Abstract
In mammals, the fatty acid transport proteins (FATP1 through FATP6) are members of a highly conserved family of proteins, which function in fatty acid transport proceeding through vectorial acylation and in the activation of very long chain fatty acids, branched chain fatty acids and secondary bile acids. FATP1, 2 and 4, for example directly function in fatty acid transport and very long chain fatty acids activation while FATP5 does not function in fatty acid transport but activates secondary bile acids. In the present work, we have used stable isotopically labeled fatty acids differing in carbon length and saturation in cells expressing FATP2 to gain further insights into how this protein functions in fatty acid transport and intracellular fatty acid trafficking. Our previous studies showed the expression of FATP2 modestly increased C16:0-CoA and C20:4-CoA and significantly increased C18:3-CoA and C22:6-CoA after 4hr. The increases in C16:0-CoA and C18:3-CoA suggest FATP2 must necessarily partner with a long chain acyl CoA synthetase (Acsl) to generate C16:0-CoA and C18:3-CoA through vectorial acylation. The very long chain acyl CoA synthetase activity of FATP2 is consistent in the generation of C20:4-CoA and C22:6-CoA coincident with transport from their respective exogenous fatty acids. The trafficking of exogenous fatty acids into phosphatidic acid (PA) and into the major classes of phospholipids (phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidyserine (PS)) resulted in distinctive profiles, which changed with the expression of FATP2. The trafficking of exogenous C16:0 and C22:6 into PA was significant where there was 6.9- and 5.3-fold increased incorporation, respectively, over the control; C18:3 and C20:4 also trended to increase in the PA pool while there were no changes for C18:1 and C18:2. The trafficking of C18:3 into PC and PI trended higher and approached significance. In the case of C20:4, expression of FATP2 resulted in increases in all four classes of phospholipid, indicating little selectivity. In the case of C22:6, there were significant increases of this exogenous fatty acids being trafficking into PC and PI. Collectively, these data support the conclusion that FATP2 has a dual function in the pathways linking the transport and activation of exogenous fatty acids. We discuss the differential roles of FATP2 and its role in both fatty acid transport and fatty acid activation in the context of lipid homeostasis.
Keywords: fatty acid transport protein 2, FATP2, fatty acid activation, fatty acid trafficking, lipid synthesis
Introduction
Monitoring the transport and trafficking of exogenous fatty acids has been difficult as transport is rapid and tightly linked to downstream metabolism. Further, the tools required to distinguish externally derived fatty acids and trace their metabolic fate apart from the endogenous pools are just being developed. Emerging evidence is consistent with the notion that members of the fatty acid transport protein (FATP) family function in the selective transport and activation of different classes of fatty acids (e.g., saturated (SFA), monounsaturated (MUFA), polyunsaturated (PUFA) and highly unsaturated (HUFA)), which are subsequently directed into discrete metabolic pools to satisfy the cellular needs of specific cell types. FATPs are integral membrane-bound proteins found in both the plasma membrane and endoplasmic reticulum, several of which facilitate the uptake and activation of exogenous long chain fatty acids [1,2,3,4]. The FATPs are related to the long chain acyl CoA synthetases (Acsl) and share common features including the highly conserved ATP/AMP binding domain, which is required for fatty acid activation [5,6,7,8,9]. There is evidence that several members of the FATP family partner with specific Acsls in the vectorial acylation of exogenous fatty acids [10,11]. We have recently shown FATP2 has a preference for generating acyl-CoA derivatives derived from exogenous n-3 fatty acids, which are preferentially trafficked into PI and to a lesser extent PC [12]. These findings suggest FATP2 is part of a metabolic pathway linking the activation and trafficking of exogenous n-3 fatty acids into different classes of phospholipids. In the present work, we extended these studies using cells overexpressing FATP2 (FATP2 OE) to trace import and metabolism using different classes of stable isotopically labeled fatty acids. Direct infusion ESI/MS/MS was utilized to trace their incorporation into the downstream glycerophospholipid pools (phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidyserine (PS)). These studies provide, for the first time, a comprehensive overview of exogenous fatty acid trafficking into the major classes of phospholipids in response to the expression of FATP2. The patterns of acyl-CoA formed following incubation with exogenous deuterium [D] or 13C labeled fatty acids, from our previous studies, coupled with tracer enrichment into different phospholipid pools in this study, were consistent with a dual function for FATP2 in long chain fatty acid transport and very long chain fatty acid activation. For the transport and activation of the long chain fatty acids, FATP2 likely partners with Acsl. The transport and activation of very long chain HUFAs is consistent with a FATP2-dependent process. In both cases, the activated fatty acids are trafficked into the major classes of phospholipids following the synthesis of PA. These data demonstrate different exogenous fatty acids result in unique patterns of phospholipid incorporation, which change upon FATP2 expression.
