Abstract
Our previous study demonstrated that an impaired sonic hedgehog (Shh) pathway contributed to cardiac dysfunction in type 1 diabetic mice with myocardial infarction (MI). The present study aimed to test the hypothesis that oxidative stress may contribute to the impaired Shh pathway and cardiac dysfunction in type 1 diabetic mice with MI. Streptozotocin-induced type 1 diabetic mice (C57/Bl6, male) and rat neonatal cardiomyocytes were used in the present study. Mice were randomly assigned to undergo ligation of the coronary artery or pseudosurgery. A potent antioxidant Tempol was administered in vivo and in vitro. Cardiac function was assessed by echocardiography, capillary density by immunohistochemisty, percentage of myocardial infarct using Massons trichrome staining, reactive oxygen species detection using dihydroethidium dye or 2,7-dichlorofluorescein diacetate probe and protein expression levels of the Shh pathway by western blot analysis. The antioxidant Tempol was shown to significantly increase myocardial protein expression levels of Shh and patched-1 (Ptc1) at 7–18 weeks and improved cardiac function at 18 weeks in type 1 diabetic mice, as compared with mice receiving no drug treatment. Furthermore, myocardial protein expression levels of Shh and Ptc1 were significantly upregulated on day 7 after MI, and capillary density was enhanced. In addition, the percentage area of myocardial infarct was reduced, and the cardiac dysfunction and survival rate were improved on day 21 in diabetic mice treated with Tempol. In vitro, treatment of rat neonatal cardiomyocytes with a mixture of xanthine oxidase and xanthine decreased protein expression levels of Shh and Ptc1 in a concentration-dependent manner, and Tempol attenuated this effect. These results indicate that oxidative stress may contribute to an impaired Shh pathway in type 1 diabetic mice, leading to diminished myocardial healing and cardiac dysfunction. Antioxidative strategies aimed at restoring the endogenous Shh pathway may offer a useful means for improving diabetic cardiac function.
Keywords: diabetes, myocardial infarction, sonic hedgehog pathway, oxidative stress
Introduction
The incidence of myocardial infarction (MI) is higher in patients with diabetes than that in patients without diabetes, and recovery is often slower, resulting in higher mortality rates (1). The etiology of diabetes-associated impaired myocardial healing and cardiac dysfunction is multifaceted (2–7) and remains to be completely elucidated.
It has previously been demonstrated that the sonic hedgehog (Shh) pathway is critical to the growth and repair of cardiovascular systems during embryonic and postnatal development and adult life (8). Shh signaling occurs via interaction of the Shh protein with its receptor, patched-1 (Ptc1), which terminates the inhibition of the smoothened receptor. This subsequently leads to activation of the transcription factor Gli, which induces the expression of downstream target genes, including Ptc1 itself (9–11).
Numerous studies have suggested that the Shh pathway is endogenously upregulated in MI, and Shh gene transfer following an MI resulted in the preservation of left ventricular function (12,13). Our previous study reported that the Shh pathway is impaired in diabetic mice following MI, which contributes to diminished myocardial healing and the exacerbation of cardiac dysfunction (14); however, the mechanisms underlying the impaired Shh pathway in diabetes remain unclear.
Oxidative stress is well documented in the diabetic state and leads to diabetic complications, particularly diabetic cardiovascular diseases. Reactive oxygen species (ROS) have been confirmed as the major source of oxidative stress (15). Previous studies have demonstrated that tissue and blood levels of ROS are higher in numerous human diseases, including atherosclerosis, chronic inflammation, neurodegenerative disease, diabetes and various types of cancer (16–20). In addition, it has been suggested that excessive ROS production may interfere with normal signaling pathways and play a key pathogenic role in disease (21).
The present study aimed to test the hypothesis that oxidative stress in type 1 diabetic mice contributes to the impaired Shh pathway that leads to exacerbated cardiac dysfunction, and that this may be rescued by protecting the Shh pathway using the antioxidant Tempol.
Materials and methods
Animals and experimental design
Adult male C57BL/6 mice (Guangdong Medical Laboratory Animal Center, Foshan, China), weighing ~20 g and aged 6–7 weeks, were used in the present study. The mice were handled in accordance with the principles of the Animal Management Rule of the Ministry of Health, China (acceptance no. 2011-62), and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85–23, revised, 1996). Mice were maintained at 21–23°C, with a humidity of 40–55% and a 12-h light cycle (lights on 06:00–18:00), with free access to food and water.
The mice were divided into six groups: i) Control (CON), ii) diabetes (DM), iii) diabetes plus Tempol (DM + Tempol), iv) control plus MI (CMI), v) diabetes plus MI (DMI), and vi) DM plus Tempol and MI (DTMI). The control mice were administrated drinking water, which is a solvent of Tempol in vivo.
The rat neonatal cardiomyocytes were divided into seven groups: i) Control (CON), ii) xanthine oxidase (XO, 1 U/l)/xanthine (X, 0.5 mM), iii) XO (1.5 U/l)/X (0.5 mM), iv) XO (2 U/l)/X (0.5 mM), v) XO (2 U/l)/X (0.5 mM) + Tempol (0.1 mM), vi) XO (2 U/l)/X (0.5 mM) + Tempol (0.5 mM), and vii) Tempol (0.5 mM). The control cells were treated with sterilized and purified water, which is the solvent of Tempol in vitro.
