Abstract
Recently, there has been progress in the treatment of chronic myeloid leukemia (CML). However, novel therapeutic strategies are required in order to address the emerging problem of imatinib resistance. Histone deacetylase inhibitors (HDACi) and proteasome inhibitors are promising alternatives, and may be amenable to integration with current therapeutic approaches. However, the mechanisms underlying the interaction between these two agents remain unclear. The present study assessed the cytotoxic effect of the HDACi, suberoylanilide hydroxamic acid (SAHA), in combination with the proteasome inhibitor, MG-132, in imatinib-sensitive K562 and imatinib-resistant K562G cells, and investigated the mechanism underlying this effect. Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method and protein expression levels were determined by western blotting. Reactive oxygen species (ROS) generation levels were observed under a fluorescence microscope The results indicated that SAHA and MG-132 act in a synergistic manner to induce cell death in K562 and K562G cells. This effect was associated with Bcr-Abl downregulation and the production of ROS. Notably, the ROS scavenger, N-acetyl-L-cysteine, almost fully reversed the cell death and Bcr-Abl downregulation that was induced by the combination of SAHA and MG-132. By contrast, the pan-caspase inhibitor, z-VAD-fmk, only partially reversed the cell death induced by these two drugs in CML cells. These results indicated that increased intracellular ROS levels are important in the induction of cell death and the downregulation of Bcr-Abl. In conclusion, the present results suggested that combined SAHA and MG-132 may be a promising treatment for CML.
Keywords: suberoylanilide hydroxamic acid, MG-132, chronic myeloid leukemia, reactive oxygen species, Bcr-Abl
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disorder, characterized by the expression and abnormal tyrosine kinase activity of the Bcr-Abl protein (1). The current recommended first line therapy for patients with newly diagnosed CML, is imatinib. However, resistance to imatinib is a significant problem, despite the good clinical results achieved with this tyrosine kinase inhibitor in the treatment of CML (2). Therefore, the identification of novel therapeutic agents, in addition to an understanding of their underlying mechanisms, is necessary in order to improve the outcome in patients with CML.
Histone deacetylase inhibitors (HDACi) represent a novel class of anticancer agents that are currently under investigation in preclinical models and in phase I–III clinical trials (3,4). Suberoylanilide hydroxamic acid (SAHA) is an orally bioavailable, well-tolerated pan-HDACi, which exhibits strong anticancer activity in hematological and solid malignancies (3–7). Recently, SAHA has been shown to exhibit anti-CML activity, when administered either alone or in combination with other agents (8–10).
Proteasome inhibitors represent a relatively recently identified class of antineoplastic agents, which function by interfering with the catalytic 20S core of the proteasome, thereby preventing the elimination of diverse cellular proteins that have been targeted for degradation. There are a number of classes of proteasome inhibitors, including peptide aldehydes, such as MG-132 (11,12). Recently, it has been reported that certain proteasome inhibitors, such as bortezomib (also termed velcade, MG-341 and PS-341), inhibit survival and induce apoptosis in imatinib-resistant Bcr-Abl cells (13). Furthermore, enhanced lethality towards CML cells, of a regimen combining HDACi and proteasome inhibitors, has also been described (11).
Evidence that HDACi promote the antileukemic activity of certain proteasome inhibitors, particularly in CML cells (11,14), led to the hypothesis that SAHA and MG-132 may function synergistically to kill CML cells. Previous studies have indicated that the combination of SAHA and MG-132 may induce cell death in imatinib-sensitive K562 cells (11). However, the mechanism underlying this effect remains unknown and, to the best of our knowledge, there is no data on combined SAHA and MG-132 treatment in imatinib-resistant CML cells. The present study investigated the combined effects of SAHA and MG-132, in imatinib-sensitive K562 cells and imatinib-resistant K562G cells in vitro, and attempted to elucidate the mechanisms underlying these effects.