Materials And Methods
Cell growth, expression of FATP2, and incubation of different classes of fatty acids
Stable HEK293 cells allowing the regulated expression of FATP2 or transformed with the vector (control) have been previously described [12]. Cells were routinely maintained in DMEM medium containing 10% FBS; expression of FATP2 was accomplished by the addition of tetracycline to a final concentration of 2µg/ml for 48h; the cells are harvested and then subjected to analytical studies as detailed below.
Quantitative PCR and western blotting to monitor FATP2 expression in the stable HEK293 cell lines
Quantitative RT-PCR was used to monitor the expression of FATP2 following over expression as previously detailed using SYBR Green [13]. The FATP2 mRNA levels were normalized to human (β-actin mRNA for calculations of relative abundance.
FATP2, which contains a c-myc epitope tag was routinely monitored using a c-myc antibody by western blot analysis using chemiluminescence or using dual mode imaging (LiCor, Odyssey FC). Protein abundance was determined relative to the β-actin control.
Stable isotope labeling
Forty-eight hours after induction of FATP2 expression, cells were starved for 1h in serum depleted MEM and subsequently incubated individually with 50µM [U-13C]- or deuterated [D]-labeled fatty acids for 4h (([U-13C] C16:0 (Cambridge Isotope Laboratories, Inc.), [U-13C] C18:1 (Medical Isotopes, Inc.), [U-13C] C18:2 (n-6) (Cambridge Isotope Laboratories, Inc.), [U-13C] C18:3 (n-3) (Medical Isotope, Inc.), [D8] C20:4 (n-6) (Isotec/Sigma-Aldrich), or [D5] C22:6 (n-3) (Isotec/Sigma-Aldrich)). Following incubation with the 13C or [D]-labeled fatty acids, cells were rapidly cooled to 4°C, washed with cold 100µM fatty acid free BSA in Hanks buffer and subjected to the analytical procedures detailed below.
ESI/MS/MS analysis of stable isotopically labeled phospholipids
Total lipids were extracted from cells labeled with the different isotopically labeled fatty acids using methods established by Bligh and Dyer [14]. A mixture of phospholipid Internal standards (PC 13/13, and PI 16/16) was routinely added to each sample during the first lipid extraction step for subsequent analyte quantification. Phospholipids were analyzed using a linear ion trap quadrupole mass spectrometer (QTRAP) as previously detailed [15,16,17]. The mass spectra of phospholipid classes (PC, PE, PI, PG, PS and PA) were acquired in the positive ion mode monitoring specific fragmentation pathways for each of the phospholipids that involve the polar head group of the molecule. PC, PE, PI, PS, and PA molecular species were analyzed by neutral loss scanning. In these experiments the QTRAP scanned for ions that had a neutral loss of the corresponding head group specific to each class. Table 1 provides the experimental parameters and internal standard mixture details used to quantify each phospholipid class. Fatty acid composition was determined for phospholipid molecular species that exhibited a mass that corresponded to a species that was labeled with a stable isotope labeled fatty acid. Fatty acid fragments from these unique peaks were analyzed by MS/MS scans to confirm fatty acid incorporation into species of each phospholipid class. Analyte monitoring and quantification were ducted as previously detailed [18].
TABLE 1.
