Abstract
It has been proposed that the Notch signaling pathway may serve a pivotal role in cellular differentiation, proliferation and apoptosis. However, the function of Notch signaling in gastric cancer stem cells (GCSCs) is largely unknown. The present study aimed to delineate the role of the Notch1 pathway in GCSCs and during epithelial-mesenchymal transition (EMT). Flow cytometry was used to isolate CD44+ cells from the human gastric cancer cell line, MKN45. CD44+ cells displayed the characteristics of CSCs and exhibited higher Notch1 expression compared with CD44− cells. To investigate the role of the Notch1 pathway in GCSCs, CD44+ cells were treated with the γ-secretase inhibitor DAPT. DAPT treatment inhibited the expression of the Notch1 downstream target Hes1 and EMT markers, suppressed the properties of CSCs and impaired the invasion and proliferation capabilities of CD44+ cells. In addition, intraperitoneal treatment with DAPT effectively inhibited the growth of CD44+ cell xenograft tumors. The present study indicated that CD44+ GCSCs possess the characteristics of CSCs and that the Notch1 pathway serves a critical role in the maintenance of CSCs and EMT.
Keywords: Notch1, gastric cancer, cancer stem cells, epithelial-mesenchymal transition
Introduction
Gastric cancer (GC) is one of the most common types of cancer and the second highest cause of cancer mortality worldwide (1). A large number of gastric cancer patients are diagnosed once the tumor has metastasized and has reached an advanced stage (2). Clinicians treat patients using conventional and targeted therapies, but these methods have little therapeutic effect.
A large number of molecular markers are associated with the metastasis of tumors, and one of the most important factors leading to this malignancy is epithelial mesenchymal transition (EMT), which is characterized by the gain of stem cell properties and the promotion of tumor invasion and metastasis (3,4). The features of EMT include the loss of the adhesion molecule epithelial-cadherin (E-cadherin) and gain of mesenchymal tissue markers.
The cancer stem cell (CSC) hypothesis has been proposed to explain the pathogenesis of a number of types of cancer (5). The notable properties of CSCs are their ability for self-renewal and cell proliferation, they can initiate tumor formation, self-renewal, differentiation and cause cancer recurrence and metastasis. Furthermore, CSCs are more chemoresistant and radioresistant than their differentiated, daughter cancer cells, which may be the reason for treatment failure for malignant tumors.
Notch signaling, which serves a pivotal role in cellular differentiation, proliferation and apoptosis, is disrupted in several malignancies and offers a potential target for therapeutic intervention. Abnormal activation of Notch1 signaling has been observed in gastric cancer cells (6) and correlates with colony-forming ability and xenografted tumor growth (7). Inhibition of Notch1 signaling with a γ-secretase inhibitor resulted in a significant reduction in GBM cell growth in vitro and in vivo (3). In addition, the Notch1 signaling pathway is also critical in maintaining the characteristics of CSCs and is associated with the self-renewal of various types of CSCs, such as breast and pancreatic cancer (8). However, the role of Notch1 signaling in gastric CSCs (GCSCs) is not clear.
The present study aimed to examine the role of Notch1 in GCSCs by treating those cells with the γ-secretase inhibitor DAPT. In addition, the role of Notch1 signaling in EMT within GCSCs was investigated.
Materials and methods
Cells and animals
The human gastric cancer cell line MKN-45 was purchased from the Type Culture Collection of the Chinese Academy of Sciences (TCCCA; Shanghai, China). The cell line was cultured in RPMI-1640 (GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, GE Healthcare Life Sciences) and were maintained at 37°C in a 5% CO2 atmosphere.
A total of 50 4-week-old female nude mice were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Science (Shanghai, China). The mice were maintained in cages (5 mice/cage) in a room with a constant temperature (22±1°C) and a dark-light cycle. The present study was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of Chongqing Medical University. The protocol was approved by the Committee on the Ethics of Animal Experiments of Chongqing Cancer Institute (Chongqing, China).
Preparation of CD44+ and CD44− MKN45 cells for in vitro and in vivo analysis of tumorigenicity. CD44+ and CD44− populations were sorted from the human gastric cancer cell line, MKN45. For fluorescence-activated cell sorting (FACS), 5–10×106 cells were harvested and incubated for 30 min at room temperature with a 10-fold dilution of the following antibodies: Anti-CD44-fluorescein isothiocyanate rat monoclonal antibody and anti-CD44-PE (eBioscience, San Diego, CA, USA). Then, the cells were detected using a FACS-LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). The cells were routinely sorted twice and reanalyzed for purity (XDP, Beckman-Coulter).
For in vivo experiments, CD44+ and CD44− cells were resuspended in PBS and were injected subcutaneously into the limbs of mice. Groups of mice were inoculated with CD44+ or CD44− cells at 1×103, 3×103, 1×104 and 5×104 (5 mice/group), and tumor growth was monitored every 2 days after the second week of inoculation. Another 2 groups of mice were injected with 5×104 CD44+ MKN45 cells for intraperitoneal treatment with γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-ananyl]-S-phenyglycine t-butyl ester (DAPT, Sigma-Aldrich, St. Louis, MO, USA). For in vitro experiments, the sorted cells were cultured in RPMI-1640 and assessed by western blotting, proliferation, self-renewal, tumor-initiation, migration and invasion assays.