Materials and methods
Cell culture and reagents
The K562 imatinib-sensitive human CML cell line and the K562G imatinib-resistant human CML cell line were maintained in RPMI 1640 medium (HyClone Laboratories, Inc., Logan, UT, USA) with 10% fetal bovine serum (HyClone Laboratories, Inc.) at 37°C in a 5% CO2 humid atmosphere, as previously described (15). SAHA was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). MG-132 and the pan-caspase inhibitor, z-VAD-fmk, were obtained from Sigma-Aldrich (St. Louis, MO, USA). N-acetyl-L-cysteine (NAC) was obtained from Beyotime Company (Shanghai, China).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell viability was determined using an MTT assay. In brief, K562 or K562G cells were cultured in 96-well plates at a density of 2×104 cells/ml, then treated with or without SAHA (0.5–8.0 µM; 24–48 h), MG-132 (200–800 nM; 24–48 h) and z-VAD-fmk (20 µM; 1 h). Subsequently, 20 µl MTT (Sigma-Aldrich) solution (5 mg/ml) was added into each well and the cells were incubated for a further 4 h. The supernatant was removed, 200 µl dimethyl sulfoxide (Amresco, Solon, OH, USA) was added and the absorbance at 570 nm was detected using an enzyme-linked immunosorbent assay plate reader (Model 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Colony formation assay
For the leukemic cell colony formation assays, K562 cells were suspended in Iscove modified Dulbecco's medium (Stem Cell Technologies, Vancouver, BC, Canada), containing 10% fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin and 100 µg/ml; Sigma-Aldrich), and were then treated with or without SAHA (2 µM) and/or MG-132 (200 nM). Subsequently, the cells were plated in 20% FBS and 0.8% methylcellulose. The cells were cultured 7 days and observed under a light microscope (Olympus, Tokyo, Japan).
Analysis of ROS generation
Intracellular ROS generation was determined using the Reactive Oxygen Species Assay kit (Beyotime, Shanghai, China), according to the manufacturer's instructions. Rosup (50 mg/ml; Beyotime Institute of Biotechnology, Shanghai, China) was used as a positive control. Cells were pretreated with or without NAC (2 mM) for 1 h, followed by co-treatment with SAHA and MG-132 for 24 h. Cells were then incubated with DCFH-DA (10 µM) for 30 min at 37°C, washed twice with phosphate-buffered saline (PBS), harvested and then suspended in PBS. A drop of stained cell suspension was placed on a slide for the immediate observation of fluorescence, using a fluorescence microscope (IX71; Olympus Corporation, Tokyo, Japan).
Western blot analysis
Following administration of SAHA (1–4 µM) for 3–24 h and MG-132 (200–400 nM) for 6–24 h with or without pretreatment with NAC (2 mM) for 1 h, cells were washed twice with PBS and lysed in a radioimmunoprecipitation assay buffer (0.5 M Tris-HCl, pH 7.4; 1.5 M NaCl; 2.5% deoxycholic acid; 10% NP-40; and 10 mM EDTA). Equal quantities of total cell lysates were separated on sodium dodecyl-sulfate-polyacrylamide gels containing 6–12% acrylamide gradients, and then transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% non-fat milk and incubated with primary antibodies at 4°C overnight in Tris-buffered saline with Tween-20 (10 mM Tris-HCl, pH 8, 150 mM NaCl, 0.1% Tween-20). The membranes were then incubated for 2 h with horseradish peroxidase-conjugated goat anti-rabbit (cat no. 7074) and horse anti-mouse (cat no. 7076) secondary antibodies purchased from Cell Signaling Technology, Inc. (Beverly, CA, USA) at a dilution of 1:2,000. The following primary antibodies were used at a dilution of 1:1,000: Rabbit anti-human polyclonal c-Abl (cat no. 2862), rabbit anti-human polyclonal phosphorylated-Bcr (p-Bcr; cat no. 3901), mouse anti-human monoclonal caspase-9 (cat no. 9508), rabbit anti-human polyclonal caspase-3 (cat no. 9662), rabbit anti-human monoclonal poly adenosine diphosphate (ADP)-ribose polymerase (PARP; cat no. 9532) and rabbit anti-human monoclonal acetylated histone H3 (cat no. 9649), which were obtained from Cell Signaling Technology, Inc. Anti-c-Abl p-Bcr are subsequently referred to as Bcr-Abl and p-Bcr-Abl, respectively. Rabbit anti-human polyclonal anti-β-actin (cat no. sc-7210) was obtained from Santa Cruz Biotechnology, Inc. and used at a dilution of 1:200. Signals were detected using an ECL kit (Amersham, Little Chalfont, UK).
