Abstract
Platelet-derived growth factor (PDGF)-C and PDGF-D are frequently upregulated in human cancers and play important roles in tumor progression, angiogenesis and metastasis. However, the distribution, frequency and prognostic value of PDGF-C and PDGF-D expression in gastric cancer have not been clarified. The present study evaluated the association between expression of PDGF-C and PDGF-D, clinicopathological factors and outcomes, in patients with gastric cancer. Gastric adenocarcinoma tumor samples were obtained from 204 patients who underwent curative gastrectomy between 2003 and 2007. The expression of PDGF-C and PDGF-D was analyzed by immunohistochemical staining. High expression of PDGF-C and PDGF-D was detected in 114 (56%) and 151 (74%) tumors, respectively. PDGF-D expression was significantly associated with tumor depth (P=0.039), histopathology (P<0.01), tumor stage (P=0.01) and recurrence (P<0.01), whereas PDGF-C expression correlated only with histopathology (P=0.05). High PDGF-D expression was also associated with significantly shorter relapse-free survival (RFS) time (P<0.01), whilst high PDGF-C expression was associated with marginally, but not significantly, shorter RFS (P=0.10). On multivariate analysis, high PDGF-D expression was determined to be an independent prognostic factor (hazard ratio, 3.3; 95% confidence interval, 1.20–9.4; P=0.02). These findings indicate that high PDGF-D expression is strongly associated with tumor progression, recurrence, distant metastasis and poor outcomes in patients with gastric cancer. PDGF-D may therefore be an independent prognostic factor and a novel therapeutic target.
Keywords: gastric cancer, platelet-derived growth factor C, platelet-derived growth factor D, prognosis
Introduction
Gastric cancer is the fourth leading cause of cancer-related mortality worldwide. There are ~989,600 new cases and 738,000 mortalities per year, accounting for ~8% of new cancer cases (1). Complete surgical resection is the only potentially curative treatment for localized gastric cancer. However, clinical outcomes of patients with advanced gastric cancer remain poor. The majority of patients with advanced gastric cancer experience recurrence or metastasis despite curative resection (2) and, even with intensive chemotherapy, the median survival time of patients with recurrent or metastatic disease is ≤13 months (3).
Receptor tyrosine kinases, including human epidermal growth factor receptor (HER) and vascular endothelial growth factor, are important in cancer progression and are associated with survival in patients with gastric cancer (4–11). A number of anticancer drugs designed to inhibit signaling pathways of tyrosine kinases have been evaluated in patients with unresectable or metastatic gastric cancer (12–14); however, only trastuzumab (anti-HER2) has been demonstrated to be effective (15). Furthermore, only 23–24% of cases of gastric cancer exhibit overexpression of HER2, the target of trastuzumab (16,17). Thus, an improved understanding of the molecular pathogenesis involved in tumor progression and survival is necessary to establish more effective therapeutic targets and to improve outcomes in patients with gastric cancer.
Platelet-derived growth factors (PDGFs) are receptor tyrosine kinases that regulate diverse cellular functions, including cell proliferation, transformation, migration and embryonic development (18). PDGFs consist of four different polypeptide chains (PDGF-A, −B, −C and −D) that are assembled into disulfide-bonded dimers via homodimerization of heterodimers in order to play their functional role. So far, four homodimers (PDGF-AA, −BB, −CC and −DD) and one heterodimer (PDGF-AB) have been described (19). PDGF isoforms exert their biological functions by activating two structurally related receptor tyrosine kinases, PDGF receptors (PDGFRs) α and β. Upon binding of dimeric PDGF to PDGFR-α and −β, dimerization and activation of these receptors occurs. The receptors may combine to generate homo- or heterodimers, resulting in three possible combinations: PDGFR-αα, PDGFR-ββ and PDGFR-αβ, which have different affinities for the four PDGFs. Activated PDGF-C is a high affinity ligand for PDGFR-α homodimers, but fails to bind to and activate PDGFR-β homodimers. By contrast, activated PDGF-D is a high affinity ligand for PDGFR-β homodimers, but fails to bind to and activate PDGFR-α homodimers (20). PDGF-C and PDGF-D are also expressed in a number of types of tumor and various tumor cell lines, and are associated with tumor progression and angiogenesis (21,22). PDGF-C overexpression is observed in glioblastoma, Ewing family sarcoma and lung carcinoma cell lines (22–24), whilst PDGF-D is frequently upregulated in prostate, lung, renal, ovarian, brain and pancreatic cancers (21,22,25–29). Although PDGF-D overexpression has been observed in gastric cancer tissues when compared with normal tissues (30), the distribution, frequency and prognostic value of PDGF-D and PDGF-C expression in gastric cancer have not been clarified.