Drug and treatment
For in vitro experiments, DAPT was prepared as a 10 µM stock in DMSO (Sigma-Aldrich). CD44+ and CD44− cells were treated with DMSO or DAPT (10 µM) and were analyzed after 72 h. Animals were treated intraperitoneally with a drug concentration of 10 mg/kg/body weight or with the vehicle (control) once daily for 5 weeks, using a 3-days-on and 4-days-off intermittent-dose schedule, as described previously (9).
Spheroid colony formation assay
Cells were seeded into each well (20 cells per well) of ultra-low-attachment 48-well plates (Beyotime Institute of Biotechnology, Shanghai, China) and supplemented with 300 µl of RPMI-1640 plus 40 ng/ml bFGF and 20 ng/ml EGF (Invitrogen Life Technologies, Carlsbad, CA, USA). After 4 weeks, the total number of spheroid colonies/well were counted.
Cell chemosensitivity examination
Cells cultured in medium were incubated and treated with 5-fluorouracil (6 mM) (Sigma-Aldrich). After 48 h of exposure to the chemotherapeutic agents, 20 ml of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT, Sigma-Aldrich) solution (0.5 mg/ml) was added for an additional 4 h before 100 ml dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added for 15 min. The plates were then shaken gently for 5 min and measured at 570 nm using a spectrophotometer. A total of 5 wells were assayed for each condition.
Migration and invasion assays
The cells were added to the upper chambers, and the lower chambers were filled with 750 ml of RPMI-1640 media with 10% FBS. The cells were incubated for 24 h at 37°C in 5% CO2. After 24 h, the non-migrated/non-invading cells were removed from the upper sides, and the migrated/invaded cells that were on the lower sides of the inserts were stained. The absorbance of the wells were read at 560 nm using a RF-5301PC fluorescence spectrometer (Shimadzu Corporation, Kyoto, Japan) according to the manufacturer's protocol.
Immunoblotting
Total protein for immunoblots was extracted from cells using RIPA lysis buffer (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. After the protein extracts were quantified using a BCA protein assay, equivalent amounts of lysates were resolved by 10% SDS polyacrylamide gel electrophoresis (Beyotime Institute of Biotechnology) and transferred onto a polyvinylidene fluoride (PVDF) membrane (Beyotime Institute of Biotechnology), which was then blocked in 5% non-fat milk in TBST (Beyotime Institute of Biotechnology) for 1 h at 4°C. Then the blots were incubated with primary antibodies overnight at 4°C and washed with PBST 3 times (each time for 5 min), subsequently incubated with HRP-conjugated secondary antibodies for 1 h at room temperature and washed with PBST 3 times (each time for 5 min). The signal was detected using an enhanced chemiluminescence reagent (Millipore, Billerica, MA, USA).
The mouse monoclonal antibodies against GAPDH and Snail were purchased from BD Biosciences (Franklin Lakes, NJ, USA). The rabbit monoclonal antibodies against ZO1 N-cadherin, E-cadherin and Vimentin were purchased from Abcam (Abcam; Cambridge, UK). The monoclonal goat anti-rabbit IgG and goat anti-mouse Horseradish peroxidase (HRP)-conjugated antibodies were purchased from Santa Cruz Biotechnology.
The antibodies were diluted in 5% non-fat milk as follows: anti-Notch1, 1:1,200; anti-Hes1, 1:10,000; anti-E-cadherin, 1:1,200; anti-N-cadherin, 1:1,200; anti-vimentin, 1:1,200; anti-Snail, 1:500; anti-ZO1, 1:500; anti-GAPDH, 1:500; and HRP-conjugated IgG, 1:7,000.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was purified from cell lines using RNAiso (Takara Bio, Inc., Otsu, Japan), and cDNA was synthesized using the Synthesis Kit (Takara Bio, Inc.). RT-qPCR was performed using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with SYBR® Premix Ex Taq II (Takara Bio, Inc.). The PCR conditions were as follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, then 60°C for 30 sec, and the data were normalized against the β-actin RNA. The sequences of the PCR primers for each of the gene transcripts were as follows: Notch1, sense 5′-TGC CGA ACC AAT ACA ACC CTC-3′ and anti-sense 5′-TGG TAG CTC ATC ATC TGG GACA-3′; Hes1, sense 5′-GTG CAT GAA CGA GGT GAC CC-3′ and anti-sense 5′-GTA TTA ACG CCC TCG CAC GT-3′; β-actin, sense 5′-CCA CGA AAC TAC CTT CAA CTCC-3′ and anti-sense 5′-GTG ATC TCC TTC TGC ATC CTGT-3′.
Histological examination
Tumor tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin and then sectioned and stained with hematoxylin and eosin (HE, Sigma-Aldrich). Histological differences were examinedusing an Optical Microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
All the experiments were repeated 3 times, and the results were analyzed using the SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation (SD). Group comparisons were performed using the t-test, the nonparametric test and one-way analysis of variance. Differences were considered statistically significant when P<0.05.
