Abstract
Background
Despite the limited success after decades of intensive research and development efforts, vaccination still represents the most promising strategy to significantly reduce the disease burden in malaria endemic regions. Besides the ultimate goal of inducing sterile protection in vaccinated individuals, the prevention of transmission by so-called transmission blocking vaccines (TBVs) is being regarded as an important feature of an efficient malaria eradication strategy. Recently, Plasmodium falciparum GAP50 (PfGAP50), a 44.6 kDa transmembrane protein that forms an essential part of the invasion machinery (glideosome) multi-protein complex, has been proposed as novel potential transmission-blocking candidate. Plant-based expression systems combine the advantages of eukaryotic expression with a up-scaling potential and a good product safety profile suitable for vaccine production. In this study we investigated the feasibility to use the transient plant expression to produce PfGAP50 suitable for the induction of parasite specific inhibitory antibodies.
Results
We performed the transient expression of recombinant PfGAP50 in Nicotiana benthamiana leaves using endoplasmatic reticulum (ER) and plastid targeting. After IMAC-purification the protein yield and integrity was investigated by SDS-PAGE and Western Blot. Rabbit immune IgG derived by the immunization with the plastid-targeted variant of PfGAP50 was analyzed by immune fluorescence assay (IFA) and zygote inhibition assay (ZIA). PfGAP50 could be produced in both subcellular compartments at different yields IMAC (Immobilized Metal Affinity Chromatography) purification from extract yielded up to 4.1 μg/g recombinant protein per fresh leaf material for ER-retarded and16.2 μg/g recombinant protein per fresh leave material for plasmid targeted PfGAP50, respectively. IgG from rabbit sera generated by immunization with the recombinant protein specifically recognized different parasite stages in immunofluorescence assay. Furthermore up to 55 % inhibition in an in vitro zygote inhibition assay could be achieved using PfGAP50-specific rabbit immune IgG.
Conclusions
The results of this study demonstrate that the plant-produced PfGAP50 is functional regarding the presentation of inhibitory epitopes and could be considered as component of a transmission-blocking malaria vaccine formulation.
Electronic supplementary material
The online version of this article (doi:10.1186/s12896-015-0225-x) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium falciparum, Sexual stage, Gametes, Agroinfiltration, Plant-made vaccines, Plastid targeting
Background
Still affecting millions of people around the world, predominantly in developing countries [1], malaria is one of the most relevant poverty-related infectious tropical diseases. Even though prophylactic and therapeutic agents exists, they are not broadly available and affordable for the local populations. Measures of vector elimination and control by draining mosquito breeding pools, application of insecticides, and bed nets have not been sufficiently effective in many cases. Additionally, increasing resistances of the parasite and the vector against both, chemical control measures and medical treatments are a growing problem [2, 3]. Therefore, the availability of a vaccine is being regarded as an essential component of a successful malaria eradication strategy [4]. Despite intense research efforts no vaccine that provides robust sterile protection against malaria is available today. Involving two different hosts, mosquito and man, the life cycle of P. falciparum is complex and features three different stages. As summarized in the WHO rainbow table [5], the majority of proteins used in the context of malaria vaccine candidates today and in the past, are either pre-erythrocytic, or blood stage antigens like PfCSP [6], proteins from the merozoite surface protein family (PfMSPn) [7], PfAMA1 [8] and other blood stage surface proteins [9]. After successful completion of clinical trials GSK’s circumsporozoite protein (CSP)-based pre-erythrocytic stage vaccine Mosquirix® [10] received a positive opinion from EMA and is expected to enter the market soon, following WHO recommendation and clearance by respective national regulators. Even though being by far the most advanced malaria vaccine, the impact of Mosquirix® is expected to be limited by moderate efficacy (only up to 60–70 % of the vaccinees protected) and relatively short-lived protection [11]. Besides vaccines targeting the pre-erythrocytic stage of the parasite and therefore aiming at the induction of sterile protection by preventing the initial establishment of the infection, there are at least two other types of vaccines. Blood stage vaccines could be useful to suppress manifestation of clinical symptoms caused by high parasite load in the blood of the patients, while so called transmission blocking vaccinestarget the sexual stages of the parasite to prevent proliferation in, or trafficking through the mosquito and thereby inhibit the transmission of the parasites from infected to healthy individuals. In the context of malaria eradication efforts and after a recent update of the Malaria Vaccine Technology Roadmap in 2013 [12], transmission-blocking vaccines have recently received elevated attention. The first and most advanced transmission-blocking malaria vaccine candidate is Pfs25, a 24 kDa post-fertilization macrogamete/zygote surface antigen featuring four epidermal growth factor (EGF)-like domains [13, 14]. It has been shown in different studies that immunization with recombinant Pfs25 induces antibodies with strong transmission-blocking activity [15–18]. Being exclusively expressed in parasite stages that develop after fertilization within the mosquito and thereby being naturally not exposed to the human immune system the highly conserved Pfs25 represents an excellent target for vaccine induced transmission-blocking antibody responses. Other potentially transmission-blocking vaccine candidates in development are the pre-fertilization gamete/gametocyte antigens Pfs230 and Pfs48/45. In contrast to Pfs25 these proteins have been shown to be targets of natural immune responses [19] since they are already expressed on gametocytes that (within infected erythrocytes) occur in the human host.
PfGAP50, a 44.6 kDa transmembrane protein, forms an essential part of the actin-myosin motor complex driven invasion machinery (glideosome) associated to the multi-protein complex called inner membrane complex (IMC) [20–23]. Detailed investigations on the role of PfGAP50 during sexual stage development presented by Simon et al. [24] indicate that the protein relocates from the IMC to the plasma membrane during gametocyte activation and gametocyte egress from erythrocytes triggered by change of temperature, pH and presence of xanthurenic acid encountered within the mosquito midgut after a blood meal. Since in this work it has also been shown that PfGAP50 may protect the gametes from complement-mediated lysis by binding the human complement Factor H, the protein can be regarded as novel transmission-blocking candidate.
Plant-based transient expression systems are robust, fast and scalable platforms capable of oxidative folding, assembly of multimeric proteins and high level expression [25]. Several pharmaceutically relevant proteins like monoclonal antibodies [26], therapeutic enzymes [27] as well as antigens, including malaria vaccine candidates [28–33] have been produced successfully by transient plant expression. The Agrobacterium tumefaciens transient plant expression system offers the chance to compare the efficiency of recombinant protein expression in various subcellular compartments (ER, cytoplasm, plastids) with different features regarding oxidative folding or post-translational modifications [34, 35].
Here, we report the successful plant-based production and characterization of the novel TBV candidate PfGAP50. The plastid targeted PfGAP50 was obtained at high quality by one-step IMAC purification and used for the immunization of rabbits. The resulting rabbit immune IgG preparations were used in different in vitro assays to confirm the induction of antibodies that recognize PfGAP50 in the native context.
