Abstract
The aim of this study was to investigate the effects of emodin on the proliferation of human breast cancer cells Bcap-37 and ZR-75-30. Cell viability following emodin treatment was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The effects of emodin on apoptosis were determined by flow cytometry using Annexin V-fluorescein isothiocyanate and propidium iodide staining. Quantitative polymerase chain reaction and western blot analysis were used to determine changes in the expression of apoptotic genes and protein, respectively. The effect of emodin on the invasiveness of breast cancer cells was evaluated by Matrigel invasion assay. Treatment of breast cancer cells Bcap-37 and ZR-75-30 with emodin was observed to inhibit the growth and induced apoptosis in a time- and dose-dependent manner. Emodin reduced the level of Bcl-2 and increased levels of cleaved caspase-3, PARP, p53 and Bax. These findings indicate that emodin induces growth inhibition and apoptosis in human breast cancer cells. Emodin may be a potential therapeutic agent for the treatment of breast cancer.
Keywords: emodin, breast carcinoma, apoptosis, growth inhibition
Introduction
Breast cancer is a common malignant tumor and accounts for the majority of cancer mortality in females (1). Breast cancer has an estimated incidence of 1,676,633 and had an estimated mortality rate of 521,817 in 2012 worldwide (2). In China, there has been an increasing incidence of breast cancer in recent decades (3). In 2012, it was estimated that ~48,000 females would succumb to breast cancer. To date, chemotherapy has been the mainstay for the treatment of breast cancer. However, the side effects and drug resistance associated with chemotherapy have limited its effectiveness for the treatment of breast cancer (4,5). A number of natural extractions have demonstrated a wide range of biological activity and low toxicity in animal models, and have been considered as an alternative method of breast cancer treatment (6,7).
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is a biologically active anthraquinone identified in the roots and bark of a number of plants. Emodin is also observed naturally in a number of widely used Chinese medicinal herbs, including Rheum officinale (8) and Polygonam cuspidatummedicine (9). It has been widely studied for its antibacterial, diuretic, immunosuppressive, anti-inflammatory and vasorelaxant effects. It has been reported that emodin induces apoptosis of several types of human cancer cells (10–13) by modulating various signaling pathways. However, the biological mechanisms by which emodin induces cytotoxicity remain largely unknown. Furthermore, the biological effects of emodin on breast cancer cells remain to be determined.
In this study, the effects of emodin on the proliferation and apoptosis of breast cancer cells were assessed. In addition, the mechanism by which emodin mediates these biological effects was elucidated. We demonstrated that emodin inhibited the proliferation and induced apoptosis of Bcap-37 and ZR-75-30 breast cancer cells. These observations suggest that emodin may be a potential agent in the treatment of breast cancer.
Materials and methods
Drugs and antibodies
Emodin was purchased from Sigma-Aldrich (St. Louis, MO, USA). A stock solution of emodin (10 mg/ml) was prepared in dimethyl sulfoxide (DMSO) and further diluted in culture medium. DMSO (0.05%) was used as vehicle control in all experiments. The antibodies used for western blot analysis were as follows: anti-cleaved caspase-3 (#9661), anti-Bcl-2 (#2876), anti-Bax (#2772), anti-PARP (#9532), anti-p53 (#2527) and anti-β-actin (#4970). All antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture
Human breast cancer cells Bcap-37 and ZR-75-30 were obtained from the Cell Resource Center of Shanghai Life Science Research Institute, Chinese Academy of Sciences and cultured in RPMI-1640 medium (Invitrogen Corporation, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Scientific Hyclone, Logan, UT, USA), 100 µg/ml streptomycin and 100 U/ml penicillin at 37°C in a humidified incubator in an atmosphere of 5% CO2.
Cell viability assay
Cells were trypsinized and seeded into 96-well plates (Corning, Tewksbury, MA, USA) at a density of 1×104 cells per well and incubated overnight. The cells were replenished with fresh medium containing various concentrations (0, 10, 20, 40 and 80 µM) of emodin for 24, 48 and 72 h. At each time point, 10 ml of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) solution was added into each well and incubated for an additional 4 h at 37°C. The culture medium was then removed, and 100 µl DMSO was added into each well. The absorbance was recorded at 490 nm on a microplate reader (BioTek, Winooski, VT, USA). The results represent the average values of three experiments. Each experiment was performed at least three times.