Results
CD44+ cells isolated from the MKN45 cell line display the characteristics of CSCs
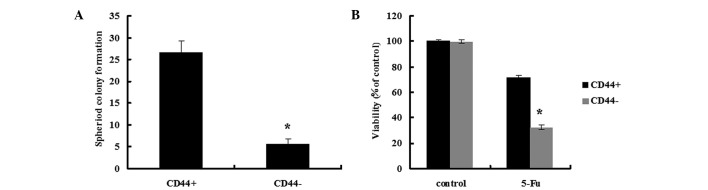
Tumors contain a small number of CSCs that have self-renewal and tumor-initiating abilities (10). In the spheroid colony formation assay, the CD44+ MKN45 cells formed a greater number of spheroids compared with CD44− MKN45 cells (Fig. 1A; P<0.05). In the tumorigenicity assay, nude mice were injected with 1×103 to 5×104 CD44+ or CD44− MKN45 cells. Transplantation of 1×103, 5×103 or 1×104 CD44− cells consistently failed to form tumors in all mice, while 5×104 CD44− cells resulted in tumor formation in 1/5 mice. In contrast, transplantation of 1×103 CD44+ cells failed to form tumors in all mice, however, the transplantation of 5×103, 1×104 or 5×104 CD44+ cells into nude mice resulted in tumor formation in 2/5, 4/5 or 5/5 mice, respectively (Table I).
Figure 1.
The spheroid-forming ability, colony formation and chemotherapy susceptibility in CD44+ and CD44− cells isolated from MKN45 cells. (A) CD44+ MKN45 cells formed a greater number of spheroids compared with CD44− MKN45 cells. (B) CD44+ MKN45 cells were more chemoresistant compared with CD44− cells. Data are presented as the mean ± SD; n=5. *P<0.05.
Table I.
Tumorigenicity of CD44+ and CD44− cells in nude mice.
| Cell numbers of injection | ||||
|---|---|---|---|---|
| CD44+/− | 1×103 | 5×103 | 1×104 | 5×104 |
| CD44+ cells | 0/5 | 2/5 | 4/5 | 5/5 |
| CD44− cells | 0/5 | 0/5 | 0/5 | 1/5 |
CD44+ and CD44− cells were isolated separately and injected subcutaneously into nude mice. Tumor formation was observed for 8 weeks after injection.
Since chemotherapy resistance is a common characteristic of CSCs, the susceptibility of CD44+ and CD44− MKN45 cells susceptibility to 5-fluorouracil (5-FU) treatments was assessed, which is generally used for the treatment of GC (Fig. 1B). CD44+ cells were more chemoresistant compared with CD44− cells and exhibited a cell survival rate of 71.5±2.0% after 48-h incubation, compared with 32.6±1.9% for CD44− cells (P<0.05).
These data indicate that CD44+ gastric cancer cells were tumorigenic and possessed CSC characteristics.
The Notch1 signaling pathway was activated in CD44+ MKN45 cells
To explore the role of the Notch1 pathway in CSCs, the expression of Notch1 and its downstream target Hes1 was assessed in CD44+ and CD44− MKN45 cells. Notch1 and Hes1 expression levels were higher in CD44+ cells compared with in CD44− cells (Fig. 2). These data demonstrated that the Notch1 signaling pathway was activated in GCSCs.
Figure 2.
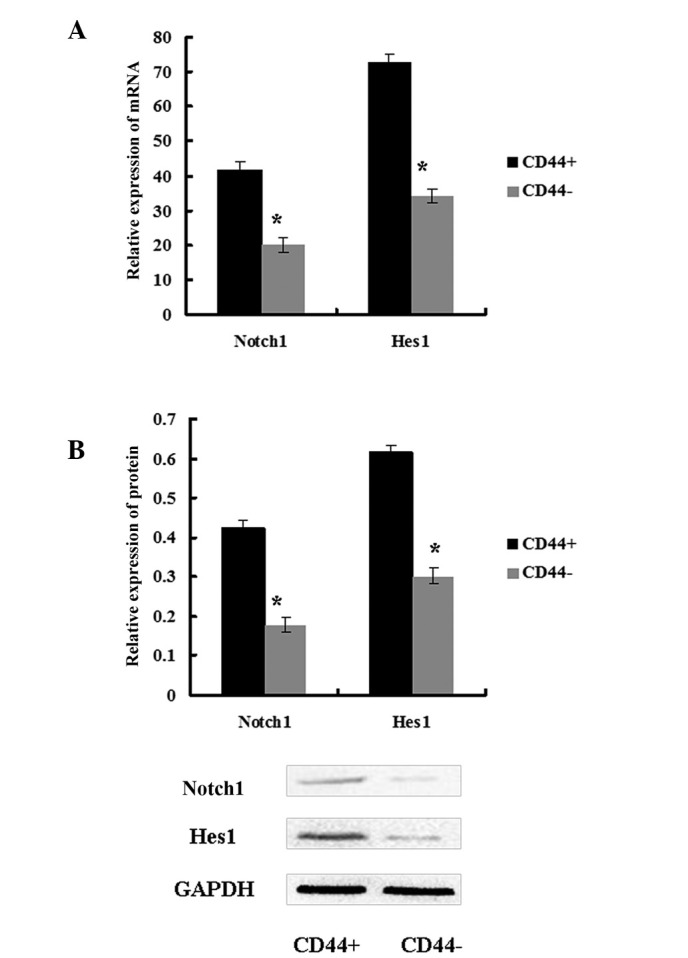
The Notch1 signaling pathway was activated in CD44+ MKN45 cells. In CD44+ MKN45 cells, the (A) mRNA and (B) protein expression levels of Notch1 and Hes1 were increased in CD44+ cells compared with in CD44− cells. Data are presented as the mean ± SD; n=5. *P<0.05.