Results
Cloning the PfGAP50 expression constructs
The cDNA coding for the extracellular domain of PfGAP50 (Fig. 1a) was cloned into the binary plant expression vectors pTRAkc-ERH and pTRAkc-cTPH. For the cloning we used cDNA encoding amino acids Q26 to R369 featuring the PfGAP50 without the native N-terminal signal peptide (to be replaced by the plant-specific targeting signals) and without the transmembrane domain to enhance the solubilty of the protein targeted to either the ER or plastid. The resulting pTRAkc-GAP50-ERH (Fig. 1b) construct features sequences for an N-terminal signal peptide (for targeting the protein to the secretory pathway), and a C-terminal His6-tag (for affinity purification and detection) followed by a SEKDEL ER-retrieval signal (for ER-retention). The second expression construct pTRAkc-GAP50-cTPH (Fig. 1b) provides an N-terminal chloroplast targeting peptide and a C-terminal His6-tag.
Fig. 1.
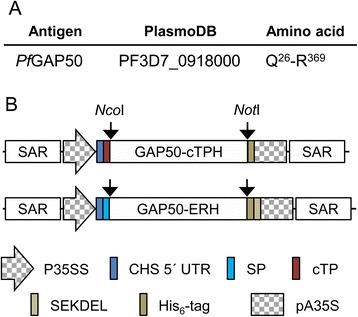
Gene IDs and plant expression cassettes. a Names, accession numbers and amino acid sequence range of PfGAP50 (for further details refer to the methods section). b Schematic drawing of the expression cassettes in the plant binary expression vector pTRAkc (not to scale). SAR: scaffold attachment region; P35SS: Cauliflower mosaic virus 35S promoter with duplicated 35S enhancer region; CHS 5ʹ UTR: 5ʹ untranslated region of the Petroselinum crispum chalcone synthase gene; SP: transit peptide sequence of the murine antibody 24 heavy chain [60]; TP: chloroplast targeting signal from small subunit of RuBisCO from Solanum tuberosum; GAP50: PfGAP50; His6-tag: six histidine tag for IMAC purification; SEKDEL: ER-retention signal. pA35S: Cauliflower mosaic virus 35S polyadenylation signal. Relevant restriction sites are indicated
Transient expression and purification of PfGAP50 in N. benthamiana
Both expression constructs were used for transient plant expression of PfGAP50. The recombinant protein was purified by IMAC from plant extracts prepared from infiltrated N. benthamiana leaves 5 days post-infiltration (dpi). The experiment was repeated independently three times for both constructs and a mock purification using wild type material was carried out once to simplify the identification of potential contaminants. Figure 2a and b exemplarily show the SDS-PAGE and immunoblot analysis of one experiment per construct (SDS-PAGE featuring the samples from all replicates is shown in additional file 1). The simple one-step purification procedure using three step elution (10 mM, 100 mM and 250 mM) yielded PfGAP50 proteins at different levels of purity and high integrity. As shown in Fig. 2a and b, elution at 10 mM Imidazole only yielded few host cell proteins (to a large proportion presumably the large subunit of Ribulose-1,5-bisphosphat-carboxylase/-oxygenase (RuBisCo), 56 kDa) and no detectable PfGAP50-ERH or PfGAP50-cTPH (as shown by His6-specific immunoblot), at 100 nM we detected host cell proteins at higher abundance as well as detectable amounts of target proteins. The elution fraction E3 (250 mM Imidazole) in both cases contained the highest amount of PfGAP50 and one major host cell protein band running at the size of the large subunit of RuBisCo. Yields were determined by densitometric analysis of SDS-PAGE lanes containing the E3 fractions against a BSA standard curve derived from 4 different concentrations of BSA. The clear differences in yield between the ER-retarded and the chloroplast-targeted variant of the protein were observed in several other experiments (data not shown). Figure 2c shows the average E3 yields obtained after three expression and purification experiments of PfGAP50-cTPH (16.2 ± 1.8 μg/g fresh leaf weight (FLW), 7 g leaf material for each repeat) and PfGAP50-ERH (4.1 ± 0.98 μg/g FLW, 4.5 g leaf material for each repeat). The differences are statistically significant P = 0.0005. The higher accumulation levels of intact PfGAP50 also correlated with the achieved relative abundance calculated by densitometric analysis of SDS-PAGE image (Additional file 1) for each purification experiment. For both PfGAP50 variants (PfGAP50-cTPH and PfGAP50-ERH) the highest purity was obtained in the third elution step (E3 fraction) at 250 mM imidazole, while the previous step at 100 mM imidazole still contained a number of host cell proteins, presumably the large subunit of the RuBisCo together with the recombinant protein. This protein was also present as the major contamination in the E3 fraction. In E3 PfGAP50-cTPH was obtained at a relative abundance of 73 % ± 8.5 while PfGAP50-ERH was less pure (32.3 % ± 3.1). The corresponding E3 fraction from an up-scaled infiltration experiment (PfGAP50-cTPH) was used for the immunization of rabbits as described in the methods section.
Fig. 2.
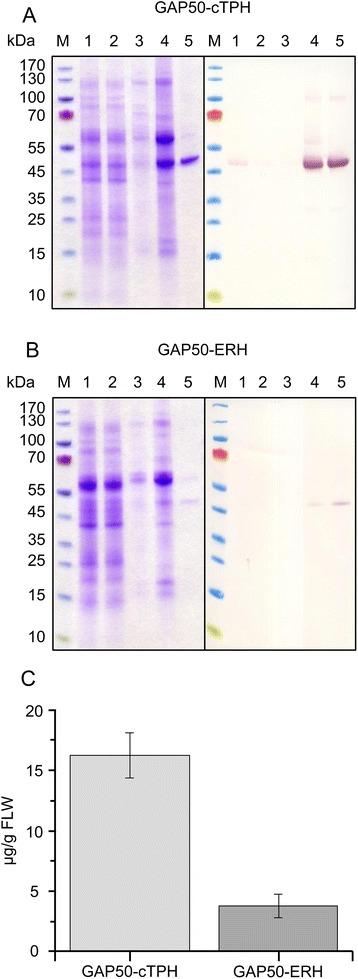
SDS-PAGE/immunoblot analysis of plant produced PfGAP50. a: Purification of plastid-targeted PfGAP50 (PfGAP50-cTPH). For purification, 7.0 g infiltrated leaf material were used. Reducing SDS-PAGE (left panel) and immunoblot (right panel). b: Purification of ER-retarded PfGAP50 (PfGAP50-ERH). For purification, 4.5 g infiltrated leaf material were used. Reducing SDS-PAGE (left panel) and immunoblot (right panel). M: Prestained protein marker (Page Ruler Fermentas), 1: 3 μL load (plant extract), 2: 3 μL flow-through, 3: 6 μL elution step 1 (10 mM imidazole), 4: 6 μL elution step 2 (100 mM imidazole), 5: 6 μL elution step 3 (250 mM imidazole). Western blot was detected with rabbit anti-His6 serum and alkaline phosphatase labeled goat anti rabbit serum. c: Plot of mean values and standard deviation of the yields for finally purified (E3) PfGAP50-cTPH and PfGAP50-ERH, determined by densitometric analysis (against BSA equivalents) of SDS-PAGE from three independent replicates (SDS-PAGE shown in additional file 1)
Determination of antigen-specific titers
To initially assess the immunogenicity and parasite inhibitory efficacy of the recombinant PfGAP50-cTPH, two rabbits were immunized using a hyper immunization protocol. Endpoint IgG titers against PfGAP50 were determined in serum samples taken on days 0, 35, 63 and 91 as described in the methods section. As shown in Fig. 3, the resulting titers are moderate for both rabbits with a maximum antibody titer observed after day 63 with 1:2.6 x 104 for rabbit number two. The titers did not increase after subsequent boosts.
