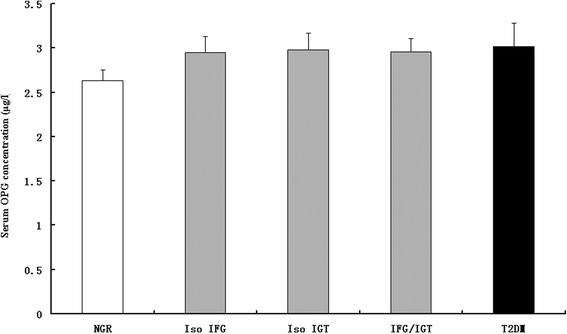
Adjusted means (±SEM) of serum OPG concentrations in subjects with NGR, IGR (isolated IFG [Iso IFG], isolated IGT [Iso IGT] and IFG/IGT) and type 2 diabetes mellitus (T2DM). The covariables included age, sex, BMI, current smoking, current alcohol intake, menopausal status and the family history of diabetes. Subjects with type 2 diabetes mellitus and IGR (isolated IFG, isolated IGT and IFG/IGT) had higher concentrations of OPG than those with NGR (both p < 0.0001). There was no significant difference among the subgroups of IGR (p = 0.35) and between the IGR and type 2 diabetes mellitus groups (p = 0.61)
As shown in Table 3, increased OPG was associated with a higher risk of hyperglycaemia. The higher level of OPG had a higher risk of impaired glucose regulation (OR 1.108, 95 % CI 1.009–1.117, p = 0.01) after adjustment for sex, age, current smoking and alcohol intake, family history of diabetes, BMI, waist/hip ratio, HOMA-IR, triacylglycerol, total cholesterol, HDL- and LDL-cholesterol; the corresponding OR for both impaired glucose regulation and type 2 diabetes was 1.121 (95 % CI 1.101–1.141, p = 0.0005).
Table 3.
The risk of impaired glucose regulation and type 2 diabetes associated with a 0.1 μg/l increase in serum OPG
| Model | Adjustment | IGR (n = 730) | p valuea | Impaired glucose regulation and type 2 diabetes (n = 1,057) | p valueb |
|---|---|---|---|---|---|
| Model 1 | Unadjusted | 1.143 (1.112–1.174) | <0.0001 | 1.146 (1.127–1.167) | <0.0001 |
| Model 2 | Adjusted for age, gender, current smoking, current alcohol intake and family history of diabetes | 1.137 (1.108–1.166) | <0.0001 | 1.141 (1.115–1.168) | <0.0001 |
| Model 3 | Further adjusted for BMI and waist/hip ratio, based on Model 2 | 1.134 (1.104–1.165) | <0.0001 | 1.136 (1.107–1.166) | <0.0001 |
| Model 4 | Further adjusted for HOMA-IR, based on Model 3 | 1.133 (1.103–1.164) | <0.0001 | 1.132 (1.103–1.161) | <0.0001 |
| Model 5 | Further adjusted for serum triacylglycerol, total cholesterol, HDL- and LDL-cholesterol, based on Model 4 | 1.108 (1.009–1.117) | 0.01 | 1.121 (1.101–1.141) | 0.0005 |
Values are ORs (95 % CI)
aFor the risk of IGR, we defined participants with normal glucose regulation as 0 (n = 599) and IGR as 1 (n = 730), excluding patients with type 2 diabetes (n = 327) from the analysis
bFor the risk of combined IGR and type 2 diabetes, we defined participants with NGR as 0 (n = 599) and combined IGR and type 2 diabetes as 1 (n = 1,057)
Serum OPG and the risk of albuminuria
We also found that serum OPG concentrations were higher from 2.7 μg/l in participants with normal albumin excretion, to 3.2 μg/l in those with microalbuminuria, and to 3.5 μg/ml in those with macroalbuminuria (p < 0.01); and the difference remained statistically significant after adjustment for sex, age, BMI, waist circumference, waist/hip ratio, HOMA-IR, current smoking and alcohol intake, serum triacylglycerol, plasma glucose, blood pressure and use of antihypertensive drugs; there was no significant difference between the micro- and macroalbuminuria groups (p = 0.69; Fig. 2). Higher serum OPG was associated with high risk of microalbuminuria and combined micro- and macroalbuminuria (Table 4). The logistic regression analysis also showed that a 0.1 μg/l increase in OPG level was associated with an increased risk of microalbuminuria and combined micro- and macroalbuminuria of 2.5 % (p = 0.02) and 2.6 % (p = 0.005), respectively, even after adjustment for sex, age, smoking habit and alcohol intake, BMI, waist/hip ratio, HOMA-IR, estimated glomerular filtration rate, triacylglycerol, total cholesterol, and HDL- and LDL-cholesterol (Table 4).
