Abstract
The liver is a unique organ in the body as it has significant roles in both metabolism and innate immune clearance. Hepatocytes in the liver carry a nearly complete complement of drug metabolizing enzymes, including numerous cytochrome P450s. While a majority of these enzymes effectively detoxify xenobiotics, or metabolize endobiotics, a sub-portion of these reactions result in accumulation of metabolites that can cause either direct liver injury or indirect liver injury through activation of inflammation. The liver also contains multiple populations of innate immune cells including the resident macrophages (Kupffer cells), a relatively large number of natural killer cells, and blood-derived neutrophils. While these cells are primarily responsible for clearance of pathogens, activation of these immune cells can result in significant tissue injury during periods of inflammation. When activated chronically, these inflammatory bouts can lead to fibrosis, cirrhosis, cancer or death. This Chapter will focus on interactions between how the liver processes xenobiotic and endobiotic compounds through the cytochrome P450 system, and how these processes can result in a response from the innate immune cells of the liver. A number of different clinically relevant diseases, as well as experimental models, are currently available to study mechanisms related to the interplay of innate immunity and cytochrome P450 mediated metabolism. A major focus of the chapter will be to evaluate currently understood mechanisms in the context of these diseases as a way of outlining mechanisms that dictate the interactions between the P450 system and innate immunity.
Keywords: Inflammation, cytochrome P450, CYP7A1, alcohol, neutrophil, acetaminophen
Introduction
Numerous metabolic processes including metabolism of both endobiotics and xenobiotics take place in the liver. A large number of these reactions are carried out by the cytochrome P450 family of proteins (CYPs). While a majority of these processes result in largely non-toxic quantities of metabolites with favorable excretion profiles, a sub-portion of these reactions result in toxic compounds that can directly elicit liver damage. Other compounds generated from CYPs can result in activation of the inflammatory response that can either exacerbate, or help to rectify ongoing liver damage. Moreover, inflammation during liver injury can affect expression and activity of CYPs, potentially altering ongoing drug metabolism and affecting toxicity by reducing or enhancing metabolism. The goal of this Chapter will be to discuss how CYP mediated activation of a number of different compounds affects immune cells either natively present, or recruited to the liver, as well as how persistent inflammation and cytokine production can alter expression levels of CYPs. This chapter will focus on clinically relevant models of liver injury and inflammation as model systems by which we can discern specific mechanisms related to how the CYP system interacts with cytokines produced by inflammatory cells and the different immune cells. As the liver has a well-defined and critical role in innate immunity, the focus of the chapter will be on interactions with the innate immune system, although adaptive immunity will discussed in brief. As the CYP system is discussed in detail throughout this book, the innate immune system present in the liver will be discussed as a preface to the Chapter.
Innate Immune Cells in the Liver
Kupffer Cells
While the liver’s involvement in metabolism is well established, the liver also has a significant role in innate immunity via clearance of pathogens and pathogenic particles from sinusoidal blood. The primary mediator of this action is the endogenous macrophage present in liver sinusoids, the Kupffer cell (reviewed in Dixon et al., 2013). Kupffer cells are the first line of defense against xenobiotics and immunoreactive material that enters the liver from the gut. Kupffer cells primarily respond to pathogenic material by identifying and phagocytizing the material, removing it from the blood. In addition to phagocytosis of pathogens, Kupffer cells can also remove dead and dying endogenous cellular material from the bloodstream, facilitating a final barrier against potentially hazardous material before it enters systemic circulation. While it has long been known that Kupffer cells recognize and phagocytose pathogens in the hepatic sinusoids, recent research has entailed new signaling pathways by which Kupffer cells can recognize and react to pathogen associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) (Pallson-McDermott and O’Neill, 2004). Kupffer cells express a number of Toll-Like Receptor proteins (TLR1–9) that can bind bacterial or viral products such as LPS, CpG DNA, single stranded RNA and more (reviewed in Akira and Takeda, 2004). The binding of LPS to TLR4 is a classic example of the initiation of this signaling pathway. LPS in serum is recognized by LPS binding protein (LBP) and CD14, which then mediate the binding of this complex to TLR4 present on macrophages in the sinusoids (Chow et al., 1999). This activates a signaling pathway that recruits the protein MyD88, an adaptor protein for TLRs (Kawai et al., 1999); although MyD88 independent effects can also occur (Kawai et al., 2001). This signaling pathway results in the activation of NF-κB and the generation of numerous cytokines, chemokines and other inflammatory mediators (Jiang et al., 2000). This serves to both activate other surrounding macrophages, hepatocytes and innate immune cells, as well as recruit neutrophils and other peripheral mediators of innate immunity. In addition to PAMPs, Kupffer cells can also recognize damage associated molecular patterns (DAMPs) through these same TLRs. Release of DAMPs occurs both actively from activated macrophages (Tang et al., 2007) and passively from necrotic cells (Scaffidi et al., 2002) and largely activates the same signaling mechanisms via the same TLRs as the corresponding PAMP. Thus, activation of Kupffer cells can occur due to both increased exposure to pathogenic material and increased exposure to pro-inflammatory cellular debris during liver injury.