The superoxide scavenger Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy; 3 mM; Sigma-Aldrich, St. Louis, MO, USA) was administered in the drinking water at the onset of diabetes until the end of the experiment. Mice were sacrificed using sodium pentobarbital (100 mg/kg; Guangzhou Qihua Medical Equipment Co., Ltd., Guangzhou, China). The hearts of the mice were harvested at various time-points of diabetes (7, 10, 14 and 18 weeks), in order to analyze the protein expression levels of the Shh pathway and the oxidative stress levels. The surgery to induce MI was performed 7 weeks after the induction of diabetes. The heart tissue was harvested on day 7 after MI in order to detect the expression levels of the Shh pathway-associated proteins using western blot analysis. A total of 21 days after MI, echocardiography was performed to assess ventricular function, and immunohistochemical analysis was conducted to evaluate capillary density. In addition, Masson's trichrome staining was used to assess the percentage area of myocardial infarct.
Induction of type 1 diabetes
Adult male C57/BL6 mice (6–7 weeks of age) received an intraperitoneal (i.p.) injection of streptozotocin (STZ; Sigma-Aldrich) dissolved in sterile citrate buffer (0.05 mol/l sodium citrate, pH 4.5, 45 mg/kg). The mice were administered STZ or citrate buffer (control) for 5 days consecutively during the first week of the study. Blood samples were collected from the vena caudalis. Whole blood glucose levels were measured using a glucose analyzer [OneTouch® UltraMini® Blood Glucose Monitoring system; Johnson & Johnson Medical (China) Ltd., Shanghai, China]. Mice with a blood glucose level ≥16.7 mmol/l were considered diabetic and were used for subsequent MI experiments.
Induction of MI
Adult male C57/BL6 mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The mice were orally intubated with a 22G intravenous catheter (Guangzhou Qihua Medical Equipment Co., Ltd.) and subjected to artificial ventilation using a respirator (Tai Meng Technology Co., Ltd., Chengdu, China). A small, oblique thoracotomy was performed lateral to the left intercostal line in the third costal space, in order to expose the heart. Following the opening of the pericardium, the proximal left anterior descending artery branch of the left coronary artery was ligated using 8-0 polypropylene sutures (Guangzhou Qihua Medical Equipment Co., Ltd.) under a dissecting microscope (Carl Zeiss AG, Oberkochen, Germany). The sham-operated animals had an untied left anterior descending artery; with suture material in place but the ligature not tightened.
Echocardiography
Transthoracic echocardiography was performed using a VisualSonics system (Vevo® 2100; Fujifilm VisualSonics, Inc., Toronto, ON, Canada) equipped with a 25-MHz imaging transducer. The anesthesia of the mice was maintained with 2% isoflurane gas (RWD Life Science Co., Ltd., Shenzhen, China) with an inflow rate of 0.5–1.5 ml/min during the echocardiographic examination. The left ventricle (LV) was analyzed under parasternal long- and short-axis views, with Doppler images for LV systolic function, LV cavity diameter, wall thickness, diastolic function and LV end-systolic and end-diastolic volume determination.
Assessment of the percentage of myocardial infarct (Masson's trichrome staining)
The hearts of the mice were perfusion-fixed with 10% buffered formalin (Sigma-Aldrich), horizontally sectioned between the point of ligation and the apex and then embedded in paraffin. The paraffin sections were stained with Masson's trichrome staining solution (Beyotime Institute of Biotechnology, Shanghai, China). The percentage area of myocardial infarct in the Masson's trichrome-stained tissue sections was determined using Image Pro-Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). The levels of fibrosis and total LV area were measured, and the area of myocardial infarct was expressed as a final percentage.
Immunohistochemistry for the determination of capillary density
The hearts of the mice were horizontally sectioned between the point of ligation and the apex and embedded in optimal cutting temperature compound (OCT; Sakura Finetek Japan Co., Ltd., Tokyo, Japan). Briefly, for the assessment of capillary density, the OCT sections were stained with rat polyclonal anti-CD31/platelet endothelial cell adhesion molecule-1 antibody (1:100; 553708; BD Pharmingen, San Diego, CA, USA), followed by incubation with a secondary anti-rat antibody (1:5,000; sc-2006; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Sections were staining using a 3,3-diaminobenzidine kit (K3468; Dako, Glostrup, Denmark) according to the manufacturer's instructions. The antigen-antibody interaction was visualized using 3,3′-diaminobenzidine (DAB) substrate using a DAB Substrate kit (Dako). Counts of capillary density per square millimeter of the border zone of infarcted myocardium (MI group) or the free wall of the LV (Sham group) were conducted using Image Pro-Plus software (Media Cybernetics, Inc.).
ROS detection in the myocardium
At each time-point of diabetes, the mice were sacrificed and heart tissue sections were harvested and directly embedded in OCT, as stated previously. Superoxide production in the heart was detected using dihydroethidium (DHE) staining (Sigma-Aldrich). Frozen heart sections (10-µm) were incubated with 10 µM DHE for 45 min at 37°C in a humidified chamber protected from the light. The average fluorescence intensity of the nuclei was then analyzed using Image Pro-Plus software (Media Cybernetics, Inc.).