Apoptosis analysis
Apoptosis was evaluated by Annexin V-fluorescein isothiocyanate (Annexin V-FITC; KeyGEN Biotech, Nanjing, China). Cells were treated with emodin (0, 10 and 40 µM) for 24 h. Then, 5×105 cells were collected, resuspended in 500 ml binding buffer containing an additional 5 µl Annexin V-FITC and 5 µl propidium iodide, and incubated for 15 mins at room temperature without direct sunlight. Samples were analyzed by flow cytometry (BD Pharmingen, San Diego, CA, USA). Experiments were performed at least three times.
Quantitative polymerase chain reaction (qPCR)
Breast cancer cells were treated with emodin (0, 10 and 40 µM) for 24 h. Total RNA was extracted by the RNAiso Plus kit (Takara, Dalian, China). First-strand complementary DNA (cDNA) was synthesized using the PrimeScript RT reagent kit (Takara) according to the instructions. Amplification of the cDNA for PCR was performed in a reaction volume of 20 µl which included 2 µl cDNA. The primer sequences used were as follows: p53 (forward, ATCCTCACCATCATCACACTGG; reverse, ACAAACACGCACCTCAAAGC); Bcl-2 (forward, CAAATGCTGGACTGAAAAATTGTA; reverse, TATTTTCTAAGGACGGCATGATCT); Bax (forward, GACACCTGAGCTGACCTTGG; reverse: AGGAAGTCCAGTGTCCAGC); β-actin (forward, CTGGGACGACATGGAGAAAA, reverse: AAGGAAGGCTGGAAGAGTGC). The PCR cycling conditions were as follows: 95°C for 30 sec followed by 40 cycles at 95°C for 5 sec, and annealing at 60°C for 30 sec. Each experiment was repeated three times. β-actin was used as an internal reference gene to normalize gene expression. Relative gene expression was analyzed by the comparative threshold cycle (Ct) method. The value was used to plot the expression of apoptotic genes by 2−∆∆Ct.
Matrigel invasion assay
Transwell chambers (Corning) were used to assess the invasiveness of breast cancer cells. Two chambers were separated by Matrigel-coated polycarbonate membrane (6.5-mm diameter inserts with an 8-µm pore size). Cells were treated with FBS-free medium for 24 h, and 5×105 cells/ml with various concentrations of emodin (0, 10 and 40 µM) in a total volume of 200 µl were placed in the upper chamber. A total of 500 µl RPMI-1640 medium and 10% FBS were added to the lower chamber. After 24 h, the cells remaining on the upper surface of the filter were removed with a cotton swab. The membrane was fixed with methanol and then stained with crystal violet. Cell migration was measured by a cell staining count in five separate areas using a light microscope (Olympus BX41; Olympus Inc., Center Valley, PA, USA). Each experiment was performed at least three times.
Western blot analysis
Western blot analysis was used to detect the levels of apoptotic protein. Breast cancer cells were treated with various concentrations of emodin (0, 10 and 40 µM) for 24 h. Cells were harvested and lysed in lysis buffer (Beyotime Biotech, Shanghai, China). The total protein concentration of the cell was determined by the bicinchoninic acid assay (KeyGEN Biotech). Forty micrograms of protein were separated by electrophoresis on 10% sodium dodecyl sulphate-polyacrylamide gel. The gel was then transferred to polyvinylidene fluoride membranes, and then blocked for 2 h with Tris-buffered saline and Tween-20 containing 5% non-fat milk. Next, the membranes were incubated with the primary antibodies at 4°C overnight. This was followed by incubation at room temperature with the appropriate horseradish peroxidase-conjugated secondary antibodies for 2 h. The results were detected using an electrochemiluminesence kit (Pierce, Rockford, IL, USA).
