Abstract
Microvesicles (MVs) in body fluids participate in a variety of physical and pathological processes, and are regarded as potential biomarkers for numerous diseases. Flow cytometry (FCM) is among the most frequently used techniques for MV detection. However, different handling methods unavoidably cause pre-analytical variations in the counts and sizes of MVs determined by FCM. The aim of the present study was to investigate the effect of centrifugation, storage conditions and anticoagulant on MV measurements. Blood samples were obtained from 13 healthy donors, including 4 women and 9 men. Calcein-AM staining was used to label MVs and assess the impact of pre-analytical preparation, including centrifugation, and storage conditions on MV measurements obtained using FCM. The range of factors investigated for comparison included: Platelet-free plasma (PFP) stored at −80°C for 1 or 4 weeks; MVs stored at 4°C for 3–4 days or 1 week; MVs frozen at −80°C for 1 or 4 weeks; and anticoagulants, either heparin or ethylenediaminetetraacetic acid (EDTA). No statistically significant differences in MV counts were detected between the two centrifugation speeds (16,000 and 20,500 × g) or among the three centrifugation times (15, 30 and 60 min) investigated. Similarly, no significant differences were noted in MV counts between the two anticoagulants tested (heparin and EDTA). However, the storage of PFP or MVs in heparin-anticoagulated plasma for different periods markedly affected the detected MV counts and size distribution. The counts and sizes of MVs from EDTA-anticoagulated plasma were only affected when the MVs were frozen at −80°C for 4 weeks. In conclusion, calcein-AM is able to efficiently identify MVs from plasma and may be an alternative to Annexin V for MV staining. EDTA preserves the MV counts and size more accurately compared with heparin under calcein-AM staining. PFP centrifuged at 16,000 × g for 15 min is sufficient to isolate MVs, which enables the batch processing of samples. PFP, rather than MVs alone, appears to be the preferable mode of sample storage, as MVs stored in PFP were less affected by storage temperature and duration. The present study provides a methodology for MV collection, storage and isolation, to facilitate further investigation of MVs as biomarkers in disease.
Keywords: microvesicles, calcein-AM, flow cytometry, pre-analytical preparation
Introduction
Microvesicles (MVs) are a heterogeneous group of membrane vesicles, with a size range of 100–1,000 nm, which are shed by various cell types in physiological and pathological conditions (1). MVs have been identified in a number of body fluids, including the plasma, serum, urine, saliva, breast milk, amniotic fluid and ascites (2–7). The counts and contents of MVs in circulation depend on the cells of origin and the stimuli that trigger MV production. Studies observing plasma by flow cytometry (FCM) have revealed correlations between MV counts and other characteristics of human hematologic disorders (8), cardiovascular (9) and autoimmune diseases (10), and cancer (11,12). Therefore, circulating MVs represent promising biomarkers and may have diagnostic and prognostic value for diseases.
However, due to different methods used for collection, storage and isolation, pre-analytical variations in MV detection are unavoidable (13–16). A number of anticoagulants are used in blood samples collected for MV analysis, including heparin (13,17), acid-citrate-dextrose (18), ethylenediaminetetraacetic acid (EDTA) (14) and sodium citrate (19). Previous studies have reported that heparin and EDTA lead to conflicting results of MV count (15,16). In addition, different centrifugation speeds and times are used to isolate MVs; however, the effect of different centrifugation conditions on MV count are unclear (8,20). Furthermore, storage status, time and temperature may markedly affect MV counts, as observed in previous studies (13,15). Recent studies have demonstrated that MV size distribution may differ in response to various forms of stress in vitro (20,21), indicating the significance of MV size in diseases. However, the effects of sample collection procedures and processing on MV size distribution have not been well-characterized.
The aim of the present study was to investigate the impact of pre-analytical sample preparation procedures and conditions on the counts and size distribution of MVs assessed using FCM.
Materials and methods
Reagents
Calcein-AM (C1430) was obtained from Life Technologies (Carlsbad, CA, USA) and used for MV staining as previously described (20). Calibration beads of 1 µm (L1030) and 3 µm (LB30) were purchased from Sigma-Aldrich (St. Louis, MO, USA) to define the MV gate and calculate MV counts. A submicron bead calibration kit (no. 832) was obtained from Bangs Laboratories, Inc. (Fishers, IN, USA) to verify the resolution capabilities of the flow cytometer and define the size distribution of MVs. Phosphate-buffered saline (PBS) solution (SH30256.01B) was purchased from HyClone Corporation (Logan, UT, USA), and filtered through a 0.22-µm filter (EMD Millipore, Billerica, MA, USA) to minimize interference from particles in PBS.