Parameters of standard phospholipids analyzed by direct infusion ESI/MS methods. The parameters of the internal mixture corresponding to each class of phospholipid species are shown. All ions were monitored in the positive ion mode. PC – phosphatidylcholine; PI – phosphatidylinositol; PE p phosphatidylethanolamine; PS – phosphatidylserine; PG – phosphatidylglycerol; PA phosphatidic acid; PL – phospholipid; IS – internal standard; HG – head group; Prec – precursor scan; NL – neutral loss scan.
| PL Class | Internal Standard | IS Mass | Adduct | IS Formula | HG Fragment | ESI-MS scan mode (HG fragment mass) |
|---|---|---|---|---|---|---|
| PC | PC 13/13 | 650.47 | [M+H]+ | C34H68O8PN | phosphocholine | Prec (184) |
| PI | PI 16/16 | 828.52 | [M+NH4]+ | C41H79O13P | phosphoinositol | NL (277) |
| PE | PE 15/15 | 664.48 | [M+H]+ | C35H70O8PN | phosphoethanolamine | NL (141) |
| PS | PS 12/12 | 624.50 | [M+H]+ | C30H58O10PN | phosphoserine | NL(185) |
| PG | PG 12/12 | 628.38 | [M+NH4]+ | C30H59O10P | phosphoglycerol | NL (189) |
| PA | PA 16/18:1 | 692.49 | [M+Nh4]+ | C37H71O8P | phosphate | NL (115) |
Results
Profiles of HEK293 cells overexpressing FATP2
Using qPCR, we demonstrated the addition of 2µg/ml tetracycline increased the expression of FATP2 46-fold in the FATP2 OE cells relative to the control. When we monitored the expression of FATP2 protein in the HEK293 cells using the myc antibody, it was, likewise significantly overexpressed. We have previously shown expression of FATP2 in the HEK293 cells results in a three-fold increase in long chain fatty acid transport and a two-fold increase in very long chain fatty acid activation [12].
Trafficking of saturated, monounsaturated, polyunsaturated, and highly unsaturated fatty acids into phosphatidic acid (PA) in response to overexpression of FATP2
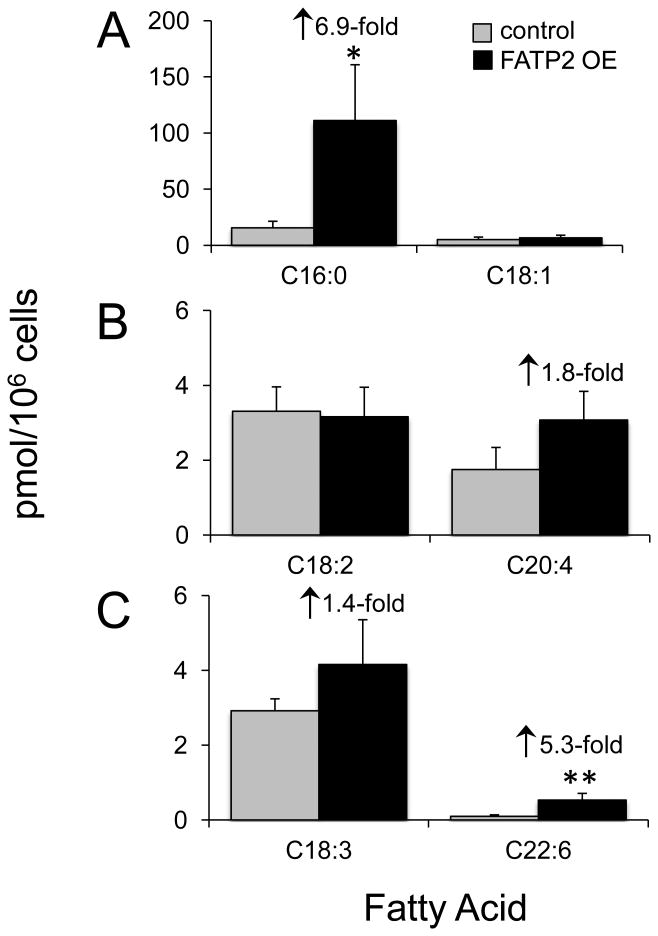
PA is the simplest of the diacylglycerol phospholipid species and is a common precursor to all glycerophospholipid species. Therefore, we addressed whether the expression of FATP2 influenced the trafficking of different classes of exogenous fatty acids into the PA pool (Figure 1). Consistent with the formation of acyl-CoA from our previous studies, these results showed increases in the trafficking of 13C16:0 (6.9-fold) and [D5]C22:6 (5.3-fold) into PA were significant and dependent upon FATP2 expression (Figures 1A and 1C). We also addressed the trafficking of 13C18:1, 13C18:2, 13C18:3 and [D8]C20:4 into PA. Our results did not reveal any difference in C18:1 and C18:2, which may suggest the flux of these fatty acid through the PA pool are likely to be rapid in the 4h time-frame used. Our studies did show that both C18:3 and C20:4 were increased in PA, which like the increased levels of their respective acyl-CoAs were consistent with FATP2 expression.