Statistical analysis
All assays were performed independently in triplicate. All values were expressed as mean ± standard deviation and analyzed by Student's t-test using SPSS 19.0 software (IBM SPSS, Armonk, MY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of emodin on the viability of breast cancer cells
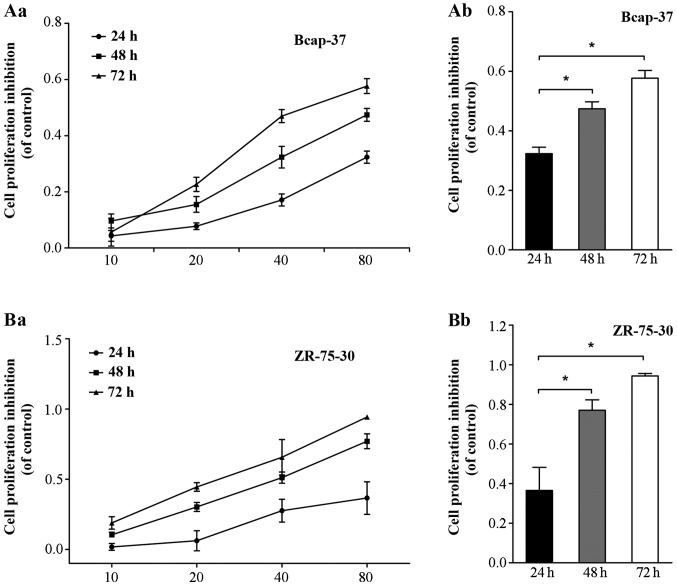
The effects of emodin on the growth of Bcap-37 and ZR-75-30 cells were measured by MTT assay. As shown in Fig. 1, emodin significantly inhibited the growth of Bcap-37 and ZR-75-30 cells in a time- and dose-dependent manner. The highest rates of decreased growth were observed using at least 10 µmol/l emodin for 72 h (Fig. 1Aa and Ba; P<0.05). This result suggests that emodin inhibited the proliferation of Bcap-37 and ZR-75-30 cells.
Figure 1.
Effects of emodin on cell viability of Bcap-37 and ZR-75-30 cells. (Aa and Ab) Inhibitory effect of emodin on proliferation of Bcap-37 cells. (Ba and Bb) Inhibitory effect of emodin on proliferation of ZR-75-30 cells. *P<0.05 vs. control.
Effects of emodin on apoptosis
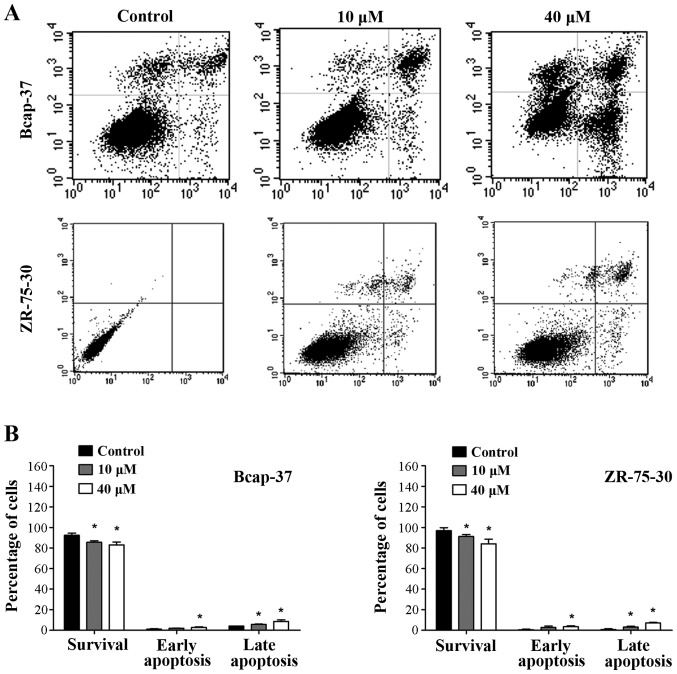
To determine whether the growth inhibition by emodin is associated with apoptosis, flow cytometry analysis was performed. Significant apoptosis induced by emodin was observed in Bcap-37 and ZR-75-30 cells in a concentration-dependent manner (Fig. 2; P<0.05). Apoptotic rates were highest when using emodin at 40 µM. These results indicate that emodin induced apoptosis of breast cancer cells.