Fig. 3.
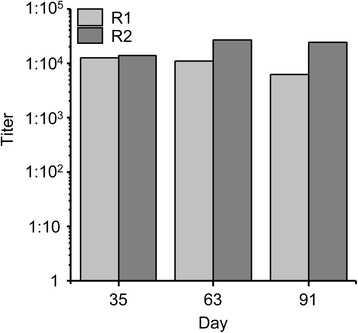
PfGAP50-specific titers in rabbit immune sera. Immune sera were analyzed by direct ELISA after the first (day 35), the second (day 63) and third boost (day 91). The threshold for titer definition was twofold the background signal obtained from the pre-immune sera. R1: rabbit 1, R2: rabbit 2
Immunofluorescence assay
An IFA was used to determine the reactivity of PfGAP50-specific rabbit immune IgG against P. falciparum schizonts, gametocytes and gametes. As shown in Fig. 4, rabbit antibodies raised against PfGAP50 specifically labeled the surfaces of the three different stages. Co-labeling with mouse antibodies against PfMSP1 and Pfs25 was used for stage-specific counterstaining. No fluorescence signal was observed when NRS was used for immunolabeling in control experiments.
Fig. 4.
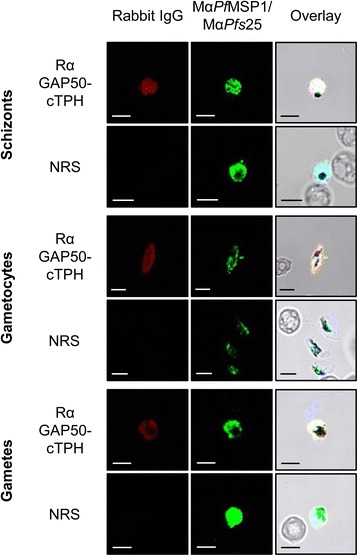
Indirect immunofluorescence assays demonstrating the reactivity of PfGAP50 specific rabbit immune IgG against different stage P. falciparum parasites. IFAs were performed on schizonts, gametocytes and gametes, using PfGAP50 specific rabbit immune IgG or IgG purified from neutral rabbit serum (NRS) as a negative control. Mouse anti-Pfs25 and anti-PfMSP1 antibodies were used to co-label the sexual-stage (Pfs25) and blood stage (PfMSP1) parasites, respectively. Mouse antibodies were visualized with Alexa Fluor 488-conjugated secondary antibodies (green) and rabbit antibodies with Alexa Fluor 594-conjugated secondary antibodies (red). Bar = 5 μm
Zygote inhibition assay (ZIA)
The ability of PfGAP50-specific rabbit immune IgG to inhibit zygote development was measured using a ZIA. As shown in Fig. 5, we observed up to 55 % zygote inhibition activity when using 1 mg/mL of the purified rabbit IgG (bleed2, day 63 and bleed 3, day 91 from both rabbits, R1 and R2, respectively).
Fig. 5.
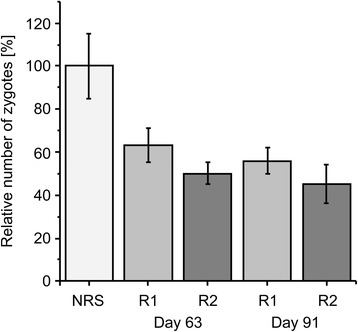
Zygote inhibition assay (ZIA) with PfGAP50 specific rabbit immune IgG. The transmission-blocking potential of the PfGAP50-cTPH specific rabbit immune IgG (rabbit 1 (R1) and rabbit 2 (R2)) from 2nd (day 63) and 3rd bleed (day 91) was assessed in a ZIA experiment in comparison with IgG purified from neutral rabbit serum (NRS) as a negative control. Inhibition was calculated as the reciprocal value of zygote numbers in comparison with the negative control. Error bars were derived by generating mean and SD of the values obtained for immune IgG from the two individual rabbits using data from three technical replicates
Discussion
Mosquirix (RTS’S), a pre-erythrocytic malaria vaccine candidate based on PfCSP presented on the surface of Hepatitis B virus-like particles is now approaching final regulatory approval. Anyhow, data from the respective clinical trials indicate that this vaccine will not match the efficacy and the sustainability of vaccines against other infectious diseases and suggests further efforts towards improved malaria vaccine formulations eventually featuring additional antigens from the blood and the sexual stages of the parasite to introduce functionalities that complement the strategy of inducing sterile protection by immune responses against pre-erythrocytic antigens. In this context a vaccine component that provides transmission blocking activity is being regarded as a valuable contribution to the goal of malaria eradication.
Here, we describe the transient plant-based production and characterization of PfGAP50 as transmission-blocking vaccine candidate antigen. We have used the A. tumefaciens-based transient plant expression platform successfully in previous studies for the expression and characterization of different P. falciparum antigens from the pre-erythrocytic, the blood and the sexual stage as single proteins [28] or as fusions [33, 36]. Along this line, and driven by the overall vision of implementing this platform for the rapid and cost efficient production of vaccine antigens in the context of malaria vaccine R&D and finally even clinical material, we were interested to evaluate the feasibility to use the transient plant-based expression to produce PfGAP50 suitable for the induction of parasite-specific inhibitory antibodies.
Bosch et al. [37] have produced folded, soluble PfGAP50 in the cytosol of E. coli for structural studies. These results indicate that PfGAP50 may not depend on an oxidative environment like the plant endoplasmatic reticulum (ER) for correct folding. Therefore, making use of the possibility to easily address different subcellular compartments within the plants, we generated two expression constructs featuring PfGAP50, one ER-retarded and the other one targeted to the plastids. Even though plastids lack oxidative conditions and the complex, chaperon-based folding machinery of the secretory pathway constituted by the ER and the Golgi apparatus, high-level expression of recombinant proteins has been achieved in plastids for several examples with transient, targeting-based [35, 38, 39] and stable, transplastomic approaches [40].
