Abstract
Background
Few reports have discussed life-threatening bleeding that occurs postoperatively in patients who have undergone thyroid surgery. In this article, we discuss the causes, treatment measures, and possible ways of preventing this severe complication.
Material/Methods
From Jan 2002 to Dec 2014 we retrospectively analyzed 7 patients who developed life-threatening bleeding after undergoing thyroid surgery at our center.
Results
Among the group of 7 patients, there was 1 case of superior thyroid artery hemorrhage (STAH), 5 cases of carotid blowout syndrome (CBS), and 1 case of tracheo-innominate fistula (TIF). The STAH was caused by unreliable ligation. All the cases of CBS and TIF were caused by surgical wound infection. Six patients were transferred to the operating room immediately; open surgical treatment was performed on these 6 patients. Out of these 6 patients, 1 patient did not survive the operation, and hemorrhage was successfully controlled in 3 patients. The remaining 2 patients again experienced bleeding even after undergoing open surgery. Only 1 patient developed long-term neurological complications.
Conclusions
Infection is the most common cause of life-threatening bleeding that occurs postoperatively in some patients who have undergone thyroid surgery. Early surgical intervention can save the lives of these patients without causing any severe neurological complications.
MeSH Keywords: Bleeding Time, General Surgery, Retrospective Studies
Background
Thyroid carcinoma is the most common endocrine malignancy. Among women, it is the fifth most common malignancy [1]. The global incidence of thyroid carcinoma has been growing rapidly in recent years. As a result, there is an exponential increase in number of operations performed to treat thyroid carcinoma globally. Thus, the postoperative complications of these operations have attracted the attention of researchers all over the world.
The most common complications of thyroid operations are as follows: hypoparathyroidism, damage to the laryngeal nerves, and postoperative hemorrhage [2,3]. According to the data of our center, the incidence of postoperative cervical hematoma is 0.85%. Moreover, 88.7% cases of cervical hematoma occurred within 12 hours postoperatively [4]. However, most of these postoperative hemorrhages were not too acute to threaten patients’ life in a short time. Among patients whose primary site was in the hypopharynx or oropharynx, the incidence of life-threatening cervical bleeding was more common postoperatively [5]. To the best of our knowledge, among the patients who underwent thyroid surgery, life-threatening bleeding occurred in very cases postoperatively.
In this study, we have reviewed our center’s data from Jan. 2002 to Dec. 2014. In this period, we came across 7 patients who suffered life-threatening bleeding after undergoing thyroid surgery. We have retrospectively analyzed the clinical histories and documents of these 7 patients in order to summarize the causes of life-threatening bleeding that occurred postoperatively. Thereafter, we have described the steps that must be taken to prevent life-threatening bleeding in patients undergoing thyroid surgery. We have also discussed the treatment measures used for handling such cases.
Material and Methods
From Jan. 2002 to Dec. 2014, 15 764 patients underwent thyroid surgery at our hospital. Among them, 13 485 patients were diagnosed with thyroid cancer. After undergoing thyroid surgery, 167 patients (1.06%) developed postoperative bleeding, so debridement was performed on these patients. However, only 7 patients (4.2% of postoperative bleeding, 0.44‰ of patients who underwent thyroid surgery) developed acute life-threatening hemorrhages. This group of 7 patients consisted of 2 males and 5 females in the age group of 35–68 years. Out of these 7 patients, 4 cases were pathologically diagnosed with thyroid papillary carcinoma (PTC); 1 case was diagnosed with poorly differentiated follicular carcinoma; and the remaining 2 patients were diagnosed with poorly differentiated carcinoma.
The medical records of these 7 cases were reviewed. Five patients had received prior treatments. In this group, 5 cases had undergone operations; 2 cases had received radioiodine treatment, while 2 cases had been treated with external beam radiotherapy (Table 1). The TNM-staging and operations were carried out at our center. The pathological results of the 7 patients are summarized in Table 2.
Table 1.