Figure 2.
Effects of emodin on apoptosis of Bcap-37 and ZR-75-30 cells. (A) Emodin-induced apoptosis analyzed by flow cytometry at doses of 0, 10 and 40 µM for 24 h. (B) Emodin-induced apoptosis rate shown by bar graph. *P<0.05 vs. control.
Effects of emodin on caspase activation and Bcl-2 family members
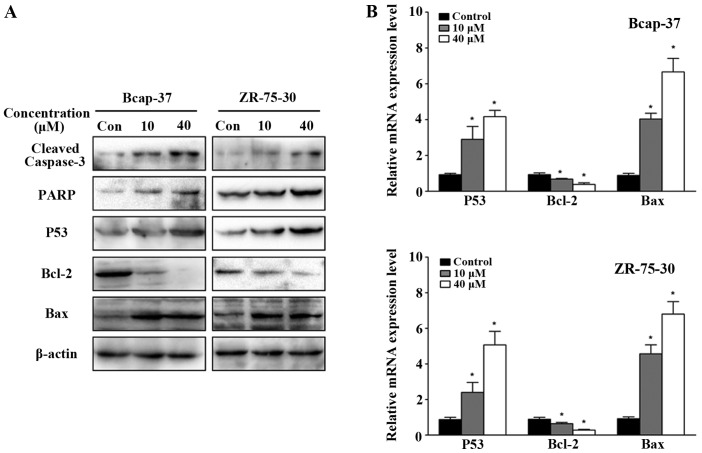
To investigate the effects of emodin on the molecular mechanisms associated with apoptosis in breast cancer cells, the expression of apoptosis-related proteins was analyzed by western blot analysis. Emodin treatment downregulated Bcl-2 expression while increasing caspase-3 activation, PARP, p53 and Bax levels in breast cancer cells in a concentration-dependent manner (Fig. 3A). Similar observations were made with respect to the mRNA level by qPCR, with significant increases in Bax levels, and decreases in p53 and Bcl-2 (Fig. 3B; P<0.05). These observations provide further support that emodin induces apoptosis of breast cancer cells.
Figure 3.
Effects of emodin on apoptosis-related protein and mRNA in Bcap-37 and ZR-75-30 cells. (A) Alteration of cleaved caspase-3, PARP, p53, Bcl-2 and Bax protein following treatment with emodin (0, 10 and 40 µM) for 24 h. (B) Relative mRNA expression level of p53, Bcl-2 and Bax. *P<0.05 vs. control.
Effects of emodin on cell invasion
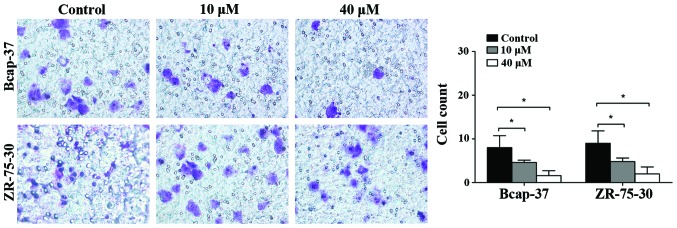
The effects of emodin on the invasiveness of breast cancer cells were assessed by the Matrigel invasion assay. As shown in Fig. 4, emodin decreased the number of Bcap-37 and ZR-75-30 cells invading through the Matrigel at 24 h in a concentration-dependent manner. Transwell assay demonstrated that the invasiveness of Bcap-37 and ZR-75-30 cells was suppressed by emodin.
Figure 4.
Effects of emodin on invasion of Bcap-37 and ZR-75-30 cells. *P<0.05 vs. control.