Blood sample collection
Optimization of MV detection was performed using samples from healthy individuals. Written informed consent was obtained from 13 donors (female, 4; male, 9; age range, 22–33 years; median age, 26 years) under a protocol approved by the local Institutional Review Board of Tongji Medical College (Wuhan, China). All participants fasted for 10 h prior to sample collection. A total of 26 blood samples were collected from the 13 donors and 21-gauge needles were used to place the samples in BD vacutainers (BD Biosciences, Franklin Lakes, NJ, USA) of which the inner wall were sprayed with heparin or EDTA. There were 2 samples from each donor and 2 ml per sample. These samples were used for the evaluation of the effect of storage conditions and anticoagulant on MV counts and size distribution. To assess the impact of centrifugation speed and time, 8 specimens were collected from healthy individuals (female, 4; male, 4; age range, 24–26 years; median age, 25 years) and placed in BD vacutainer tubes with heparin sprayed on the inner wall, 2 ml per sample. For all samples, the first 3 ml of blood collected following venepuncture was discarded. All samples were centrifuged within 2 h from collection.
Preparation of platelet-free plasma (PFP)
PFP was obtained from anticoagulated blood as previously described (15) with certain modifications. Briefly, whole blood samples were centrifuged at 2,500 × g for 30 min at 20°C, and plasma was collected and centrifuged for an additional 30 min at 2,500 × g. Next, the supernatant was collected and aliquots of 100 µl PFP were stored at −80°C for 1 or 4 week until use, or MVs were isolated immediately.
Isolation of MVs
MVs were isolated from PFP, that was stored at −80°C and thawed rapidly to 37°C prior to use (13). The PFP was centrifuged at 20,500 × g for 60 min at 4°C to obtain a MV pellet, as described by Ghosh et al (8). The centrifuge tube was tipped to discard the supernatant, leaving the pellet at the bottom undisturbed. The MV pellet was resuspended in 100 µl PBS by gentle vortexing for 20 sec for immediate analysis using FCM, or storage at 4°C for 3–4 days or 1 week or or −80°C for 1 or 4 week prior to analysis. Unless otherwise indicated, MVs freshly obtained from PFP without any storage were used as controls.
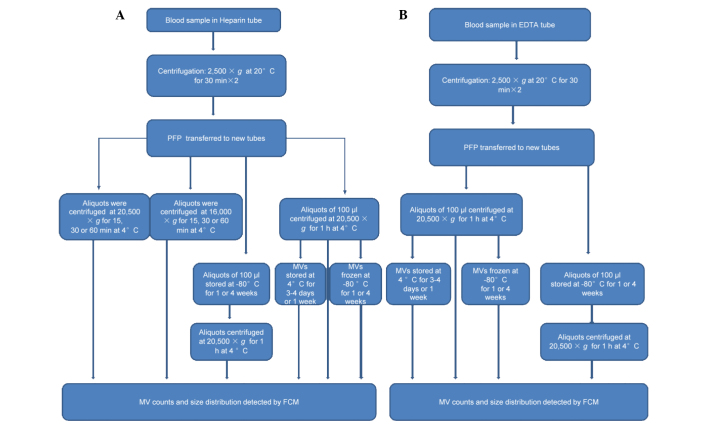
In order to investigate the effects of centrifugation speed and duration on MV analysis, PFP from heparin-anticoagulated blood was immediately centrifuged at 4°C and 16,000 or 20,500 × g, for 15, 30 or 60 min to isolate MVs. MVs obtained at 20,500 × g were regarded as the control in this section. The workflow is outlined in Fig. 1.
Figure 1.
Sample treatment flowchart showing the experimental strategy for evaluating variables of collection, storage and isolation for MV counts and size distribution using FCM detection. (A) Blood samples were collected in BD Vacutainers tubes with heparin sprayed on the inner wall and centrifuged twice at 2,500 × g for 30 min to obtain PFP. A number of aliquots were centrifuged at 16,000 or 20,500 × g for 15, 30 or 60 min at 4°C to obtain MVs, which were analyzed immediately to evaluate the influences of centrifugation speed and time on MV count and size distribution. Other PFP aliquots were centrifuged at 20,500xg for 1 h at 4°C to obtain MVs, or stored at −80°C for various periods prior to isolation of the MVs. Subsequently, these MVs were analyzed immediately or stored at 4 or −80°C for various periods until required for analysis using FCM. (B) Blood samples were collected in BD Vacutainers tubes with EDTA sprayed on the inner wall. Following double centrifugation at 2,500 × g for 30 min the supernatants were transferred to other tubes. Aliquots of 100 µl were centrifuged at 20,500 × g for 1 h at 4°C to obtain MVs, or stored at −80°C for various periods prior to isolation of the MVs. MVs were analyzed immediately, or stored at 4 or −80°C for various periods prior to analysis by FCM. EDTA, ethylenediaminetetraacetic acid; PFP, platelet-free plasma; MV, microvesicle; FCM, flow cytometry.
FCM analysis of MVs
Data were acquired and analyzed using a BD LSR II flow cytometer (BD Biosciences) equipped with FACSDiva software. Forward scatter (FSC) and side scatter (SSC) of light were set in a logarithmic scale, and the fluorescence channels were set at logarithmic gain. Calibration beads were used to set the MV gate and to calculate the MV counts.