In our study we observed significantly higher yields for recombinant PfGAP50 (±16.2 ± 1.9 μg/g FLW) targeted to the plastids when compared to PfGAP50 (±4.1 ± 0.98 μg/g FLW) accumulated in the ER. These values were calculated by quantification of the finalmaterial (partially purified by IMAC) and related to yield after extraction and purification. The differences in achieved purities (73 % ± 8.5 for PfGAP50-cTPH and 32.3 % ± 3.1 for PfGAP50-ERH) resulted from a constant amount (around 1-2 μg/g FWL) of a predominant co-purified host cell protein (presumably the large subunit of RuBisCo).
The observation that a 4-fold higher yield could be achieved by chloroplast targeting compared to the ER-retarded version of PfGAP50 was not predictable, but indicates that this strategy should be generally considered when using plant expression systems for the production of recombinant proteins at optimal yields. In a study on the transient and stable plant-based expression of different HIV-I antigen constructs Meyers et al. [41] also compared ER-retardation with chloroplast targeting and found large protein-specific differences in yields, including up to 20-fold higher (HIV-I p17/p24) or 4-fold lower yields (HIV-I p24), by chloroplast targeting compared to ER-retardation. These results also emphasize the versatility of the transient plant expression system as a tool in vaccine candidate development and evaluation, since different properties and requirements of heterologous proteins like vaccine candidate antigens can be addressed by simply testing the respective proteins in the context of different subcellular targeting options.
The yields of 16 and 4 μg/g FLW were calculated for comparative purposes only considering the PfGAP50 amounts found in the elution fraction E3. Since it is obvious that also the fraction E2 contains relevant amounts of PfGAP50 (especially for PfGAP50-cTPH, where E2 seems to contains at least the same amount as E3) it can be speculated that by developing a more advanced extraction and purification strategy [42, 43] it should be possible to significantly increase the yield of PfGAP50-cTPH towards levels of 30-50 μg/g FLW. These assumptions can be used to briefly asses the question of economic perspectives of the production of this transmission-blocking vaccine candidate by transient expression in plants. As basis for this estimation one can refer to the numbers provided by Tusé et al. [44] in an article about the manufacturing economics of plant-made biologics. In this context the per/dose production costs (upstream) for a recombinant butyrylcholinesterase manufactured by transient plant-based expression for therapeutic purposes were calculated as 474 $ (production costs upstream) per dose based on the following variables (Facility capacity: 25 kg recombinant protein/year at expression levels of 500 μg/g, Overall Yield: 100 μg/g FLW, 1 dose: 400 mg). Adapting this calculation to a 25 μg dose, the per/dose production costs for PfGAP50 at an overall yield of 50 μg/g would be 0.06 $ or 0.19 $ at an overall yield (as observed in this study under pre-process development conditions) of 16 μg/g at a capacity of 32 000 000–100 000 000 doses per year. These numbers can be combined with previously calculated numbers presented by [45] assuming that downstream costs will typically account for around 80 % of the total production costs. For the PfGAP50 scenario this would mean between 0.30 $ (yield 50 μg/g) and 0.95 $ (yield 16 μg/g). With these total production costs it should be possible to match a vaccine price that would provide cost effectiveness in a malaria endemic region, given a sufficient efficacy of the vaccine. Based on models [46] and empirical data [47] the cost effectiveness of a pre-erythrocytic vaccine (for the model) and more specifically RTS,S (within a trial setting) has been calculated. These complex calculations do rely on a large number of factors, including efficacy, transmission rate, and look at the socio-economic impact of mild, as well as severe infections and therefore should be regarded as a very rough estimate, when being transferred to another scenario. Anyhow, together with the results from another modeling approach that also involves transmission blocking vaccines [48] it seems reasonable to assume cost effectiveness of a decent transmission blocking vaccine at a vaccine price somewhere between 2 and 10 $, and therefore roughly 10-times higher than the assumed total production costs for PfGAP50 derived from a calculation that was working with a production facility able to produce 32 000 000 to 100 000 000 doses per year.
Because of the better yields and higher purity, the plastid-targeted PfGAP50-cTPH was chosen for rabbit immunization studies. Using a standard hyper immunization protocol moderate titers below 1:1x105were observed in both rabbits. In contrast to other rabbit immunizations we conducted with plant-produced P. falciparum antigens under the same conditions [28, 36], in this study we did not observe an increase of antigen-specific titers after the second and/or the third boost. In contrast, for rabbit R1 the titers even slightly decreased over the course of the immunization period. There are different possible explanations for this somehow unexpected result (e.g. lack of suitable T-helper epitopes, immune suppressive activity, influences of animal housing conditions) but given the low number of animals (two) used in this study the observation should probably not be overestimated.
The ability of PfGAP50-cTPH-specific rabbit IgG to recognize PfGAP50 in its native context was assessed by immunofluorescence assays. In these experiments, a staining of blood stage (schizonts) as well as sexual stage parasites (gametocytes and gametes) was observed. Because in schizonts and gametocytes before activation the PfGAP50 is part of the actin-myosin motor complex driven invasion machinery (glideosome) associated to the multi-protein complex called inner membrane complex (IMC), the cell membranes were permeabilized by saponin washing upon preparation of these samples. These results correlate well with what has been observed by Simon et al. [24] after mouse immunization studies performed with recombinant PfGAP50-GST fusions produced in E. coli and confirm that properly folded PfGAP50 could be produced by transient plant-based expression.
To initially investigate the parasite inhibitory and thereby transmission-blocking potential of the PfGAP50-cTPH-specific antibodies a zygote inhibition assay was performed by the addition of PfGAP50-cTPH-specific rabbit immune IgG to gametocyte cultures. The observed reduction of zygote numbers after 16 h of incubation between 37 % and 55 % is in good accordance with the 44 % reduction observed by Simon et al. [24] with PfGAP50-specific murine antibodies, providing another indication that the plant-produced PfGAP50 is functional regarding the presentation of inhibitory epitopes and is generally suitable as component of a transmission-blocking malaria vaccine formulation.