Discussion
Emodin is a natural anthraquinone and an inhibitor of tyrosine kinase (14). Emodin is derived from rhubarb and numerous other plants, and has demonstrated antibacterial, antiviral, anti-inflammatory and anticancer effects (15). However, previous studies have revealed that emodin inhibits cell growth in several types of cancer cells by regulating genes related to apoptosis, oncogenesis, proliferation and cancer cell invasion and metastasis (10,16,17). Notably, emodin inhibited epithelial-mesenchymal transition, suggesting that emodin may have anticancer effects (18). Additionally, a study demonstrated that emodin enhanced anticancer effects when used in combination with gemcitabine (19). However, little is known about the effects of emodin in breast cancer cells. In this study, we assessed the effects of emodin on the growth, apoptosis and invasiveness of Bcap-37 and ZR-75-30 breast cancer cells.
In this study, emodin induced apoptosis of Bcap-37 and ZR-75-30 cells in a dose- and time-dependent manner. Furthermore, flow cytometric analysis demonstrated a significant increase in the number of Bcap-37 and ZR-75-30 cells in the early and late apoptosis phase. Emodin also inhibited the invasiveness of these cells, as demonstrated by the Matrigel invasion assay. These findings suggest that emodin functions by inhibiting the growth and invasion of tumor cells.
Cell apoptosis is an autonomous cell death process that regulates the development and homeostasis of multicellular organisms (20). A key strategy for drug development is to induce apoptosis of cancer cells while minimizing the damage to normal and healthy cells (21). The induction of apoptosis in cancer cells has been described as a key mechanism in anticancer therapy (22,23).
Apoptosis is regulated by the Fas and mitochondrial signaling pathways (24). The tumor suppressor gene p53 plays a significant role in genomic stability, anticancer activity, protection against malignant transformation, and induction of apoptosis (25,26). p53 is a transcriptional factor that upregulates the expression of downstream genes involved in cell cycle arrest, senescence, autophagy and apoptosis. The p53 protein is also involved in apoptosis, partly by inducing Bax expression (27). Bcl-2 is an inhibitor of the mitochondrial apoptotic pathway, which prevents the release of cytochrome c and caspase activation by blocking the effects of pro-apoptotic proteins (28). Among the Bcl-2 gene family, the apoptosis-promoting protein Bax and the anti-apoptotic protein Bcl-2 play a crucial role in regulating cell apoptosis (29,30), which depends on the occurrence and severity of apoptosis (30). In our study, a dose-dependent decrease in Bcl-2 expression and an increase in Bax expression was observed following emodin treatment in Bcap-37 and ZR-75-30 cells. These observations demonstrate that emodin-induced apoptosis of Bcap-37 and ZR-75-30 cells was triggered by the downregulation of Bcl-2 and the upregulation of Bax. Previous studies have demonstrated that the mitochondrial membrane potential stimulates the opening of the mitochondrial membrane, resulting in the release of cytochrome c into the cytoplasm, caspase activation and degradation of key intracellular proteins, which leads to apoptosis (31,32).
Caspase-3 exists in the cytoplasm as an inactive zymogen. When activated by the external apoptotic signals, caspase-3 induces the inactivation of a number of key proteases in the cytoplasm, cell nucleus and cytoskeleton, which subsequently induces apoptosis (33). The cleavage of apoptosis-related proteins, caspase-9, caspase-3 and PARP is accompanied by cytochrome c release. In this study, the cleavage of caspase-3 expression and the alteration of PARP were in accordance with the tendency of cell apoptosis (34).
In summary, we have demonstrated in the present study that emodin significantly inhibited the growth and invasiveness, and induced the apoptosis of human breast cancer cells in a dose- and time-dependent manner. These processes were also associated with caspase and PARP activation, increased levels of p53 and Bax, and decreased levels of Bcl-2. Further studies are needed in the future in order to elucidate the biological and molecular mechanisms of emodin for the treatment of breast cancer. Overall, this study suggests that emodin is a potential therapeutic agent for the treatment of breast cancer.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 81172199 and 81272920).