The treatment history of all the seven patients.
| Case | Operation history (times) | Radioiodine treatment* (times) | External beam radiotherapy |
|---|---|---|---|
| 1 | 1 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 3 | 3 | 0 |
| 4 | 0 | 0 | 0 |
| 5 | 2 | 0 | 42 Gy |
| 6 | 3 | 0 | 70 Gy |
| 7 | 3 | 2 | 0 |
The exact radiological dose can’t be confirmed.
Table 2.
Surgical method, TNM-staging, and pathological results of the seven cases.
| Case | Surgical method | TNM-staging | Pathological result | ||
|---|---|---|---|---|---|
| T | N | TNM | |||
| 1 | The residual lobe resection of the thyroid gland + subtotal contralateral lobe resection | 3 | 1a | III | PTC |
| 2 | Total thyroidectomy + central & ipsilateral neck dissection | 1 | 1b | IVa | PTC |
| 3 | Total thyroidectomy + central & bilateral neck dissection + tracheotomy | 4a | 1b | IVa | PTC |
| 4 | Total thyroidectomy + bilateral neck dissection +superior mediastinum lymphadenectomy through partial sternotomy + sleeve-resection of cervical trachea lesions | 4a | 1b | IVc | PTC |
| 5 | Recurrence of thyroid area resection + resection of cervical trachea lesions + selective ipsilateral neck dissection | 4a | 1b | IVa | Poorly differentiated carcinoma |
| 6 | Residual thyroidectomy + resection of the hypopharynx, cervical esophagus + reconstruction with free jejunal flap | 4a | 1b | IVa | Poorly differentiated carcinoma |
| 7 | Resection of cervical recurrence, larynx, segmental trachea + tracheotomy | 4a | 1b | IVc | Poorly differentiated follicular thyroid carcinoma with neuroendocrine differentiation |
Results
Out of the 7 patients, 1 patient developed hemorrhage in the superior thyroid artery, while 5 patients developed carotid blowout syndromes (CBS). Only 1 patient had been diagnosed with tracheo-innominate artery fistula (TIF). Interestingly, in patients with CBSs, all the bleeding sites were located in the lower segment or the root of the right carotid artery. The hemorrhage of the superior thyroid artery was detected in the patient who started coughing severely about 1 hour after undergoing surgery. The remaining 6 patients developed hemorrhages within 3 to 21 days after undergoing thyroid operations. Before developing massive hemorrhages, 2 patients suffered herald bleeding that stopped spontaneously. Out of the 7 patients, 4 patients developed obvious signs of hemorrhages, such as severe cough, defecation, and sputum-sucking nursing. One patient developed severe cough that acted as an external force and blew off the ligature. As a result, this patient developed hemorrhage in the superior thyroid artery. The remaining 6 patients were diagnosed with massive hemorrhages, which were caused by surgical wound infection that developed due to various reasons. Table 3 summarizes the 7 hemorrhages in detail.
Table 3.
The detail information of the seven hemorrhages.
| Case | Hemorrhage site | Time of occurrence (after surgery) | Herald bleeding (before the hemorrhage) | Obvious inducement | Main cause |
|---|---|---|---|---|---|
| 1 | Right superior thyroid arterial | 1.5 hs | No | Severe cough | Falling off of ligature |
| 2 | Root of right carotid artery | 8 ds | No | Defecation | Surgical wound infection (caused by leukopenia, chylous leakage) |
| 3 | Root of right carotid artery | 11 ds after surgery | 8 ds | Severe cough | Surgical wound infection (caused by cervical esophageal fistula) |
| 4 | Posterior wall of innominate artery | 6 ds | 4ds | No | Surgical wound infection (caused by Tracheal anastomotic fistula and subcutaneous pneumatosis) |
| 5 | Lower segment of right carotid artery | 5 ds | No | No | Surgical wound infection (caused by Tracheal anastomotic fistula) |
| 6 | Lower segment of right carotid artery | 21 ds | No | No | Surgical wound infection and carotid artery exposure |
| 7 | Root of Right carotid artery | 2 ds and 8 hs | No | Sputum-sucking nursing | Surgical wound infection (caused by malnutrition and pharynoesophageal anastomotic fistula) |
The 7 patients were provided with emergency bedside treatment. This included opening the surgical wound, keeping the airway open, and compressing suspicious bleeding site. Six patients were immediately sent to the operating room (OR). Under the effect of general anesthesia, debridement was performed on these patients. However, the fifth patient died before reaching the OR. This patient died due to respiratory and circulatory complications.