In order to distinguish true events from electronic noise and increase the specificity of MV detection, events in the MV gate were further discriminated by labeling with calcein-AM (20). MVs were defined as particles <1.0 µm in diameter that exhibited positive staining for calcein-AM. Individual MV samples were labeled with 0.5 µl calcein-AM (5 µmol/l) for 25–30 min in the dark and diluted to a final volume of 300 µl. The time and concentration employed was optimized by titration. MV samples prepared in PBS without calcein-AM were used as negative controls.
For calculation of MV counts, 3-µm calibration beads (0.5 µl) were added immediately prior to analysis. Gain settings were adjusted to place the beads in the top right corner for scatter. The equation MV=GMV × TC/(GTC × V) was used to calculate the absolute counts of MVs in single staining, where GMV is the number of events in the MV gate, GTC is the number of events in the 3-µm calibration bead gate and TC is the number of beads added to the sample of volume V (15,22,23). Data acquisition stopped when the number of events in GTC reached 100,000. The original data analysis was performed using FACSDiva software (version 6.1.2; BD Biosciences), and FlowJo software (version 7.6.2; Treestar, Inc., Palo Alto, CA, USA) was applied to analyze the size distribution.
Statistical analysis. Statistically significant differences were compared among groups using the independent-samples t-test. Analysis was performed using SPSS statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA). Two-sided P-values were used throughout. P<0.05 was considered to indicate a statistically significant difference.
Results
Data acquisition, gating and counting strategy
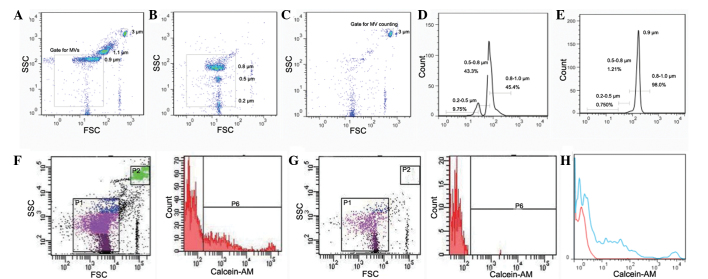
Scatter events from size calibration beads of 0.2, 0.5, 0.8, 1 and 3 µm were resolved from instrument noise using the BD LSR II flow cytometer (Fig. 2). Inspection of the scatter plot indicates that 0.2 µm is the lower limit for bead detection (Fig. 2B). Calibration beads of 0.2, 0.5 and 0.8 µm were additionally used to define the size distribution of MVs. As shown in Fig. 2D, MVs may be divided into three size ranges of 0.2–0.5, 0.5–0.8 and 0.8–1.0 µm in diameter. Fig. 2E is the histogram of the data presented in Fig. 2A, verifying the applicability of size distribution defined by the small beads.
Figure 2.
Gating and counting strategy. Representative dot plots and histograms illustrate gating and counting strategy for flow cytometric analysis of MVs. (A) 1 µm (a mixture of 0.9 and 1.1 µm) beads from Sigma-Aldrich were used to set the MV gate and establish appropriate instrument settings. The MV gate was defined as <1.0 µm. (B) A small bead calibration kit was used to verify the resolution capabilities of the flow cytometer, and to define the size distribution of MVs. (C) Representative dot plot for the 3-µm beads used for MV counting. (D) Histogram of size distributions from the data in Fig. 2B. (E) Histogram of size distribution from the data in Fig. 2A, verifying the applicability of Fig. 2D. (F) Representative sample of MV counting from the same individual as for the data in Fig. 2G. (G) An individual sample of MVs without calcein-AM staining was used as the calcein-AM negative control. (H) Histogram of the data in Fig. 2F, normalized against Fig. 2G. Red lines indicate data from Fig. 2G and blue lines indicate data from Fig. 2F. SSC, side scatter; FSC, forward scatter.
In the present study, MVs were defined as particles that were <1.0 µm in diameter and positive for calcein-AM staining. As shown in Fig. 2F, events in the P6 gate were regarded as MVs from plasma. MV counts were calculated by reference to added 3-µm calibration beads. According to the product information from Sigma-Aldrich, the number of particles per microliter of these beads is 6.8×106, and 0.5 µl beads were added per experimental sample. Thus, the number of particles added per sample was 3.4×106. Data acquisition stopped when events in the P2 gate (3-µm calibration beads gate) reached 100,000; events in the P6 (MV) gate were recorded simultaneously. The number of MVs was calculated according to the formula described in the methods section.