The γ-secretase inhibitor DAPT attenuated the self-renewal, tumor-initiating, migration and invasion abilities of CD44+ MKN45 cells
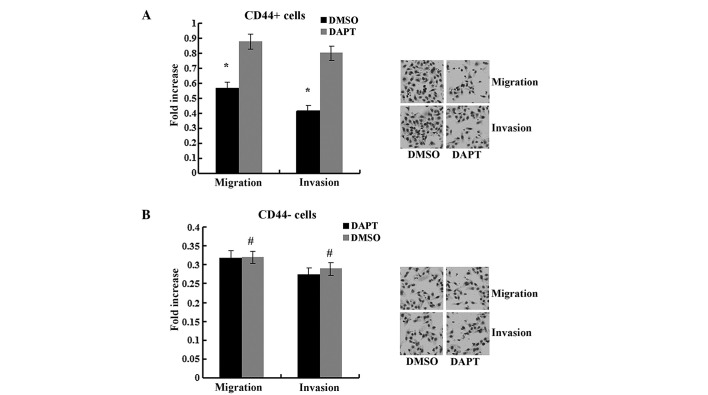
It has previously been demonstrated that Notch1 signaling serves a role in stem cell renewal and cell fate determination in neural, hematopoietic and embryonic stem cells (4). To further determine the effect of the Notch1 pathway, CD44+ and CD44− cells were treated with DAPT. As presented in Fig. 3, DAPT treatment suppressed the expression of the Notch1 downstream target Hes1 in CD44+ cells (P<0.05) but not in CD44− cells (P>0.05). The migration and invasion abilities were impaired by DAPT in CD44+ cells compared to cells treated with DMSO (Fig. 4, P<0.05) but not in CD44− cells (P>0.05). In the spheroid colony formation assay, CD44+ cells that were treated with DAPT formed fewer spheroids compared with cells treated with DMSO (Fig. 5; P<0.05), and results from the MTT assay demonstrated that the chemotherapy susceptibility of CD44+ cells that were treated with DAPT was upregulated compared with cells treated with DMSO (Fig. 6; P<0.05). However, those changes in migration and chemotherapeutic susceptibility were not observed in CD44− MKN45 cells (Figs. 5 and 6; P>0.05). These data demonstrated that the γ-secretase inhibitor DAPT suppressed the Notch1 signaling pathway and inhibited the self-renewal, tumor-initiating, migration and invasion abilities and improved chemotherapy susceptibility of CD44+ MKN45 cells.
Figure 3.
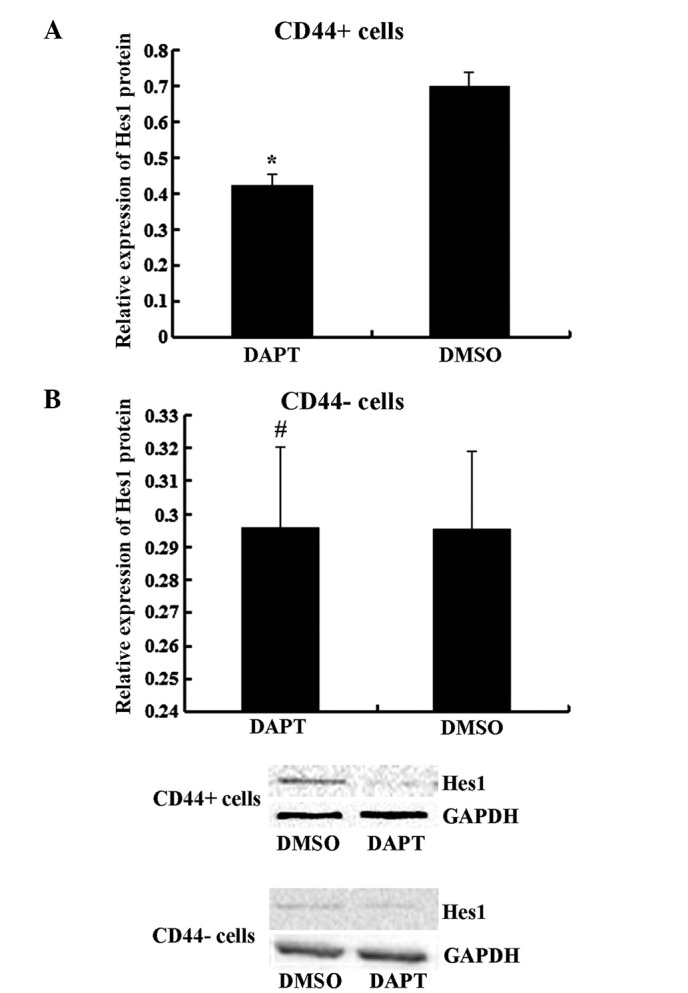
Hes1 were analyzed in (A) CD44+ and (B) CD44− MKN45 cells by immunoblotting after treatment with DAPT or DMSO. Data are presented as the mean ± SD; n=5. *P<0.05, #P>0.05.