The superior thyroid artery of the first case was ligated once more to control bleeding. In the remaining 5 patients with CBS and TIF, the treatment measures were as follows: the ruptures of carotid arteries were sewn and mended in 2 cases; the carotid artery was ligated in 1 case; and innominate, carotid, and subclavian arteries were ligated in 2 cases. To cover the large arteries, we used 3 pectoralis major myocutaneous flaps and 1 sternocleidomastoid muscle flap. All the hemorrhages of these 5 patients were under control as they received emergency treatment in OR. Among the 5 patients who were rescued successfully, hemorrhages recurred once again in 1 patient. Moreover, there was 1 patient who suffered hemorrhages 3 times. To control bleeding of these recurrent hemorrhages, we resected the head of the clavicle of 1 patient. The sternum of the other patient was split to gain adequate access to the vessel. Finally, the innominate arteries were ligated in both the patients. Table 4 shows the various treatments and their corresponding outcomes in these patients.
Table 4.
The treatment received by patients and their corresponding outcome.
| Case | Emergency treatment at bedside | Emergency treatment at operation room | Results of emergency treatment | Subsequent treatment | Results of subsequent treatment |
|---|---|---|---|---|---|
| 1 | Transferred to OR | Ligated superior thyroid artery | Controlled | – | – |
| 2 | Transferred to OR | Ligatured innominate artery, carotid artery and subclavian artery | Recurrent bleeding 3 days and 10 days later | Sternotomy and ligation of innominate artery, carotid artery and subclavian artery | Controlled |
| 3 | Transferred to OR | Ligatured carotid artery and subclavian artery + esophagostomy + reconstructed with sternocleidomastoid muscle flap | Died of respiratory and circulatory failure | – | – |
| 4 | Transferred to OR | Ligatured innominate artery, carotid artery and subclavian artery + tracheostomy + reconstructed with the pectoralis major myocutaneous flap | Controlled | – | – |
| 5 | Died of respiratory and circulatory failure at bedside | – | – | – | – |
| 6 | Transferred to OR | Sew and mended the crevasse of carotid arteries + reconstructed with the pectoralis major myocutaneous flap | Controlled | – | – |
| 7 | Transferred to OR | Sew and mended the crevasse of carotid arteries + reconstructed with the pectoralis major myocutaneous flap | Rebleeded 29 hours later | Resection of clavicular head and ligation of innominate artery, carotid artery and subclavian artery | Controlled |
Among the 5 patients who finally survived, 3 patients underwent innominate artery ligation. One patient suffered from a short-term delirious state, while another patient developed muscle weakness in the lower left limb within in a few days after the operation. This patient did not have any neurological complication. Although these 2 patients recovered gradually, 1 patient suffered from mild muscle weakness of the left lower extremity (Grade 4) 2 months after the surgery. The neurological complications of the 5 surviving patients are summarized in Table 5.
Table 5.
Neurological complications of surviving patients.
| Case | Short-term complications | Long-term complications |
|---|---|---|
| 1 | No | No |
| 2 | Short-term delirious state 5 days after the hemorrhage | No |
| 4 | Muscle weakness of the left lower limb (Grade 2) | Muscle weakness of the left lower extremity(Grade 4) |
| 6 | No | No |
| 7 | No | No |
Discussion
There is no exact definition for life-threatening bleeding that occurs postoperatively. In this study, the postoperative bleeding is defined as life-threatening bleeding under any of the following 2 conditions: i) the patient bleeds more than 500 ml within 5 minutes after the surgery; and ii) the hemorrhage would become life-threatening if emergency treatment is not provided within a few minutes. The life-threatening bleeding usually occurs postoperatively in patients who have undergone operations for treating head and neck squamous cell carcinomas, such as laryngeal carcinoma, hypopharyngeal carcinoma, and cervical esophageal carcinoma.[5] Postoperative cervical hemorrhages occur in some patients who have undergone thyroid surgery. In these patients, there is minimal bleeding due to hematomas [4,6,7], which are generally not life-threatening with timely treatment (usually several quarters). The post-operative bleeding becomes life-threatening in very few cases after undergoing thyroid surgery. We have reported such cases to draw attention to this rare complication.