Cell culture
The primary culture of cardiomyocytes was performed according to previously described methods (22). Briefly, rats were sacrificed by cervical dislocation and sterilized with 75% alcohol. The chests of new-born rats were opened using ophthalmic scissors and the hearts were dissected with ophthalmic forceps. Hearts from the 1-day-old Sprague Dawley rats (Guangdong Medical Laboratory Animal Center) were dissected, minced and placed in a petri dish. The tissue was trypsinized at 37°C in D-Hank's Balanced Salt Solution (HBSS; Gibco Life Technologies, Carlsbad, CA, USA). Following centrifugation (1,200 × g, 10 min), the cells were collected and suspended in Dulbecco's modified Eagle's medium (Gibco Life Technologies), supplemented with 15% fetal bovine serum (Gibco Life Technologies), 100 U/ml penicillin (Sigma-Aldrich) and 100 µg/ml streptomycin (Sigma-Aldrich). The cells were then incubated at 37°C for 1 h. The cells were diluted to 5×106 cells/ml, plated in a 60-mm petri dish (Corning Incorporated, Corning, NY, USA) and cultured for 48 to 72 h in medium supplemented with 0.1 mmol/l bromodeoxyuridine (Sigma-Aldrich), in order to prevent the proliferation of non-myocytes. Subsequently, a series of chemicals (XO/X and the superoxide dismutase homolog Tempol), all purchased from Sigma-Aldrich, were used to treat the cells. The rat neonatal cardiomyocytes were treated with the mixture of XO/X for 4 or 24 h, and Tempol was administered at 1 h prior to XO/X.
ROS detection in rat neonatal cardiomyocytes
Intracellular ROS levels were assessed using the ROS-specific probe 2′,7′-dichlorofluorescein diacetate (DCF-DA; Molecular Probes Life Technologies, Carlsbad, CA, USA). On culture day 4, the cultured cardiomyocytes were washed with HBSS (Gibco Life Technologies) and then incubated with DCF-DA (5 µmol/l) in HBSS at 37°C. Following a 1-h incubation, the cardiomyocytes were further washed with HBSS. In each case, five randomly selected fields in each well were selected for examination. Data were collected using a fluorescence reader (Carl Zeiss AG) at excitation and emission wavelengths of 485 and 530 nm, respectively. The average fluorescence intensity was then analyzed using Image Pro-Plus 6.0 software (Media Cybernetics, Inc.).
Western blot analysis
Western blot analysis was used to detect the protein expression levels of Shh and Ptc1. Cells were washed with phosphate-buffered three times, then collected using sodium dodecyl sulfate (SDS) sample buffer containing 62.5 mmol/l Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mmol/l dithiothreitol and 0.1% bromphenol blue. Cells were incubated for 5 min at 95°C, cooled on ice for 5 min and stored at −20°C until required. Cell lysates were quantified using a Bicinchonininic Acid Protein Assay Kit (Beyotime Institute of Biotechnology) and 40-µg protein samples were separated on 10% SDS-PAGE and transferred to PVDF membranes using a semi-dry electroblot chamber. Proteins in the gel were visualized with Coomassie brilliant blue staining. Membranes were blocked in Tris-buffered saline (pH 7.4) containing 0.1% Tween-20 and 5% non-fat dry milk for 1 h at room temperature. The membranes were incubated with anti-Shh (1:1,000; 06–1106; EMD Millipore, Billerica, MA, USA), anti-Ptc1 (1:1,000; 06–1102; EMD Millipore) and anti-β-actin (1:1,000; sc-130657; Santa Cruz Biotechnology, Inc.) primary antibodies at 4°C overnight, as recommended by the manufacturer. Subsequently, the membranes underwent incubation with goat anti-rabbit IgG horse radish peroxidase-conjugated secondary antibodies (1:5,000; sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The blots were visualized using a SuperSignal® West Pico Chemiluminescent substrate (Pierce Biotechnology, Inc., Rockville, MD, USA), and molecular band intensity was determined via densitometry (Quantity One 1-D Analysis Software; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data were analyzed using the statistical software GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA), and all values are expressed as the mean ± standard error of the mean. The differences between two groups were analyzed using the Student's unpaired t-test, and differences between three or more groups were evaluated via one-way analysis of variance with Bonferroni correction. P≤0.05 was considered to indicate a statistically significant difference.