Kupffer cells produce significant amounts of the apoptotic cytokine tumor necrosis factor alpha (TNF-α). TNF-α induced apoptosis occurs through activation of the death receptor TNF receptor 1 (TNFR1) on the surface of a diverse array of cells, including hepatocytes (Yoon and Gores, 2002). TNFR-1 contains an intracellular death domain which can oligomerize and recruit multiple proteins such as Fas associated death domain (FADD), TNF receptor associated death domain (TRADD) and procaspase 8 to form a death induced cell signaling or DISC complex (Kischkel et al., 1995, 2001). In type I cells, those cells that require no mitochondrial amplification, this initiates an apoptotic cascade featuring activation of subsequent caspase such as caspase-3, and eventual cleavage of DNA by caspase activated DNAses (reviewed in Chen and Goeddel. 2002). Although certain stimuli can trigger apoptosis through a type I mechanism (Schlungel et al., 2009), hepatocytes generally act as type II cells and require mitochondrial amplification in order to fully undergo apoptosis (Bajt et al., 2000; Scaffidi et al., 1998; Yin et al., 1999). Caspase-8 cleaves Bid protein, which then translocates to the mitochondria (Yin et al., 1999). This results in release of mitochondrial proteins such as smac/diablo and cytochrome c (Liu et al., 1996; Du et al., 2000), which when combined with ATP form a complex called the apoptosome that can activate caspase 9 and initiate procaspase-3 cleavage and amplify the apoptotic signal (reviewed in Jaeschke and Lemasters, 2003). Thus, Kupffer cells are capable of releasing TNF-α to induce a potent apoptotic signal. TNF-α is the major cytokine triggering apoptosis during galactosamine/endotoxin induced liver injury (Rothe et al., 1993) and may serve as a source of liver injury in other models as well.
Activation of Kupffer cells also results in the production of reactive oxygen species (ROS) through the production of superoxide by NADPH oxidase (Jaeschke and Farhood, 1991; Jaeschke, 2011). Kupffer cell mediated oxidant stress occurs largely in the hepatic vasculature where the Kupffer cells are located; however, enough oxidant stress is generated to directly damage hepatocytes, as inactivation of Kupffer cells is protective against ischemia-reperfusion injury in the liver (Jaeschke and Farhood, 1991; Jaeschke et al., 1991). Kupffer cells are also capable of generating peroxynitrite through the expression of inducible nitric oxide synthase (Billiar et al., 1990); although there is limited evidence for the role of reactive nitrogen compounds in the pathophysiology of hepatic ischemia-reperfusion injury (Jaeschke et al., 1992; Wang et al., 1995). As such, production of ROS from Kupffer cells by NADPH oxidase is presumed to be the primary cause of Kupffer cell mediated liver injury in relevant models. Kupffer cells do contain small amounts of cytochrome P450 2E1 (Koivisto et al., 1996), which metabolizes a number of hepatotoxicants including acetaminophen (McGill and Jaeschke, 2013) and ethanol (Lieber, 1997), although most of the CYP2E1 activity is present in hepatocytes (Koivisto et al., 1996). Thus, the major contribution of Kupffer cells to these models of injury is likely a combination of their phagocytic ability, their capacity for ROS production, their ability to generate pro-apoptotic molecules such as TNF-α and their generation of other pro-inflammatory and anti-inflammatory cytokines during liver injury.