Figure 4.
DAPT treatment impaired the migration and invasion abilities of (A) CD44+ cells, but not in (B) CD44− cells. Data are presented as the mean ± SD; n=5. *P<0.05, #P>0.05.
Figure 5.
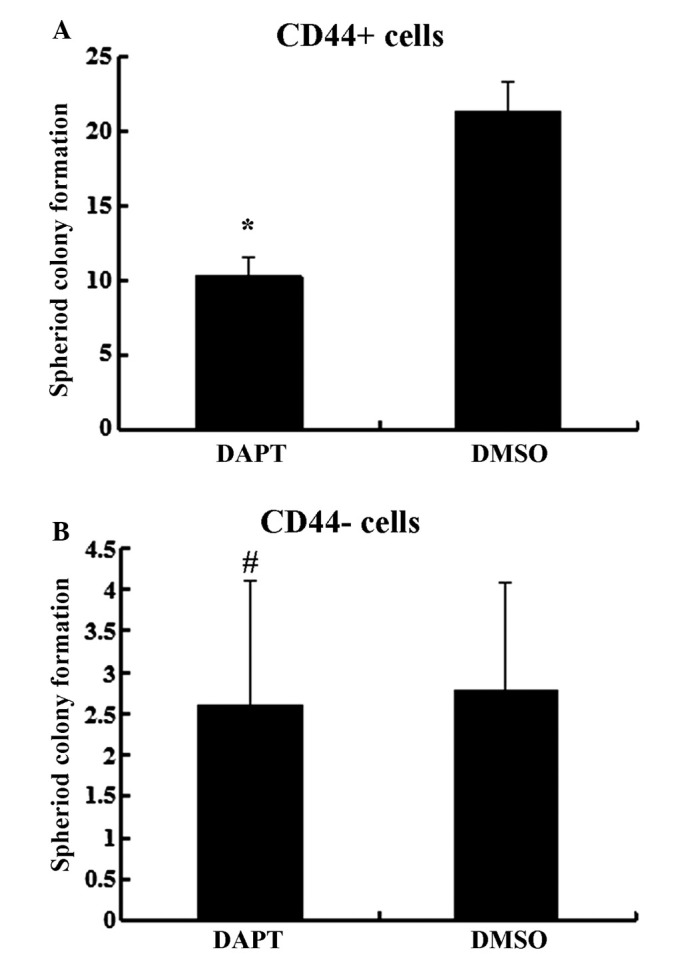
DAPT treatment inhibited spheroid formation by (A) CD44+ cells, but not in (B) CD44− cells. Data are presented as the mean ± SD; n=5. *P<0.05, #P>0.05.
Figure 6.
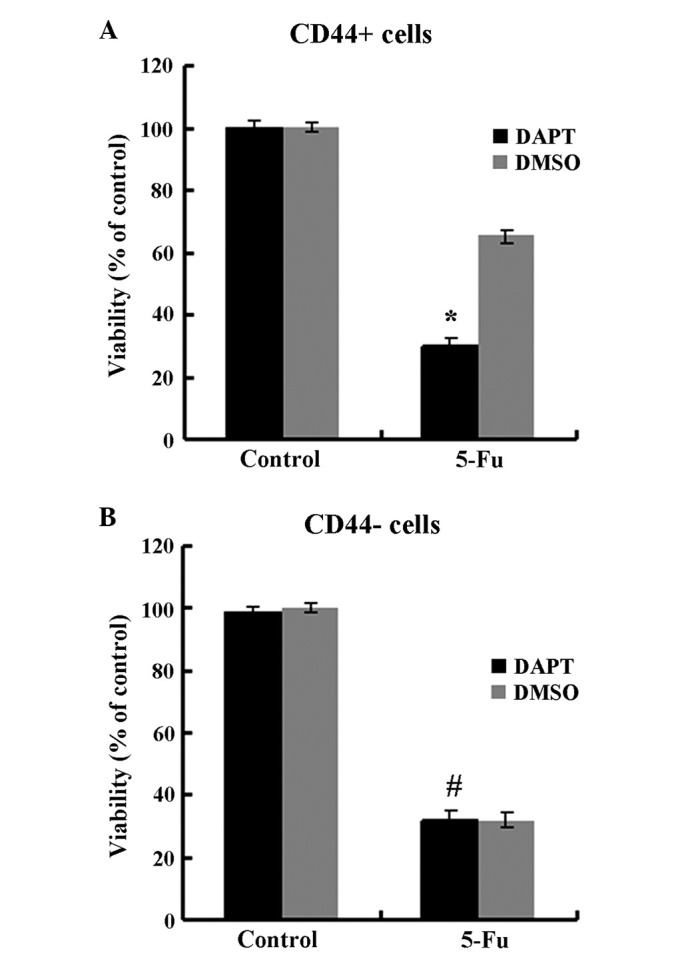
The chemotherapy susceptibility of CD44+ cells treated with DAPT was up-regulated, but not in CD44- cells. Data are presented as the mean ± SD; n=5. *P<0.05, #P>0.05.