Causes
The life-threatening bleeding does occur in some patients after undergoing thyroid surgery. In general, these patients have undergone multiple treatments as they are diagnosed with thyroid carcinoma at an advanced stage. The pathologic differentiation is quite poor in these cases [5]. In this study, 5 patients had undergone various treatments, including multiple operations, radioiodine treatment, and external beam radiotherapy. After being subjected to multiple operations and radiation, the tissue of the surgical wound lacked proper blood circulation. As a result, it was difficult to heal the surgical wound of these patients. Another important risk factor was the advanced stage of this disease. In our group, there were 5 cases with T4 disease. In these patients, we not only resected the thyroid gland but also performed partial or total resection of the following organs: hypopharynx, larynx, cervical trachea, and cervical esophagus. The surgical wound was easily contaminated by the bacteria residing in the upper respiratory and digestive tract, which were also opened while performing the surgery. Owing to the incidence of anastomotic fistula after surgery, the operative wounds, where the great arteries lost the protection of their surrounding connective tissue, were totally contaminated by the resident bacteria. In some cases, the tumor surrounded the carotid sheath, so it had to be scraped carefully while performing surgery. This decreased the vascular wall’s ability to resist infection to some degree. Therefore, patients with multiple treatments and advanced diseases should be treated carefully to prevent the occurrence of life-threatening bleeding postoperatively.
Previous studies have reported that after undergoing thyroid surgery, patients have to be monitored for the first 24 hours, especially the first 12 hours. In this high-risk period, patients are quite prone to hemorrhage [7]. The most frequent bleeding sites are the surface of the strap muscle, superior thyroid vessel, and the end of the recurrent laryngeal nerve [4]. In patients treated with thyroid surgery, the life-threatening bleeding occurs unpredictably after a considerable period of time [8,9]. However, the case diagnosed with hemorrhage in the superior thyroid artery was an exception as life-threatening bleeding occurred just 1 hour after the surgery. In the other 6 cases, hemorrhages occurred within 3–21 days after the operations. Five hemorrhages occurred at common carotid artery, while 1 hemorrhage occurred at an innominate artery. Interestingly, in patients with CBSs, all the bleeding sites were located in the lower segment or the root of right carotid artery, so they were clinically similar to the case with TIFs. In this group, 1 patient experienced herald bleeding 4 days before suffering life-threatening bleeding postoperatively. Another patient also experienced herald bleeding 8 days before developing life-threatening bleeding postoperatively.
Infection is the major reason for life-threatening bleeding that occurs postoperatively in patients who undergo thyroid operations [9,10]. At the same time, unreliable ligation of arteries could also be another cause of this life-threatening bleeding. In our group, the first case suffered from a hemorrhage in the superior thyroid artery 1 hour after undergoing the surgery. This patient developed severe cough, which blew off the ligature; this observation was confirmed while performing debridement of the surgical wound. The superior thyroid artery is the major artery that supplies blood to the thyroid gland. Its hemorrhage caused the wound to become swollen within seconds; the hemorrhage of this artery also oppressed the trachea within seconds. However, this serious phenomenon has been seldom reported in previous studies.
In the remaining 6 cases, life-threatening bleeding occurred postoperatively due to surgical wound infection. Cervical and pharyngoesophageal anastomotic fistula were the causes of infections in the third and seventh cases, respectively. Tracheal anastomotic fistula and subcutaneous pneumatosis were the causes of infection in fourth and fifth cases, respectively. Esophageal fistula and tracheal anastomotic fistula were not detected in the sixth case. However, while being subjected to radical radiation therapy, the surgical wound of the sixth case underwent continuous necrosis, exposing the carotid artery. The ligature of the artery blew out finally. Total thyroidectomy was performed along with central and ipsilateral neck dissection on the second patient in this group. However, the surgical procedure did not involve the upper respiratory or gastrointestinal tract. But, owing to preoperative leukopenia and postoperative chylous leakage, infection finally developed in the second case. Six days after the surgery, we detected the infection in the second patient after draining the necrotic material from the tube. On the 8th day after surgery, this patient developed massive hemorrhage.