Taken together the results encourage the investigation of chloroplast targeting for other malaria vaccine constructs using the transient expression system. This would be especially interesting for antigens like Pfs25, PfAMA1, PfMSP3 or others that contain potential N-linked glycosylation sites which are not post-translationally modified in the native context because Plasmodium lacks the molecular machinery for N-linked glycosylation [49]. In most cases when potentially N-glycosylated P.falciparum antigens are produced in eukaryotic expression hosts N-glycosylation is being prevented by mutation of the NxT/S motifs in the amino acid sequence of the proteins [50]. Another option that has been applied for the generation of diversity covering variants of PfAMA1 [51] is the identification of allelic variations that do not contain the motive at a respective site, allowing to use a native P.falciparum sequence instead of an artificial one resulting from the introduced mutation of the N or the T/S residue. Using chloroplast targeting, it is possible to express the fully native, non-glycosylated protein sequence in an environment that is suitable for the expression of at least some folded proteins as already shown for the expression of the disulfide-rich P.falciparum sexual stage vaccine antigens Pfs25 and Pfs28 in the chloroplasts of the eukaryotic green microalgae, Chlamydomonas reinhardtii by Gregory et al. [52]. Another advantage of chloroplast targeting is the option to attach the recombinant antigens to starch granules by fusion to the granule bound starch synthase (GBSS), which enables the presentation of the antigen in the context of starch granules offering alternative purification strategies as well as potentially improved immunogenicity by the particulate format. This concept has been successfully proven with Plasmodium berghei and Plasmodium falciparum antigens expressed in a starch granule bound format in C. reinhardtii by Dauvillée et al. [53] and also the high immunogenicity of RTS,S the clinically most advanced malaria vaccine candidate based on the presentation of the pre-erythrocytic antigen PfCSP on the surface of hepatitis B virus S antigen (HBs Ag) based virus like particles, suggests the presentation of malaria antigens in a particulate format. In this context it should be also mentioned that plants also offer the possibility to express virus like particles from plant virus coat protein fusion proteins [54].
Conclusion
The results of this study demonstrate that PfGAP50 can be successfully produced in plants by transient transfection, and that significantly higher levels can be obtained by chloroplast targeting. The plant-produced PfGAP50 is functional regarding the presentation of inhibitory epitopes, can be used to induce parasite inhibitory antibodies and therefore should be considered as an interesting component of a transmission-blocking malaria vaccine formulation. Based on these promising results, we will focus on further optimizing the protein expression and purification as well as on strategies to improve the immunogenicity of the recombinant protein or its formulation before proceeding towards more detailed studies involving more animals and additional, more detailed, functional characterization of the protein itself as well as corresponding immune IgG in different parasite inhibition assays.
Methods
Bacteria, plants and parasites
Agrobacterium tumefaciens strain GV3101 : : pMP90RK [GmR, KmR, RifR] [55] and Nicotiana benthamiana plants were used for the production of the recombinant protein by agroinfiltration. Freshly prepared P. falciparum parasites strain NF54 were used for immunofluorescence assay (IFA) and zygote inhibition assay (ZIA) procedures. P. falciparum parasites were cultivated as previously described [56].
Plant expression constructs
The cDNAs encoding the P. falciparum strain 3D7 PfGAP50 extracellular domain without the signal peptide and GPI-anchor sequence was obtained as synthetic gene, codon-optimized for N. benthamiana from GeneArt (LifeTechnologies, Darmstadt, Germany). The PfGAP50 sequence was introduced via NcoI/NotI cloning either into the binary vector pTRAkc-ERH [57] between the signal peptide sequence and a His6-tag followed by the SEKDEL signal for endoplasmic reticulum (ER) retention [58] or into pTRAkc-cTPH, a modified variant of the binary vector pTRAk-(rbcs)cTP [59] between a plastid targeting signal sequence and a His6-tag (H). The resulting plasmids were named pTRAkc-GAP50-cTPH and pTRAkc-GAP50-ERH. All cloning steps were verified by DNA sequencing.
Transient expression in N. benthamiana
The pTRAkc-GAP50-cTPH and pTRAkc-GAP50-ERH vector were introduced into A. tumefaciens by electroporation using a Multiporator (Eppendorf AG, Hamburg, Germany) according to the manufacturer’s instructions. Recombinant A. tumefaciens carrying pTRAkc-GAP50-cTPH or pTRAkc-GAP50-ERH were used for the transient expression of PfGAP50 either as ER-retarded or as chloroplast targeted variant. Detailed cultivation and infiltration procedures have been reported elsewhere [28, 31, 33].
Extraction of total soluble protein from N. benthamiana leaves
The infiltrated N. benthamiana leaves were harvested 5 days post infiltration (dpi) and total soluble protein was extracted as previously described [33].
Immobilized metal affinity chromatography purification (IMAC)
After pH adjustment, the clarified extract was loaded onto a disposable column filled with 1 ml of Ni2+-charged Chelating Sepharose (GE Healthcare, Solingen, Germany). After washing with PBS (10 column volumes), bound proteins were eluted in a three-step gradient using PBS containing 10 mM, 100 mM and 250 mM imidazole.
SDS-PAGE and immunoblot analysis
SDS-PAGE and immunoblot analysis was performed as previously described [33]. The PfGAP50 variants (PfGAP50-ERH: ER-retarded and PfGAP50-cTPH chloroplast-targeted) were detected with rabbit anti-His6 antiserum (GenScript, Piscataway, NY) and an alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Europe Ltd., Suffolk, UK) both diluted 1:5000 in PBS. BSA standard (NEB) at 900, 600, 300 and 150 ng was used for comparative densitometric quantification of purified PfGAP50. The evaluation was performed using AIDA software (Raytest, Straubenhardt, Germany).
Rabbit immunization, titer determination and IgG purification
Rabbits were housed, immunized and sampled by Biogenes GmbH (Berlin, Germany), according to national animal welfare regulations. The animal facilities and protocols were reviewed and approved by: Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei MecklenburgVorpommern (LALLF M-V) (Approval No: 7221.3-2-030-13). To isolate the blood after immunization according to national regulations the animals were anesthetized using Ventranquil, stunned using a captive bolt device and exsanguinated by throat cut. Two rabbits were hyper immunized intramuscularly with the plant-derived and purified PfGAP50-cTPH formulated with a proprietary Biogenes adjuvant (an oil in water emulsion containing lipopolysaccharides) on days 0, 7, 14, 28, 49 and 77. 200 μg of antigen were used for prime and 100 μg for the five consecutive boosts. Serum samples were taken on days 35, 63 and 91. For titer determination, the samples from the three bleeds, as well as pre-immune serum, were tested for reactivity with the plant-derived PfGAP50-cTPH by enzyme-linked immunosorbent assay. Titer determination and IgG purification by Protein A was performed as previously described [28]. End-point titers were determined as the highest dilution that gave the double the value of the background (day 0, pre-immune serum).
Immunofluorescence assay (IFA)
IFAs were carried out on three different P. falciparum stages (schizonts, mature gametocytes, and macrogametes) by immunolabeling with 10 μg/mL purified PfGAP50-cTPH-specific rabbit IgG (day 63) or 10 μg/mL IgG purified from normal rabbit serum as previously described [33]. The sexual stages were co-labeled with antibodies against PfMsp1 (schizonts) or Pfs25 (mature gametocytes and macrogametes).