Statistical analysis
Data are presented as the mean ± standard deviation. Data were compared using Student's t-test. All data were analyzed using SPSS software (version 11.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
SAHA induces cell death, downregulates expression of Bcr-Abl and upregulates acetylation of histone H3 in K562 and K562G cells
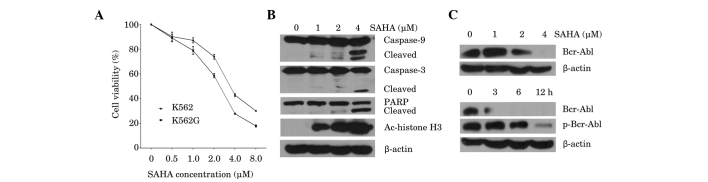
In order to assess the effects of SAHA on cell viability in imatinib-sensitive K562 cells and imatinib-resistant K562G cells, cell proliferation was assessed using an MTT assay. Cells were treated for 48 h with increasing doses of SAHA, from 0.5–8 µM. SAHA inhibited the viability of K562 and K562G cells in a dose-dependent manner, with 50% inhibition (IC50) values at 48 h, of 3.93 and 2.42 µM, respectively (Fig. 1A), which indicates that K562 and K562G cells are both sensitive to SAHA. Subsequently the induction of the apoptotic pathway by SAHA was investigated. As shown in Fig. 1B, SAHA triggered dose-dependent cleavage of caspase-9 and caspase-3, followed by PARP cleavage. Marked induction of apoptosis was observed at the highest dose of SAHA (4 µM), as evidenced by increased cleavage of caspase-3 and PARP. In accordance with this result, 4 µM SAHA significantly inhibited the expression of Bcr-Abl (Fig. 1C; upper panel). Measurement of the protein expression and phosphorylation status of Bcr-Abl in K562 cells at different time points following treatment with 4 µM SAHA, demonstrated that inhibition of Bcr-Abl occurred after 3 h of treatment, while downregulation of p-Bcr-Abl was observed at 6 h (Fig. 1C; lower panel). Furthermore, exposure to SAHA for 24 h increased the acetylation of histone H3 in K562 cells in a dose-dependent manner (Fig. 1B). These results indicated that SAHA is cytotoxic to CML cells, and is associated with downregulation of the Bcr-Abl oncoprotein and upregulation of histone H3 acetylation.
Figure 1.
Effects of SAHA on the K562 and K562G CML cell lines. (A) K562 and K562G cells were treated with SAHA (0.5–8 µM) for 48 h. Cell viability was measured using an MTT assay. Data are presented as the mean ± standard deviation of three independent experiments. (B) K562 cells were treated with SAHA (1–4 µM) for 24 h. Western blot analysis was performed, using antibodies against caspase-9, caspase-3, PARP and Ac-histone H3. β-actin was used as a loading control. A representative blot from three independent experiments is shown. (C) K562 cells were treated with SAHA (1–4 µM) for 24 h (upper panel) or with 4 µM SAHA for 3 h, 6 h or 12 h (lower panel). Western blot analysis for Bcr-Abl and p-Bcr-Abl was performed. β-actin was used as a loading control. A representative blot from three independent experiments is shown. SAHA, suberoylanilide hydroxamic acid; CML, chronic myeloid leukemia; Ac-histone, acetylated histone; PARP, poly adenosine diphosphate-ribose polymerase.