References
- 1.Sanguinetti A, Polistena A, Lucchini R, Monacelli M, Avenia S, Conti C, Barillaro I, Rondelli F, Bugiantella W, Avenia N. Breast cancer in older women: What factors affect the treatment? Int J Surg. 2014;12(Suppl 2):S177–S180. doi: 10.1016/j.ijsu.2014.08.346. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. International Agency for Research on Cancer. Lyon, France: 2013. [Dec 12;2013 ]. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Accessed. [Google Scholar]
- 3.Zeng H, Zheng R, Zhang S, Zou X, Chen W. Female breast cancer statistics of 2010 in China: Estimates based on data from 145 population-based cancer registries. J Thorac Dis. 2014;6:466–470. doi: 10.3978/j.issn.2072-1439.2014.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin G, Zhu W, Yang L, Wu J, Lin B, Xu Y, Cheng Z, Xia C, Gong Q, Song B, Ai H. Delivery of siRNA by MRI-visible nanovehicles to overcome drug resistance in MCF-7/ADR human breast cancer cells. Biomaterials. 2014;35:9495–9507. doi: 10.1016/j.biomaterials.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Amirzada MI, Ma X, Gong X, Chen Y, Bashir S, Jin J. Recombinant human interleukin 24 reverses Adriamycin resistance in a human breast cancer cell line. Pharmacol Rep. 2014;66:915–919. doi: 10.1016/j.pharep.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Bright-Gbebry M, Makambi KH, Rohan JP, Llanos AA, Rosenberg L, Palmer JR, Adams-Campbell LL. Use of multivitamins, folic acid and herbal supplements among breast cancer survivors: the black women's health study. BMC Complement Altern Med. 2011;11:30. doi: 10.1186/1472-6882-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spazzapan S, Crivellari D, Bedard P, Lombardi D, Miolo G, Scalone S, Veronesi A. Therapeutic management of breast cancer in the elderly. Expert Opin Pharmacother. 2011;12:945–960. doi: 10.1517/14656566.2011.540570. [DOI] [PubMed] [Google Scholar]
- 8.Li WY, Chan SW, Guo DJ, Chung MK, Leung TY, Yu PH. Water extract of Rheum officinale Baill. induces apoptosis in human lung adenocarcinoma A549 and human breast cancer MCF-7 cell lines. J Ethnopharmacol. 2009;124:251–256. doi: 10.1016/j.jep.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Wu X, Chen M, Duan W, Sun L, Yan M, Zhang L. Emodin induces apoptosis through caspase 3-dependent pathway in HK-2 cells. Toxicology. 2007;231:120–128. doi: 10.1016/j.tox.2006.11.064. [DOI] [PubMed] [Google Scholar]
- 10.Li WY, Ng YF, Zhang H, Guo ZD, Guo DJ, Kwan YW, Leung GP, Lee SM, Yu PH, Chan SW. Emodin elicits cytotoxicity in human lung adenocarcinoma A549 cells through inducing apoptosis. Inflammopharmacology. 2014;22:127–134. doi: 10.1007/s10787-013-0186-4. [DOI] [PubMed] [Google Scholar]
- 11.Li WY, Chan RY, Yu PH, Chan SW. Emodin induces cytotoxic effect in human breast carcinoma MCF-7 cell through modulating the expression of apoptosis-related genes. Pharm Biol. 2013;51:1175–1181. doi: 10.3109/13880209.2013.782322. [DOI] [PubMed] [Google Scholar]
- 12.Sui JQ, Xie KP, Zou W, Xie MJ. Emodin inhibits breast cancer cell proliferation through the ERα-MAPK/Akt-cyclin D1/Bcl-2 signaling pathway. Asian Pac J Cancer Prev. 2014;15:6247–6251. doi: 10.7314/APJCP.2014.15.15.6247. [DOI] [PubMed] [Google Scholar]
- 13.Pooja T, Karunagaran D. Emodin suppresses Wnt signaling in human colorectal cancer cells SW480 and SW620. Eur J Pharmacol. 2014;742:55–64. doi: 10.1016/j.ejphar.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Wei WT, Lin SZ, Liu DL, Wang ZH. The distinct mechanisms of the antitumor activity of emodin in different types of cancer (Review) Oncol Rep. 2013;30:2555–2562. doi: 10.3892/or.2013.2741. [DOI] [PubMed] [Google Scholar]