Results
Treatment with the antioxidant Tempol improves cardiac function in type 1 diabetic mice
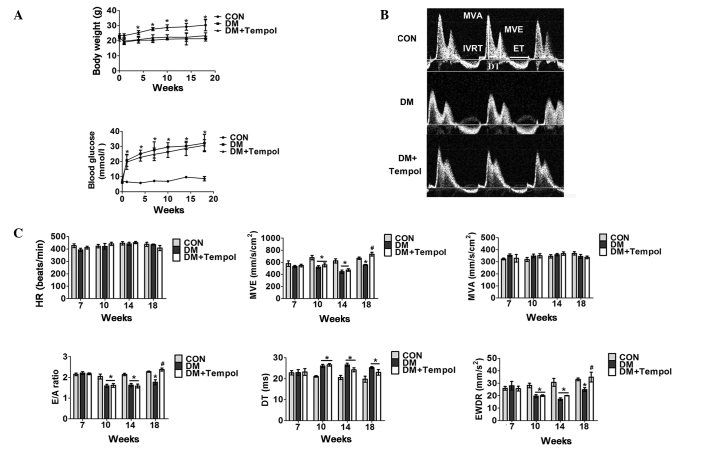
Five days of low-dose STZ treatment resulted in 18 weeks of sustained hyperglycemia and body weight loss throughout the present study (Fig. 1A). An echocardiographic assessment of cardiac function was performed from 7 to 18 weeks after STZ treatment. The superoxide scavenger Tempol (3 mM) was administered in the drinking water at the onset of diabetes and was continued for 18 weeks. Cardiac dysfunction was detected in the diabetic mice between 10 and 18 weeks. Treatment with Tempol between 0 and 18 weeks significantly increased E-wave velocity (31.92%), the E- to A-wave ratio (34.83%) and the E-wave deceleration rate (40.02%), and decreased isovolumetric relaxation time (19.32%) and ejection time (14.55%) in diabetic mice at 18 weeks, as compared with the untreated diabetic mice (Fig. 1B and C).
Figure 1.
Treatment with the antioxidant Tempol improves cardiac function in type 1 diabetic mice. (A) Quantitative analysis of body weight (upper panel) and whole blood glucose concentration (lower panel) in control (CON), diabetic (DM) and DM + Tempol groups (n=5). (B) Representative transmittal Doppler flow profile of the CON and DM groups at 18 weeks. (C) Quantitative analysis of heart rate (HR), E-wave velocity (MVE), mitral valve area (MVA), E- to A-wave ratio (E/A), E-wave detection time (DT), E-wave deceleration rate (EWDR), isovolumetric relaxation time (IVRT), and ejection time (ET) of the CON and DM groups at the 7-, 10-, 14- and 18-week time-points (n=5). Values are presented as the mean ± standard error of the mean. *P≤0.05 vs. the CON group; #P≤0.05 vs. the DM group.
Treatment with the antioxidant Tempol reduces myocardial ROS levels in type 1 diabetic mice
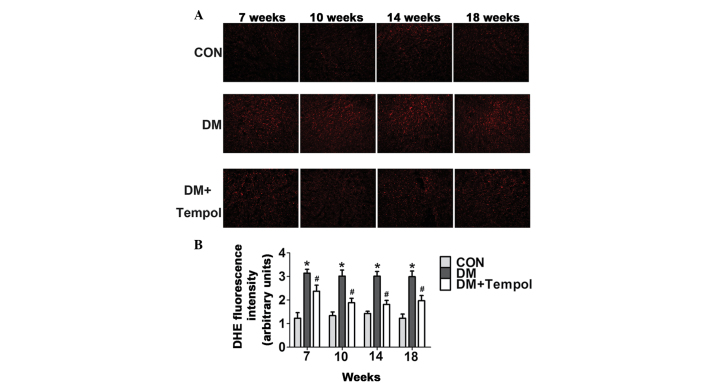
Myocardial ROS levels were quantified by DHE staining at the 7-, 10-, 14- and 18-week time-points of diabetes. The ROS levels were significantly increased in the diabetic mice during this time. Treatment with Tempol significantly decreased ROS levels (by 24.42, 37.50, 39.79 and 34.12%, respectively) at 7, 10, 14 and 18 weeks, as compared with the untreated diabetic mice (Fig. 2).
Figure 2.
Treatment with the antioxidant Tempol reduces myocardial reactive oxygen species levels in type 1 diabetic mice. (A) Representative images of immunostaining for oxidative dihydroethidium (DHE, red) in the nuclei of the myocardium. Scale bar, 100 µm. The density of fluorescence was measured using Image Pro-Plus software. (B) Quantitative analysis of fluorescence density (n=5). Values are presented as the mean ± standard error of the mean. *P≤0.05 vs. the control (CON) group; #P≤0.05 vs. the diabetic (DM) group.
Treatment with the antioxidant Tempol ameliorates the impaired myocardial Shh pathway in type 1 diabetic mice
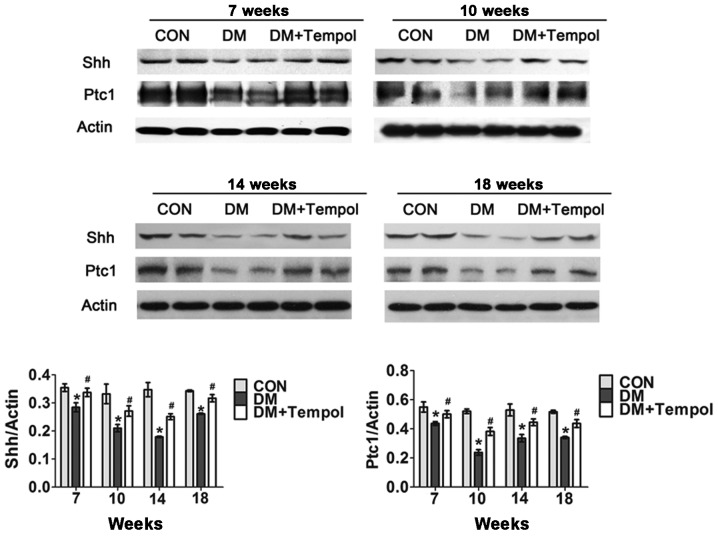
Myocardial Shh and Ptc1 protein expression levels were detected via western blot analysis at the 7-, 10-, 14- and 18-week time-points of diabetes. The Shh pathway was significantly impaired in the diabetic mice during this time. Treatment with Tempol significantly increased the protein expression levels of Shh (by 15.06, 60.67, 32.57 and 28.18%, respectively) and Ptc1 (by 18.67, 29.12, 40.22 and 21.14%, respectively) at 7, 10, 14 and 18 weeks, as compared with the untreated diabetic mice (Fig. 3).
