Abstract
The functions of different regulatory T cell (Treg) types in cancer progression are unclear. Recently, expression of the transcription factor Helios was proposed as a marker for natural (non-induced) Tregs. The present study investigated the clinical significance of Helios expression in patients with non-small cell lung cancer (NSCLC). We enrolled 64 patients with NSCLC, of whom 45 were treated surgically and 19 received chemotherapy because of advanced/recurrent disease. Their peripheral blood mononuclear cells were examined by flow cytometry. From the 45 surgery patients, we matched 9 patients with recurrent disease with 9 stage-matched patients without recurrence (n=18), compared their specimens immunohistochemically for tumor infiltrating lymphocytes (TILs) and analyzed these data against clinicopathological factors. Helios expression in Foxp3+ Tregs was 47.5±13.3% in peripheral blood and 18.1±13.4% in tumor specimens. Percentage of Helios− Tregs among CD4+ T cells were significantly higher in the cancer patients (2.4%), especially those with stage IA disease (2.6%) than in healthy donors (1.5%; P<0.001). Patients with low levels of Helios expression in Tregs among their TILs had significantly poorer survival (P=0.038). Helios− Tregs may affect immune suppression, even in early stage NSCLC; they could also be a useful prognostic biomarker in patients with NSCLC, and possibly a novel cancer immunotherapy target.
Keywords: regulatory T cells, Helios, non-small cell lung cancer, Ikaros gene family, Foxp3
Introduction
Lung cancer is the leading cause of cancer death worldwide (1). Although some molecular-targeted drugs provide longer survival time than cytotoxic chemotherapeutic agents (2–6), their efficacy is limited. Earlier cancer immunotherapies such as several types of vaccines have failed to show clinical effectiveness because the mechanism of immunosuppression has not been fully understood (7). Immune checkpoint inhibitors are widely studied in cancer immunotherapy. These agents, including cytotoxic T lymphocyte-associated antigen (CTLA)-4, PD-1 and PD-L1 inhibitors show 10.2–24% overall response rates in lung cancer patients in early-phase clinical trials (8–12), several agents that target other checkpoint proteins are now in clinical trial pipeline (13). Control of immunosuppression will be an important aspect of effective cancer immunotherapy.
Regulatory T cells (Tregs) have been widely studied in the context of immunosuppression (14). Tregs are characterized by expression of transcription factor Foxp3 (15–17) and are important in the cancer immunosuppressive mechanism. We also reported that Treg levels significantly increase in patients with non-small cell lung cancer (NSCLC), and high peripheral Treg levels correlate with postoperative disease recurrence (18). However, how Tregs expand in cancer and how they influence tumor immunology is unclear.
The Tregs are a heterogeneous population and consist of at least two subsets: natural Tregs (nTregs) and induced or adaptive Tregs (iTregs) (19). The nTregs originate in the thymus and are thought to recognize self-antigens (20). iTregs develop from conventional naïve T cell precursors at extra-thymic sites by exposure to TGF-β and retinoic acid (21). Because these two Treg types are currently indistinguishable, their relative contributions in tumor immunology are unclear. However, expression of the transcription factor Helios, a member of the Ikaros gene family, was recently proposed as a marker for nTreg cells (22). Thereafter, several reports showed iTregs also express Helios in vitro and in vivo, which suggests that, rather than a definite marker of nTregs, Helios could be a marker of T cell activation (23–26). Furthermore, Helios− Tregs reportedly display greater suppressive capacity than do Helios+ Tregs (27). Thus, the significance of Helios expression in Tregs is controversial, and its clinical impact in patients with cancer is totally unclear. The percentage of Helios+ Tregs was reported as significantly higher in peripheral blood of patients with renal cell carcinoma (RCC) than in healthy donors (HDs) (28). On the other hand, in patients with premalignant respiratory papilloma (PRP), the percentage of Helios+ Tregs was significantly reduced among tumor infiltrating lymphocytes (TILs) compared with blood (29). However, to the best of our knowledge, this is the first study of Helios+ or Helios− Tregs in patients with lung cancer. In trying to understand the clinical influence of Helios expression in patients with NSCLC in the context of other investigations, we found that Helios− Tregs were increased among peripheral Tregs in patients with NSCLC, which implies that Helios− Tregs in the tumor microenvironment might be clinically important in tumor progression.