Intraperitoneal treatment with DAPT effectively inhibited the growth of CD44+ MKN45 cell xenograft tumors
Given the ability of DAPT to inhibit CD44+ MKN45 cells in vitro, the role of DAPT was tested in a nude mouse model. CD44+ MKN45 cells were subcutaneously injected into the flanks of mice, and intraperitoneal treatment with either DAPT or DMSO was initiated when the tumor volume reached a size of 10 mm3. All mice were treated for 5 weeks, using an established, 3-days-on and 4-days-off, intermittent dose schedule (9). Xenograft tumors grew continuously in vehicle-treated animals, whereas DAPT treatment significantly inhibited tumor growth (Fig. 7; P<0.05).
Figure 7.
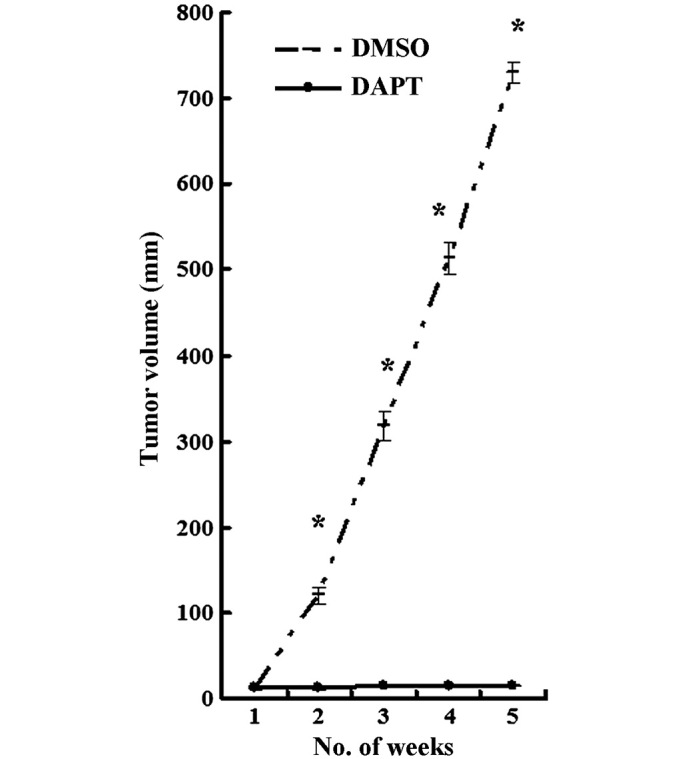
Intraperitoneal treatment with DAPT effectively inhibited the growth of CD44+ xenograft tumors. The tumor growth volume was significantly reduced in the treated DAPT compared to that of the control group. Data are presented as the mean ± SD; n=5. *P<0.05.
The γ-secretase inhibitor DAPT prevented epithelial-mesenchymal transition in CD44+ MKN45 cells
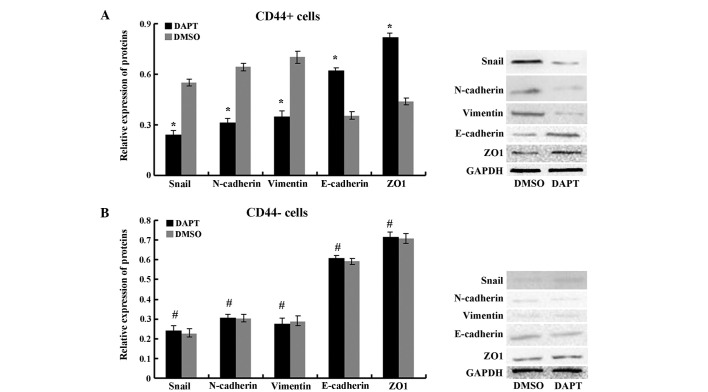
To further explore the molecular mechanism of the inhibition of DAPT on EMT in CSCs, the expression of EMT markers was examined. Western blot analysis demonstrated that the protein expression levels of the epithelial markers E-cadherin and ZO1 were upregulated, and expression of the mesenchymal markers N-cadherin, Vimentin and Snail were downregulated in CD44+ cells that were treated with DAPT compared with cells treated with DMSO. The expression levels of these EMT markers were not changed by DAPT treatin CD44− MKN45 cells (Fig. 8; P>0.05). Altogether, these results indicated that the γ-secretase inhibitor DAPT could impair EMT in CD44+ MKN45 cells (Fig. 8).
Figure 8.
EMT markers were analyzed in (A) CD44+ and (B) CD44− MKN45 cells by immunoblotting after treatment with DAPT or DMSO. Data are presented as the mean ± SD; n=5. *P<0.05, #P>0.05.