Figure 3.
Treatment with the antioxidant Tempol ameliorates the impaired myocardial sonic hedgehog (Shh) pathway in type 1 diabetic mice. Western blot analysis of myocardial Shh and patched-1 (Ptc1) protein was performed at 7, 10, 14 and 18 weeks after streptozotocin injection (n=5). Data are presented as the mean ± standard error of the mean. *P≤0.05 vs. the control (CON) group; #P≤0.05 vs. the diabetic (DM) group.
Treatment with the antioxidant Tempol ameliorates the impaired myocardial Shh pathway in type 1 diabetic mice with MI
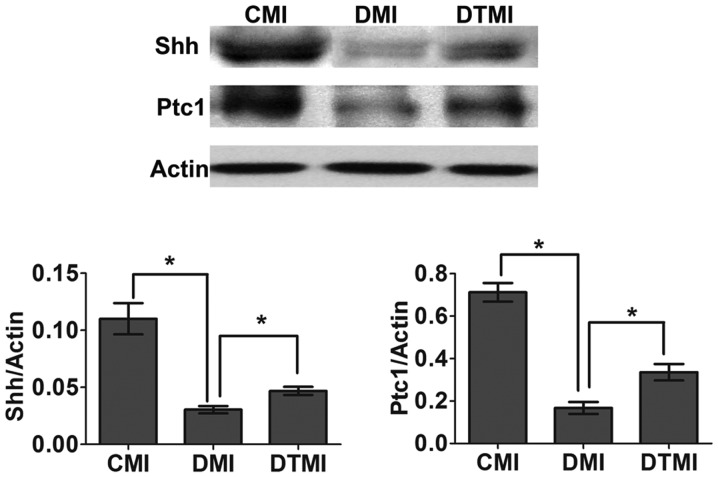
The surgery to induce MI was performed 7 weeks after the induction of diabetes, and western blot analysis was performed 7 days after MI. Tempol was administered in the drinking water at the onset of diabetes and continued for 8 weeks (7 weeks for diabetes and 1 week for diabetes after MI). On day 7 after MI, the Shh pathway was impaired in the DMI group, whereas treatment with Tempol significantly increased Shh and Ptc1 protein expression levels (by 71.04 and 95.07%, respectively) in the DTMI group, as compared with the DMI group (Fig. 4).
Figure 4.
Treatment with the antioxidant Tempol ameliorates the impaired myocardial sonic hedgehog (Shh) pathway in type 1 diabetic mice with myocardial infarction (MI). Western blot analysis was performed to detect the protein expression levels of Shh and patched-1 (Ptc1) in the border area of the infarcted heart 7 days after MI (n=5). Data are presented as the mean ± standard error of the mean. *P≤0.05. CMI, control MI group; DMI, diabetic mice with MI; DTMI, DMI group treated with Tempol.
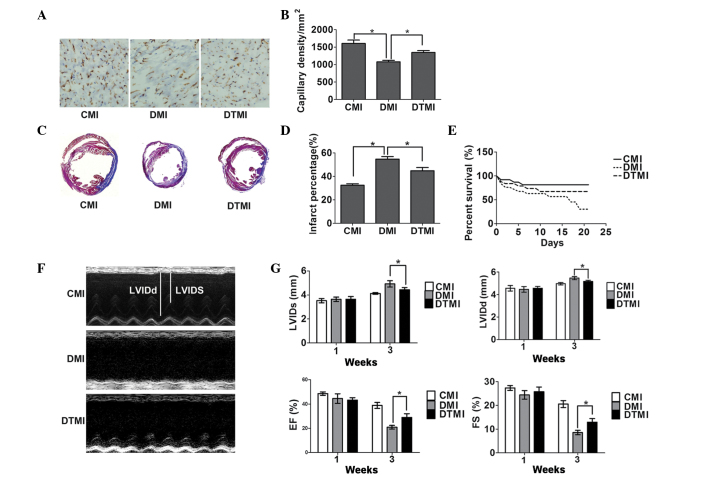
Treatment with the antioxidant Tempol enhances capillary density and reduces the percentage area of myocardial infarct in type 1 diabetic mice with MI
Surgery to induce MI was performed 7 weeks after the induction of diabetes, and histochemical analysis was performed 21 days after MI. Tempol was administered in the drinking water at the onset of diabetes and continued for 10 weeks (7 weeks for diabetes and 3 weeks for diabetes after MI). On day 21 after treatment, Tempol significantly increased capillary density by 25.75% and reduced the percentage area of myocardial infarct by 20.18% in the DTMI group, as compared with the DMI group (Fig. 5A-D).