Materials and methods
Patients
We enrolled 64 patients with non-small cell lung cancer (NSCLC) who were treated at Fukushima Medical University Hospital in 2008, including 45 who were treated by surgery and 19 who were treated by chemotherapy because of advanced or recurrent disease (Table I). Disease staging was evaluated according to the International Staging System for Lung Tumors, 7th edition (30).
Table I.
The baseline demographics of the patients.
| Characteristics | N=64 (%) |
|---|---|
| Age (years) | |
| Mean ± SD | 67±8 |
| Gender | |
| Female | 27 (42) |
| Male | 37 (58) |
| Pathological stage | |
| IA | 22 (34) |
| IB | 16 (25) |
| IIA | 4 (6) |
| IIB | 3 (5) |
| III | 0 (0) |
| IV (recurrence included) | |
| Chemotherapy | |
| 1st line | 6 (9) |
| 2nd line | 6 (9) |
| 3rd or later line | 7 (11) |
| Pathological classification | |
| Adenocarcinoma | 51 (80) |
| Squamous cell carcinoma | 10 (16) |
| Others | 3 (5) |
| EGFR mutation | |
| Positive | 22 (34) |
| Negative | 31 (48) |
| Unknown | 11 (17) |
EGFR, epidermal growth factor receptor.
Patient specimens
Peripheral blood mononuclear cells (PBMCs)
Peripheral blood samples were withdrawn prior to treatment by surgery or chemotherapy. Samples were also taken from 10 HDs. PBMCs were isolated from peripheral venous blood (20–30 ml) using Ficoll-Paque density gradient centrifugation, and then cryopreserved at −80ºC. The PBMCs were thawed for flow cytometry, washed in AIM-V medium (Invitrogen, Carlsbad, CA, USA), counted in the presence of trypan blue dye to evaluate viability and used immediately.
Tumor tissues
Tumor tissues were obtained from patients who i) were suffering from primary NSCLC with confirmed stage (T1-T3, pN0-pN2 and pM0); ii) underwent curative surgery but did not receive any preoperative treatment; and iii) had available clinical follow-up data. All patients had at least 5-year follow-up information for the present study. As 9 patients had recurrent diseases, we selected 9 other recurrence-free patients with matched pathological stages (i.e., 18 patients out of the 45 treated surgically) to examine their immunohistochemical differences in Helios expression in the tumor microenvironment.
Flow cytometry
Cell-surface and intracellular staining procedures were performed as previously described (31). Surfaces of 100 μl of cells (1×106) were stained using 10 μl fluorescein isothiocyanate-conjugated anti-CD25 and peridinin chlorophyll-conjugated anti-CD4 (eBiosciences, San Jose, CA, USA). Isotype control, mouse IgG1, was included in all the experiments. For intracellular staining, cells were saponized, washed in cold flow cytometry staining buffer and stained with phycoerythrin-conjugated anti-human Foxp3, its isotype control rat IgG2a (eBiosciences), Adenomatous polyposis coli anti-mouse/human Helios, and its isotype control Armenian Hamster IgG (BioLegend, Inc., San Diego, CA, USA). Flow cytometry was performed using FACSCanto II (BD Biosciences). Acquisition and analysis gates were restricted to the lymphocyte gate, as determined by their characteristic forward and side scatter properties. Flow data were analyzed using FlowJo software, version 7.6.5 (FlowJo, LLC, Ashland, OR, USA).
Immunohisitochemistry
We cut 3-μm microtome sections from paraffin-embedded lung cancer specimens and performed immunoperoxidase staining by the avidin-biotin-peroxidase complex method. The sections were dewaxed in xylene and dehydrated through an alcohol gradient. Endogenous peroxidase activity was quenched by 20-min incubation with 0.3% (v/v) solution of hydrogen peroxidase (Wako Pure Chemical Industries Ltd., Osaka, Japan) in 100% methanol. Following incubation in 5% dried skimmed milk in phosphate-buffered saline (PBS) for 30 min at room temperature, the sections were incubated overnight at 4ºC with primary monoclonal antibody to Helios (1:50; GTX115629; GeneTex, Inc., Irvine, CA, USA), to Foxp3 protein (1:100; ab20034; Abcam Inc., Tokyo, Japan), CD4 (1:50; NCL-CD4-1F6; Leica Microsystems, Wetzlar, Germany). The primary antibody was then detected using biotinylated secondary anti-rabbit IgG antibody (E0431; Dako, Glostrup, Denmark), or anti-mouse IgG antibody (BA-2000; Vector Laboratories, Burlingame, CA, USA) by the avidin-biotin complex method. The sections were washed several times in PBS after each step and counterstained with Mayer's hematoxylin (Muto Pure Chemicals, Co., Ltd., Tokyo, Japan), dehydrated through an alcohol gradient and mounted on glass slides.