MV counts and size distribution are not affected by centrifugation speed or time
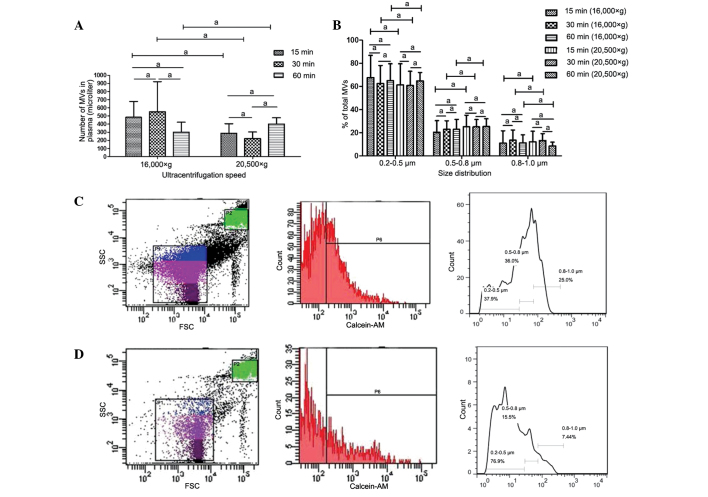
Various centrifugation speeds and times have been used in previous studies for MV isolation (8,13,20); however, the effect of these variations in the parameters on MV counts and size distribution is unclear. In the present study, the differences arising from the various methods used for MV isolation were investigated. MVs isolated by centrifugation at 16,000 or 20,500 × g for 15, 30 or 60 min were analyzed using FCM. No statistically significant differences were detected in MV counts or size distribution between samples prepared at different centrifugation speeds, or among samples centrifuged for different times at identical speeds (Fig. 3).
Figure 3.
Impact of centrifugation speed and duration on MV counts and size distribution. Differences in (A) MV count and (B) size distribution, respectively. Also, representative dot plot and histogram showing the effects of centrifugation speed on MV count and size distribution for samples from a single individual are presented: (C) Samples centrifuged at 20,500 × g; and (D) samples centrifuged at 16,000 × g. Data are expressed as the mean ± standard error of the mean. aNo statistically significant difference. MV, microvesicle; SSC, side scatter; FSC, forward scatter.
Counts and size of MVs from heparin-anticoagulated samples vary with storage conditions
A number of studies have reported that heparin is able to preserve MV counts (15,16). Therefore, in the present study, heparin was used as the primary anticoagulant in collected samples. In order to investigate the effect of storage conditions on MV counts and size distribution, the PFP or MVs were stored for different periods at various temperatures until analysis (Fig. 4A, C, E and F).
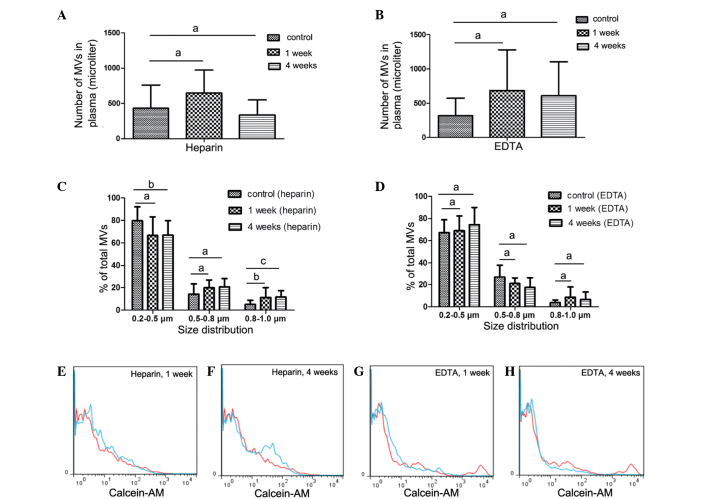
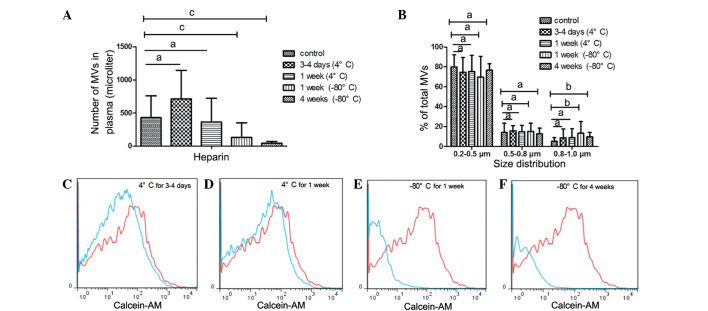
Figure 4.
Impact of PFP storage at −80°C for 1 or 4 weeks on MV counts and size distribution. Impact of (A) heparin and (B) EDTA on MV counts, as well as impact of (C) heparin and (D) EDTA on MV size distribution. Representative histograms of MV counts vs. control samples from a single individual are also shown (red lines, control; blue lines, experiment). Platelet-free plasma (PFP) from heparin-anticoagulated plasma at (E) 1 week and (F) 4 weeks. PFP from EDTA-anticoagulated plasma at (G) 1 week and (H) 4 weeks. Control samples were collected and centrifuged immediately to isolate MVs (i.e. there was no prior storage of the PFP). Data are expressed as the mean ± standard error of the mean. aNo statistically significant difference; bP<0.05; cP<0.01. MV, microvesicle; EDTA, ethylenediaminetetraacetic acid.