In this study, we have easily figured out the measures that can be used to prevent this severe complication. The high-risk patients must be prescribed antibiotics to prevent pre-operative infection. Then, during the operation, superior thyroid arteries should be ligatured firmly and checked over to prevent hemorrhage. While resecting the tracheal sleeve, we must ensure proper blood supply in anastomosis. This would reduce the tension in anastomosis. Thus, the occurrence of anastomotic fistula can be prevented. If anastomotic fistula or wound infection is detected after surgery, the surgical wound should be opened immediately. Then, the necrotic materials should be removed to prevent it from infiltrating the great arteries and causing bleeding.
Emergency treatment at bedside
Emergency treatment should be provided to patients at bedside and OR. This would help in arresting the life-threatening bleeding that occurs in some patients after undergoing thyroid surgery. The emergency treatment at bedside is provided to stabilize the patients, who are then immediately transferred to OR. The patient develops life-threatening bleeding in any of the following conditions: rapid-progressing hematoma, massive hemorrhage from incision, and tracheostoma. The life-threatening bleeding that occurs postoperatively is totally different from the common hemorrhage that occurs in patients after undergoing thyroidectomy. The emergency treatment provided at bedside includes the following features: (1) airway protection; (2) temporary control or reduction in the bleeding by compressing; (3) transfer of the patient to OR; and (4) setting up venous channels to correct hemorrhagic shock [8]. The first thing to do at bedside is keep the airway unobstructed. With this objective, we hyperinflate the cuff of the tracheostomy tube; this has been a successful measure in 85% of the cases as it prevents blood aspiration temporarily [11]. If tracheotomy had not been performed, then the surgical incision would have opened without hesitation and the trachea would have got suppressed, thereby limiting airflow. Digital pressure may be used to minimize blood loss. Utley [12] has reported that when the artery is compressed against the posterior surface of the manubrium by a finger, hemorrhage is controlled and reduced. At this stage, we have adequate time for transferring these patients into OR. However, vein access should be set up simultaneously. Furthermore, fluids and blood must be administered rapidly to correct acute hemorrhagic shock. The mortality rate of patients provided with emergency treatment at bedside is very high; Jones [11] have reported that only 31% cases with tracheo-innominate artery fistula could be successfully handled by providing emergency treatment at bedside. In our group, 6 patients successfully revived after receiving emergency treatment at bedside; however, 1 patient died of respiratory and circulatory failure even after receiving emergency treatment at bedside. Furthermore, the life-threatening bleeding of these 6 patients was controlled within a short span when they were shifted to OR. Therefore, although the success rate of emergency treatment provided at bedside is low, it is extremely necessary in such cases.
Emergency treatment at OR
To control bleeding in the superior thyroid artery, we once again ligated the artery. In the first case, the hemorrhage was successfully controlled after performing the ligature of the artery.
It was quite challenging to tackle the remaining 5 cases with CBS. The single case with TIF complication was also quite challenging. We first enlarged the incision to explore and confirm the bleeding site of these cases. In the 5 cases with CBS, all the hemorrhage sites were located at the root or lower segment of carotid artery. As a result, we could not easily expose the bleeding site in these 5 cases. In the single case with TIF, we again encountered some difficulties while exposing the bleeding site. In some of these cases, median sternotomy or clavicular head resection was performed to gain an adequate access to the vessel [13,14].