MG-132 synergistically interacts with SAHA to induce cell death, inhibit Bcr-Abl and mediate acetylation of histone H3 in K562 and K562G cells
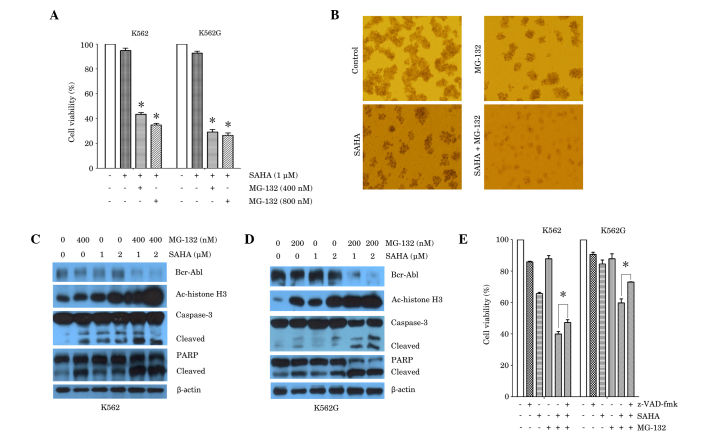
Subsequently, the combined effects of SAHA and MG-132 on K562 and K562G cells were examined, using different combinations of doses of each of these agents (Fig. 2A). Exposure of K562 cells to low dose SAHA (1 µM) in addition to MG-132 (400 or 800 nM) for 24 h, resulted in a significant reduction in cell viability compared with SAHA treatment alone. Similar results were obtained in K562G cells (Fig. 2A). Further experiments were conducted in order to determine whether co-treatment with SAHA and MG-132 altered CML cell colony formation in vitro. The results demonstrated that treatment of K562 cells with SAHA (2 µM) or MG-132 (200 nM) alone, inhibited colony formation, compared with untreated control. However, a combination of SAHA (2 µM) and MG-132 (200 nM), resulted in a greater reduction in colony formation than that observed with either treatment alone (Fig. 2B). The effects of co-treatment of cells with SAHA and MG-132 were then examined in association with the expression of Bcr-Abl, histone acetylation protein and the apoptotic proteins, caspase-3 and PARP. Western blot analysis was performed 24 h after treatment with SAHA and MG-132 alone or in combination. The results demonstrated that treatment with SAHA (1 or 2 µM) or MG-132 (400 nM) alone exerted a minimal effect or no effect on Bcr-Abl expression. However, K562 cells exposed to combined treatment, exhibited a marked reduction in Bcr-Abl protein expression (Fig. 2C). Furthermore, combined treatment with SAHA (1 or 2 µM) and MG-132 (400 nM) resulted in a marked increase in the expression of the acetylated histone H3 protein, compared with either treatment alone. In accordance with these results, combined treatment resulted in increased cleavage of caspase-3 and PARP, compared with either treatment alone (Fig. 2C). The results in K562G cells also demonstrated that following exposure to SAHA (1 or 2 µM) and/or MG-132 (200 nM) for 24 h, combined treatment resulted in a pronounced downregulation of Bcr-Abl expression, upregulation of acetylated histone H3 expression, and increase in cleavage of caspase-3 and PARP, compared with either treatment alone (Fig. 2D). Furthermore, the pan-caspase inhibitor, z-VAD-fmk, partially reversed the cell death induced by combined treatment with SAHA and MG-132, in K562 and K562G cells (Fig. 2E). This suggested an involvement of caspase-independent cell death induced in combined treatment with SAHA and MG-132.
Figure 2.