Zygote inhibition assay (ZIA)
The zygote inhibition potential of the PfGAP50-cTPH antisera was investigated by zygote inhibition assay (ZIA) as described in detail by Simon et al. [24]. PfGAP50-cTPH-specific rabbit immune IgG purified from day 63 and day 91 were used at concentrations of 1 mg/mL in the final assay volume. The numbers of zygotes were counted in triplicate using a hemocytometer.
Acknowledgments
P. falciparum NF54, and MRA-1000 deposited by M. Dowler, WRAIR, were obtained through the MR4 as part of the BEI Resources Repository, NIAID, NIH. Many thanks to Markus Sack for valuable discussions and input during the revision of the manuscript. This study was supported by the Fraunhofer Future Foundation via the project “Innovative technologies to manufacture ground-breaking biopharmaceutical products in microbes and plants”.
Abbreviations
- PfGAP50
Plasmodium falciparum glideosome associated protein 50
- TBV
Transmission-blocking vaccine
- ERH
ER-retention sequence with His6-tag
- cTPH
Plastid targeting sequence with His6-tag
- IMC
Inner membrane complex
- IFA
Immunofluorescence assay
- TBA
Transmission-blocking assay
- IMAC
Immobilized metal affinity chromatography
- FLW
Fresh leaf weight
- P35SS:
Cauliflower mosaic virus 35S promoter with duplicated 35S enhancer region
- CHS 5ʹ UTR
5ʹ untranslated region of the Petroselinum crispum chalcone synthase gene
- SP
Transit peptide sequence of the murine antibody 24 heavy chain [60]
- TP
Chloroplast targeting signal from small subunit of RuBiSCO from Solanum tuberosum
- His6-tag
Six histidine tag for IMAC purification
- SEKDEL
ER-retention signal
- pA35S
Cauliflower mosaic virus 35S polyadenylation signal
- NRS
Neutral rabbit serum
Additional file
SDS-PAGE based quantification (densitometric against a BSA standard) of Pf GAP50 variants in IMAC elution fractions (three independent replicate each). Mock purification of wt N. benthamiana extract was included. Proteins were eluted from the IMAC column using three elution steps (elution 1 (E1): 10 mM imidazole; elution 2 (E2): 100 mM imidazole and elution 3 (E3): 250 mM imidazole). 6 μl of each sample was loaded under reducing conditions. 1: 150 ng BSA/slot; 2: 300 ng BSA/slot; 3: 600 ng BSA/slot; 4: 900 ng BSA/slot; 5–7: E1-3 from mock purification; 8–10: E1-3 from PfGAP50-ERH repeat 1; 11–13: E1-3 from PfGAP50-ERH repeat 2; 14–16: E1-3 from PfGAP50-ERH repeat 3; 17–19: E1-3 from PfGAP50-cTPH repeat 1; 20–22: E1-3 from PfGAP50-cTPH repeat 2; 23–25: E1-3 from PfGAP50-cTPH repeat 3. (PDF 156 kb)
Footnotes
Veronique Beiss and Holger Spiegel contributed equally to this work.
Competing interest
AR and RF are inventors of a patent application entitled: Novel malaria transmission-blocking vaccines, Application number: US 20150191518 A1. VB, HS, AB, MS and SS declare to have no financial or non-financial conflict of interest.
Authors’ contributions
VB Participated in the design of the experiments, carried out the cloning, the expression, protein purification and the immunization study, participated in the analysis of the data in drafting the manuscript. HS participated in the design of the experiments, the data interpretation and drafted the manuscript. AB participated in the design of the experiments and analysis, participated in data interpretation and helped drafting the manuscript. MS participated in the design of the experiments and performed the zygote inhibition assays and participated in data interpretation. AR participated in the study design, the data interpretation and revised the manuscript. SS participated in study design and coordination and helped to draft the manuscript. RF conceived the study and participated in its design and revised the manuscript. All authors have read and approved the manuscript.
Contributor Information
Veronique Beiss, Email: veronique.beiss@ime.fraunhofer.de.
Holger Spiegel, Email: holger.spiegel@ime.fraunhofer.de.
Alexander Boes, Email: alexander.boes@ime.fraunhofer.de.
Matthias Scheuermayer, matthias.scheuermayer@googlemail.com.
Andreas Reimann, Email: andreas.reimann@ime.faunhofer.de.
Stefan Schillberg, Email: stefan.schillberg@ime.fraunhofer.de.
Rainer Fischer, Email: rainer.fischer@ime.fraunhofer.de.
References
- 1.WHO . Malaria Report. Geneva, Switzerland: World Health Organization; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairhurst RM, Nayyar GM, Breman JG, Hallett R, Vennerstrom JL, Duong S, et al. Artemisinin-resistant malaria: research challenges, opportunities, and public health implications. Am J Trop Med Hyg. 2012;87(2):231–241. doi: 10.4269/ajtmh.2012.12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plowe CV. The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg. 2009;103(Suppl 1):S11–14. doi: 10.1016/j.trstmh.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targett GA, Greenwood BM. Malaria vaccines and their potential role in the elimination of malaria. Malaria J. 2008;7. [DOI] [PMC free article] [PubMed]
- 5.Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379(9814):413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 6.Mian-McCarthy S, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BGNO, et al. A Phase 3 Trial of RTS, S/AS01 Malaria Vaccine in African Infants. New Engl J Med. 2012;367(24):2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Withers MR, McKinney D, Ogutu BR, Waitumbi JN, Milman JB, Apollo OJ et al. Safety and reactogenicity of an MSP-1 malaria vaccine candidate: A randomized phase Ib dose-escalation trial in Kenyan children. Plos Clin Trials. 2006;1(7). [DOI] [PMC free article] [PubMed]
- 8.Faber BW, Younis S, Remarque EJ, Garcia RR, Riasat V, Walraven V, et al. Diversity Covering AMA1-MSP1(19) Fusion Proteins as Malaria Vaccines. Infect Immun. 2013;81(5):1479–1490. doi: 10.1128/IAI.01267-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamborrini M, Stoffel SA, Westerfeld N, Amacker M, Theisen M, Zurbriggen R et al. Immunogenicity of a virosomally-formulated Plasmodium falciparum GLURP-MSP3 chimeric protein-based malaria vaccine candidate in comparison to adjuvanted formulations. Malaria J. 2011;10. [DOI] [PMC free article] [PubMed]