The purpose of the current study was to evaluate the association between the expression of PDGF-C and PDGF-D, clinicopathological factors and outcomes in patients with gastric cancer.
Materials and methods
Patients
The study group comprised 204 patients with primary gastric adenocarcinomas who underwent curative gastrectomy (R0) between January 2003 and December 2007 at the Department of Esophagogastric Surgery, Tokyo Medical and Dental University Hospital (Tokyo, Japan). Patient characterisitcs are summarized in Table I. No patient received anticancer treatment prior to surgery. Each tumor was classified according to the tumor-node-metastasis classification criteria recommended by the Union for International Cancer Control (31). All patients were evaluated for recurrent disease by diagnostic imaging, including computed tomography, ultrasonography and endoscopy, every 3–6 months. The median follow-up time was 60 months (range, 5–111 months). Recurrent disease was diagnosed in 51 patients (25%). There were 48 mortalities (24%) due to metastatic gastric cancer, and 11 (5%) due to other diseases in the absence of recurrence. This study was approved by the Institutional Review Board of Tokyo Medical and Dental University. Written informed consent was obtained from all patients.
Table I.
Characteristics of the studied patients (n=204).
| Characteristic | Value |
|---|---|
| Age, years; median (range) | 64 (21–92) |
| Gender, n (%) | |
| Male | 156 (76) |
| Female | 48 (24) |
| Main location, n (%) | |
| Upper third of stomach | 43 (21) |
| Middle/lower third of stomach | 161 (79) |
| WHO pathological type, n (%) | |
| Differentiated | 104 (51) |
| Undifferentiated | 100 (49) |
| Depth of invasion, n (%) | |
| T1a | 12 (6) |
| T1b | 75 (37) |
| T2 | 30 (15) |
| T3 | 37 (18) |
| T4 | 50 (25) |
| Lymph node metastasis, n (%) | |
| Positive | 91 (45) |
| Negative | 113 (55) |
| TNM stage, n (%) | |
| IA | 73 (36) |
| IB | 33 (16) |
| IIA | 19 (9) |
| IIB | 17 (8) |
| IIIA | 19 (9) |
| IIIB | 21 (10) |
| IIIC | 22 (11) |
WHO, World Health Organization; TNM, tumor-node-metastasis.
Immunohistochemical staining of PDGF-C and PDGF-D
Immunohistochemical staining was conducted using the Simple Stain MAX PO method with a Histofine Simple Stain MAX PO (MULTI) (Nichirei Biosciences, Inc., Tokyo, Japan). The goat polyclonal IgG antibody against human PDGF-C (#sc-18228) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and the rabbit polyclonal antibody against human PDGF-D (#PAB4843) was purchased from Abnova (Taipei, Taiwan). All available hematoxylin and eosin-stained slides of the surgical specimens were reviewed. For each case, representative paraffin blocks were selected for immunohistochemical studies. The 4 µm-thick sections were cut from formalin-fixed, paraffin-embedded tissue blocks. Following deparaffinization and rehydration in graded concentrations of ethanol, antigen retrieval treatment was performed at 98°C (microwave) for 15 min in a pH 9.0 retrieval solution (Nichirei Biosciences, Inc.) prior to treatment with 3% hydrogen peroxide (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 15 min to quench endogenous peroxidase activity. The slides were incubated with the primary antibodies against PDGF-C (dilution, 1:50) or PDGF-D (dilution, 1:50) overnight at 4°C. Sections were incubated with peroxidase-labeled anti-goat or anti-rabbit antibodies [Histofine Simple Stain MAX PO (G) or (MULTI); #414161 and #424152; Nichirei Biosciences, Inc.] for 30 min at room temperature. Peroxidase activity was detected with diaminobenzidine (Histofine Simple Stain DAB solution; Nichirei Biosciences, Inc.). The slides were counterstained with 1% Mayer's hematoxylin (Wako Pure Chemical Industries, Ltd.). Expression levels of PDGF-C and PDGF-D were evaluated based on cytoplasmic staining intensity and positive frequency, and were classified into two groups (high expression or low expression). Staining intensity was scored into four grades: 0 (none), 1 (weak positive), 2 (moderate positive) or 3 (strong positive). Staining extent (positive frequency) was scored into four grades: 0, <25%; 1, 25% to <50%; 2, 50% to <75%; or 3, ≥75%. Composite scores were derived by adding the intensity score to the extent score. For statistical analysis, composite scores of ≥4 were defined as high expression, and scores of <4 were considered low expression. Normal tissues from the same patients were used as controls. In negative controls, the antibodies were replaced by normal goat or rabbit IgG (Santa Cruz Biotechnology, Inc.). Colorectal cancer and hepatocellular carcinoma tissues, which exhibit high expression, served as positive controls.