For each specimen, we took micrographs of 10 randomly selected fields with a microscope (IX73; Olympus, Co., Tokyo, Japan), a CCD camera (DP73; Olympus), and counted the positively-stained lymphocytes at high-power fields (HPF, ×400). We made sure to select the same field for each stain (CD4, Foxp3 and Helios).
Statistical analysis
In peripheral Tregs, first we found percentages of Foxp3+ Helios+ cells, Foxp3+ Helios− cells in CD4+ T cells, and Helios+ and Helios− cells among CD4+ Foxp3+ cells. In Treg TILs, we counted the number of CD4+ T cells immunohistochemically. Then we counted Foxp3+ cells in CD4+ T cells, Helios+ and Helios− cells in CD4+ Foxp3+ cells, and analyzed associations between Helios expressions and clinicopathological factors, which were evaluated using Pearson's χ2 test. Differences between groups were evaluated for statistical significance using the Student's t-test. Survival curves were drawn according to the Kaplan-Meier method. We compared recurrence-free survival (RFS) and overall survival (OS) between groups of patients who expressed high and low Helios levels among their CD4+ Foxp3+ cells by log-rank test. All analyses were performed using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered to be statistically significant.
Ethics statement
The present study was approved by the Ethics Committee of the Fukushima Medical University (no. 2075). Written informed consent was also obtained from all the patients.
Results
Helios expression in peripheral Treg
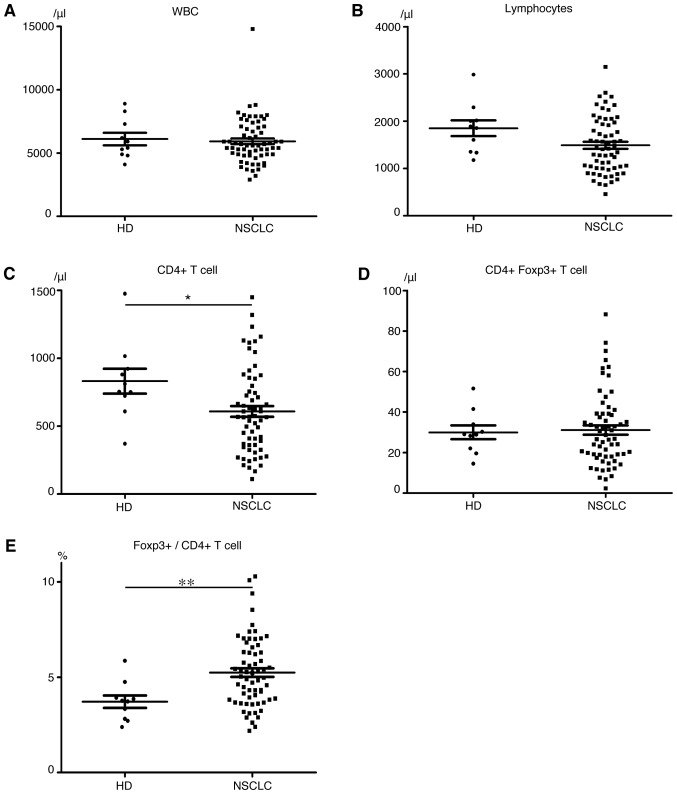
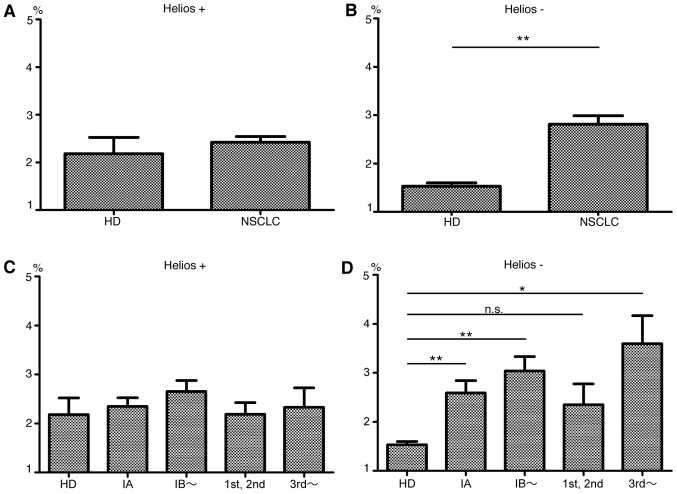
We first compared the expression of Foxp3 and Helios among CD4+ T cells in peripheral blood of 64 patients with NSCLC and 10 HDs. A representative flow cytometry plot of CD4, Foxp3 and Helios expression is shown in Fig. 1. The HDs and NSCLC patients had approximately equal numbers of white blood cells (Fig. 2A) and lymphocytes (Fig. 2B), but the cancer patients had lower CD4+ T cell levels (P=0.04, Fig. 2C). Although levels of CD4+ Foxp3+ T cells (Fig. 2D) did not significantly