Discussion
CSCs have been identified in a number of malignancies and are functionally defined by their ability to undergo self-renewal and produce differentiated progeny (11,12). CSCs may display certain properties and they may be isolated based on cell surface-marker profiles (13). CSCs exhibit increased resistance against conventional chemotherapy (14,15), and they may initiate tumors at limiting dilutions in animals (5). A number of previous studies have validated the CSC hypothesis by isolating CSCs from gastric cancer patients, and CD44 has been identified as a surface marker of GCSCs (16–20). In the present study, CD44+ MKN45 cells were sorted by FACS. CD44+ cells exhibited increased resistance against chemotherapy and formed a greater number of spheroids in vitro and tumors in vivo, which indicated that CD44+ MKN45 cells possessed properties of CSCs.
Numerous studies have focused specifically on the signaling pathways that may mediate the resistance of CSCs (21,22). Notch signaling is involved in the development and progression of several solid tumors (23). In addition, Notch1 signaling is implicated in the self-renewal of various types of CSCs, including breast cancer, medulloblastoma and pancreatic cancer (8). Notch1 activation induces up-regulation of Hes1 expression, which dictated cell fate decisions in hematopoietic stem and progenitor cells (24). In the present study, the role of the Notch signaling pathway was investigated in GCSCs. Gastric cancer cell line MKN45 was derived from gastric cancer metastatic tissue (25). In addition, Notch1 expression was upregulated in MKN45 cells compared with other cells derived from non-metastatic gastric cancer in our previous studies (26). Therefore, MKN45 cells were selected for the present study. In the present study, the expression levels of Notch1 and its downstream target Hes1 were higher in CD44+ MKN45 cells compared with CD44− cells, which is in accordance with previous studies (27).
The interaction of Notch ligands with their receptors promotes a γ-secretase-dependent cleavage of the Notch receptor and releases the Notch intracellular domain (NICD), which results in activation of the pathway and induces target genes such as Hes1. Therefore, suppressing γ-secretase function with the γ-secretase inhibitor DAPT blocks the Notch signaling pathway (9). To explore the function of Notch1 signaling, CD44+ and CD44− cells were treated with DAPT. Hes1 expression was downregulated in CD44+ cells. Meanwhile, the self-renewal, tumor-initiation, migration and invasion abilities of CD44+ cells were inhibited, and chemotherapy susceptibility was improved. Treatment with DAPT resulted in a significant inhibition of gastric CD44+ cells in vivo. However, those phenomena did not appear in CD44− cells. These data indicate that the Notch1 pathway may contribute to maintaining properties of GCSCs and their ability for migration and invasion.
To explore the molecular mechanisms of DAPT's effect on GCSCs, the expression of EMT markers were determined. During EMT, epithelial cells lose many of their epithelial characteristics, such as the expression of E-cadherin and ZO1, and instead, acquire properties that are typical for mesenchymal cells such as the expression of vimentin. DAPT treatment inhibited the expression of Notch1 signaling pathway members and its downstream target Hes1, which reduced Snail expression and impaired EMT in CD44+ cells: These results were in accordance with other previous studies (28,29). It has been proposed that transformed epithelial cells may activate embryonic programs for epithelial plasticity and switch from sessile and epithelial phenotypes to motile and mesenchymal phenotypes (30). Therefore, the induction of EMT may lead to the invasion of surrounding stroma, intravasation, dissemination and colonization of distant sites (31). Under the CSC hypothesis, sustained metastatic growth requires the dissemination of a CSC from the primary tumor followed by its reestablishment at a secondary site (32). In EMT, cancer cells acquire the ability to invade the surrounding microenvironment and, thus, may lead to relapse and metastases (33).
The findings of the present study support the idea that the CD44+ population in human gastric cancer possess properties of CSCs and corroborate the CSC hypothesis. In addition, the role of the Notch1 signaling pathway in GCSCs was demonstrated and inhibition by DAPT was shown to impair properties of GCSCs and the process of EMT. In the future, clinical investigations are needed to confirm these results and to establish DAPT as part of the treatment for gastric cancer patients.