Statistical analysis
The χ2 test was used to test possible associations between the expression of PDGF-C or PDGF-D and clinicopathological factors. It was also used to assess correlations between PDGF-C and PDGF-D expressions. Kaplan-Meier curves were plotted to assess the association between PDGF-C and PDGF-D expression and relapse-free survival (RFS). Survival curves were compared using the log-rank-test. P<0.05 was considered to indicate statistical significance. A multivariate Cox proportional hazards regression model was used to assess the prognostic significance of PDGF-C and PDGF-D expression and a number of clinicopathological factors. Statistical analysis was conducted using SPSS software, version 20 (IBM SPSS, Armonk, NY, USA).
Results
PDGF-C and PDGF-D immunostaining
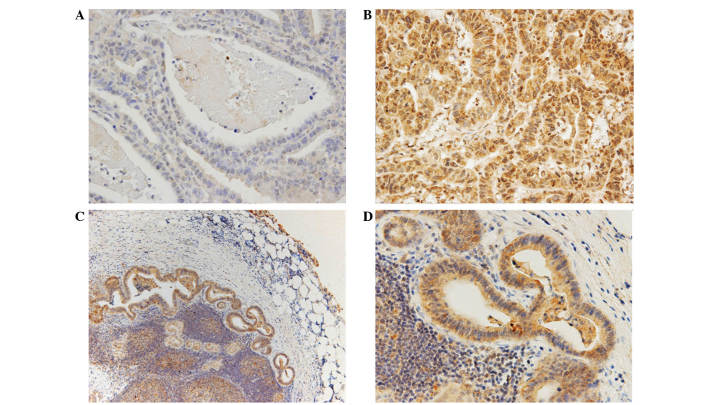
PDGF-C expression was predominantly located in the cytoplasm, with some in the nucleus of the tumor cells, whilst PDGF-D expression was observed only in the cytoplasm (Figs. 1 and 2). Adjacent non-malignant tissue exhibited weak or no staining of either protein. High expression of cytoplasmic PDGF-C and PDGF-D was detected in 114 (56%) and 151 (74%) samples, respectively. Expression of both PDGF-C and PDGF-D was observed in 98 (48%) tumors, while 37 (18%) tumors exhibited low expression of PDGF-C and PDGF-D. PDGF-C expression correlated with PDGF-D expression (P<0.01). The expression of PDGF-C and PDGF-D was also evaluated in 89 metastasis-positive lymph nodes; 81% of the samples were found to exhibit high expression of PDGF-C, and 87% displayed high expression of PDGF-D. These frequencies were higher than those observed in the primary tumors. However, there was no significant association between expression of either PDGF-C of PDGF-D in the primary tumor and that in metastatic lymph nodes (Table II).
Figure 1.
Representative immunostaining of gastric carcinomas for platelet-derived growth factor C: (A) none; (B) strong positive; (C,D) staining in metastatic lymph nodes (magnification, ×400).
Figure 2.
Representative immunostaining of gastric carcinomas for platelet-derived growth factor D: (A) none; (B) moderate positive; (C,D) staining in metastatic lymph nodes (magnification, ×400).
Table II.