Figure 1. Trafficking of isotopically labeled fatty acids into phosphatidic acid (PA) in HEK293 cells expressing FATP2 (FATP2 OE).
FATP2 OE or control cells were labeled individually (50µM) with 13C16:0, 13C18:1, 13C18:2 (n-6), 13C18:3 (n-3), [D8]C20:4 (n-6), or [D5]C22:6 (n-3) for 4h, and isotopically phosphatidic acid (PA) determined using ESI/MS as detailed under “Experimental Procedures;” saturated fatty acids and monounsaturated fatty acids (A), n-6 fatty acids (B) and n-3 fatty acids (C). Fold differences relative to the vector control are shown; the error bars represent the standard error of the mean (n=4). **, p ≤ 0.01, *, p ≤0.05 relative to the vector control (paired t test).
Trafficking of saturated, monounsaturated, polyunsaturated, and highly unsaturated fatty acids into different classes of glycerophospholipids in response to overexpression of FATP2
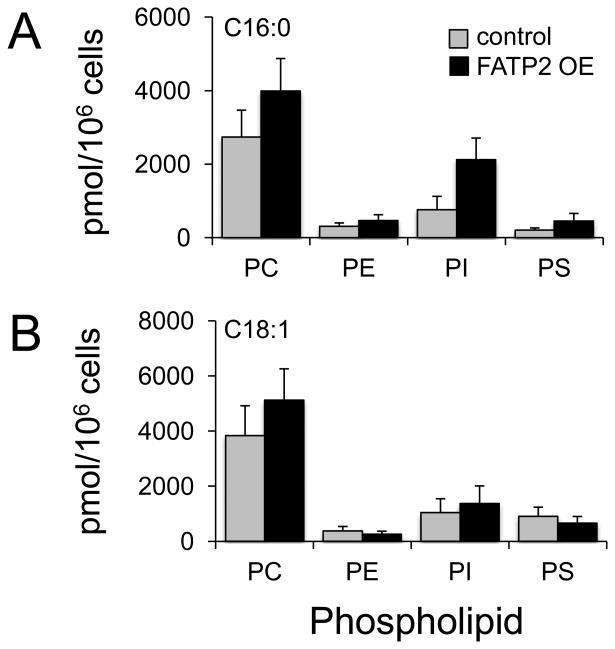
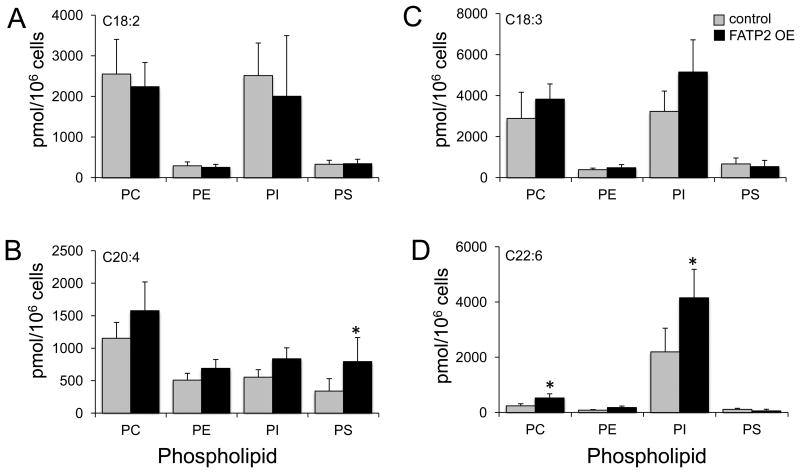
PA is a pivotal intermediate in phospholipid synthesis leading to the synthesis of PI though the formation of CDP-diacylglycerol and PC, PE and PS through diacylglycerol and the Kennedy pathway. To address the flux of PA labeled with exogenous fatty acids into PC, PE, PS, and PI, we initially focused on the 13C16:0, and 13C18:1. We observed consistent increases into PC and PI and little to no changes in PE and PS when FATP2 was expressed relative to the control (Figure 2). We next addressed the fate of exogenous n-6 fatty acids (13C18:2 and [D8]C20:4) into the different phospholipid species (Figures 3A and 3B). Of particular note were the lack differences between the FATP2 OE and control cell lines for exogenous 13C18:2, which mirrored its lack of incorporation into PA. For the n-6 HUFA, [D8]C20:4, there were increases, ranging from 25 to 50%, in incorporation into PC, PE, PI and PS. The increased trafficking of this exogenous fatty acid was more pronounced for PS, but overall there was no apparent selectivity resulting from the expression of FATP2 in the trafficking of this n-6 fatty acid into the major classes of phospholipids (Figure 3B). We next completed parallel studies using the n-3 fatty acids (13C18:3 and [D5]C22:6) and showed an enrichment into PI and to a lesser extent into PC, which was consistent with earlier studies (Figures 3C and 3D). In the case of [D5]C22:6, there were significant increases of incorporation into both the PC and PI when FATP2 was expressed. Given that PI comprises only 5-10% of total cellular phospholipid while PC is nearly 50% of the total cellular phospholipid, the selective trafficking of these n-3 fatty acids into the PI pool appears especially significant. PI is known to be enriched in the very long chain polyunsaturated fatty acids, including C22:6. An important observation from these studies was that the expression of FATP2 resulted in very different patterns of incorporation of the n-6 and n-3 HUFAs, C20:4 and C22:6 respectively, into the four classes of phospholipids. For C20:4, there was no apparent selectivity of incorporation, while for C22:6, there was a clear preference for PC and PI (Figures 3B and 3D).