Fig. 2.
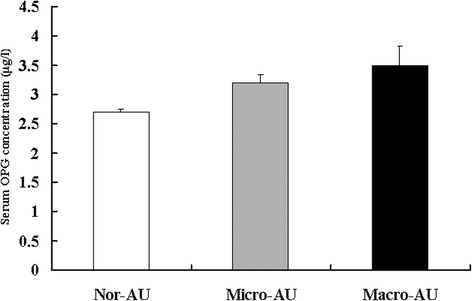
Adjusted means (±SEM) of serum OPG concentrations in participants with normal urinary albumin excretion (Nor-AU) (n = 1,539), microalbuminuria (Micro-AU) (n = 103) and macroalbuminuria (Macro-AU) (n = 14). The co-variables included gender, age, BMI, waist circumference, waist/hip ratio, HOMA-IR, current smoking and alcohol intake, serum triacylglycerol, plasma glucose, blood pressure, menopausal status and use of antihypertensive drugs. There was no significant difference between the groups with micro- and macroalbuminuria (p = 0.69), while the OPG level was significantly higher in the group with microalbuminuria than in the normal group (p < 0.01)
Table 4.
The risk of micro- and macroalbuminuria associated with a 0.1 μg/l increase in serum OPG
| Model | Adjustment | Microalbuminuria (n = 103) | p valuea | Micro- and macroalbuminuria (n = 117) | p valueb |
|---|---|---|---|---|---|
| Model 1 | Unadjusted | 1.042 (1.026–1.058) | <0.0001 | 1.039 (1.022–1.056) | <0.0001 |
| Model 2 | Adjusted for sex, current smoking, current alcohol intake, ACEI/ARB use and family history of diabetes | 1.032 (1.017–1.046) | <0.0001 | 1.034 (1.019–1.050) | <0.0001 |
| Model 3 | Further adjusted for BMI and WHR, based on Model 2 | 1.027 (1.010–1.045) | 0.0007 | 1.029 (1.012–1.046) | <0.0001 |
| Model 4 | Further adjusted for HOMA-IR, based on Model 3 | 1.026 (1.010–1.042) | 0.0009 | 1.028 (1.012–1.044) | <0.0001 |
| Model 5 | Further adjusted for GFR, based on Model 4 | 1.29 (1.013–1.046) | 0.006 | 1.32 (1.018–1.046) | 0.0003 |
| Model 6 | Further adjusted for lipid profiles, based on Model 5 | 1.025 (1.006–1.044) | 0.02 | 1.026 (1.008–1.044) | 0.005 |
Values are ORs (95% CI)
aFor the risk of microalbuminuria, we defined participants with normal urinary albumin excretion as 0 (n=1,539) and microalbuminuria as 1 (n=103), excluding macroalbuminuria (n=14) from the logistic regression analyses
bFor the risk of combined micro- and macroalbuminuria, we defined participants with normal urinary albumin excretion as 0 (n=1,539) and combined micro- and macroalbuminuria as 1 (n=117)
Discussion
In this study, we found a strong association between serum OPG levels and the risk of impaired glucose regulation in a large Chinese cohort aged 40 years or older. Serum OPG concentrations were higher in the presence of isolated IFG, isolated IGT, combined IFG and IGT, and type 2 diabetes. Moreover, this association is independent of potential confounding factors. Serum OPG was also associated with a higher risk of microalbuminuria independent of various confounding factors.
Serum OPG levels were positively correlated with age, 2 h OGTT plasma glucose, HbA1c, triacylglycerol, Log10 ACR and blood cell count. However, in our study, there was no significant difference in the levels of OPG with respect to the duration of the disease, in contrast to the data published by other study groups [16]. Key findings in our study were that increased OPG levels increased the risk for hyperglycaemia, including impaired glucose regulation and newly diagnosed type 2 diabetes, after excluding the effects of age, gender, central obesity, HOMA-IR, family history of type 2 diabetes and the levels of serum lipid. However, given the large sample in this study, the observed association between OPG and glycemic status is relative small and of marginal statistical significance. Certainly prospective studies with solid clinical end points are urgently needed to clarify whether a high OPG level plays a causal role in the development of impaired glucose regulation.