Association between PDGF-C and PDGF-D expression, primary tumor and metastatic lymph nodes.
| Metastatic lymph nodes | |||
|---|---|---|---|
| Primary tumor | Low | High | P-value |
| PDGF-C | 0.88 | ||
| Low | 6 | 24 | |
| High | 11 | 48 | |
| PDGF-D | 0.92 | ||
| Low | 2 | 12 | |
| High | 10 | 65 | |
PDGF, platelet-derived growth factor.
Clinicopathological parameters and expression of PDGF-C and PDGF-D
High expression of PDGF-C and PDGF-D was observed more often in differentiated-type tumors than in undifferentiated-type tumors (P=0.05). High PDGF-C expression tended to be associated with distant metastasis and recurrence (P=0.07). High PDGF-D expression significantly correlated with gender, tumor depth, tumor stage and distant metastasis and recurrence (P=0.02, P=0.04, P=0.01 and P<0.01, respectively) and tended to be associated with lymph node metastasis (P=0.07) (Table III).
Table III.
Clinicopathological factors and expression of PDGF-C and PDGF-D.
| PDGF-C expression, n | PDGF-D expression, n | |||||
|---|---|---|---|---|---|---|
| Variables | Low (n=90) | High (n=114) | P-value | Low (n=53) | High (n=151) | P-value |
| Age, years | 0.9 | 0.19 | ||||
| <70 | 60 | 75 | 39 | 96 | ||
| ≥70 | 30 | 39 | 14 | 55 | ||
| Gender | 0.59 | 0.02 | ||||
| Male | 70 | 85 | 34 | 121 | ||
| Female | 20 | 29 | 19 | 30 | ||
| Histopathology | 0.05 | <0.01 | ||||
| Differentiated | 37 | 63 | 16 | 84 | ||
| Undifferentiated | 53 | 51 | 37 | 67 | ||
| Depth of invasion | 0.11 | 0.04 | ||||
| T1 | 44 | 43 | 29 | 58 | ||
| T2/T3/T4 | 46 | 71 | 24 | 93 | ||
| Lymph node metastasis | 0.14 | 0.07 | ||||
| N0 | 55 | 58 | 35 | 78 | ||
| N1/N2/N3 | 33 | 53 | 18 | 73 | ||
| Recurrence | 0.07 | <0.01 | ||||
| Absent | 73 | 80 | 49 | 104 | ||
| Present | 17 | 34 | 47 | |||
| TNM stage | 0.15 | 0.01 | ||||
| I | 58 | 62 | 39 | 81 | ||
| II/III | 32 | 52 | 14 | 70 | ||
PDGF, platelet-derived growth factor; TNM, tumor-node-metastasis.
Prognostic significance of PDGF-C and PDGF-D expression
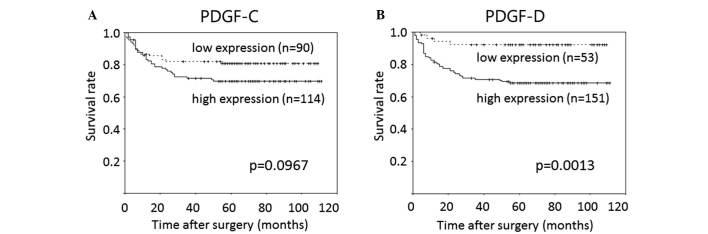
High PDGF-D expression was associated with significantly shorter RFS time relative to the low expression group (mean, 81 vs. 101 months; P<0.01), whilst high PDGF-C expression was associated with marginally, but not significantly, shorter RFS compared with the low expression group (mean, 82 vs. 90 months; P=0.10). The prognostic relevance of high PDGF-C and PDGF-D expression was assessed using a multivariate proportional hazards regression model adjusted for the established clinical prognostic factors (i.e., histopathology, tumor depth, lymph node metastasis). High PDGF-D expression was determined to be an independent prognostic factor [hazard ratio (HR), 3.6; 95% confidence interval (CI), 1.3–10.4; P=0.02], whereas PDGF-C was not (P=0.48). Histopathology (HR, 1.8; 95% CI, 1.0–3.3; P=0.05), tumor depth (HR, 9.5; 95% CI, 2.2–41.0; P<0.01) and lymph node metastasis (HR, 5.4; 95% CI, 2.2–13.1; P<0.01) were also independent prognostic factors (Table IV).
Table IV.