Previous studies have described various methods to control CBS or TIF, including direct suture, resection, and ligation of innominate artery [15]. In addition, endovascular stent graft or endovascular embolization has been successfully performed on cases with CBS or TIF in previous studies [10,14,16–18]. Traditionally, there have been 2 broad approaches to tackle the erosion of innominate and carotid artery: i) interrupting the blood flow through the artery and ii) maintaining the blood flow [14]. In case of TIF, the survival rate of patients whose blood flow was interrupted through the innominate artery was greater than that of patients treated by maintaining the blood flow. In a review by Jones [11] et al., the survival rates of cases treated by interrupting the blood flow was significantly higher (77% survival) than that of patients whose blood flow was maintained (6% survival). In our research, the fourth case developed TIF after undergoing thyroid surgery; we performed ligation of both proximal and distal innominate artery of this patient. The ends of the carotid and subclavian arteries were also sewn. At the same time, the wound was repeatedly washed, and the infected necrotic tissues were debrided thoroughly. Luckily, the hemorrhage was controlled effectively, and there was no recurrence of bleeding in this case.
Traditionally, open surgery is the main treatment for patients with CBS. The conventional open surgery approaches include direct suture, resection, and ligation [9]. In the mid-1980s, a novel endovascular treatment for CBS was the use of balloon embolization [17]. This therapy was a safer and effective alternative to surgical ligation. However, in the 5 patients with CBS, all the hemorrhage sites were located in the root or the lower segment of the carotid artery, which was close to the innominate artery. As a result, the chances of developing furcation were high. Therefore, endovascular intervention was more challenging and unreliable. The use of any synthetic materials in the infected area would have easily led to life-threatening bleeding of fatal nature. In our group, all the 4 patients with CBS underwent open surgery in OR after receiving emergency treatment. The ligation of carotid artery was carried out in the second and third cases, while the crevasse was directly sutured in sixth and seventh cases. Unfortunately, the third case could not survive during the operation. The bleeding of sixth case was successfully controlled as there was no recurrence of bleeding. However, there was recurrence of bleeding in the second and seventh case 29 hours after undergoing open surgery. Furthermore, ten days after receiving first aid, the second case suffered bleeding once more. Finally, to control the bleeding, we ligated the innominate, carotid, and subclavian arteries of second and seventh cases. Our outcome suggests that ligation of innominate artery must be carried out to stop life-threatening bleeding that occurs postoperatively in patients who develop CBS or TIF complications after undergoing thyroid surgery.
Viable adjacent tissues, such as pectoralis major myocutaneous flap and sternocleidomastoid muscle flap should be used to cover and protect the blood vessels [19]. Prophylactic intravenous antibiotics should be administered to avoid mediastinitis and sternal dehiscence.
Neurological complications
Because of collateral flow through the external carotid artery, thyrocervical trunk, and vertebral vessels, ligation of the innominate artery was recommended in the management of TIF. Researchers were of the view that this approach would not lead to significant neurological or vascular complications [20]. Jones et al. [11] have reported that 22 patients survived the initial operation, and only 1 patient developed neurological deficits. Neurological events may be reduced by adequately replacing blood perioperatively. At the same time, the intraoperative and postoperative blood pressure should be maintained at a slightly higher level to overcome cerebral ischemia.
In our group, 3 patients who underwent ligation of innominate arteries survived surgical management. After the ligation, they were administered intravenous fluids to increase blood volume and improve microcirculation. Then, 2 of them developed neurological deficits. The second case developed a short-term delirious state after receiving first aid; however, this patient recovered from that state within 2 months. The fourth case developed grade 2 muscle weakness in the left lower limb, and the MRI scan revealed multiple cortical infarctions in his right frontal lobe, temporal lobe, parietal, and occipital cortex (Figures 1, 2). After 2 months, this patient’s left lower extremity recovered greatly and the patient showed grade 4 muscle weakness, which was milder than the original grade 2 muscle weakness. Therefore, only 1 patient (33.3%) presented long-term neurological complications.
Figure 1.