There have also been a number of studies in healthy and obese populations revealing a negative association between OPG and insulin resistance. An inverse relationship between OPG and insulin resistance was reported in an aging male population [25] and in healthy premenopausal obese women [13]. In contrast, OPG was positively correlated with insulin resistance in an obese population and in men with type 2 diabetes mellitus. Insulin resistance could be the potential mechanism for increased serum lipid levels. We found a striking association between serum OPG and lipid levels, especially levels of triacylglycerol.
Our results also indicated that participants with a higher severity of albuminuria tended to have a higher OPG expression. We also found the independent association of OPG with risk of microalbuminuria, which coincides with the findings from previous study [26]. In the present study, the prevalence of microalbuminuria increased correspondingly and significantly with the increase of serum OPG concentration. In light of those and our results, OPG appears to be a more sensitive marker in the diabetes state and may play a vital role in diabetic nephropathy. Xiang et al [27] demonstrated that OPG was increased in microalbuminuric and macroalbuminuric type 2 diabetes mellitus patients as compared with normoalbuminuric type 2 diabetes mellitus patients. Taken together with the results of this study, these findings suggest that OPG might play an important role in diabetic nephropathy, especially when albuminuria becomes severe. OPG is produced by a variety of cell types including endothelial cells and smooth muscle cells. Therefore, the origin of the increased serum OPG levels in diabetic subjects with microvascular disease is uncertain.
In Chang’s study, which found the elevated osteoprotegerin and tumor necrosis factor related apoptosis inducing-ligand (TRAIL) concentration was associated with diabetic nephropathy. Lorz et al. explored the role of TRAIL in diabetic kidney. They demonstrated that TRAIL mRNA was upregulated in the tubulointerstitium of patients with diabetic nephropathy. The same authors observed that a high-glucose medium, characteristic of diabetes, sensitized tubular cells and podocytes to the proapoptotic effects of TRAIL suggesting that TRAIL-induced cell death could play an important role in the progression of diabetic nephropathy [28].
Type 2 diabetes and its complications were associated with wide pathophysiological pathways, including chronic low-grade systemic inflammation. We also found a significant relation between OPG and inflammation, as indicated by the levels of white blood cell counts, in our study. It has been demonstrated that OPG also positive correlated with inflammatory markers (CRP and TNF-α) [4]. These findings suggested that OPG might play a causal role in the pathogenesis of inflammation [29].
To our knowledge, this is the first study to evaluate the relationship between OPG levels and impaired glucose regulation in a large-scale population. Most potential confounders were carefully controlled, which limited the possibility of residual confounding effects. Furthermore, OPG concentrations were measured in duplicate, and the field study was completed within 2 months to minimize seasonal influences on biomarkers and other lifestyle factors. However, due to the cross-sectional nature of the present study, admittedly, we could not determine whether OPG plays a causal role in the pathogenesis of type 2 diabetes. Also, it has yet to be seen whether our results in middle-aged and older Chinese subjects can be generalized to younger populations or other ethnic groups. In the present study, we could not demonstrate any correlation between serum OPG and the presence of macrovascular disease. However, only a few patients in our study had symptoms or signs of macrovascular disease, and as our main focus was on microvascular disease, no invasive tests for macrovascular disease were performed.
Conclusion
We have found that elevated serum OPG levels are strongly and independently associated with impaired glucose metabolism and were independently association with a higher risk of microalbuminuria in Chinese adults. Although longitudinal studies are needed, our findings provide novel insights into the potential role of OPG in the pathogenesis of type 2 diabetes as well as in the prevention and management of impaired glucose regulation.
Ethics approval
Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine. The methods were carried out in accordance with the approved guidelines.
Acknowledgements
This work was supported by grants from the Shanghai Science and Technology Commission (15411953200,10411956600, 14ZR1427400), National Natural Science Foundation of China (81000332, 81300667,81170322, 81370953, 81370935), Shanghai Health System Outstanding Young Talents Training Program (XYQ2013098), Shanghai Education Committee Key Program (14zz110), National Clinical Research Center for Metabolic Diseases (2013BAI09B13) and National Key New Drug Creation and Manufacturing Program of Ministry of Science and Technology (2012ZX09303006-001). We thank the field workers for their contribution and the participants for their cooperation.