Figure 2. Trafficking of isotopically labeled saturated and monounsaturated fatty acids into the major classes of phospholipids in HEK293 cells expressing FATP2 (FATP2 OE).
FATP2 OE or control cells were labeled individually (50µM) with 13C16:0, or 13C18:1 for 4h, and isotopically labeled phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS) determined using ESI/MS as detailed under “Experimental Procedures;” C16:0 (A), C18:0 (B), and C18:1 (C). The error bars represent the standard error of the mean (n=4). *, p ≤ 0.05 relative to the vector control (paired t test).
Figure 3. Trafficking of isotopically labeled n-6 and 2-3 fatty acids into the major classes of phospholipids in HEK293 cells expressing FATP2 (FATP2 OE).
FATP2 OE or control cells were labeled individually (50µM) with 13C18:2 (n-6), [D8]C20:4 (n-6), 13C18:3 (n-3) or [D5]C22:6 (n-3) for 4h, and isotopically labeled phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS) determined using ESI/MS as detailed under “Experimental Procedures;” 13C18:2 (n-6) (A), [D8]C20:4 (n-6) (B), 13C18:3 (n-3) (C) and [D5]C22:6 (n-3) (D). The error bars represent the standard error of the mean (n=4). *, p ≤ 0.05 relative to the vector control (paired t test).
Discussion
The fatty acid transport proteins have been shown to be involved in both long chain fatty acid transport and very long chain fatty acid activation. In this regard, their role in fatty acid homeostasis is, in part, linked to the movement of exogenous fatty acids into the cell through the formation of specific acyl CoA pools, which are subsequently trafficked into complex lipid synthesis primarily through phosphatidic acid. Our previous studies, along with others, have shown that FATP1, FATP2, and FATP4 function in both long chain fatty acid transport and very long chain fatty acid activation [2,4,12,13,19,20,21].
In the present study, we focused attention on FATP2, which we have previously shown functions in long chain fatty acid transport and when expressed in HEK293 cells increases acyl CoA species derived from exogenous C16:0, C18:3, C20:4 and C22:6 [2,12]. While the expression of FATP2 only slightly, but reproducibly, increased both C16:0-CoA and C20:4-CoA derived from exogenous fatty acids, the formation of acyl CoA from C18:3 and C22:6 was significant. These initial studies supported the conclusions that the biochemical activities of FATP2 (transport and activation) were distinct and consistent with our studies defining a splice variant of FATP2, studies using FATP chimeras, and directed mutagenesis of the yeast FATP (Fat1p) showing the transport and activation activities were distinguishable [9,12,20]. Of particular note from these earlier studies were the findings showing the expression of FATP2 increased the formation of acyl CoA from the exogenous long chain fatty acids C16:0 and C18:3 despite lacking the acyl CoA synthetase specificity for the long chain fatty acids. By comparison, both C20:4-CoA and C22:6-CoA, derived from exogenous fatty acids, were increased, which is likely reflective of the very long chain acyl CoA synthetase activity of FATP2.