Synergistic effect of SAHA and MG-132 in the K562 and K562G CML cell lines. (A) K562 cells and K562G cells were treated with SAHA (1 µM) or SAHA plus MG-132 (400 nM or 800 nM) for 24 h. Cell viability was measured using an MTT assay. *P<0.0001 vs. SAHA alone. (B) K562 cells were treated with SAHA (2 µM), MG-132 (200 nM) or SAHA plus MG-132 for 7 days. Colony formation was observed using a light microscope. Representative images are shown. (C) K562 cells were treated with SAHA (1 µM or 2 µM), MG-132 (400 nM) or SAHA plus MG-132 (400 nM) for 24 h. Western blot analysis was performed, using antibodies against Bcr-Abl, Ac-histone H3, caspase-3 and PARP. β-actin was used as a loading control. (D) K562G cells were treated with SAHA (1 µM or 2 µM), MG-132 (200 nM) or SAHA plus MG-132 (200 nM) for 24 h. Western blot analysis was performed, using antibodies against Bcr-Abl, Ac-histone H3, caspase-3 and PARP. β-actin was used as a loading control. Representative blots from three independent experiments are shown. (E) K562 cells and K562G cells were treated with SAHA (1 µM), MG-132 (200 nM) alone, or SAHA plus MG-132 for 24 h, prior to which cells were cultured in the presence or absence of z-VAD-fmk (20 µM) for 1 h. Cell viability was measured using an MTT assay. *P<0.05. Data are presented as the mean ± standard deviation of three independent experiments. SAHA, suberoylanilide hydroxamic acid; CML, chronic myeloid leukemia; Ac-histone, acetylated histone; PARP, poly adenosine diphosphate-ribose polymerase.
ROS scavenger NAC reverses the effect of combined SAHA and MG-132 treatment on cell death, generation of ROS and Bcr-Abl downregulation, in K562 and K562G cells
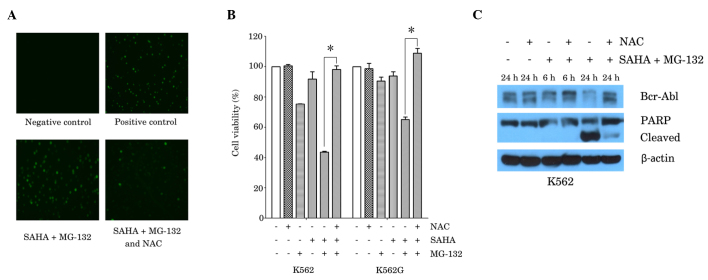
It has been reported that SAHA and proteasome inhibitors, such as MG-132 and NPI-0052, induce ROS-dependent cell death in cancer cells (14,16). In order to elucidate whether the generation of ROS by co-treatment with SAHA and MG-132 contributes to cell death in CML cells, K562 cells were treated with SAHA (1 µM) plus MG-132 (200 nM), with or without pretreatment with the ROS scavenger, NAC (2 mM). Measurements of intracellular ROS were determined by fluorescence microscopy, following staining with a fluorescence probe. Compared with the negative control, combined treatment with SAHA and MG-132 resulted in a marked increase in the level of ROS, and this effect was attenuated in the presence of NAC (Fig. 3A). In accordance with these results, the MTT assay demonstrated that NAC (2 mM) almost fully reversed the reduction in cell viability that was induced by combined treatment with SAHA (1 µM) and MG-132 (200 nM) in K562 and K562G cells (Fig. 3B). Furthermore, the reduction in Bcr-Abl expression and in the activation of PARP induced by SAHA and MG-132, were also reversed by treatment with NAC (2 mM; Fig. 3C).
Figure 3.
ROS scavenger, NAC, reversed the effects of the combination of SAHA and MG-132 in the K562 and K562G CML cell lines. (A) K562 cells were treated with SAHA (1 µM) plus MG-132 (200 nM) for 24 h, with or without 1 h pretreatment with NAC (2 mM), Rosup (50 mg/ml) was used as a positive control. ROS generation was examined using a fluorescence microscope. Representative images from three independent experiments are shown. (B) K562 cells and K562G cells were treated with SAHA (1 µM) or MG-132 (200 nM) alone, or with SAHA plus MG-132 for 48 h, prior to which cells were cultured in the presence or absence of NAC (2 mM) for 1 h. Cell viability was measured using MTT assay. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.0001. (C) K562 cells were cultured with SAHA (1 µM) plus MG-132 (200 nM) for 6 h or 24 h, with or without pretreatment with NAC (2 mM) for 1 h. Western blot analysis was performed, using antibodies against Bcr-Abl and PARP. β-actin was used as a loading control. A representative blot from three independent experiments is shown. SAHA, suberoylanilide hydroxamic acid; CML, chronic myeloid leukemia; Ac-histone, acetylated histone; PARP, poly adenosine diphosphate-ribose polymerase; NAC, N-acetyl-L-cysteine.