Neutrophils
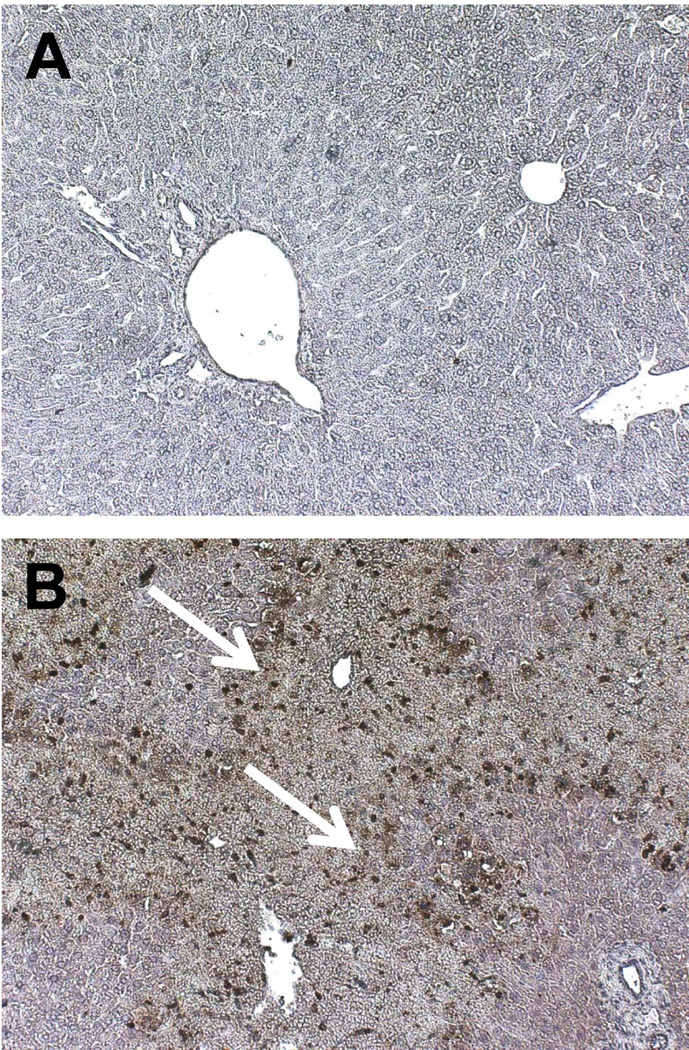
Polymorphonuclear leukocytes (PMNs), or neutrophils, are the major circulating population of leukocytes in the body. Neutrophils are generated in the bone marrow, but due to the large vascular volume in the sinusoids, and the liver being a low-flow organ, significant numbers of neutrophils are present in the sinusoids at any given time (Figure 1). Neutrophils generally do not induce liver injury while sitting in the hepatic vasculature, and can accumulate to significant degrees before any onset of injury (Chosay et al., 1997). Neutrophil extravasation coincides with increases in liver injury parameters in a number of models, and as blockade of neutrophil extravasation or neutrophil recruitment is protective, neutrophils have been implicated in the injury process of a number of a different liver pathologies including obstructive cholestasis (Gujral et al., 2003; 2004c; Yang et al., 2014), ischemia-reperfusion injury (Jaeschke et al., 1990; Jaeschke and Woolbright, 2012), non-alcoholic steatohepatitis (Harley et al., 2014), alcoholic hepatitis (Bertola et al., 2013), galactosamine/endotoxin-mediated liver injury (Jaeschke et al., 1991, 1998), and shock-induced liver trauma (Leung et al., 2014). Recruitment of neutrophils is thought to take place through multiple different mechanisms dependent on the etiology of the injury. Numerous pathologies feature release of cytokines and chemokines that can actively recruit neutrophils via their respective receptors (Bajt et al., 2001; Lentsch et al., 1998). DAMPs released from dying cells have been suggested to recruit neutrophils to areas of injury during liver intoxication as well (Imaeda et al., 2009). This process is thought to occur through DAMP mediated activation of a recently identified complex called the inflammasome, which helps mediate leukocyte recruitment in a number of models (Kubes and Mehal., 2012); although the degree to which these activities can contribute to liver injury remains controversial in certain models (Williams et al., 2010a,b, 2011; Jaeschke et al., 2012b). Activation of TLRs by DAMP products stimulates formation of an inflammasome complex. A classic example of this is the multi-protein complex containing apoptosis-associated speck-like protein containing a CARD (PYCARD), a NACHT, LRR and PYD domains-containing protein 3 protein (NALP3), and caspase-1 – or the NALP3 inflammasome (Schroder and Tschopp, 2010). The caspase-1 activity of this complex cleaves pro-IL-1β to generate the active cytokine IL-1β, which binds to IL-1 receptors and stimulates neutrophil recruitment. Once recruited to the hepatic sinusoids, neutrophils must extravasate into the hepatic parenchyma for neutrophil mediated cell killing, which is typically mediated by interactions between adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) present on hepatocytes and sinusoidal endothelial cells, and β2 integrins present on neutrophils (Jaeschke and Smith, 1997). Extravasated neutrophils can exacerbate or initiate hepatic injury through release of reactive oxygen species (ROS) generated by NADPH oxidase as well as degranulation and release of cytotoxic enzymes (Jaeschke, 2011). Neutrophils are known to express the enzyme myeloperoxidase, which can generate hypochlorous acid