Prognostic factors according to a multivariate Cox proportional hazards regression model for relapse free survival.
| Variables | Patients, n | Univariate analysis P-value | Hazard ratio (95% confidence interval) | Multivariate analysis P-value |
|---|---|---|---|---|
| Age | 0.70 | |||
| <70 years | 135 | |||
| ≥70 years | 69 | |||
| Gender | 0.87 | |||
| Male | 155 | |||
| Female | 49 | |||
| Histopathology | <0.01 | 1.8 (1.0–3.3) | 0.05 | |
| Differentiated | 100 | |||
| Undifferentiated | 104 | |||
| Tumor depth | <0.01 | 9.5 (2.2–41.0) | <0.01 | |
| T1 | 87 | |||
| T2/T3/T4 | 117 | |||
| Lymph node metastasis | <0.01 | 5.4 (2.2–13.1) | <0.01 | |
| N0 | 113 | |||
| N1/N2/N3 | 91 | |||
| PDGF-C expression | 0.10 | 0.8 (0.4–1.5) | 0.48 | |
| Low | 90 | |||
| High | 114 | |||
| PDGF-D expression | <0.01 | 3.6 (1.3–10.4) | 0.02 | |
| Low | 53 | |||
| High | 151 |
PDGF, platelet-derived growth factor.
Discussion
The present study demonstrated that high PDGF-D expression was significantly associated with tumor depth, recurrence, distant metastasis and poor survival in patients with gastric cancer, whereas high PDGF-C expression tended to be associated (non-significantly) with distant metastasis, recurrence and shorter RFS.
PDGF-D is frequently upregulated in various types of cancer and plays an important role in tumor progression, angiogenesis and metastasis through multiple oncogenic pathways, including the phosphatidylinositol 3-kinase/Akt, nuclear factor-κB (NF-κB), extracellular signal-regulated kinase, mammalian target of rapamycin, mitogen-activated protein kinase and Notch pathways (12,25,26,29). Wang et al (26) demonstrated that PDGF-D was associated with cancer invasion and angiogenesis in pancreatic carcinomas via the regulation of Notch-1 and NF-κB signaling. Ustach et al (27) demonstrated that PDGF-D expression markedly accelerated tumor growth in prostate carcinoma cells, suggesting the potential oncogenic activity of PDGF-D. Xu et al (29) reported that overexpression of PDGF-D in renal cell carcinoma cells promoted tumor growth, angiogenesis and metastasis. These data suggest that PDGF-D overexpression may be associated with human cancer progression. Accordingly, the present results support the idea that high expression of PDGF-D in cancer may be important in tumor progression.
Furthermore, PDGF-D may also be associated with the epithelial-to-mesenchymal transition (EMT), an important process for tumor metastasis, via a number of signaling pathways, including Notch and NF-κB (32–34). Kong et al (32) reported that high expression of PDGF-D was significantly associated with the induction of EMT in prostate cancer cells.
As PDGF-D exerts oncogenic activity via the regulation of tumor cell growth, invasion and metastasis, PDGF-D signaling pathways are a potential therapeutic target for the treatment of human cancers. Notably, Kong et al (25) reported that blocking the expression and activation of PDGF-D in prostate cancer cells led to the inhibition of cell proliferation, invasion and angiogenesis. In addition, Zhao et al (35) reported that silencing PDGF-D using RNA interference significantly attenuated the proliferation and invasion of gastric cancer cells that overexpressed PDGF-D. Furthermore, Lokker et al (22) demonstrated that blocking PDGF-D/PDGFR signaling inhibited survival and mitogenic pathways in glioblastoma cell lines and prevented glioma formation in a nude mouse xenograft model. However, antagonizing PDGF-D via small-molecule inhibitors or neutralizing antibodies has not been evaluated in human cancer. The current results suggest that PDGF-D may be a therapeutic target for advanced or metastatic gastric cancer. Furthermore, PDGF-D overexpression was detected in 85% of advanced gastric cancers in the present study, indicating that antagonizing PDGF-D may be a useful therapeutic strategy.