Discussion
While the introduction of the Bcr-Abl kinase inhibitor, imatinib, into the clinical armamentarium, represents an important advance in the treatment of CML, the development of drug resistance constitutes a significant barrier to curing this disease (2,17). The present study demonstrated that treatment with the HDACi, SAHA (0.5–8 µM), induced cell death in imatinib-sensitive K562 cells, as well as imatinib-resistant K562G cells, in a dose-dependent manner. The proteasome inhibitor, MG-132, significantly and synergistically enhanced low-dose SAHA-induced cell death and inhibited colony formation, suggesting a synergistic effect of combined SAHA and MG-132 treatment in CML cells in vitro.
The level of acetylated histones is a useful intermediary marker of HDACi activity (18). SAHA induces the accumulation of acetylated histones in the chromatin, and it has been reported that exposure to SAHA increased the acetylation of histone H3 in K562 cells (8). In accordance with this, the present study showed that SAHA (1–4 µM) increased the acetylation of histone H3 in a dose-dependent manner in K562 cells. Furthermore, combined SAHA and MG-132 treatment resulted in a significant increase in acetylated histone H3 expression, compared with SAHA alone, suggesting that MG-132 enhances the activity of SAHA in CML cells by enhancing histone acetylation.
Bcr-Abl is frequently the causative agent in CML and is known to be associated with drug resistance (1). It has been reported that SAHA enhances sensitivity to imatinib in CML cells by decreasing the level of the Bcr-Abl protein and that this effect is associated with apoptosis (8). The combination of SAHA with the proteasome inhibitor, bortezomib, also exhibits an association with Bcr-Abl downregulation and apoptosis (11). The current study showed that SAHA (2–4 µM) significantly inhibited the expression of Bcr-Abl in CML cells. This is in accordance with a previous study, which reported that exposure to 2.0 µM SAHA for 48 h downregulated the expression of Bcr-Abl in K562 cells, in association with the attenuation of auto-tyrosine phosphorylation of Bcr-Abl (8). The present study also demonstrated that co-treatment of SAHA and MG-132 induced significant downregulation of Bcr-Abl, compared with either drug alone, in K562 and K562G cells, suggesting that the synergistic effect of SAHA and MG-132 on cell death in CML cells is associated with Bcr-Abl downregulation. The mechanisms underlying the development of clinical resistance to the imatinib, appear to involve either increased expression of the Bcr-Abl protein through gene amplification (19) or the development of mutations in the Bcr-Abl catalytic domain, which interfere with imatinib binding to Bcr-Abl (20). The present finding that combined treatment with SAHA and MG-132 effectively induces cell death in the imatinib-resistant K562G cell line, in association with downregulation of Bcr-Abl expression, raises the possibility that this combination may be of value in patients exhibiting the former type of drug resistance. Furthermore, SAHA (1–4 µM) was shown to activate caspase-9, caspase-3 and PARP cleavage in K562 cells, in a dose dependent manner. Activation of caspase-9 and caspase-3 is hypothesized to irreversibly commit a cell to apoptosis. PARP is the substrate of caspase-3, and activation of caspase-3 thus commits a cell to apoptosis (21). The present results suggest that SAHA-induced cell death in CML cells is result of an increase in apoptosis. Furthermore, MG-132 enhances SAHA-induced caspase activation in K562 and K562G cells, suggesting that the CML cell death induced by SAHA in combination with MG-132, is associated with caspase-dependent apoptosis. However, the pan-caspase inhibitor, z-VAD-fmk, only resulted in the partial reversal of cell death of K562 and K562G cells, indicating that caspase-independent mechanisms also lead to cell death in combined SAHA and MG-132 treatment.