from hydrogen peroxide and chloride anions. HOCl modified proteins are detectable in a number of different liver pathologies with significant neutrophil involvement and are a direct marker of neutrophil-mediated oxidant stress and neutrophil mediated liver injury (Gujral et al., 2003, 2004a,b; Hasegawa et al., 2005; Ullen et al., 2013). Through release of ROS and degranulation, neutrophils create a potent localized oxidant stress that can kill hepatocytes (Jaeschke, 2011). The potential for a neutrophil mediated hepatic cell death in vivo will be discussed in detail in future sections of this chapter, as well as, the biology and physiology behind neutrophil recruitment during multiple models of liver injury.
Figure 1.
Ly6B positive neutrophils present 24 hours after control saline (A) or acetaminophen overdose in mice (B). Neutrophils localize to areas of injury in this model; however, neutrophils are constantly present in the sinusoids as in A. White arrows points to areas of neutrophil accumulation.
Natural Killer and Natural Killer T Cells
The liver also contains a significant number of natural kill cells (previously known as Pit cells) which act as an effector population in the liver (Godfrey et al., 2000). This includes multiple subtypes such as NK, NKT, iNKT, and γδT (Lysakova-Devine and O’Farrelly, 2014). Natural killer cells are capable of producing multiple cytokines including interferon-gamma (IFN-γ), and TNF-α and additionally may be capable of inducing cell death in hepatic parenchymal cells via release of enzymes such as perforin and granzymes (Vermiljen et al., 1999). Release of a number of these cytokines can affect survival of other local effector populations, including neutrophils, by either inducing or inhibiting cell death in these populations via release of IL-4 or IFN- γ (Wang et al., 2013). The effect of NK and NKT cells on liver injury is an ongoing topic of research (Tian et al., 2013.)
Other Immune Cells of the Liver
Dendritic cells are an antigen presenting class of innate immune cells and classically thought of as a link between innate immunity and adaptive immunity. While their role in liver toxicity has not been studied to the same extent as other innate immune populations, a few studies have been done that indicate dendritic cells may have a role in certain P450 mediated liver injuries, and subsequent inflammation. Depletion of dendritic cells can exacerbate acetaminophen toxicity (Connolly et al., 2011). This is likely due to increased formation of cytokines such as IL-6 shown to be pro-regenerative in human patients (Antoniades et al., 2006). It should be noted that dendritic cell depleted mice had higher levels of neutrophils in their non-parenchymal fraction after dendritic cell depletion; however, this was excluded a cause of increased injury (Connolly et al., 2011). Myeloid derived suppressor cells (MDSC) may also be involved in some forms of drug-induced immune mediated liver injury as a recent study indicates that MDSCs may link innate and adaptive immunity and play a key role in immune tolerance to molecules such as halothane (Chakraborty et al., 2015). Further work is required in these cell populations before a full understanding of how they function and how they affect interactions between P450s and the innate immune system.