differ between patients and HDs, the percentage of Foxp3+ cells among CD4+ T cells was significantly higher in patients (HDs, 3.72%; patients, 5.24%; P=0.001, Fig. 2E). Helios expression in Foxp3+ cells was 47.5±13.3% in patients and 55.9±12.3% in HDs (P=0.07). Among CD4+ Foxp3+ T cells, patients had significantly higher Helios− subpopulation levels (P<0.0001) but Helios+ cell levels did not significantly differ between HDs and patients (Fig. 3A and B). These results indicate that expanded Treg levels in NSCLC patients are mainly Helios− cells. Notably, even patients with early-stage (stage IA) disease had significantly higher percentages of Helios− Treg among their CD4+ T cells than did HDs (HDs, 1.5%; all NSCLC patients, 2.4%; stage IA patients, 2.6%; P=0.0005). The Helios− subpopulation, but not the Helios+ subpopulation, tended to increase with cancer progression (Fig. 3C and D). We divided patients in high and low Helios-expressing groups by median Helios expression among peripheral CD4+ Foxp3+ T cells (50.3%), and compared clinicopathological features, but found no significant differences (Table II).
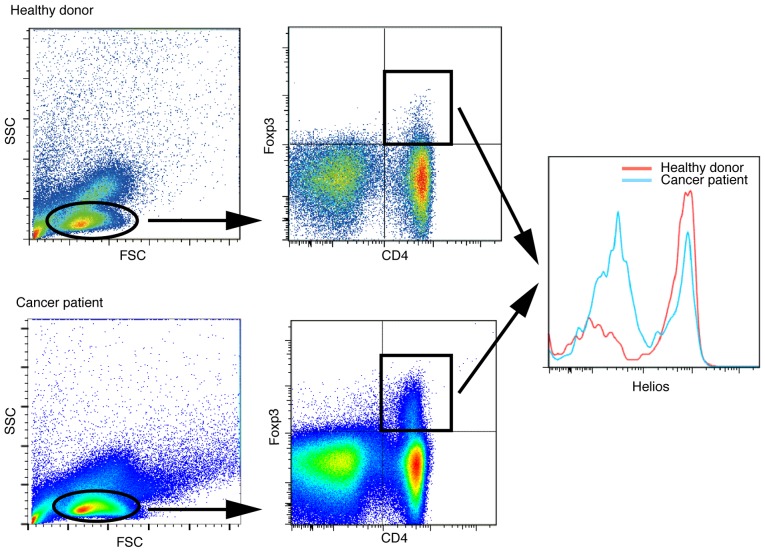
Figure 1.
Representative flow cytometry plots of healthy donors and NSCLC patients. NSCLC patients had more Helios− cells among their CD4+ Foxp3+ T cells than did healthy donors.
Figure 2.
Peripheral blood data. Numbers of (A) white blood cells, (B) lymphocytes, (C) CD4+ T cells, (D) CD4+ Foxp3+ T cells (/mm3). (E) Rates of Foxp3+ cells among CD4+ T cells (%). Mean ± SEM. HD, healthy donors; NSCLC, non-small cell lung cancer patients; *P<0.05; **P<0.01.
Figure 3.
Rates of Helios+ Foxp3+, Helios− Foxp3+ cells among CD4+ T cells of HD and NSCLC. Data of HD and NSCLC patients in panels (A and B) were divided into four groups: preoperative stage IA; preoperative stage ≥IB; 1st or 2nd line chemotherapy; and ≥3rd line or more chemotherapy for recurrence in panels (C and D), respectively. Mean ± SEM. HD, healthy donors; NSCLC, non-small cell lung cancer patients; *P<0.05; **P<0.01.
Table II.