Previously we demonstrated specific classes of exogenous fatty acids were identified as their respective acyl-CoAs following transport in cells expressing FATP2 [12]. Using these initial studies as a foundation, and as detailed in this work, we traced the trafficking of exogenous fatty acids into PA and then into PC, PE, PS and PI in cells expressing FATP2. Several findings linking our previous studies and the present study were noteworthy and of significance. The first was that exogenous C16:0 routinely resulted in increased C16:0-CoA, but these increases were subtle and only approached significance [12]. The 6.9-fold incorporation of 13C16:0 into PA when FATP2 was expressed suggests a rapid flux through its acyl CoA intermediate into PA. The same trends held for 13C18:3, [D8]C20:4, and [D5]C22:6, which when FATP2 was expressed, the resultant acyl-CoAs were increased over the control, which were then incorporated into PA. In the case of exogenous 13C18:1 and 13C18:2, there were no changes in the respective acyl CoA pools, which was reflected by no changes in the fate of these fatty acids in PA in the cells expressing FATP2.
The metabolic fate of the different classes of fatty acids into the four major classes of phospholipids in cells expressing FATP2 offered some interesting insights into their intracellular trafficking and suggests there may be differential channeling of exogenous fatty acids based on carbon length and degree of unsaturation. For the long chain fatty acids, the expression of FATP2 resulted in slightly higher levels of incorporation into PC and PI, but with little changes in PE and PS. The trafficking of the PUFA 13C18:2 into PC, PE, PS, and PI did not change with FATP2 expression, which was consistent with results showing no changes in 13C18:2-CoA formation and its incorporation into PA. The n-6 fatty acid [D8]C20:4 was increased in all four classes of phospholipids following FATP2 expression suggesting little selectivity in trafficking through PA and into PC, PE, PS, and PI. In tracing the fate of the exogenous n-3 fatty acids into downstream phospholipids, differential patterns were more apparent. For 13C18:3, there was a notable increase in the trafficking into PC and PI. Perhaps the most interesting patterns of incorporation were found for [D5]C22:6. The overall patterns of incorporation of this n-3 fatty acid into PC, PE, PI and PS was distinct when compared to the other classes of fatty acids.
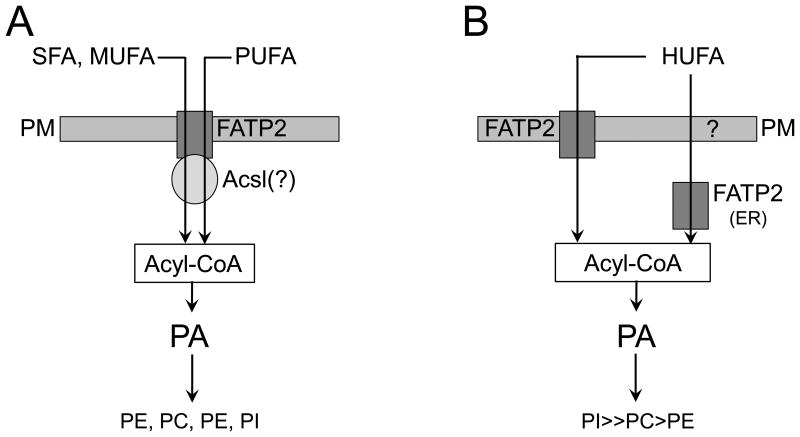
Taken together, these data supports the conclusion that fatty acid uptake is coupled with activation and that FATP2 potentiates this process. These studies, for the first time, provide a comprehensive overview addressing the mechanistic role of FATP2 in the metabolic flux of different classes of exogenous fatty acids following the formation of acyl-CoA, through PA and into the major classes of phospholipids. On the basis of the data presented in this study along with earlier studies on members of the FATP family of proteins, it is apparent that FATP2 functions in both long chain fatty acid transport and very long chain fatty acid activation. In this regard, FATP2 resides in a pathway linking the selective trafficking of different classes of exogenous fatty acids into distinct classes of phospholipids. In the context of long chain fatty acid transport, the increases of at least C16:0-CoA and C18:3-CoA derived from exogenous fatty acids, which are then incorporated into PA in response to expression of FATP2 is consistent with its interaction with a specific long chain acyl CoA synthetase (Acsl) to facilitate vectorial acylation. Since the specificity of acyl-CoA synthetase activity of FATP2 is for very long chain fatty acids (15,17,35), we propose the increase in the general pool of acyl-CoA species reported is primarily due to the coupled import activation of FATP2 and Acsl (e.g., for SFA, MUFA) together or FATP2 (e.g., for HUFA) alone. Figure 4 proposes the differential roles of FATP2 in the context of fatty acid transport, activation and metabolic channeling of the different classes of exogenous fatty acids.