Cross-Talk between Liver CYPs and Inflammation after Exposure to Toxicants
Alcoholic Liver Injury
Excessive consumption of alcohol is a major source of morbidity and mortality, especially in the West (Gao and Bataller, 2011). Alcoholic liver injury is a progressive injury with a majority of patients experiencing simple steatosis, and no further liver dysfunction. A sub-population of these patients undergo a profound change from largely benign steatosis to an inflammatory state called steatohepatitis, which can then progress to fibrosis, cirrhosis, and eventually cancer. At any point during this progression, patients can also enter a clinically distinct syndrome referred to as alcoholic hepatitis, wherein the liver goes into an acute inflammatory state with severe liver dysfunction and liver injury. As bouts of alcoholic hepatitis occur simultaneously with ongoing steatohepatitis, it is presumed that alcoholic hepatitis can help drive the injury process towards fibrosis and cirrhosis. A significant quantity of research has focused on how metabolism of alcohol by cytochrome P450s and other drug metabolizing enzymes stimulates the onset of alcoholic steatohepatitis and alcoholic hepatitis.
The role of cytochrome P450 enzymes in alcohol induced liver injury has been somewhat controversial, especially that of CYP2E1 (Tsukamoto, 2000). Alcohol is metabolized primarily via alcohol dehydrogenase and aldehyde dehydrogenase to the relatively harmless chemical acetic acid (Lieber, 2005); although a portion of alcohol is metabolized by CYP2E1. Protein levels of CYP2E1 are also induced by alcohol both in the liver (Lu and Cederbaum, 2008) and in the gut (Hakkak et al., 1996). CYP2E1 metabolizes millimolar quantities of ethanol into micromolar quantities of acetaldehyde, a more reactive, and more toxic intermediate. During this process CYP2E1 also produces ROS that may contribute to the total ethanol induced oxidative stress (Lu and Cederbaum, 2008). Autoantibodies against CYP2E1 have been found in alcoholic patients suggesting a potential pro-immunogenic role (Clot et al., 1996). However, there was surprisingly little difference in liver histology and serum AST levels between CYP2E1−/− mice and their WT counterparts when ethanol was administered via intragastric feeding (Kono et al., 1999). Moreover, administration of a cytochrome P450 inhibitor did not affect oxidative stress, indicating the vast majority of oxidative stress occurs independently of cytochrome P450 function (Isayama et al., 2003). Instead, most of the oxidative stress is likely caused by other sources such as NADPH oxidase (Kono et al., 2001) and inducible nitric oxide synthase (McKim et al., 2003) expressed largely in inflammatory cells such as Kupffer cells and recruited neutrophils (Arteel, 2003). Thus, most likely, CYP2E1 contributes minimally to oxidative stress during chronic alcohol exposure.