Characteristics of patients with NSCLC by levels of Helios expression in their regulatory T cells in PBMCs (N=64) and tumor sites (N=18).
| PBMC Helios expression | TIL Helios expression | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristics | High n=32 (50%) |
Low n=32 (50%) |
P-value high vs. low |
High n=9 (50%) |
Low n=9 (50%) |
P-value high vs. low |
| Age (years) | ||||||
| <65 | 10 (31) | 14 (44) | 0.3 | 4 (44) | 1 (11) | 0.1 |
| ≥65 | 22 (69) | 18 (56) | 5 (56) | 8 (89) | ||
| Gender | ||||||
| Female | 14 (44) | 13 (41) | 0.8 | 6 (67) | 4 (44) | 0.3 |
| Male | 18 (56) | 19 (59) | 3 (33) | 5 (56) | ||
| Pathological stage | ||||||
| I | 18 (56) | 20 (63) | 0.5 | 9 (100) | 4 (44) | 0.02 |
| II | 2 (6) | 2 (6) | 0 (0) | 2 (11) | ||
| III | 3 (9) | 0 (0) | 0 (0) | 3 (17) | ||
| IV (recurrence included) | 9 (28) | 10 (31) | 0 (0) | 0 (0) | ||
| Pathology | ||||||
| Adenocarcinoma | 26 (81) | 25 (78) | 0.8 | 7 (78) | 9 (100) | 0.2 |
| Squamous cell carcinoma | 5 (16) | 5 (16) | 2 (22) | 0 (0) | ||
| Others | 1 (3) | 2 (6) | 0 (0) | 0 (0) | ||
| EGFR mutation | ||||||
| Positive | 15 (47) | 7 (22) | 0.1 | 4 (44) | 5 (56) | 0.3 |
| Negative | 13 (41) | 18 (56) | 3 (33) | 4 (44) | ||
| Unknown | 4 (13) | 7 (22) | 2 (22) | 0 (0) | ||
PBMC, peripheral blood mononuclear cell; TIL, tumor infiltrating lymphocyte; EGFR, epidermal growth factor receptor.
Helios expression in Treg TILs
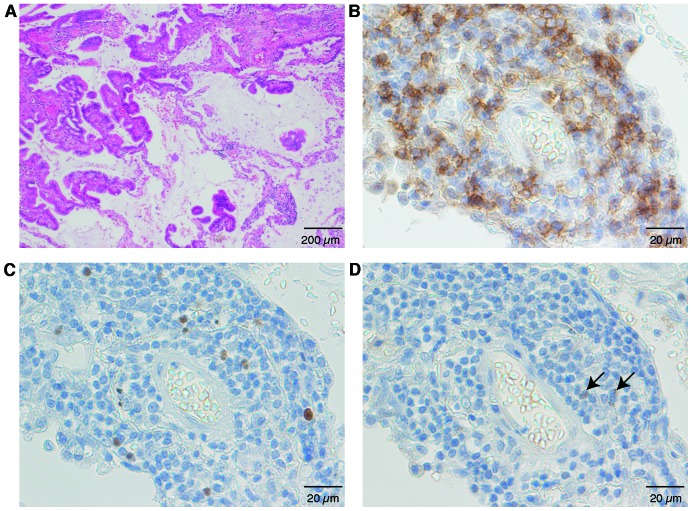
Next we evaluated Tregs in the tumor microenvironment, and found Foxp3+ among CD4+ T cells 27.1±12.3% (mean ± SD); Helios+ among Foxp3+ T cells: 18.1±13.4%; and Helios+ Foxp3+ among CD4+ T cells: 5.3±5.2% (Fig. 4). CD4+ T cells existed in clusters (Fig. 4A and B), with Foxp3+ cells scattered around the CD4+ T cells (Fig. 4C). Few Helios+ cells were at the same site (Fig. 4D), which indicated that most CD4+ Foxp3+ cells at the tumor sites were Helios−. Thus, the Helios+ Tregs percentage was low among both TIL Tregs and peripheral Tregs. We saw a weak but not significant relationship between Helios expression in peripheral-blood Tregs and that in Treg TILs (Fig. 5). Clinicopathological parameters were analyzed against TILs (Table II). When the patients were divided by median Helios+ percentage in their TIL CD4+ Foxp3+ T cells (16.3%) into high- and low-Helios expressing groups, the low Helios expressing group had significantly more advanced-stage disease (P=0.02).
Figure 4.
Representative micrographs of tumor tissue. Stained by (A) H&E; (B) anti-CD4 antibody; (C) anti-Foxp3 antibody; and (D) anti-Helios antibody in the same section of tumor tissue. Black arrow, positive cells (D).
Figure 5.