ROS are involved in cell death. They provoke apoptosis and necrotic cell death (22), and also regulate autophagy (23). The current study demonstrated that the cell death induced by the combination of SAHA and MG-132, involved the generation of ROS, and the ROS scavenger, NAC, significantly blocked cell death, in addition to ROS formation. Activation of the cleaved form of PARP was also significantly reduced by treatment with NAC, suggesting that ROS is involved in the induction of cell death by SAHA and MG-132, and that apoptosis is at least partially a result of ROS-induced cell death under these conditions. However, further investigation is required in order to clarify whether other forms of cell death, such as autophagy and necrosis, lead to cell the death of CML cells as a result of combined treatment with SAHA and MG-132.
To date, the association between Bcr-Abl and ROS remains unclear. A number of reports have suggested that the increases in ROS generation may stem from Bcr-Abl kinase inhibition and that ROS levels are increased downstream of Bcr-Abl (24,25). However, in the present study, NAC reversed the cell death and Bcr-Abl downregulation induced by combined treatment of SAHA and MG-132, which indicates that the increase in ROS is upstream of Bcr-Abl downregulation and mediates the synergistic effect of SAHA and MG-132 in CML cells.
In conclusion, the present study demonstrates potent anti-CML activity of combined treatment with SAHA and MG-132 in vitro and elucidates the underlying mechanisms of this effect, which are associated with Bcr-Abl downregulation and increased ROS production. The current study also established ROS as an upstream regulator of Bcr-Abl. These findings provide a rationale for further investigation into the use of combined SAHA and MG-132 treatment in CML.
Acknowledgements
This study was supported by the Doctoral Fund of Ministry of Education of China (grant no. 20120101110010), the National Natural Science Foundation of China (grant nos. 81070419 and 81200384), Zhejiang Provincial Natural Science Foundation of China (grant no. R2090392), Funds of Science Technology Department of Zhejiang Province (grant nos. 2012C13021-2 and 2012C37103), Zhejiang Leading Team of S&T Innovation (grant no. 2011R50015) and the Fund of Health Bureau of Zhejiang Province (grant no. 2010SSA006).
References
- 1.Yang C, Yang J, Sun M, Yan J, Meng X, Ma T. Alantolactone inhibits growth of K562/adriamycin cells by downregulating Bcr/Abl and P-glycoprotein expression. IUBMB Life. 2013;65:435–444. doi: 10.1002/iub.1141. [DOI] [PubMed] [Google Scholar]
- 2.Melo JV, Chuah C. Novel agents in CML therapy: Tyrosine kinase inhibitors and beyond. Hematology Am Soc Hematol Educ Program. 2008:427–435. doi: 10.1182/asheducation-2008.1.427. [DOI] [PubMed] [Google Scholar]
- 3.Carafa V, Nebbioso A, Altucci L. Histone deacetylase inhibitors: Recent insights from basic to clinical knowledge & patenting of anti-cancer actions. Recent Pat Anticancer Drug Discov. 2011;6:131–145. doi: 10.2174/157489211793980088. [DOI] [PubMed] [Google Scholar]
- 4.Lemal R, Ravinet A, Moluçon-Chabrot C, Bay JO, Guièze R. Histone deacetylase inhibitors in the treatment of hematological malignancies. Bull Cancer. 2011;98:867–878. doi: 10.1684/bdc.2011.1409. (In French) [DOI] [PubMed] [Google Scholar]
- 5.O'Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, MacGregor-Cortelli B, Curly T, Moskowitz C, Portlock C, Horwitz S, et al. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006;24:166–173. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- 6.Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors-development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S, Tanaka K, Sakimura R, Okada T, Nakamura T, Li Y, Takasaki M, Nakabeppu Y, Iwamoto Y. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer Res. 2008;28:1585–1591. [PubMed] [Google Scholar]