Abbreviations
- HOMA-IR
Homeostasis model assessment of insulin resistance
- BMI
Body mass index
- IFG
Isolated impaired fasting glucose
- IGR
Impaired glucose regulation
- IGT
Isolated impaired glucose tolerance
- NGR
Normal glucose regulation
- OPG
Osteoprotegerin
- eGFR
estimated glomerular filtration rate
Footnotes
Yixin Niu and Zhen Yang contributed equally to this work.
Competing interest
The authors declared that there is no duality of interest associated with this manuscript.
Authors’ contributions
Conceived and designed the experiments: LQ, QS. Performed the experiments: ZY, YN, XL. Analyzed the data: ZY. Contributed reagents/materials/analysis tools: WZ, SL, HZ, LZ, YX, GN. Wrote the paper: ZY, YN. All authors read and approved the final manuscript.
Contributor Information
Li Qin, Email: qinli@medmail.com.cn.
Qing Su, Email: suqingxinhua@163.com.
References
- 1.Kiechl S, Werner P, Knoflach M, Furtner M, Willeit J, Schett G. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Expert Rev Cardiovasc Ther. 2006;4:801–11. doi: 10.1586/14779072.4.6.801. [DOI] [PubMed] [Google Scholar]
- 2.Karatolios K, Pankuweit S, Goettsch C, Hofbauer LC, Timmesfeld N, Al-Fakhri N, et al. Osteoprotegerin (OPG) and TNF-related apoptosis-inducing ligand (TRAIL) levels in malignant and benign pericardial effusions. Clin Biochem. 2012;45:237–42. doi: 10.1016/j.clinbiochem.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Suliburska J, Bogdanski P, Gajewska E, Kalmus G, Sobieska M, Samborski W. The association of insulin resistance with serum osteoprotegerin in obese adolescents. J Physiol Biochem. 2013;69:847–53. doi: 10.1007/s13105-013-0261-8. [DOI] [PubMed] [Google Scholar]
- 4.Yaturu S, Rains J, Jain SK. Relationship of elevated osteoprotegerin with insulin resistance, CRP, and TNF-alpha levels in men with type 2 diabetes. Cytokine. 2008;44:168–71. doi: 10.1016/j.cyto.2008.07.471. [DOI] [PubMed] [Google Scholar]
- 5.Nabipour I, Kalantarhormozi M, Larijani B, Assadi M, Sanjdideh Z. Osteoprotegerin in relation to type 2 diabetes mellitus and the metabolic syndrome in postmenopausal women. Metabolism. 2010;59:742–7. doi: 10.1016/j.metabol.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Ashley DT, O’Sullivan EP, Davenport C, Devlin N, Crowley RK, McCaffrey N, et al. Similar to adiponectin, serum levels of osteoprotegerin are associated with obesity in healthy subjects. Metabolism. 2011;60:994–1000. doi: 10.1016/j.metabol.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Altinova AE, Toruner F, Akturk M, Bukan N, Yetkin I, Cakir N, et al. Relationship between serum osteoprotegerin, glycemic control, renal function and markers of atherosclerosis in type 2 diabetes. Scand J Clin Lab Invest. 2011;71:340–3. doi: 10.3109/00365513.2011.570868. [DOI] [PubMed] [Google Scholar]
- 8.Park JS, Cho MH, Nam JS, Yoo JS, Ahn CW, Cha BS, et al. Effect of pioglitazone on serum concentrations of osteoprotegerin in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2011;164:69–74. doi: 10.1530/EJE-10-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmaz Y, Yonal O, Kurt R, Oral AY, Eren F, Ozdogan O, et al. Serum levels of osteoprotegerin in the spectrum of nonalcoholic fatty liver disease. Scand J Clin Lab Invest. 2010;70:541–6. doi: 10.3109/00365513.2010.524933. [DOI] [PubMed] [Google Scholar]
- 10.Pepene CE, Ilie IR, Marian I, Duncea I. Circulating osteoprotegerin and soluble receptor activator of nuclear factor κB ligand in polycystic ovary syndrome: relationships to insulin resistance and endothelial dysfunction. Eur J Endocrinol. 2011;164:61–8. doi: 10.1530/EJE-10-0720. [DOI] [PubMed] [Google Scholar]
- 11.Akinci B, Celtik A, Yuksel F, Genc S, Yener S, Secil M, et al. Increased osteoprotegerin levels in women with previous gestational diabetes developing metabolic syndrome. Diabetes Res Clin Pract. 2011;91:26–31. doi: 10.1016/j.diabres.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Gannagé-Yared MH, Yaghi C, Habre B, Khalife S, Noun R, Germanos-Haddad M, et al. Osteoprotegerin in relation to body weight, lipid parameters insulin sensitivity, adipocytokines, and C-reactive protein in obese and non-obese young individuals: results from both cross-sectional and interventional study. Eur J Endocrinol. 2008;158:353–9. doi: 10.1530/EJE-07-0797. [DOI] [PubMed] [Google Scholar]