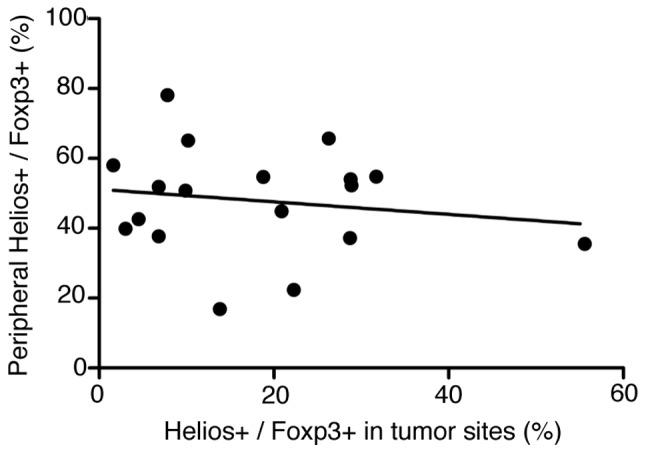
The relationship between Helios expression in peripheral blood Tregs and that in tumor site. They were weakly correlated. R2=0.03.
Survival analysis according to Helios expression in Treg
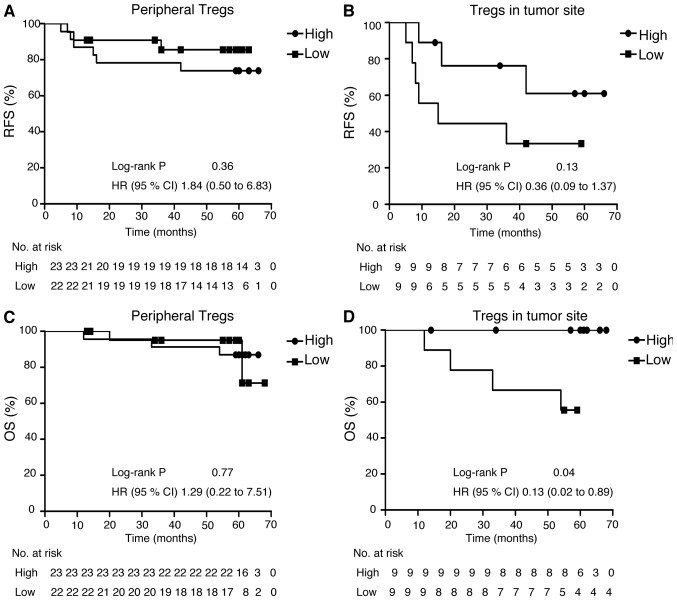
We further analyzed survival outcomes for patients with NSCLC by their Helios expression in Treg among TILs. For the 45 patients who provided PBMCs, median follow-up was 1666 days; relapse occurred in 9 patients and 5 patients died during follow-up. For the 18 patients who provided the specimens for analysis of Treg TILs, the median follow-up was 1525 days; this group included the same patients who relapsed and who died in the former group. The patients who provided tumor specimens did not significantly differ in RFS or OS from those who provided only PBMCs. Patients with lower Helios expression among their Treg TILs had significantly poorer OS (P=0.038), but not significantly poorer RFS (Fig. 6).
Figure 6.
Survival curve of patients who expressed high or low levels of Helios in CD4+ Foxp3+ T cells. (A) Recurrence-free survival (RFS) and (B) overall survival (OS) by peripheral CD4+ Foxp3+ T cells. (C) RFS and (D) OS by CD4+ Foxp3+ T cells in tumor sites.
Discussion
To date, very few reports have addressed Helios expression in peripheral CD4+ Foxp3+ T cells. According to these previous reports, the percentage of Helios+ Tregs in patients with RCC was ~60% (28) and in PRP was 66.1±6.5% (29). In the present study, we show that Helios expression among CD4+ Foxp3+ cells was 47.5±13.3% in patients with NSCLC, lower than previous data but almost compatible. The percentage of Helios+ among CD4+ Foxp3+ T cells was significantly lower in these patients than those of HDs (NSCLC, 47.5%; HD, 55.9%). This is the first report of Helios expression status in Tregs from patients with NSCLC; it was significantly decreased in patients with NSCLC than in HDs. Interestingly, even higher percent-ages of Helios− Tregs were seen in advanced-stage NSCLC. These results show expanded Tregs in patients with NSCLC to be Helios− cells, which implies that Helios− Tregs mediate immunosuppression in NSCLC. These results are inconsistent with the earlier reports on RCC and PRP, and suggest that the role of these cells in immunosuppression may vary between the type of tumor. However, these data are still limited; further studies are needed to understand these findings.