- 10.Wilby KJ, Lau TT, Gilchrist SE, Ensom MH. Mosquirix (RTS, S): A Novel Vaccine for the Prevention of Plasmodium falciparum Malaria. Ann Pharmacother. 2012;46(3):384–393. doi: 10.1345/aph.1Q634. [DOI] [PubMed] [Google Scholar]
- 11.Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kabore W, et al. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorthy VS, Newman RD, Okwo-Bele JM. Malaria vaccine technology roadmap. Lancet. 2013;382(9906):1700–1701. doi: 10.1016/S0140-6736(13)62238-2. [DOI] [PubMed] [Google Scholar]
- 13.Fries HCW, Lamers MBAC, Vandeursen J, Ponnudurai T, Meuwissen JHET. Biosynthesis of the 25-Kda Protein in the Macrogametes Zygotes of Plasmodium-Falciparum. Exp Parasitol. 1990;71(2):229–235. doi: 10.1016/0014-4894(90)90025-8. [DOI] [PubMed] [Google Scholar]
- 14.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, et al. A Vaccine Candidate from the Sexual Stage of Human Malaria That Contains Egf-Like Domains. Nature. 1988;333(6168):74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 15.Kaslow DC, Bathurst IC, Isaacs SN, Keister DB, Moss B, Barr PJ. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant Pfs25. Mem Inst Oswaldo Cruz. 1992;87(Suppl 3):175–177. doi: 10.1590/S0074-02761992000700028. [DOI] [PubMed] [Google Scholar]
- 16.Lobo CA, Dhar R, Kumar N. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect Immun. 1999;67(4):1688–1693. doi: 10.1128/iai.67.4.1688-1693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura K, Keister DB, Muratova OV, Sattabongkot J, Long CA, Saul A. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/Pvs25 is a direct and predictable function of antibody titer. Malaria J. 2007;6. [DOI] [PMC free article] [PubMed]
- 18.Wu YM, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S et al. Phase 1 Trial of Malaria Transmission Blocking Vaccine Candidates Pfs25 and Pvs25 Formulated with Montanide ISA 51. Plos One. 2008;3(7). [DOI] [PMC free article] [PubMed]
- 19.Bousema T, Roeffen W, Meijerink H, Mwerinde H, Mwakalinga S, van Gemert GJ, et al. The dynamics of naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs230 & Pfs48/45 in a low endemic area in Tanzania. Plos One. 2010;5(11):e14114. doi: 10.1371/journal.pone.0014114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, et al. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281(8):5197–5208. doi: 10.1074/jbc.M509807200. [DOI] [PubMed] [Google Scholar]
- 21.Yeoman JA, Hanssen E, Maier AG, Klonis N, Maco B, Baum J, et al. Tracking Glideosome-Associated Protein 50 Reveals the Development and Organization of the Inner Membrane Complex of Plasmodium falciparum. Eukaryot Cell. 2011;10(4):556–564. doi: 10.1128/EC.00244-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. Functional Dissection of the Apicomplexan Glideosome Molecular Architecture. Cell Host Microbe. 2010;8(4):343–357. doi: 10.1016/j.chom.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Sanders PR, Cantin GT, Greenbaum DC, Gilson PR, Nebl T, Moritz RL, et al. Identification of protein complexes in detergent-resistant membranes of Plasmodium falciparum schizonts. Mol Biochem Parasit. 2007;154(2):148–157. doi: 10.1016/j.molbiopara.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Simon N, Lasonder E, Scheuermayer M, Kuehn A, Tews S, Fischer R, et al. Malaria Parasites Co-opt Human Factor H to Prevent Complement-Mediated Lysis in the Mosquito Midgut. Cell Host Microbe. 2013;13(1):29–41. doi: 10.1016/j.chom.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Fischer R, Schillberg S, Buyel JF, Twyman RM. Commercial Aspects of Pharmaceutical Protein Production in Plants. Curr Pharm Design. 2013;19(31):5471–5477. doi: 10.2174/1381612811319310002. [DOI] [PubMed] [Google Scholar]
- 26.Phoolcharoen W, Bhoo SH, Lai HF, Ma JL, Arntzen CJ, Chen Q, et al. Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnol J. 2011;9(7):807–816. doi: 10.1111/j.1467-7652.2011.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geyer BC, Muralidharan M, Cherni I, Doran J, Fletcher SP, Evron T, et al. Purification of transgenic plantderived recombinant human acetylcholinesterase-R. Chem Biol Interact. 2005;157–158:331–334. doi: 10.1016/j.cbi.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 28.Boes A, Spiegel H, Edgue G, Kapelski S, Scheuermayer M, Fendel R, et al. Detailed functional characterization of glycosylated and nonglycosylated variants of malaria vaccine candidate PfAMA1 produced in Nicotiana benthamiana and analysis of growth inhibitory responses in rabbits. Plant Biotechnol J. 2015;13(2):222–234. doi: 10.1111/pbi.12255. [DOI] [PubMed] [Google Scholar]
- 29.Farrance CE, Chichester JA, Musiychuk K, Shamloul M, Rhee A, Manceva SD, et al. Antibodies to plantproduced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum Vaccines. 2011;7:191–198. doi: 10.4161/hv.7.0.14588. [DOI] [PubMed] [Google Scholar]
- 30.Farrance CE, Rhee A, Jones RM, Musiychuk K, Shamloul M, Sharma S, et al. A Plant-Produced Pfs230 Vaccine Candidate Blocks Transmission of Plasmodium falciparum. Clin Vaccine Immunol. 2011;18(8):1351–1357. doi: 10.1128/CVI.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feller T, Thom P, Koch N, Spiegel H, Addai-Mensah O, Fischer R et al. Plant-Based Production of Recombinant Plasmodium Surface Protein Pf38 and Evaluation of its Potential as a Vaccine Candidate. Plos One. 2013;8(11). [DOI] [PMC free article] [PubMed]
- 32.Ma C, Wang LN, Webster DE, Campbell AE, Coppel RL. Production, characterisation and immunogenicity of a plant-made Plasmodium antigen-the 19 kDa C-terminal fragment of Plasmodium yoelii merozoite surface protein 1. Appl Microbiol Biot. 2012;94(1):151–161. doi: 10.1007/s00253-011-3772-7. [DOI] [PubMed] [Google Scholar]
- 33.Voepel N, Boes A, Edgue G, Beiss V, Kapelski S, Reimann A, et al. Malaria vaccine candidate antigen targeting the pre-erythrocytic stage of Plasmodium falciparum produced at high level in plants. Biotechnol J. 2014;9(11):1435–1445. doi: 10.1002/biot.201400350. [DOI] [PubMed] [Google Scholar]
- 34.Twyman RM, Schillberg S, Fischer R. Optimizing the Yield of Recombinant Pharmaceutical Proteins in Plants. Curr Pharm Design. 2013;19(31):5486–5494. doi: 10.2174/1381612811319310004. [DOI] [PubMed] [Google Scholar]