Figure 4. Proposed role of FATP2 in fatty acid transport and activation.
Two scenarios are provided, one for long chain fatty acids (LCFA (SFA, MUFA, PUFA), left) and one for highly unsaturated fatty acids (HUFA, right). The LCFA saturated (SFA), monounsaturated fatty acids (MUFA), and the polyunsaturated fatty acids (PUFA) are transported across the plasma membrane (PM) by a FATP2-dependent process and are activated by a specific long chain acyl CoA synthetase (Acsl (?)) to generate specific pools of acyl-CoA, which then enter the phospholipid biosynthetic pathway through PA. In this scenario, the exogenous fatty acids that are trafficked into PA end up in the different classes of phospholipid with little selectivity. In the scenario on the right, the HUFAs (e.g., C20:4 and C22:6) enter the cell and are activated by FATP2; it is unknown whether this occurs at the PM or the endoplasmic reticulum (ER). For FATP2 mediated activation to take place at the ER, these fatty acids must traverse the PM through a mechanism that has not been fully defined (?).
While speculative at this point, different FATPs may interact with distinct acyl CoA synthetases and acyl transferases in directional metabolic trafficking of exogenous fatty acids. FATP1 and the yeast FATP, Fat1p, have been shown to specifically interact with acyl CoA synthetase [10,11]. In the case of FATP1, for example, there is also evidence this isoform is part of a complex, which includes diacylglycerol acyltransferase that is involved in the expansion of the ER-lipid droplet interface [22]. For FATP2, our evidence suggests there may be some type of interaction with a specific acyl transferase (e.g., glycerol-3-phosphate acyl transferase or lyso PA acyltransferase) during the synthesis of phosphatidic acid.
Roles of FATP2 in fatty acid transport/activation contribute to lipid homeostasis
Use of 13C- and D-labeled fatty acids provide novel insights into FATP2 function
FATP2-dependent trafficking of FA into phospholipids results in distinctive profiles
FATP2 functions in the transport and activation pathways for exogenous fatty acids
Acknowledgments
This work was supported, in whole or in part, by National Institute of Health Grants RO1-GM56850 and RO1-DK07076 (to P. N. B. and C.C.D), and 1F31-DK085961 (to E. M. M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Black PN, DiRusso CC. Vectorial acylation: linking fatty acid transport and activation to metabolic trafficking. Novartis Foundation symposium. 2007;286:127–138. doi: 10.1002/9780470985571.ch11. [DOI] [PubMed] [Google Scholar]
- 2.DiRusso CC, Li H, Darwis D, Watkins PA, Berger J, Black PN. Comparative biochemical studies of the murine fatty acid transport proteins (FATP) expressed in yeast. J Biol Chem. 2005;280:16829–16837. doi: 10.1074/jbc.M409598200. [DOI] [PubMed] [Google Scholar]
- 3.Stahl A. A current review of fatty acid transport proteins (SLC27) Eur J Physiol. 2004;447:722–727. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CM, Shahl A. SLC27 fatty acid transport proteins. Mol Asp Med. 2013:516–528. doi: 10.1016/j.mam.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black PN, DiRusso CC. Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta. 2007;1771:286–298. doi: 10.1016/j.bbalip.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Stuhlsatz-Krouper SM, Bennett NE, Schaffer JE. Substitution of alanine for serine 250 in the murine fatty acid transport protein inhibits long chain fatty acid transport. J Biol Chem. 1998;273:28642–28650. doi: 10.1074/jbc.273.44.28642. [DOI] [PubMed] [Google Scholar]
- 7.Stuhlsatz-Krouper SM, Bennett NE, Schaffer JE. Molecular aspects of fatty acid transport: mutations in the IYTSGTTGXPK motif impair fatty acid transport protein function. Prostaglandins Leukot Essent Fatty Acids. 1999;60:285–289. doi: 10.1016/s0952-3278(99)80001-5. [DOI] [PubMed] [Google Scholar]