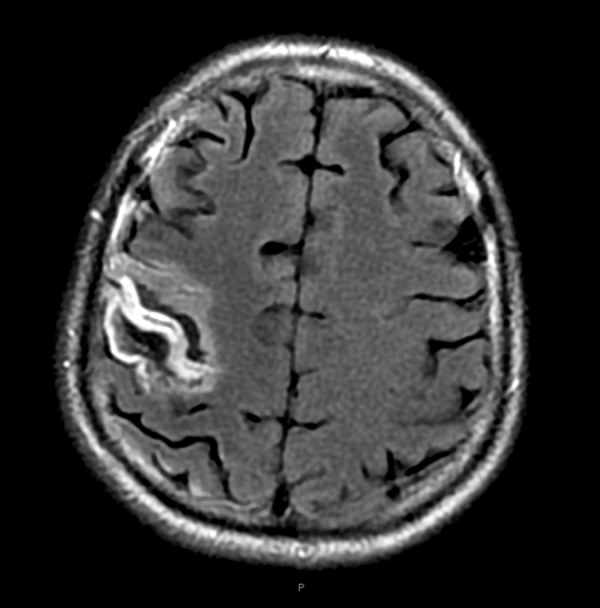
Infarction in parietal cortex.
Figure 2.
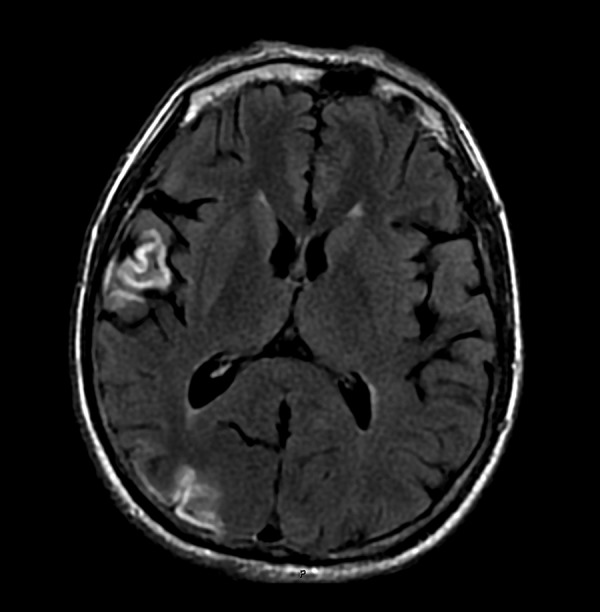
Infarction in temporal and occipital cortex.
Conclusions
Life-threatening bleeding is a rare and serious complication that develops in some patients after undergoing thyroid surgery. It is often encountered in thyroid carcinoma patients with any of the following complications: history of multiple treatments, advanced stage of disease, and poor pathological differentiation. This life-threatening bleeding that occurs in patients after undergoing thyroid operation can be classified as superior thyroid artery hemorrhage, CBS, and TIF. The bleeding site of CBS is always located at the root or lower segment of the artery, making it clinically similar to TIF. Hemorrhage of the superior thyroid artery occurs several hours after the surgery, and its development is related to the unreliable ligation and patient movement after surgery. The complications of CBS and TIF usually occur several days after the surgery, and both are related to the surgical wound infection, which is induced by events such as chylous leakage, cervical esophageal fistula, and tracheal anastomotic fistula. Some patients experienced herald bleeding before developing life-threatening bleeding postoperatively. Once the hemorrhage is detected, emergency aid at bedside helps in stabilizing the patients. Then, they are safely transferred immediately to the OR. To control the life-threatening bleeding, ligation of the innominate artery was performed in cases complicated with TIF or CBS; with this approach, the patients did not develop any significant neurological or vascular complications. Thereafter, the patients were given intravenous fluids to increase blood volume and improve microcirculation. Thus, we prevented the development of neurological complications, such as hemiplegia and mental disorders.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura Y, Shimizu K, Nagahama M, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999;84:4043–49. doi: 10.1210/jcem.84.11.6115. [DOI] [PubMed] [Google Scholar]
- 3.Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393:667–73. doi: 10.1007/s00423-008-0366-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Li Z, Liu S, et al. Risk factors for and occurrence of postoperative cervical hematoma after thyroid surgery: A single-institution study based on 5156 cases from the past 2 years. Head Neck. 2014 doi: 10.1002/hed.23868. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Chen YJ, Wang CP, Wang CC, et al. Carotid blowout in patients with head and neck cancer: associated factors and treatment outcomes. Head Neck. 2015;37:265–72. doi: 10.1002/hed.23590. [DOI] [PubMed] [Google Scholar]
- 6.Calo PG, Pisano G, Piga G, et al. Postoperative hematomas after thyroid surgery. Incidence and risk factors in our experience. Ann Ital Chir. 2010;81:343–47. [PubMed] [Google Scholar]