Among TILs, Helios expression in Foxp3+ cells was 18.1±13.4% in patients with NSCLC in this series. Helios− Tregs in tumor sites were associated with patient prognoses. Wainwright et al reported that Helios was expressed by almost 90% of Tregs in glioblastoma (32), but in only 31.2±18.51% of Tregs in PRP (29). These data also imply that the immunosuppressive status in the tumor microenvironment may depend on the type of tumor. Our findings indicate that Helios− Tregs have an essential role in immunosuppression, at least in NSCLC. According to the previous study showing that Helios could be a biomarker for nTregs (22), the present study suggests that iTregs were mainly increased in patients with NSCLC patients and might be induced in tumor microenvironment.
However, recent reports suggest that nTregs from iTregs are not distinguished only by Helios expression (25). Other molecular markers are now under study. Several promising molecules are epigenetic Treg-specific demethylated region (TSDR) modifications and Neuropilin 1. Reportedly, epigenetic modifications in the TSDR of the Foxp3 locus affect the stability of Foxp3 (33), and are thought to differ between nTregs from iTregs cells (34). Neuropilin 1 is a receptor for vascular endothelial growth factor and semaphorin family proteins (35), and is reported to be a possible marker for nTregs (36,37). In spite of these studies, a definitive marker for nTregs has not been found so far (38).
Our findings suggest that Helios− Tregs could have an important function in NSCLC. We must next elucidate the function of Helios− Tregs in immunosuppression to verify their role in cancer immune response. For such a study, we need to obtain a definitive cell-surface marker for Helios− Tregs, as Helios is a transcription factor. To solve this problem, Neuropilin 1 may be useful as mentioned above (35). Other cell-surface markers have been reported. Zabransky et al reported CD103 and glucocorticoid-induced tumor necrosis receptor (GITR) (25), and Raffin et al described IL-1RI and CCR7 (27). Currently, we are trying to separate Helios− Tregs from other CD4+ T cells using IL-1RI and CCR7, which gave 70–80% specificity (data not shown). Further studies are needed to find a definitive cell-surface marker and establish the effects of Helios− Tregs on immunosuppression.
Successful blockades of immune checkpoints suggest that immune escape mechanisms clearly contribute to lung cancer progression, and also could be targets for lung cancer treatment. To date, attempts have been made to suppress Tregs function by using anti-CD25 antibody (39–41), and by inhibiting the CTLA-4 pathway (12,42), or GITR family-related proteins (43). These trials did not work well. According to the present study, Helios− Tregs could be both a useful prognostic biomarker in NSCLC patients and a therapeutic target. If we can ascertain the mechanism of immunosuppression, we may be able to establish more powerful immunotherapy. For instance, peptide vaccine has been one of the main streams of immunotherapy. Although peptide vaccine therapy for NSCLC could not achieve a survival benefit in a late-phase studies (44), other trials, such as our multiple peptide vaccine therapy for NSCLC, have been expected to induce strong specific T cell responses (45). Combination therapy of blockades of immunosuppressive pathway, including immune checkpoints and/or Tregs that target Helios− Tregs and these peptide vaccines could be a next-generation immunotherapy.
In summary, in the present study we demonstrated the clinical impact of both local and systemic Helios− Treg cells. Systemic Helios− Tregs correlate with advanced cancer stage, and those in tumor sites are associated with poor patient prognosis. Thus, analyzing Helios expression levels in Tregs could be a useful marker for disease progression and prognosis in patients with NSCLC. Furthermore, it also could be a novel therapeutic target for various cancers. Further study is needed to understand the function of Helios− Tregs in immune suppression, and to develop therapeutic modality targeting cancer-specific Tregs.