Figure 5.
Treatment with the antioxidant Tempol ameliorates cardiac status in type 1 diabetic mice following myocardial infarction (MI). Capillary density, percentage myocardial infarct area and cardiac function were measured using immunohistochemistry, Masson's trichrome staining and echocardiography, respectively, on day 21 after MI. (A) Immunostaining for CD31 (brown) and the cell nucleus (blue) in the border zone of the infarcted myocardium. Scale bar, 100 µm. The number of vessels was measured using Image Pro-Plus. (B) Quantitative analysis of capillary density (n=5). (C) Masson's trichrome staining of the mouse hearts. Scale bar, 3 mm. (D) Percentage infarct area (n=5). (E) Survival rate (n=20). (F) Representative M-mode images. (G) Quantitative analysis of left ventricular internal dimension-systole (LVIDs), left ventricular internal dimension-diastole (LVIDd), fractional shortening (FS) and ejection fraction (EF) (n=5). Data are presented as the mean ± standard error of the mean. *P≤0.05. CMI, control MI group; DMI, diabetic mice with MI; DTMI, DMI group treated with Tempol.
Treatment with the antioxidant Tempol protects against cardiac dysfunction and enhances the survival rate in type 1 diabetic mice with MI
The cardiac function and survival rate were examined 21 days after MI. Tempol significantly enhanced the survival of DMI mice by 87.5% (Fig. 5E) and significantly prevented the enlargement of the LV end-systolic and LV end-diastolic dimensions (by 20.1 and 16.75%, respectively). In addition, Tempol reduced the fractional shortening and ejection fraction (by 38.33 and 50.40%, respectively) in the DTMI group, as compared with the DMI group (Fig. 5F and G).
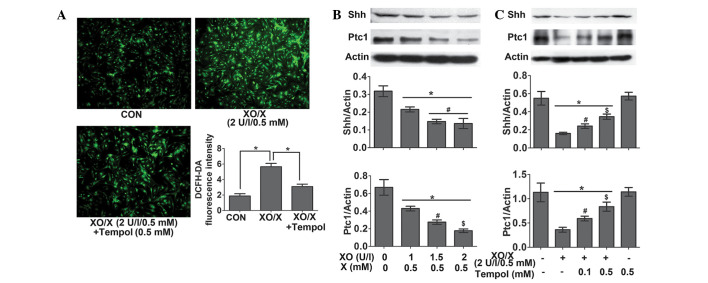
The oxidative-stress system XO/X inhibits the Shh pathway in rat neonatal cardiomyocytes
Following the 4-h treatment of rat neonatal cardiomyocytes, XO (2 U/l)/X (0.5 mM) significantly increased ROS levels by 201.26%. This effect was reversed by 45.28% following the administration of Tempol (0.5 mM) (Fig. 6A). Following the 24-h treatment of rat neonatal cardiomyocytes, XO (1–2 U/l)/X (0.5 mM) significantly decreased the protein expression levels of Shh and Ptc1 in a concentration-dependent manner (Fig. 6B); however, this effect was attenuated by treatment with Tempol (0.1 and 0.5 mM) (Fig. 6C).
Figure 6.
Xanthine oxidase (XO)/xanthine (X) inhibits the sonic hedgehog (Shh) pathway in rat neonatal cardiomyocytes. The rat neonatal cardiomyocytes were treated with XO/X for 4 or 24 h, and Tempol was administered 1 h prior to XO/X. (A) Immunofluorescence analysis of reactive oxygen species (ROS) levels was performed in rat neonatal cardiomyocytes. Representative images show immunostaining for dichlorofluorescein (green) in the cytoplasm of the myocardium. Scale bar, 100 µm. The density of fluorescence was measured using Image Pro-Plus. A quantitative analysis of fluorescence density is shown (n=4). *P≤0.05. (B) Western blot analysis of myocardial Shh and patched-1 (Ptc1) protein in rat neonatal cardiomyocytes (n=4). *P≤0.05 vs. the control; #P≤0.05 vs. XO (1 U/l)/X (0.5 mM); $P≤0.05 vs. XO (1.5 U/l)/X (0.5 mM). (C) Western blot analysis of myocardial Shh and Ptc1 protein in rat neonatal cardiomyocytes (n=4). *P≤0.05 vs. the control; #P≤0.05 vs. XO (2 U/l)/X (0.5 mM); $P≤0.05 vs. XO (2 U/l)/X (0.5 mM) + Tempol (0.1 mM). Data are presented as the mean ± standard error of the mean. CON, control.
Discussion
The present study is the first, to the best of our knowledge, to demonstrate that: i) In type 1 diabetic mice, treatment with the antioxidant Tempol can improve the impaired myocardial Shh pathway following MI and eventually ameliorate cardiac dysfunction; ii) in neonatal cardiomyocytes, administration of XO/X significantly increases ROS levels and reduces the protein expression levels of Shh pathway-associated proteins. These findings suggest that oxidative stress may contribute to the impaired Shh pathway in type 1 diabetic mice.