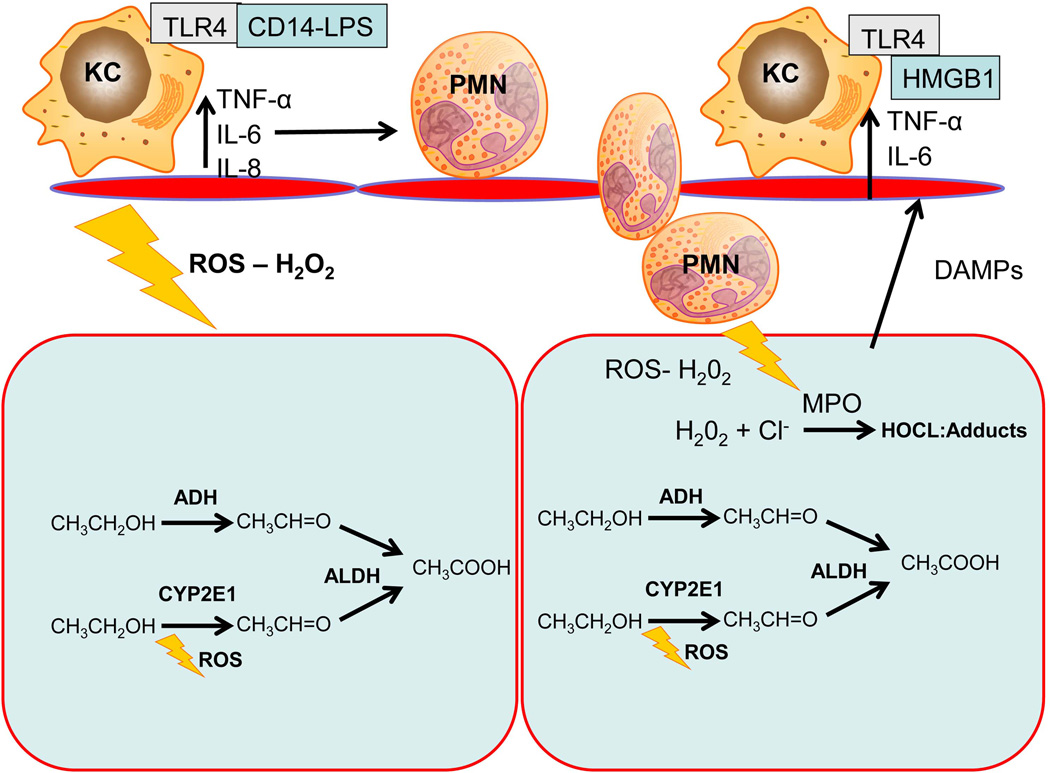
Despite these findings, recent information suggests CYP2E1 might play a role during binge ethanol intake (Abdelmeegood et al., 2013; Yun et al., 2014). Binge ethanol intake is increasingly noted as a likely contributor to alcoholic liver injury both in experimental models (Ding et al., 2010; Bertola et al., 2013) and in clinical practice (Plunk et al., 2014). Inhibition of CYP2E1 limits accumulation of triglycerides and reduces steatosis during binge alcohol treatment (Wu et al., 2012). CYP2E1 has also been presumed to enhance injury during acute binge after chronic exposure (Bertola et al., 2013). As neutrophil recruitment is limited without administration of an additional binge after chronic administration of alcohol, metabolism through CYP2E1 may be linked to neutrophil recruitment during alcoholic liver injury in murine models, although this hypothesis remains untested. As neutrophil accumulation is a well-known aspect of the human pathophysiology both in patients with alcoholic hepatitis and alcoholic cirrhosis rodent models that feature neutrophil accumulation may more accurately recapitulate the human pathophysiology (Mathews et al., 2014). Given that binge alcohol is likely to overwhelm normal enzymatic capacity and increase the contribution of alcohol inducible CYP2E1 to alcohol metabolism, future studies investigating the effect of CYP2E1 on binge and chronic + binge induced alcoholic liver injury may be warranted, especially in the context of advanced disease states, such as alcoholic hepatitis. This section is summarized in Figure 2.
Figure 2.
A simple model of alcohol induced liver injury. A majority of alcohol is metabolized by alcohol dehydrogenase to acetaldehyde and then by aldehyde dehydrogenase into acetic acid. A sub-portion of alcohol may be metabolized by CYP2E1 during binge alcohol dosing. This causes intracellular stress due to excessive acetaldehyde production. Kupffer cell activation by LPS and other DAMPs results in additional oxidative stress, as well as release of cytokines that recruit cytotoxic neutrophils. Neutrophils use NADPH to generate superoxide radical and hydrogen peroxide. Hydrogen peroxide is converted to hypochlorous acid by myeloperoxidase, which can then adduct proteins and cause significant oxidative stress. The combined oxidant stress results in hepatocyte death. TNF-α – tumor necrosis factor-α, IL – interleukin, TLR – toll like receptor, KC – Kupffer cell, LPS – lipopolysaccharide, ADH – alcohol dehydrogenase, ALDH – aldehyde dehydrogenase, DAMP – damage associated molecular pattern, H2O2 – hydrogen peroxide, MPO – myeloperoxidase, HOCl – hypochlorous acid, PMN – polymorphonuclear leukocytes, HMGB1 – high mobility group box-1, ROS – reactive oxygen species.