- 15.Xu JD, Liu S, Wang W, Li LS, Li XF, Li Y, Guo H, Ji T, Feng XY, Hou XL, et al. Emodin induces chloride secretion in rat distal colon through activation of mast cells and enteric neurons. Br J Pharmacol. 2012;165:197–207. doi: 10.1111/j.1476-5381.2011.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue H, Chen Y, Cai X, Zhao L, He A, Guo K, Zheng X. The combined effect of survivin-targeted shRNA and emodin on the proliferation and invasion of ovarian cancer cells. Anticancer Drugs. 2013;24:937–944. doi: 10.1097/CAD.0b013e328364efe0. [DOI] [PubMed] [Google Scholar]
- 17.Yaoxian W, Hui Y, Yunyan Z, Yanqin L, Xin G, Xiaoke W. Emodin induces apoptosis of human cervical cancer hela cells via intrinsic mitochondrial and extrinsic death receptor pathway. Cancer Cell Int. 2013;13:71. doi: 10.1186/1475-2867-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Way TD, Huang JT, Chou CH, Huang CH, Yang MH, Ho CT. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the β-catenin and Akt pathways. Eur J Cancer. 2014;50:366–378. doi: 10.1016/j.ejca.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Lin SZ, Wei WT, Chen H, Chen KJ, Tong HF, Wang ZH, Ni ZL, Liu HB, Guo HC, Liu DL. Antitumor activity of emodin against pancreatic cancer depends on its dual role: Promotion of apoptosis and suppression of angiogenesis. PLoS One. 2012;7:e42146. doi: 10.1371/journal.pone.0042146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muppidi J, Porter M, Siegel RM. Measurement of apoptosis and other forms of cell death. Curr Protoc Immunol. doi: 10.1002/0471142735.im0317s59. [DOI] [PubMed] [Google Scholar]
- 21.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 22.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–1424. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heath RM, Jayne DG, O'Leary R, Morrison EE, Guillou PJ. Tumour-induced apoptosis in human mesothelial cells: A mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br J Cancer. 2004;90:1437–1442. doi: 10.1038/sj.bjc.6601635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng QQ, Dong DF, Chen NZ, Wu YY, Li EX, Wang J, Wang SM. Induction of p53 expression and apoptosis by a recombinant dual-target MDM2/MDMX inhibitory protein in wild-type p53 breast cancer cells. Int J Oncol. 2013;43:1935–1942. doi: 10.3892/ijo.2013.2138. [DOI] [PubMed] [Google Scholar]
- 26.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 27.Leard LE, Broaddus VC. Mesothelial cell proliferation and apoptosis. Respirology. 2004;9:292–299. doi: 10.1111/j.1440-1843.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 28.Fang CC, Yen CJ, Tsai TJ, Chen RH, Lee PH, Tomino Y. Antibiotics induce apoptosis of human peritoneal mesothelial cells. Nephrology (Carlton) 2003;8:142–149. doi: 10.1046/j.1440-1797.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 29.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 30.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria-specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Li W. Emodin inhibits LOVO colorectal cancer cell proliferation via the regulation of the Bcl-2/Bax ratio and cytochrome c. Exp Ther Med. 2014;8:1225–1228. doi: 10.3892/etm.2014.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 33.Liu TY, Tan ZJ, Jiang L, Gu JF, Wu XS, Cao Y, Li ML, Wu KJ, Liu YB. Curcumin induces apoptosis in gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int. 2013;13:64. doi: 10.1186/1475-2867-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Wu N, Ma LN, Zhong JT, Liu G, Zheng LH, Lin XK. p38 MAPK signaling mediates mitochondrial apoptosis in cancer cells induced by oleanolic acid. Asian Pac J Cancer Prev. 2014;15:4519–4525. doi: 10.7314/APJCP.2014.15.11.4519. [DOI] [PubMed] [Google Scholar]