Counts and size of MVs from PFP stored at −80°C for 1 or 4 weeks were compared with the control samples (MVs that were isolated immediately). Fig. 4C demonstrates that MV size, but not count, was significantly affected. After 1 week of storage of PFP, MV size was larger compared with that of the control samples, with an increased percentage of 0.8–1.0 µm MVs observed compared with the control (P=0.025). After 4 weeks, the percentage of MVs distributed in the 0.8–1.0 µm group significantly increased (P=0.006), while the percentage in the 0.2–0.5 µm group decreased compared with the control (P=0.038).
In addition to PFP, MVs is another mode of storage. It was observed that freezing MVs at −80°C markedly affected the MV count and size (Fig. 5). MV counts significantly reduced after MVs were stored for 1 or 4 weeks at −80°C (1 week, P=0.006; 4 weeks, P<0.001) compared with the control, whereas the percentage of MVs in the 0.8–1.0 µm bead size group increased (1 week, P=0.018; 4 weeks, P=0.014). By contrast, MV counts and size distribution did not change after MVs were stored at 4°C for 3–4 days or 1 week (Fig. 5A and B).
Figure 5.
Effect of storage of MVs isolated from heparin-anticoagulated plasma at 4 or −80°C for different periods. Impact on (A) MV counts and (B) size distribution, respectively. Representative histograms showing the impact of MV storage on MV counts vs. a control sample from a single individual are also presented (red lines, control; blue lines, experiment). Samples were stored at 4°C for (C) 3–4 days or (D) 1 week, and at −80°C for (E) 1 week or (F) 4 weeks. The control sample was collected and centrifuged immediately to isolate MVs, which were not stored. Data are expressed as the mean ± standard error of the mean. aNo significance; bP<0.05; cP<0.01. MV, microvesicle.
MVs from EDTA-anticoagulated samples
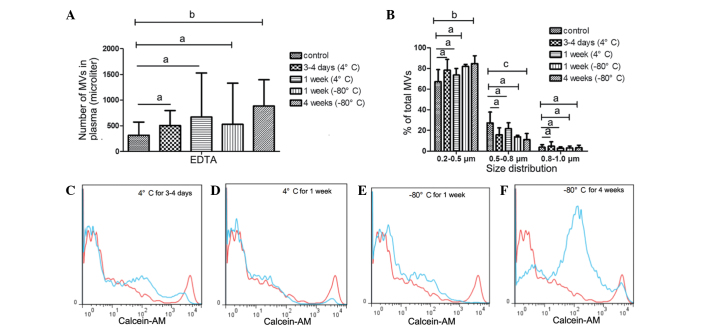
Although a number of studies (15,16) have indicated that MVs from EDTA-anticoagulated plasma do not accurately evaluate the level of circulating MVs, EDTA is the most frequently used anticoagulant in clinical settings. On the basis of the protective effect of EDTA on blood cells, it was speculated that EDTA would protect MVs from storage influences and prolong safe storage time. Furthermore, the present study aimed to determine whether EDTA was able to function as a feasible alternative to heparin.
In the EDTA-anticoagulated PFP that was stored at −80°C for 1 or 4 weeks, no statistically significant increase in MV count was detected. Similarly, the size distribution of MVs in the PFP stored at −80°C for 1 or 4 weeks was comparable with that in the control samples, as shown in Fig. 4B, D, G and H.
MVs from EDTA-anticoagulated plasma stored at 4°C for 0 days (control), 3–4 days or 1 week exhibited no statistically significant differences in MV counts. Similarly, the size distribution showed no significant differences in the MVs that were stored at 4°C for up to 1 week (Fig. 6A–D), or those frozen at −80°C for 1 week (Fig. 6A, B and E). However, MV counts and size were significantly influenced by freezing at −80°C for 4 weeks (Fig. 6A, B and F). The number of MVs increased (P=0.041 vs. control; Fig. 6A and F), whereas MV size gradually decreased. The percentage of MVs in the 0.5–0.8 µm size distribution group significantly decreased (P=0.008 vs. control) and the 0.2–0.5 µm group increased (P=0.014 vs. control; Fig. 6B).
Figure 6.
Impact of storage of MVs isolated from EDTA-anticoagulated plasma at 4 or −80°C for different periods. Impact on (A) MV counts and (B) size distribution, respectively. Representative histograms showing the impact of MV storage on MV counts vs. a control sample from the same individual are also presented (red lines, control; blue lines, experiment). Samples were stored at 4°C for (C) 3–4 days or (D) 1 week, and at −80°C for (E) 1 week or (F) 4 weeks. The control sample was collected and centrifuged immediately to isolate MVs, which were not stored. Data are expressed as the mean ± standard error of the mean. aNo significance; bP<0.05; cP<0.01. MV, microvesicle; EDTA, ethylenediaminetetraacetic acid.