The subsequent generation of ROS and accompanying oxidative stress in diabetes are hallmarks of the molecular mechanisms underlying diabetic cardiovascular disease. In diabetic cardiomyopathy, the production of ROS induces inflammation, endothelial dysfunction, cell apoptosis and myocardial remodeling (23). ROS from the hexosamine and polyol pathways impair the expression and activity of nitric oxide synthase and decrease the function of nitric oxide, which results in the induction of endothelial dysfunction. In addition, increased ROS levels may lead to cell apoptosis, via the aggregation of apoptosis signal-regulating kinase 1 and ceramide, and induce myocardial remodeling, cardiac dysfunction and arrhythmia (24). ROS also have a critical role in diabetes with cardiac ischemia. A previous study demonstrated that exogenous glutathione (an endogenous ROS-scavenging agent) was able to normalize Rac1 and hypoxia inducible factor-1α expression and ameliorate infarct size in isolated high-glucose-perfused ischemic hearts (7). In addition, administration of thioredoxin-1, an endogenous ROS-scavenging protein that is required for the maintenance of redox environments, immediately following MI reversed the reduction of vascular endothelial growth factor and heme oxygenase-1 and ameliorated myocardial healing and cardiac dysfunction in diabetic rats (5). In the present study, therefore, it was hypothesized that the oxidative stress-induced impairment of the Shh pathway in diabetes and diabetic MI may have also been associated with cardiac dysfunction.
Tempol is a redox cycling nitroxide that promotes the metabolism of numerous ROS and improves nitric oxide bioavailability. Tempol is effective in preventing various adverse consequences of oxidative stress, which is associated with numerous diseases including shock, hypertension, aging, metabolic syndrome and diabetes (25). In the present study, the effects of Tempol on oxidative stress in the Shh pathway were analyzed. Myocardial and cardiomyocyte ROS levels were subsequently detected, and the results indicated that Tempol was able to act as an effective and efficient antioxidant.
The results of the present study suggested that the antioxidant Tempol was able to improve the impaired Shh pathway and cardiac function in diabetic mice. Since previous studies have yet to establish a causal link between an impaired Shh pathway and cardiac dysfunction in diabetes, the present study was only able to report a preliminary association between the oxidative stress-induced Shh pathway inhibition and oxidative stress-induced cardiac dysfunction. Therefore, the significance of the oxidative stress-induced Shh pathway inhibition in diabetes required further exploration.
As our previous study demonstrated (14), activation of the Shh pathway in diabetic mice was impaired on day 7 after MI. Notably, the impaired Shh pathway contributed to cardiac dysfunction at 3 weeks after MI in diabetic mice. In the present study, MI was induced in order to analyze the effect and significance of diabetes-stimulated oxidative stress on the inhibition of the Shh pathway in diabetic mice with MI. MI was induced after 7 weeks of diabetes, when a markedly impaired Shh pathway was detected.
The results of the present study suggested that Tempol was able to significantly upregulate the Shh pathway in diabetic mice with MI, which was beneficial for the enhancement of capillary density, the reduction of the percentage area of myocardial infarct and the improvement in cardiac function and survival rate. These results are consistent with those observed following treatment with the Shh pathway agonist SAG1.3 in our previous study (14).
In order to eliminate the interference of various factors that may occur in vivo, the XO/X oxidative stress producer system was used in neonatal cardiomyocytes, in order to investigate the effect of oxidative stress on the Shh pathway in vitro. The results demonstrated that treatment with XO/X significantly induced the production of ROS and inhibited the Shh pathway in a concentration-dependent manner; however, Tempol attenuated these effects.
The results of the present study collectively suggest that oxidative stress can impair the endogenous Shh pathway in diabetes and weaken its ability when combined with MI, which may subsequently exacerbate cardiac dysfunction in diabetic MI.
Antioxidants used in preclinical animal studies have provided promising results (26–29); however, the explanation for why antioxidative drugs, including vitamin C and vitamin E, do not lead to satisfactory results when applied clinically is complex. Numerous studies have demonstrated that antioxidants appear promising as substrate-modifying ancillary agents for use with the major therapy (e.g. injection of angiogenic factors) in the treatment of various diseases, including diabetes, hypercholesterolemia and ischemia, since the molecular and cellular environments of these diseases are not suitable for regular therapy (30). Preliminary evidence from rodent models of hindlimb ischemia has suggested that supplementation with vitamins C and E, which prevents the excessive generation of ischemia-induced oxygen radicals, enhances the angiogenic effect of L-arginine on bone marrow cell infusion (31). Our previous study also revealed that administration of a sufficient Shh agonist inadequately activated the Shh pathway in diabetic MI (14). Therefore, further studies should consider combining long-time applied antioxidants with Shh pathway receptor agonists, Shh protein or Shh-adenoviral vector gene therapy, in order to improve the effectiveness of the treatment.
In conclusion, the present study has demonstrated that oxidative stress may contribute to the impaired Shh pathway in type 1 diabetic mice, leading to diminished myocardial healing and cardiac dysfunction. Antioxidative strategies that are aimed at restoring the endogenous Shh pathway may offer a useful means for improving cardiac function.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81173062 and 81302767), the Science and Technology Planning Project of Guangdong Province (grant no. 2014A020212361) and the Medical Scientific Research Foundation of Guangdong Province (grant no. A2015444).