- 8.Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. Journal of lipid research. 2007;48:2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Zou Z, DiRusso CC, Ctrnacta V, Black PN. Fatty acid transport in Saccharomyces cerevisiae. Directed mutagenesis of FAT1 distinguishes the biochemical activities associated with Fat1p. J Biol Chem. 2002;277:31062–31071. doi: 10.1074/jbc.M205034200. [DOI] [PubMed] [Google Scholar]
- 10.Richards MR, Harp JD, Ory DS, Schaffer JE. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J Lipid Res. 2006;47:665–672. doi: 10.1194/jlr.M500514-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Zou Z, Tong F, Faergeman NJ, Borsting C, Black PN, DiRusso CC. Vectorial acylation in Saccharomyces cerevisiae. Fat1p and fatty acyl-CoA synthetase are interacting components of a fatty acid import complex. J Biol Chem. 2003;278:16414–16422. doi: 10.1074/jbc.M210557200. [DOI] [PubMed] [Google Scholar]
- 12.Melton EM, Cerny RL, Watkins PA, DiRusso CC, Black PN. Human fatty acid transport protein 2a/very long chain acyl-CoA synthetase 1 (FATP2a/Acsvl1) has a preference in mediating the channeling of exogenous n-3 fatty acids into phosphatidylinositol. J Biol Chem. 2011;286:30670–30679. doi: 10.1074/jbc.M111.226316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandoval A, Fraisl P, Arias-Barrau E, Dirusso CC, Singer D, Sealls W, Black PN. Fatty acid transport and activation and the expression patterns of genes involved in fatty acid trafficking. Arch Biochem Biophys. 2008;477:363–371. doi: 10.1016/j.abb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney RT, Davis GJ, Noonan JA. Cardiomyopathy of unknown etiology: Barth syndrome unrecognized. Congenit Heart Dis. 2008;3:443–448. doi: 10.1111/j.1747-0803.2008.00226.x. [DOI] [PubMed] [Google Scholar]
- 16.Wakelam MJ, Pettitt TR, Postle AD. Lipidomic analysis of signaling pathways. Meth Enzymol. 2007;432:233–246. doi: 10.1016/S0076-6879(07)32010-7. [DOI] [PubMed] [Google Scholar]
- 17.Davis B, Koster G, Douet LJ, Scigelova M, Woffendin G, Ward JM, Smith A, Humphries J, Burnand KG, Macphee CH, Postle AD. Electrospray ionization mass spectrometry identifies substrates and products of lipoprotein-associated phospholipase A2 in oxidized human low density lipoprotein. J Biol Chem. 2008;283:6428–6437. doi: 10.1074/jbc.M709970200. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, Marepally SR, Nune DS, Pallakollu P, Ragan G, Roth MR, Wang L, Lushington GH, Visvanathan M, Welti R. LipidomeDB Data Calculation Environment: Online Processing of Direct-Infusion Mass Spectral Data for Lipid Profiles. Lipids. 2011;46:879–884. doi: 10.1007/s11745-011-3575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias-Barrau E, Dirusso CC, Black PN. Methods to monitor Fatty Acid transport proceeding through vectorial acylation. Meth Mol Biol. 2009;580:233–249. doi: 10.1007/978-1-60761-325-1_13. [DOI] [PubMed] [Google Scholar]
- 20.DiRusso CC, Darwis D, Obermeyer T, Black PN. Functional domains of the fatty acid transport proteins: studies using protein chimeras. Biochim Biophys Acta. 2008;1781:135–143. doi: 10.1016/j.bbalip.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krammer J, Digel M, Ehehalt F, Stremmel W, Fullekrug J, Ehehalt R. Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells. Int J Med Sci. 2011;8:599–614. doi: 10.7150/ijms.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu N, Zhang SO, Cole RA, McKinney SA, Guo F, Haas JT, Bobba S, Farese RV, Jr, Mak HY. The FATP1-DGAT2 complex facilitates lipid droplet expansion at the ER-lipid droplet interface. J Cell Biol. 2012;198:895–911. doi: 10.1083/jcb.201201139. [DOI] [PMC free article] [PubMed] [Google Scholar]