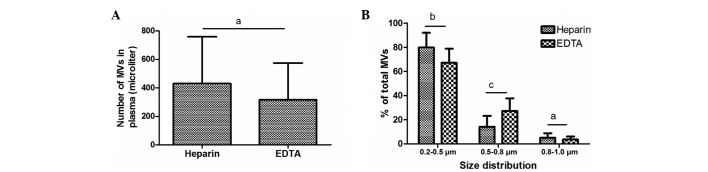
MV size distribution is affected by the anticoagulant used, but MV count is not
Previous studies have reported differences in MV counts between heparin- and EDTA-anticoagulated plasma using Annexin V staining (13,15); however, no data has been published to elucidate the impact of anticoagulant on MV counts following calcein-AM labeling. Therefore, the present study compared the counts and size distribution of MVs freshly isolated from plasma anticoagulated with heparin and EDTA. Notably, no statistically significant differences in the number of MVs were detected between the samples treated with these two anticoagulants. In the EDTA and heparin groups, MVs were predominantly distributed in the 0.2–0.5 µm size group, to a reduced degree in the 0.5–0.8 µm group and minimally in the 0.8–1.0 µm group. However, the size of MVs in the EDTA-anticoagulated plasma was increased compared with that in the heparin-anticoagulated plasma: The percentage increased in the 0.5–0.8 µm size distribution range (P=0.002) and decreased in the 0.2–0.5 µm group (P=0.032), compared with the size of MVs isolated from heparin-anticoagulated plasma (Fig. 7).
Figure 7.
Impact of anticoagulant on (A) MV counts and (B) size distribution. aNo significance; bP<0.05; cP<0.01. MV, microvesicle; EDTA, ethylenediaminetetraacetic acid.
Discussion
In the present study, the effects of pre-analytical variables, including centrifugation speed and duration, and storage conditions on MV measurement using FCM with calcein-AM staining were evaluated. In addition, the impact of heparin and EDTA anticoagulants on MV counts and size distribution were compared. The data indicated that MV counts are independent of anticoagulant, centrifugation speed and duration, but are affected by storage conditions, while the size of MVs varied with plasma anticoagulant and storage conditions.
The profile of circulating MVs released by cells correlates with the development, progression and metastasis of a variety of cancers (24–26). According to the widely accepted principle that phosphatidylserine (PS) exposure on the cell surface is a common feature of MV release, Annexin V, a specific ligand to PS, is used for MV detection by FCM in the majority of experiments (13,15,16). However, reports of the existence of Annexin V-negative MVs (22,27,28) challenge the basis of this detection approach. Furthermore, PS exposure occurs in apoptosis and necrosis, which may lead to false-positive results using FCM. Thus, it is essential to identify a novel reagent for the detection of MVs. In the present study, calcein-AM was used to identify MVs, primarily due to the fact that it is colorless and nonfluorescent until hydrolyzed in cells by nonspecific esterases, and the leakage of the hydrolyzed compound out of cells is markedly slower compared with its parent compound, referred to the product introduction of calcein-AM. In addition, calcein-AM staining is independent of PS content and calcium concentration. The latter is a major drawback of Annexin V, leading to different results in MV counts between heparin- and EDTA-anticoagulated plasma. Therefore, calcein-AM may be suitable and relatively specific for intact MV labeling. To the best of our knowledge, the present study is the first to apply the calcein-AM staining method to label clinical circulating MVs. Further investigations are required to compare the differences in MV counts and size distribution between calcein-AM and Annexin V labeling in different conditions.
Previous studies have reported (13,15) that MV counts are substantially reduced in blood when using EDTA compared with heparin; however, in the present study, no statistically significant differences in MV count were detected between EDTA and heparin using calcein-AM staining. A previous study suggested that calcium chelation by EDTA contributed to the decreased MV counts (15), while a different study attributed this difference to microvesiculation in vitro with heparin (16). The present data support the former explanation, indicating that the primary distinction between calcein-AM and Annexin V is the effect of calcium. In the present experiments that employed an MV staining reagent independent of calcium, no differences were observed in MV counts between samples treated with EDTA and heparin. EDTA has been recommended as the first line anticoagulant for hematological testing as it allows the optimal preservation of cellular components and morphology of blood cells (29). The results of the present study indicate that EDTA may be more suitable than heparin for MV detection by FCM, as EDTA preserves MV counts and size more effectively compared with heparin. Connor et al (30) suggested that, for blood samples that are not immediately processed, MV measurement should be performed using EDTA-anticoagulated samples (31), which is consistent with the results of the present study.
Isolation of MVs is a time-consuming process which increased the difficulty of large-scale sample preparation and detection. For this reason, it may be more practical and convenient if MVs could be obtained in a shorter time. The results of the present study comparing different centrifugation speeds and times may meet this requirement, thus assisting in batch processing of samples and promoting the development of MV detection. An excessive centrifugation speed may lead to fragment contamination of the MV pellet, so proper speed is critical for intact MV isolation. The present data indicated that centrifugation of PFP at 16,000 × g for 15 min was sufficient to achieve the requirements of MV isolation.