Acknowledgements
We thank E. Ohtomo, Y. Kikuta and Y. Yuda for their excellent technical assistance.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et al. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 5.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al. PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou SH, Butaney M, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: A retrospective analysis. Lancet Oncol. 2011;12:1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garon E, Balmanoukian A, Hamid O, Hui R, Gandhi L, Leighi N. Preliminary clinical safety and activity of MK-3475 monotherapy for the treatment of previously treated patients with non-small cell lung cancer. IASLC 15th World Conference on Lung Cancer; 27–31 October; Sydney, Australia. 2013. [Google Scholar]
- 10.Herbst RS, Gordon MS, Fine GD. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol. 2013;31:3000. [Google Scholar]
- 11.Horn L, Herbst R, Spiegel D. An analysis of the relationship of clinical activity to baseline EGFR status PD-L1 expression and prior treatment history in patients with non-small cell lung cancer (NSCLC) following PD-L1 blockade with MPDL3280A (anti-PDL1). IASLC 14th World Conference on Lung Cancer; July 3–7; Amsterdam, The Netherlands. 2012. [Google Scholar]
- 12.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 13.Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control. 2014;21:80–89. doi: 10.1177/107327481402100112. [DOI] [PubMed] [Google Scholar]
- 14.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: A critical review of the literature. Clin Cancer Res. 2012;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 16.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 17.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa T, Suzuki H, Yamaura T, Muto S, Okabe N, Osugi J, Hoshino M, Higuchi M, Ise K, Gotoh M. Prognostic value of peripheral and local forkhead box P3+ regulatory T cells in patients with non-small-cell lung cancer. Mol Clin Oncol. 2014;2:685–694. doi: 10.3892/mco.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: More of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 21.Bilate AM, Lafaille JJ. Induced CD4 Foxp3 regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 22.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188:976–980. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 25.Zabransky DJ, Nirschl CJ, Durham NM, Park BV, Ceccato CM, Bruno TC, Tam AJ, Getnet D, Drake CG. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS One. 2012;7:e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, Durham NM, Hipkiss EL, Pyle KJ, Wada S. A role for the transcription factor Helios in human CD4+CD25+ regulatory T cells. Mol Immunol. 2010;47:1595–1600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffin C, Pignon P, Celse C, Debien E, Valmori D, Ayyoub M. Human memory Helios− FOXP3+ regulatory T cells (Tregs) encompass induced Tregs that express Aiolos and respond to IL-1β by downregulating their suppressor functions. J Immunol. 2013;191:4619–4627. doi: 10.4049/jimmunol.1301378. [DOI] [PubMed] [Google Scholar]
- 28.Elkord E, Sharma S, Burt DJ, Hawkins RE. Expanded subpopulation of FoxP3+ T regulatory cells in renal cell carcinoma co-express Helios, indicating they could be derived from natural but not induced Tregs. Clin Immunol. 2011;140:218–222. doi: 10.1016/j.clim.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Hatam LJ, Devoti JA, Rosenthal DW, Lam F, Abramson AL, Steinberg BM, Bonagura VR. Immune suppression in premalignant respiratory papillomas: Enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression. Clin Cancer Res. 2012;18:1925–1935. doi: 10.1158/1078-0432.CCR-11-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 31.Roncador G, Brown PJ, Maestre L, Hue S, Martínez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 32.Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011;13:1308–1323. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polansky JK, Schreiber L, Thelemann C, Ludwig L, Krüger M, Baumgrass R, Cording S, Floess S, Hamann A, Huehn J. Methylation matters: Binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med Berl. 2010;88:1029–1040. doi: 10.1007/s00109-010-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ, Shevach EM. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119:2810–2818. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossignol M, Pouysségur J, Klagsbrun M. Characterization of the neuropilin-1 promoter; gene expression is mediated by the transcription factor Sp1. J Cell Biochem. 2003;88:744–757. doi: 10.1002/jcb.10384. [DOI] [PubMed] [Google Scholar]
- 36.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. s1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3 T reg cells. J Exp Med. 2012;209:1723–1742. s1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, Zheng SG. Advances in distinguishing natural from induced Foxp3+ regulatory T cells. Int J Clin Exp Pathol. 2013;6:116–123. [PMC free article] [PubMed] [Google Scholar]
- 39.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 40.Gallimore A, Sakaguchi S. Regulation of tumour immunity by CD25+ T cells. Immunology. 2002;107:5–9. doi: 10.1046/j.1365-2567.2002.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.GlaxoSmithKline plc. Update on phase III clinical trial of investigational MAGE-A3 antigen-specific cancer immunotherapeutic in non-small cell lung cancer [Internet] 2014 Apr 02; [cited 2015 March 24] Available from http://www.gsk.com/en-gb/media/press-releases/2014/update-on-phase-iii-clinical-trial-of-investigational-mage-a3-antigen-specific-cancer-immunother-apeutic-in-non-small-cell-lung-cancer/
- 45.Suzuki H, Fukuhara M, Yamaura T, Mutoh S, Okabe N, Yaginuma H, Hasegawa T, Yonechi A, Osugi J, Hoshino M, et al. Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer. J Transl Med. 2013;11:97. doi: 10.1186/1479-5876-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]