PDGF-C is also associated with tumor growth, and a number of studies have demonstrated its role in tumor growth to date (22,36,37). Lokker et al (22) reported that PDGF-C autocrine signaling may play a role in the progression of brain tumors, such as glioblastoma and medulloblastoma. Anderberg et al (35) reported that that paracrine signaling of PDGF-C accelerated tumor growth through recruitment and activation of cancer-associated fibroblasts in malignant melanoma. These findings indicate that overexpression of PDGF-C accelerates tumor growth through autocrine and paracrine signaling. In fact, Yamauchi et al (37) reported that PDGF-C overexpression in colorectal cancer was associated with significantly poorer overall survival and RFS, and was an independent risk factor for recurrence. However, in the present study, PDGF-C overexpression in gastric cancer showed no significant correlation with tumor growth, distant metastasis and recurrence, in contrast to PDGF-D overexpression. This result indicates that the role of PDGF-C overexpression may be less important than that of PDGF-D in the progression of gastric cancer. However, further investigation of the molecular function of PDGF-C in gastric cancer is required.
PDGFR-β is a receptor for PDGF-C and PDGF-D. Guo et al (38) reported that PDGFR-β was overexpressed predominantly in tumor stromal cells and was positively correlated with tumor depth, lymph node metastasis and tumor stage in gastric cancer. Considering the results of the current study, it is possible that PDGF-D accelerates tumor growth through the activation of adjacent stromal cells; however, further studies are necessary to clarify this.
In conclusion, high PDGF-C and PDGF-D expression were associated with tumor progression and poor survival in patients with gastric cancer. In particular, PDGF-D was frequently expressed in gastric cancer and was associated with tumor progression and poor prognosis. PDGF-D signaling pathways may be a prognostic factor related to recurrence following curative surgery, and could serve as a novel target for the treatment of gastric cancer.
Figure 3.
(A) Kaplan-Meier curves for relapse-free survival of patients with expression of PDGF-C. (B) Kaplan-Meier curves for relapse-free survival of patients with expression of PDGF-D. PDGF, platelet-derived growth factor.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 4.Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M. ACTS-GC group: Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000. doi: 10.1158/1078-0432.CCR-12-1318. [DOI] [PubMed] [Google Scholar]
- 5.Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F, Galizia G. Expresison of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15:69–79. doi: 10.1245/s10434-007-9596-0. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M, Inokuchi M, Takagi Y, Yamada H, Kojima K, Kumagai J, Kawano T, Sugihara K. High expression of HER3 is associated with a decreased survival in gastric cancer. Clin Cancer Res. 2008;14:7843–7849. doi: 10.1158/1078-0432.CCR-08-1064. [DOI] [PubMed] [Google Scholar]
- 7.Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Jr, Soares FA. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030–3036. doi: 10.1200/JCO.2010.33.6313. [DOI] [PubMed] [Google Scholar]
- 8.Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: Prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738–746. doi: 10.1111/j.1365-2559.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 9.García I, Vizoso F, Martín A, Sanz L, Abdel-Lah O, Raigoso P, García-Muñiz JL. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol. 2003;10:234–241. doi: 10.1245/ASO.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Hirashima Y, Yamada Y, Matsubara J, Takahari D, Okita N, Takashima A, Kato K, Hamaguchi T, Shirao K, Shimada Y, et al. Impact of vascular endothelial growth factor receptor 1, 2 and 3 expression on the outcome of patients with gastric cancer. Cancer Sci. 2009;100:310–315. doi: 10.1111/j.1349-7006.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jüttner S, Wissmann C, Jöns T, Vieth M, Hertel J, Gretschel S, Schlag PM, Kemmner W, Höcker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: Two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 13.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et al. Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators: Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 15.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER-2 positive advanced gastric or gastro-oseophageal junction cancer (ToGA): A phase 3, open-label, randomized controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 16.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al. Amplification of HER-2 in gastric carcinoma: Association with topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 17.Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65–71. [PubMed] [Google Scholar]