It may be optimal to proceed to isolation and detection of MVs immediately after plasma preparation. However, in certain cases, samples from different patients cannot be processed immediately and sample storage is necessary, which is usually as PFP or MVs (13,15). Ayers et al (13) reported that MV counts were significantly reduced in MVs that were stored at −80°C for an extended duration, which is consistent with the present results. This may be due to the adsorption of MVs to sample tubes, or MVs shrinking to a size that is below the detection limit of FCM for samples frozen at a low temperature for a long period. However, it appears that this explanation only applies to MVs from heparin-anticoagulated plasma, as the counts of MVs from EDTA-anticoagulated plasma increased in the MVs that were frozen at −80°C for 4 weeks. The underlying mechanism remains unknown; however, it is plausible that this phenomenon may be associated with the calcium-chelating properties of the anticoagulant. PFP, rather than MVs, appears to be the preferable mode of sample storage, on the basis of the present results and the findings of previous experiments (15).
A number of studies have reported that MV size distribution modulates according to diverse stimuli in vitro (20,21), indicating that MV size may be associated with disease condition or cause. Although previous studies have demonstrated the utility of electron microscopy, atomic force microscopy (AFM), dynamic light scattering (DLS) and microfluidics in measuring the size of circulating MVs (2,32,33), these detection methods are relatively time-consuming and complicated. Compared with the aforementioned methods, FCM is user-friendly and is widely used in MV detection. However, little is known about MV size distribution as determined by FCM in samples from healthy tissues, or the effects of sample preparation methods. In the present study, calibration beads were used to define MV size and assess the impact of various preparation conditions on sample size distribution. MV size was predominantly 0.2–0.5 µm in diameter, as observed using transmission electron microscopy (TEM), AFM or DLS (32,33). However, MVs isolated from different anticoagulants exhibited diverse size distributions under different conditions. MVs from heparin-anticoagulated plasma frozen at low temperature were enlarged over time and presented with reduced counts, whereas the opposite was observed in the MVs stored in EDTA-anticoagulated plasma. For MVs from heparin-anticoagulated plasma, this may be a result of the aggregation of MVs into a reduced number of larger vesicles. For MVs from EDTA-anticoagulated plasma, larger MVs may have reduced to a size that is detectable using FCM. Notably, the average size of freshly isolated (i.e. never stored) MVs from EDTA-anticoagulated plasma is increased compared with that of MVs from heparin-anticoagulated plasma. Future investigations may be required in order to further elucidate these findings.
In conclusion, the present study evaluated the impact of pre-analytical variables including anticoagulant, centrifugation speed and time, and storage conditions on MV measurements using FCM with calcein-AM staining. Analysis of these factors is essential for the development of MVs as diagnostic and prognostic biomarkers for disease.
Acknowledgements
The authors would like to thank Yong Xu and Zhihui Liang from the Institute of Biochemistry (School of Basic Medicine, Huazhong University of Science and Technology, Wuhan, China) for their assistance in FCM detection and data analysis. In addition, the authors thank the healthy volunteers for their cooperation in providing blood specimens for the present study. This study was supported by grants from the National Natural Science Foundation of China (nos. 81272624, 81300259 and 81170497).
Abbreviations
- MVs
microvesicles
- FCM
flow cytometry
- EDTA
ethylenediaminetetraacetic acid
- PBS
phosphate-buffered saline
- PFP
platelet-free plasma
- PS
phosphatidylserine
- AFM
atomic force microscopy
- DLS
dynamic light scattering
- TEM
transmission electron microscopy
References
- 1.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft BA, de Sonneville J, Yuana Y, Osanto S, Bertina R, Kuil ME, Oosterkamp TH. Determination of the size distribution of blood microparticles directly in plasma using atomic force microscopy and microfluidics. Biomed Microdevices. 2012;14:641–649. doi: 10.1007/s10544-012-9642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalton AJ. Microvesicles and vesicles of multivesicular bodies versus ‘virus-like’ particles. J Natl Cancer Inst. 1975;54:1137–1148. doi: 10.1093/jnci/54.5.1137. [DOI] [PubMed] [Google Scholar]
- 4.Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun. 2010;396:528–533. doi: 10.1016/j.bbrc.2010.04.135. [DOI] [PubMed] [Google Scholar]
- 6.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G, Altevogt P. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107:563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: Implications for disease progression. Blood. 2010;115:1755–1764. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, de Marchena E, Ahn YS. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 10.Sellam J, Proulle V, Jüngel A, Ittah M, Richard Miceli C, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM, Mariette X. Increased levels of circulating microparticles in primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11:R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toth B, Liebhardt S, Steinig K, Ditsch N, Rank A, Bauerfeind I, Spannagl M, Friese K, Reininger AJ. Platelet-derived microparticles and coagulation activation in breast cancer patients. Thromb Haemost. 2008;100:663–669. [PubMed] [Google Scholar]