- 7.Godballe C, Madsen AR, Pedersen HB, et al. Post-thyroidectomy hemorrhage: a national study of patients treated at the Danish departments of ENT Head and Neck Surgery. Eur Arch Otorhinolaryngol. 2009;266:1945–52. doi: 10.1007/s00405-009-0949-0. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Fung A, Cole IE. Innominate artery hemorrhage following tracheostomy. Otolaryngol Head Neck Surg. 2007;136:S68–72. doi: 10.1016/j.otohns.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Powitzky R, Vasan N, Krempl G, Medina J. Carotid blowout in patients with head and neck cancer. Ann Otol Rhinol Laryngol. 2010;119:476–84. doi: 10.1177/000348941011900709. [DOI] [PubMed] [Google Scholar]
- 10.Takasaki K, Enatsu K, Nakayama M, et al. A case with tracheo-innominate artery fistula. Successful management of endovascular embolization of innominate artery. Auris Nasus Larynx. 2005;32:195–98. doi: 10.1016/j.anl.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Jones JW, Reynolds M, Hewitt RL, Drapanas T. Tracheo-innominate artery erosion: Successful surgical management of a devastating complication. Ann Surg. 1976;184:194–204. doi: 10.1097/00000658-197608000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utley JR, Singer MM, Roe BB, et al. Definitive management of innominate artery hemorrhage complicating tracheostomy. JAMA. 1972;220:577–79. [PubMed] [Google Scholar]
- 13.Cooper JD. Trachea-innominate artery fistula: successful management of 3 consecutive patients. Ann Thorac Surg. 1977;24:439–47. doi: 10.1016/s0003-4975(10)63438-8. [DOI] [PubMed] [Google Scholar]
- 14.Jamal-Eddine H, Ayed AK, Al-Moosa A, Al-Sarraf N. Graft repair of tracheo-innominate artery fistula following percutaneous tracheostomy. Interact Cardiovasc Thorac Surg. 2008;7:654–55. doi: 10.1510/icvts.2007.174656. [DOI] [PubMed] [Google Scholar]
- 15.Gelman JJ, Aro M, Weiss SM. Tracheo-innominate artery fistula. J Am Coll Surg. 1994;179:626–34. [PubMed] [Google Scholar]
- 16.Cohen JE, Klimov A, Rajz G, et al. Exsanguinating tracheoinnominate artery fistula repaired with endovascular stent-graft. Surg Neurol. 2008;69:306–9. doi: 10.1016/j.surneu.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 17.Lesley WS, Chaloupka JC, Weigele JB, et al. Preliminary experience with endovascular reconstruction for the management of carotid blowout syndrome. Am J Neuroradiol. 2003;24:975–81. [PMC free article] [PubMed] [Google Scholar]
- 18.Warren FM, Cohen JI, Nesbit GM, et al. Management of carotid ‘blowout’ with endovascular stent grafts. Laryngoscope. 2002;112:428–33. doi: 10.1097/00005537-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Roh JL, Na MH, Kim KH. Treating tracheo-innominate artery fistula with interposition of a pectoralis major myocutaneous flap. Eur Arch Otorhinolaryngol. 2006;263:180–82. doi: 10.1007/s00405-005-0976-4. [DOI] [PubMed] [Google Scholar]
- 20.Lim RC, Jr, Sanderson RG, Hall AD, Thomas AN. Multiple traumatic thoracic aneurysms after nonpenetrating chest injury. Ann Thorac Surg. 1968;6:377–83. doi: 10.1016/s0003-4975(10)66040-7. [DOI] [PubMed] [Google Scholar]