- 8.Nimmanapalli R, Fuino L, Stobaugh C, Richon V, Bhalla K. Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Blood. 2003;101:3236–3239. doi: 10.1182/blood-2002-08-2675. [DOI] [PubMed] [Google Scholar]
- 9.Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, Cleveland JL. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiskus W, Wang Y, Joshi R, Rao R, Yang Y, Chen J, Kolhe R, Balusu R, Eaton K, Lee P, et al. Cotreatment with vorinostat enhances activity of MK-0457 (VX-680) against acute and chronic myelogenous leukemia cells. Clin Cancer Res. 2008;14:6106–6115. doi: 10.1158/1078-0432.CCR-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu C, Rahmani M, Conrad D, Subler M, Dent P, Grant S. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood. 2003;102:3765–3774. doi: 10.1182/blood-2003-03-0737. [DOI] [PubMed] [Google Scholar]
- 12.Guo N, Peng Z. MG132, A proteasome inhibitor, Induces apoptosis in tumor cells. Asia Pac J Clin Oncol. 2013;9:6–11. doi: 10.1111/j.1743-7563.2012.01535.x. [DOI] [PubMed] [Google Scholar]
- 13.Jagani Z, Song K, Kutok JL, Dewar MR, Melet A, Santos T, Grassian A, Ghaffari S, Wu C, Yeckes-Rodin H, et al. Proteasome inhibition causes regression of leukemia and abrogates BCR-ABL-induced evasion of apoptosis in part through regulation of forkhead tumor suppressors. Cancer Res. 2009;69:6546–6555. doi: 10.1158/0008-5472.CAN-09-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller CP, Ban K, Dujka ME, McConkey DJ, Munsell M, Palladino M, Chandra J. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110:267–277. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong Y, Liu YY, You LS, Qian WB. Perifosine induces protective autophagy and upregulation of ATG5 in human chronic myelogenous leukemia cells in vitro. Acta Pharmacol Sin. 2012;33:542–550. doi: 10.1038/aps.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park WH, Kim SH. MG132, a proteasome inhibitor, induces human pulmonary fibroblast cell death via increasing ROS levels and GSH depletion. Oncol Rep. 2012;27:1284–1291. doi: 10.3892/or.2012.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaney NB, Holyoake TL. Therapeutic targets in chronic myeloid leukaemia. Hematol Oncol. 2007;25:66–75. doi: 10.1002/hon.813. [DOI] [PubMed] [Google Scholar]
- 18.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 19.Gorre ME, Sawyers CL. Molecular mechanisms of resistance to STI571 in chronic myeloid leukemia. Curr Opin Hematol. 2002;9:303–307. doi: 10.1097/00062752-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, Sawyers CL. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/S1535-6108(02)00096-X. [DOI] [PubMed] [Google Scholar]
- 21.Sankari SL, Masthan KM, Babu NA, Bhattacharjee T, Elumalai M. Apoptosis in cancer-an update. Asian Pac J Cancer Prev. 2012;13:4873–4878. doi: 10.7314/APJCP.2012.13.10.4873. [DOI] [PubMed] [Google Scholar]
- 22.Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 23.Ling LU, Tan KB, Lin H, Chiu GN. The role of reactive oxygen species and autophagy in safingol-induced cell death. Cell Death Dis. 2011;2:e129. doi: 10.1038/cddis.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein SJ, Baldwin AS. NF-κB suppresses ROS levels in BCR-ABL(+) cells to prevent activation of JNK and cell death. Oncogene. 2011;30:4557–4566. doi: 10.1038/onc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T, Dai Y, Attkisson E, Kramer L, Jordan N, Nguyen N, Kolluri N, Muschen M, Grant S. HDAC inhibitors potentiate the activity of the BCR/ABL kinase inhibitor KW.2449 in imatinib. sensitive or. resistant BCR/ABL+leukemia cells in vitro and in vivo. Clin Cancer Res. 2011;17:3219–3232. doi: 10.1158/1078-0432.CCR-11-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]