- 12.Galindo-Hernandez O, Villegas-Comonfort S, Candanedo F, González-Vázquez MC, Chavez-Ocana S, Jimenez-Villanueva X, Sierra-Martinez M, Salazar EP. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res. 2013;44:208–214. doi: 10.1016/j.arcmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Ayers L, Kohler M, Harrison P, Sargent I, Dragovic R, Schaap M, Nieuwland R, Brooks SA, Ferry B. Measurement of circulating cell-derived microparticles by flow cytometry: Sources of variability within the assay. Thromb Res. 2011;127:370–377. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Ueba T, Haze T, Sugiyama M, Higuchi M, Asayama H, Karitani Y, Nishikawa T, Yamashita K, Nagami S, Nakayama T, et al. Level, distribution and correlates of platelet-derived microparticles in healthy individuals with special reference to the metabolic syndrome. Thromb Haemost. 2008;100:280–285. [PubMed] [Google Scholar]
- 15.Jayachandran M, Miller VM, Heit JA, Owen WG. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J Immunol Methods. 2012;375:207–214. doi: 10.1016/j.jim.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah MD, Bergeron AL, Dong JF, López JA. Flow cytometric measurement of microparticles: Pitfalls and protocol modifications. Platelets. 2008;19:365–372. doi: 10.1080/09537100802054107. [DOI] [PubMed] [Google Scholar]
- 17.Breimo ES, Østerud B. Generation of tissue factor-rich microparticles in an ex vivo whole blood model. Blood Coagul Fibrinolysis. 2005;16:399–405. doi: 10.1097/01.mbc.0000172329.66130.d2. [DOI] [PubMed] [Google Scholar]
- 18.Piersma SR, Broxterman HJ, Kapci M, de Haas RR, Hoekman K, Verheul HM, Jiménez CR. Proteomics of the TRAP-induced platelet releasate. J Proteomics. 2009;72:91–109. doi: 10.1016/j.jprot.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Jy W, Horstman LL, Jimenez JJ, Ahn YS, Biró E, Nieuwland R, Sturk A, Dignat-George F, Sabatier F, Camoin-Jau L, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–1851. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 20.Bernimoulin M, Waters EK, Foy M, Steele BM, Sullivan M, Falet H, Walsh MT, Barteneva N, Geng JG, Hartwig JH, et al. Differential stimulation of monocytic cells results in distinct populations of microparticles. J Thromb Haemost. 2009;7:1019–1028. doi: 10.1111/j.1538-7836.2009.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Wang HX, Zhu XJ, Wu PH, Chen WQ, Zou P, Li QB, Chen ZC. Serum deprivation elevates the levels of microvesicles with different size distributions and selectively enriched proteins in human myeloma cells in vitro. Acta Pharmacol Sin. 2014;35:381–393. doi: 10.1038/aps.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 23.Jayachandran M, Litwiller RD, Owen WG, Heit JA, Behrenbeck T, Mulvagh SL, Araoz PA, Budoff MJ, Harman SM, Miller VM. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am J Physiol Heart Circ Physiol. 2008;295:H931–H938. doi: 10.1152/ajpheart.00193.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada N, Tsujimura N, Kumazaki M, Shinohara H, Taniguchi K, Nakagawa Y, Naoe T, Akao Y. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta. 2014;1839:1256–1272. doi: 10.1016/j.bbagrm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Zhao P, Xu XL, Cai L, Song ZS, Cao DY, Tao KS, Zhou WP, Chen ZN, Dou KF. Annexin A2 promotes the migration and invasion of human hepatocellular carcinoma cells in vitro by regulating the shedding of CD147-harboring microvesicles from tumor cells. PLoS One. 2013;8:e67268. doi: 10.1371/journal.pone.0067268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen CT, Østergaard O, Johnsen C, Jacobsen S, Heegaard NH. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3067–3077. doi: 10.1002/art.30499. [DOI] [PubMed] [Google Scholar]
- 28.Joop K, Berckmans RJ, Nieuwland R, Berkhout J, Romijn FP, Hack CE, Sturk A. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001;85:810–820. [PubMed] [Google Scholar]
- 29.Banfi G, Salvagno GL, Lippi G. The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med. 2007;45:565–576. doi: 10.1515/CCLM.2007.110. [DOI] [PubMed] [Google Scholar]
- 30.Connor DE, Exner T, Ma DD, Joseph JE. Detection of the procoagulant activity of microparticle-associated phosphatidylserine using XACT. Blood Coagul Fibrinolysis. 2009;20:558–564. doi: 10.1097/MBC.0b013e32832ee915. [DOI] [PubMed] [Google Scholar]
- 31.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011;105:396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- 32.György B, Módos K, Pállinger E, Pálóczi K, Pásztói M, Misják P, Deli MA, Sipos A, Szalai A, Voszka I, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–e48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 33.Lawrie AS, Albanyan A, Cardigan RA, Mackie IJ, Harrison P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang. 2009;96:206–212. doi: 10.1111/j.1423-0410.2008.01151.x. [DOI] [PubMed] [Google Scholar]