Acknowledgements
The authors would like to acknowledge grant support from the National Natural Science Foundation of China (no. 81172387) and the National Natural Science Foundation of Chongqing (CSTC 2011BB5119).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;3:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Kesari S, Rooney C, et al. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010;1:822–835. doi: 10.1177/1947601910383564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells byinhibiting Wnt, Notch and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 5.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 6.Brzozowa M, Mielańczyk L, Michalski M, et al. Role of Notch signaling pathway in gastric cancer pathogenesis. Contemp Oncol (Pozn) 2013;17:1–5. doi: 10.5114/wo.2013.33765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piazzi G, Fini L, Selgrad M, et al. Epigenetic regulation of Delta-Like1 controls Notch1 activation in gastric cancer. Oncotarget. 2011;2:1291–1301. doi: 10.18632/oncotarget.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannuti A, Foreman K, Rizzo P, et al. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16:3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palagani V, El Khatib M, Kossatz U, et al. Epithelial mesenchymal transition and pancreatic tumor initiating CD44+/EpCAM+ cells are inhibited by γ-secretase inhibitor IX. PLoS One. 2012;7:e46514. doi: 10.1371/journal.pone.0046514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin B, Zeng Y, Liu G, Wang X, Wang P, Song Y. MAGE-A3 is highly expressed in a cancer stem cell-like side population of bladder cancer cells. Int J Clin Exp Pathol. 2014;7:2934–2941. [PMC free article] [PubMed] [Google Scholar]
- 11.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Alison MR, Murphy G, Leedham S. Stem cells and cancer: A deadly mix. Cell Tissue Res. 2008;331:109–124. doi: 10.1007/s00441-007-0510-7. [DOI] [PubMed] [Google Scholar]
- 13.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 14.Tan S, Chen JS, Sun LJ, Yao HR. Selective enrichment of hepatocellular cancer stem cells by chemotherapy. J Int Med Res. 2009;37:1046–1056. doi: 10.1177/147323000903700409. [DOI] [PubMed] [Google Scholar]
- 15.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 16.Han ME, Jeon TY, Hwang SH, Lee YS, Kim HJ, Shim HE, Yoon S, Baek SY, Kim BS, Kang CD, Oh SO. Cancer spheres from gastric cancer patients provide an ideal model system for cancer stem cell research. Cell Mol Life Sci. 2011;68:3589–3605. doi: 10.1007/s00018-011-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Yang K, Yu J, Meng W, Yuan D, Bi F, Liu F, Liu J, Dai B, Chen X, et al. Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived from gastric adenocarcinoma patients. Cell Res. 2012;22:248–258. doi: 10.1038/cr.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang J, Zhang Y, Chuai S, Wang Z, Zheng D, Xu F, Zhang Y, Li C, Liang Y, Chen Z. Trastuzumab (herceptin) targets gastric cancer stem cells characterized by CD90 phenotype. Oncogene. 2012;31:671–682. doi: 10.1038/onc.2011.282. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Li C, He F, Cai Y, Yang H. Identification of CD44+CD24+ gastric cancer stem cells. J Cancer Res Clin Oncol. 2011;137:1679–1686. doi: 10.1007/s00432-011-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu G, Shen J, Ou Yang X, Sasahara M, Su X. Cancer stem cells: The 'heartbeat' of gastric cancer. J Gastroenterol. 2013;48:781–797. doi: 10.1007/s00535-012-0712-y. [DOI] [PubMed] [Google Scholar]
- 21.Teng Y, Wang X, Wang Y, Ma D. Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392:373–379. doi: 10.1016/j.bbrc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Song Z, Yue W, Wei B, Wang N, Li T, Guan L, Shi S, Zeng Q, Pei X, Chen L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6:e17687. doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch U, Radtke F. Notch signaling in solid tumors. Curr Top Dev Biol. 2010;92:411–455. doi: 10.1016/S0070-2153(10)92013-9. [DOI] [PubMed] [Google Scholar]
- 24.Oh P, Lobry C, Gao J, et al. In vivo mapping of notch pathway activity in normal and stress hematopoiesis. Cell Stem Cell. 2013;13:190–204. doi: 10.1016/j.stem.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng HC, Takahashi H, Li XH, et al. Downregulated parafibromin expression is a promising marker for pathogenesis, invasion, metastasis and prognosis of gastric carcinomas. Virchows Arch. 2008;452:147–155. doi: 10.1007/s00428-007-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li LC, Peng Y, Liu YM, Wang LL, Wu XL. Gastric cancer cell growth and epithelial-mesenchymal transition are inhibited by γ-secretase inhibitor DAPT. Oncol Lett. 2014;7:2160–2164. doi: 10.3892/ol.2014.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan B, Liu L, Zhao Y, et al. Xiaotan Sanjie decoction attenuates tumor angiogenesis by manipulating Notch-1-regulated proliferation of gastric cancer stem-like cells. World J Gastroenterol. 2014;20:13105–13118. doi: 10.3748/wjg.v20.i36.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ischenko I, Seeliger H, Kleespies A, Angele MK, Eichhorn ME, Jauch KW, Bruns CJ. Pancreatic cancer stem cells: New understanding of tumorigenesis, clinical implications. Langenbecks Arch Surg. 2010;395:1–10. doi: 10.1007/s00423-009-0502-z. [DOI] [PubMed] [Google Scholar]
- 29.Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abell AN, Johnson GL. Implications of mesenchymal cells in cancer stem cell populations: relevance to EMT. Curr Pathobiol Rep. 2014;2:21–26. doi: 10.1007/s40139-013-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankar S, Nall D, Tang SN, Meeker D, Passarini J, Sharma J, Srivastava RK. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One. 2011;6:e16530. doi: 10.1371/journal.pone.0016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo W. Concise review: breast cancer stem cells: regulatory networks, stem cell niches, and disease relevance. Stem Cells Transl Med. 2014;3:942–948. doi: 10.5966/sctm.2014-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dave B, Mittal V, Tan NM, Chang JC. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2012;14:202. doi: 10.1186/bcr2938. [DOI] [PMC free article] [PubMed] [Google Scholar]