- 35.Hoppmann V, Di Fiore S, Zimmermann S, Emans N, Rademacher T, Fischer R, et al. The potato granule bound starch synthase chloroplast transit peptide directs recombinant proteins to plastids. J Plant Physiol. 2002;159(10):1061–1067. doi: 10.1078/0176-1617-00762. [DOI] [Google Scholar]
- 36.Beiss V, Spiegel H, Boes A, Kapelski S, Scheuermayer M, Edgue G, et al. Heat-precipitation allows the efficient purification of a functional plant-derived malaria transmission-blocking vaccine candidate fusion protein. Biotechnol Bioeng. 2015;112(7):1297–1305. doi: 10.1002/bit.25548. [DOI] [PubMed] [Google Scholar]
- 37.Bosch J, Paige MH, Vaidya AB, Bergman LW, Hol WGJ. Crystal structure of GAP50, the anchor of the invasion machinery in the inner membrane complex of Plasmodium falciparum. J Struct Biol. 2012;178(1):61–73. doi: 10.1016/j.jsb.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Fiore S, Li QR, Leech MJ, Schuster F, Emans N, Fischer R, et al. Targeting tryptophan decarboxylase to selected subcellular compartments of tobacco plants affects enzyme stability and in vivo function and leads to a lesion-mimic phenotype. Plant Physiol. 2002;129(3):1160–1169. doi: 10.1104/pp.010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Fiore S, Hoppmann V, Fischer R, Schillberg S. Transient gene expression of recombinant terpenoid indole alkaloid enzymes in Catharanthus roseus leaves. Plant Mol Biol Rep. 2004;22(1):15–22. doi: 10.1007/BF02773344. [DOI] [Google Scholar]
- 40.Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14(12):669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyers A, Chakauya E, Shephard E, Tanzer FL, Maclean J, Lynch A et al. Expression of HIV-1 antigens in plants as potential subunit vaccines. Bmc Biotechnol. 2008;8. [DOI] [PMC free article] [PubMed]
- 42.Buyel JF, Fischer R. Processing heterogeneous biomass Overcoming the hurdles in model building. Bioengineered. 2013;4(1):21–24. doi: 10.4161/bioe.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buyel JF, Gruchow HM, Boes A, Fischer R. Rational design of a host cell protein heat precipitation step simplifies the subsequent purification of recombinant proteins from tobacco. Biochem Eng J. 2014;88:162–170. doi: 10.1016/j.bej.2014.04.015. [DOI] [Google Scholar]
- 44.Tuse D, Tu T, McDonald KA. Manufacturing economics of plant-made biologics: case studies in therapeutic and industrial enzymes. Biomed Res Int. 2014;2014:256135. doi: 10.1155/2014/256135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilken LR, Nikolov ZL. Downstream Processing of Transgenic Plant Systems: Protein Recovery and Purification Strategies. Molecular Farming in Plants: Recent Advances and Future Prospects 2012:217–257.
- 46.Tediosi F, Hutton G, Maire N, Smith TA, Ross A, Tanner M. Predicting the cost-effectiveness of introducing a pre-erythrocytic malaria vaccine into the expanded program on immunization in Tanzania. American Journal of Tropical Medicine and Hygiene. 2006;75(2):131–143. doi: 10.4269/ajtmh.2006.75.131. [DOI] [PubMed] [Google Scholar]
- 47.Seo MK, Baker P, Ngo KNL. Cost-effectiveness analysis of vaccinating children in Malawi with RTS,S vaccines in comparison with long-lasting insecticide-treated nets. Malaria J. 2014, 13. [DOI] [PMC free article] [PubMed]
- 48.Tediosi F, Maire N, Penny M, Studer A, Smith TA. Simulation of the cost-effectiveness of malaria vaccines. Malar J. 2009;8:127. doi: 10.1186/1475-2875-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Itzstein M, Plebanski M, Cooke BM, Coppel RL. Hot, sweet and sticky: the glycobiology of Plasmodium falciparum. Trends Parasitol. 2008;24(5):210–218. doi: 10.1016/j.pt.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174(5):1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Remarque EJ, Faber BW, Kocken CHM, Thomas AW. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect Immun. 2008;76(6):2660–2670. doi: 10.1128/IAI.00170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, et al. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. Plos One. 2012;7(5):e37179. doi: 10.1371/journal.pone.0037179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dauvillee D, Delhaye S, Gruyer S, Slomianny C, Moretz SE, d'Hulst C et al. Engineering the Chloroplast Targeted Malarial Vaccine Antigens in Chlamydomonas Starch Granules. Plos One. 2010;5(12). [DOI] [PMC free article] [PubMed]
- 54.Scotti N, Rybicki EP. Virus-like particles produced in plants as potential vaccines. Expert Rev Vaccines. 2013;12(2):211–224. doi: 10.1586/erv.12.147. [DOI] [PubMed] [Google Scholar]
- 55.Koncz C, Schell J. The Promoter of Tl-DNA Gene 5 Controls the Tissue-Specific Expression of Chimeric Genes Carried by a Novel Type of Agrobacterium Binary Vector. Mol Gen Genet. 1986;204(3):383–396. doi: 10.1007/BF00331014. [DOI] [Google Scholar]
- 56.Wirth CC, Glushakova S, Scheuermayer M, Repnik U, Garg S, Schaack D, et al. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol. 2014;16(5):709–733. doi: 10.1111/cmi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rademacher T, Sack M, Arcalis E, Stadlmann J, Balzer S, Altmann F, et al. Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc Nglycans. Plant Biotechnol J. 2008;6(2):189–201. doi: 10.1111/j.1467-7652.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 58.Wandelt CI, Khan MRI, Craig S, Schroeder HE, Spencer D, Higgins TJV. Vicilin with Carboxy-Terminal Kdel Is Retained in the Endoplasmic-Reticulum and Accumulates to High-Levels in the Leaves of Transgenic Plants. Plant J. 1992;2(2):181–192. doi: 10.1046/j.1365-313x.1992.t01-41-00999.x. [DOI] [PubMed] [Google Scholar]
- 59.Nolke G, Houdelet M, Kreuzaler F, Peterhansel C, Schillberg S. The expression of a recombinant glycolate dehydrogenase polyprotein in potato (Solanum tuberosum) plastids strongly enhances photosynthesis and tuber yield. Plant Biotechnol J. 2014;12(6):734–742. doi: 10.1111/pbi.12178. [DOI] [PubMed] [Google Scholar]
- 60.Voss A, Niersbach M, Hain R, Hirsch HJ, Liao YC, Kreuzaler F, et al. Reduced Virus Infectivity in Nicotiana-Tabacum Secreting a Tmv-Specific Full-Size Antibody. Mol Breeding. 1995;1(1):39–50. doi: 10.1007/BF01682088. [DOI] [Google Scholar]