- 18.Ustach CV, Taube ME, Hurst NJ, Jr, Bhagat S, Bonfil RD, Cher ML, Schuger L, Kim HR. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Res. 2004;64:1722–1729. doi: 10.1158/0008-5472.CAN-03-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, et al. PDGF-D, new protease-actovated growth factor. Nat Cell Biol. 2001;3:517–521. doi: 10.1038/35074593. [DOI] [PubMed] [Google Scholar]
- 20.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaRochelle WJ, Jeffers M, Corvalan JR, Jia XC, Feng X, Vanegas S, Vickroy JD, Yang XD, Chen F, Gazit G, et al. Platelet-derived growth factor D: Tumorigenicity in mice and dysregulated expression in human cancer. Cancer Res. 2002;62:2468–2473. [PubMed] [Google Scholar]
- 22.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002;62:3729–3735. [PubMed] [Google Scholar]
- 23.Zwerner JP, May WA. Dominant negative PDGF-C inhibits growth of ewing family tumor cell lines. Oncogene. 2002;21:3847–3854. doi: 10.1038/sj.onc.1205486. [DOI] [PubMed] [Google Scholar]
- 24.Tejada ML, Yu L, Dong J, Jung K, Meng G, Peale FV, Frantz GD, Hall L, Liang X, Gerber HP, Ferrara N. Tumor-driven paracrine platelet-derived growth factor receptor alpha signaling is a key determinant of stromal cell recruitment in a model of human lung carcinoma. Clin Cancer Res. 2006;12:2676–2688. doi: 10.1158/1078-0432.CCR-05-1770. [DOI] [PubMed] [Google Scholar]
- 25.Kong D, Banerjee S, Huang W, Li Y, Wang Z, Kim HR, Sarkar FH. Mammalian target of rapamycin repression by 3,3′-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68:1927–1934. doi: 10.1158/0008-5472.CAN-07-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Kong D, Banerjee S, Li Y, Adsay NV, Abbruzzese J, Sarkar FH. Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-kappaB signaling. Cancer Res. 2007;67:11377–11385. doi: 10.1158/0008-5472.CAN-07-2803. [DOI] [PubMed] [Google Scholar]
- 27.Ustach CV, Taube ME, Hurst NJ, Jr, Bhagat S, Bonfil RD, Cher ML, Schuger L, Kim HR. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Res. 2004;64:1722–1729. doi: 10.1158/0008-5472.CAN-03-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ustach CV, Kim HR. Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Mol Cell Biol. 2005;25:6279–6288. doi: 10.1128/MCB.25.14.6279-6288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Tong R, Cochran DM, Jain RK. Blocking platelet-derived growth factor-D/platelet-derived growth factor receptor beta signaling inhibits human renal cell carcinoma progression in an orthotopic mouse model. Cancer Res. 2005;65:5711–5719. doi: 10.1158/0008-5472.CAN-04-4313. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Shin J, Park KH, Jeung HC, Rha SY, Noh SH, Yang WI, Chung HC. Molecular basis of the differences between normal and tumor tissues of gastric cancer. Biochim Biophys Acta. 2007;1772:1033–1040. doi: 10.1016/j.bbadis.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell; 2009. [Google Scholar]
- 32.Kong D, Wang Z, Sarkar SH, Li Y, Banerjee S, Saliganan A, Kim HR, Cher ML, Sarkar FH. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425–1435. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Ali S, Banerjee S, Bao B, Li Y, Azmi AS, Korc M, Sarkar FH. Activated K-Ras and INK4a/Arf deficiency promote aggressiveness of pancreatic cancer by induction of EMT consistent with cancer stem cell phenotype. J Cell Physiol. 2013;228:556–562. doi: 10.1002/jcp.24162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Zhao L, Zhang C, Liao G, Long J. RNAi-mediated inhibition of PDGF-D leads to decreased cell growth, invasion and angiogenesis in the SGC-7901 gastric cancer xenograft model. Cancer Biol Ther. 2010;9:42–48. doi: 10.4161/cbt.9.1.10282. [DOI] [PubMed] [Google Scholar]
- 36.Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, Eriksson U, Pietras K. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009;69:369–378. doi: 10.1158/0008-5472.CAN-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi S, Iida S, Ishiguro M, Ishikawa T, Uetake H, Sugihara K. Clinical significance of platelet-derived growth factor-C expression in colorectal cancer. J Cancer Ther. 2014;5:11–20. doi: 10.4236/jct.2014.51002. [DOI] [Google Scholar]
- 38.Guo Y, Yin J, Zha L, Wang Z. Clinicopathological significance of platelet-derived growth factor B, platelet-derived growth factor receptor-β and E-cadherin expression in gastric carcinoma. Contemp Oncol (Pozn) 2013;17:150–155. doi: 10.5114/wo.2013.34618. [DOI] [PMC free article] [PubMed] [Google Scholar]