- 13.Ugur-Altun B, Altun A. Circulating leptin and osteoprotegerin levels affect insulin resistance in healthy premenopausal obese women. Arch Med Res. 2007;38:891–6. doi: 10.1016/j.arcmed.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005;47:201–10. [PubMed] [Google Scholar]
- 15.Aoki A, Murata M, Asano T, Ikoma A, Sasaki M, Saito T, et al. Association of serum osteoprotegerin with vascular calcification in patients with type 2 diabetes. Cardiovasc Diabetol. 2013;12:11. doi: 10.1186/1475-2840-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 17.O’Sullivan EP, Ashley DT, Davenport C, Penugonda L, Kelleher G, Devlin N, et al. A comparison of osteoprotegerin with adiponectin and high-sensitivity C-reactive protein (hsCRP) as a marker for insulin resistance. Metabolism. 2013;62:34–8. doi: 10.1016/j.metabol.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Reinhard H, Nybo M, Hansen PR, Wiinberg N, Kjær A, Petersen CL, et al. Osteoprotegerin and coronary artery disease in type 2 diabetic patients with microalbuminuria. Cardiovasc Diabetol. 2011;10:70. doi: 10.1186/1475-2840-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–62. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 20.Reaction NG, Group S. Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diabetes. 2012;4:172–3. doi: 10.1111/j.1753-0407.2012.00182.x. [DOI] [PubMed] [Google Scholar]
- 21.Qin L, Yang Z, Gu H, Lu S, Shi Q, Xing Y, et al. Association between serum uric acid levels and cardiovascular disease in middle-aged and elderly Chinese individuals. BMC Cardiovasc Disord. 2014;14:26. doi: 10.1186/1471-2261-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rundle AG, Vineis P, Ahsan H. Design options for molecular epidemiology research within cohort studies. Cancer Epidemiol Biomarkers Prev. 2005;14:1899–907. doi: 10.1158/1055-9965.EPI-04-0860. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Qiu X, Shi C, Huang H, Huang J, Li M, et al. Modified glomerular filtration rate-estimating equations developed in asiatic population for chinese patients with type 2 diabetes. Int J Endocrinol. 2014;2014:521071. doi: 10.1155/2014/521071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gannage-Yared MH, Fares F, Semaant M, Khalife S, Jambart S. Circulation osteoprotegerin is correlated with lipid profile, insulin sensitivity, adiponectin and sex steroids in an ageing male population. Clin Endocrinol. 2006;64:652–8. doi: 10.1111/j.1365-2265.2006.02522.x. [DOI] [PubMed] [Google Scholar]
- 26.Chang YH, Lin KD, He SR, Hsieh MC, Hsiao JY, Shin SJ. Serum osteoprotegerin and tumor necrosis factor related apoptosis inducing-ligand (TRAIL) are elevated in type 2 diabetic patients with albuminuria and serum osteoprotegerin is independently associated with the severity of diabetic nephropathy. Metabolism. 2011;60:1064–9. doi: 10.1016/j.metabol.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Xiang GD, Pu JH, Zhao LS, et al. Association between plasma osteoprotegerin concentrations and urinary albumin excretion in type 2 diabetes. Diabet Med. 2009;26:397–403. doi: 10.1111/j.1464-5491.2009.02683.x. [DOI] [PubMed] [Google Scholar]
- 28.Lorz C, Benito-Martin A, Boucherot A, Ucero AC, Rastaldi MP, Henger A, et al. The death ligand TRAIL in diabetic nephropathy. J Am Soc Nephrol. 2008;19:904–14. doi: 10.1681/ASN.2007050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen WJ, Rijzewijk LJ, van der Meer RW, Heymans MW, van Duinkerken E, Lubberink M, et al. Association of plasma osteoprotegerin and adiponectin with arterial function, cardiac function and metabolism in asymptomatic type 2 diabetic men. Cardiovasc Diabetol. 2011;10:67. doi: 10.1186/1475-2840-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]