References
- 1.Krum H, Gilbert RE. Demographics and concomitant disorders in heart failure. Lancet. 2003;362:147–158. doi: 10.1016/S0140-6736(03)13869-X. [DOI] [PubMed] [Google Scholar]
- 2.Marfella R, Filippo CD, Portoghese M, Siniscalchi M, Martis S, Ferraraccio F, Gustafierro S, Nicoletti G, Barbieri M, Coppola A, et al. The ubiquitin-proteasome system contributes to the inflammatory injury in ischemic diabetic myocardium: The role of glycemic control. Cardiovasc Pathol. 2009;18:332–345. doi: 10.1016/j.carpath.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Shishido T, Woo CH, Ding B, McClain C, Molina CA, Yan C, Yang J, Abe J. Effects of MEK5/ERK5 association on small ubiquitin-related modification of ERK5: Implications for diabetic ventricular dysfunction after myocardial infarction. Circ Res. 2008;102:1416–1425. doi: 10.1161/CIRCRESAHA.107.168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bucciarelli LG, Ananthakrishnan R, Hwang YC, Kaneko M, Song F, Sell DR, Strauch C, Monnier VM, Yan SF, Schmidt AM, Ramasamy R. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–1951. doi: 10.2337/db07-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel SM, Thirunavukkarasu M, Penumathsa SV, Koneru S, Zhan L, Maulik G, Sudhakaran PR, Maulik N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244–1255. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Filippo C, Marfella R, Cuzzocrea S, Piegari E, Petronella P, Giugliano D, Rossi F, D'Amico M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005;54:803–810. doi: 10.2337/diabetes.54.3.803. [DOI] [PubMed] [Google Scholar]
- 7.Marfella R, D'Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, Berrino L, Rossi F, Giugliano D. Myocardial infarction in diabetic rats: Role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002;45:1172–1181. doi: 10.1007/s00125-002-0882-x. [DOI] [PubMed] [Google Scholar]
- 8.Mimeault M, Batra SK. Frequent deregulations in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies. Pharmacol Rev. 2010;62:497–524. doi: 10.1124/pr.109.002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/S0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 10.Chari NS, McDonnell TJ. The sonic hedgehog signaling network in development and neoplasia. Adv Anat Pathol. 2007;14:344–352. doi: 10.1097/PAP.0b013e3180ca8a1d. [DOI] [PubMed] [Google Scholar]
- 11.Varjosalo M, Taipale J. Hedgehog signaling. J Cell Sci. 2007;120:3–6. doi: 10.1242/jcs.03309. [DOI] [PubMed] [Google Scholar]
- 12.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, et al. Sonic hedgehog myocardial gene therapy: Tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 13.Ueda K, Takano H, Niitsuma Y, Hasegawa H, Uchiyama R, Oka T, Miyazaki M, Nakaya H, Komuro I. Sonic hedgehog is a critical mediator of erythropoietin-induced cardiac protection in mice. J Clin Invest. 2010;120:2016–2029. doi: 10.1172/JCI39896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Q, Hou N, Wang YP, He LS, He YH, Zhang GP, Yi Q, Liu SM, Chen MS, Luo JD. Impaired sonic hedgehog pathway contributes to cardiac dysfunction in type 1 diabetic mice with myocardial infarction. Cardiovas Res. 2012;95:507–516. doi: 10.1093/cvr/cvs216. [DOI] [PubMed] [Google Scholar]
- 15.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliviera-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Tabas I. Emerging roles of mitochondria ROS in atherosclerotic lesions: causation or association? J Atheroscler Thromb. 2014;21:381–390. doi: 10.5551/jat.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–969. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- 22.Hou N, Luo MS, Liu SM, Zhang HN, Xiao Q, Sun P, Zhang GS, Luo JD, Chen MS. Leptin induces hypertrophy through activating the peroxisome proliferator-activated receptor α pathway in cultured neonatal rat cardiomyocytes. Clin Exp Pharmacol Physiol. 2010;37:1087–1095. doi: 10.1111/j.1440-1681.2010.05442.x. [DOI] [PubMed] [Google Scholar]
- 23.Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: A key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88:233–240. doi: 10.1139/Y10-016. [DOI] [PubMed] [Google Scholar]
- 24.Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7:15–25. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danta CC, Piplani P. The discovery and development of new potential antioxidant agents for the treatment of neurodegenerative diseases. Expert Opin Drug Discov. 2014;9:1205–1222. doi: 10.1517/17460441.2014.942218. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y, Wang X. Antioxidant therapies for Alzheimers disease. Oxid Med Cell Longev. 2012;2012 doi: 10.1155/2012/472932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: Preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boodhwani M, Sellke FW. Therapeutic angiogenesis in diabetes and hypercholesterolemia: Influence of oxidative stress. Antioxid Redox Signal. 2009;11:1945–1959. doi: 10.1089/ars.2009.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napoli C, Williams-Ignarro S, de Nigris F, de Rosa G, Lerman LO, Farzati B, Matarazzo A, Sica G, Botti C, Fiore A, et al. Beneficial effects of concurrent autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the mouse hindlimb. Proc Natl Acad Sci USA. 2005;102:17202–17206. doi: 10.1073/pnas.0508534102. [DOI] [PMC free article] [PubMed] [Google Scholar]