Acetaminophen Induced Liver Injury
In contrast to alcohol, acetaminophen (APAP) is a classic example of cytochrome P450 mediated formation of a reactive metabolite (Potter et al., 1973; McGill and Jaeschke, 2013). While the majority of acetaminophen is either glucuronidated or sulfated and subsequently excreted, a subportion is metabolized by cytochrome P450s, largely CYP2E1, CYP2A1, and CYP3A4, into the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) (McGill and Jaeschke, 2013). As formation of the reactive metabolite NAPQI is the initiating step for liver injury, drug metabolism becomes a critical point in the assessment of APAP induced liver injury (Jaeschke et al., 2011). Inhibition of drug metabolism by potential therapeutics will prevent all future downstream sequelae, and mask actual drug effects, preventing a realistic assessment of the pharmacological action of the potential therapeutic; thus, studies where inhibition of drug metabolism occurs must be interpreted with caution. Production of NAPQI results in adduction of mitochondrial proteins by NAPQI and initiates mitochondrial oxidant stress that causes a significant portion of the injury (Dahlin et al., 1984; Jaeschke et al., 2012a). This initial oxidant stress leads to the activation (phosphorylation) of c-jun N-terminal kinase (JNK) (Gunawan et al., 2006). The phosphorylated form of JNK translocates to mitochondria and substantially enhances the mitochondrial oxidant stress (Hanawa et al., 2008; Saito et al., 2010). The amplified mitochondrial oxidant stress results in opening of the mitochondrial membrane permeability transition pore and initiates cellular necrosis (Kon et al., 2004; Gujral et al., 2002). Liver injury is further magnified by translocation of the protein Bax to the mitochondria, which results in release of mitochondrial endonucleases, such as endonuclease G, as well as release of apoptosis-inducing factor (AIF), which amplifies DNA fragmentation and enhances necrosis (Bajt et al., 2006, 2008). During necrosis, a number of cellular constituents are leaked out from hepatocytes that can initiate an immune response, including molecules such as high mobility group box-1 (HMGB1) protein (Antoine et al., 2012), ATP (Hoque et al., 2012), mitochondrial DNA (McGill et al., 2012), nuclear DNA fragments (McGill et al., 2012), and more. As these molecules exacerbate inflammation through binding of DAMP receptors such as TLR4, it has been proposed that APAP contains a second phase of injury that is independent of drug metabolism, and instead occurs through the activation of the innate immune system (Liu et al., 2006; Imaeda et al., 2009; Kubes and Mehal, 2012). While a majority of these mechanisms on intracellular dysfunction during APAP are widely accepted in the literature, there is considerable controversy over whether or not the inflammatory cascade that follows the initial cell death mediates any portion of APAP-induced liver injury (Lawson et al., 2000; Liu et al., 2006; Jaeschke, 2008; Imaeda et al., 2009; Williams et al., 2014), even though it has been established for over 30 years that modulators of inflammation can have profound effects on CYP activity (Renton and Dickson, 1984). While it is understood that inflammation and inflammatory mediators can affect APAP metabolism through drug metabolizing enzymes such as CYP2E1, there remains substantial debate over whether or not these effects are critical to the actual pathogenesis or secondary effects (Martin-Murphy et al., 2013; Feng et al., 2014; Jaeschke et al., 2012b).
Kupffer cells have been repeatedly suggested to be a major component of acetaminophen induced liver injury over the last twenty years (Michael et al., 1999; Choi et al., 2015), although this topic remains controversial due to conflicts in the data (Ito et al., 2003; Ju et al., 2002; You et al., 2013). Pretreatment of mice with gadolinium chloride, which reduces the capacity of Kupffer cells to produce ROS (Liu et al., 1995), was shown to protect against APAP (Michael et al., 1999); however, pretreatment with clodronated liposomes, a more specific macrophage toxin, has opposite results (Ju et al., 2002). Kupffer cells were presumed to contribute to injury via production of ROS via NADPH oxidase (Michael et al., 1999). However, mice with deficiency of NADPH oxidase activity had similar levels of oxidative stress compared to WT mice after APAP, indicating Kupffer cell mediated oxidative stress contributed minimally to APAP induced liver (James et al., 2003). Some of this confusion may be due to interactions between treatments and drug metabolism. It was recently proposed that APAP induced liver injury is aggravated by Kupffer cells-derived cytokines induced through the purinergic receptor P2X7, as blockade with the inhibitor A438079 was protective against APAP (Hoque et al., 2012); however, it was subsequently demonstrated that this inhibitor functions as a direct inhibitor of P450 activity, indicating the actual protective effect was mediated by inhibition of drug metabolism (Xie et al., 2013). Recent studies indicating a role for macrophages have also failed to fully examine drug metabolism effects related to treatment regimes, and thus, must be interpreted cautiously (Choi et al., 2015). Together, the preponderance of evidence is in favor of intracellular mechanisms of injury mediating a majority of APAP induced liver injury (Jaeschke et al., 2012a).
Recent studies in man have illustrated a separate role for macrophages during APAP induced liver injury. Studies in human patients indicate the macrophage population is expanded in patients, especially at sites of necrosis, and is derived of both endogenous Kupffer cells that are initially depleted after APAP overdose, and recruited monocytes that differentiate into macrophages (Antoniades et al., 2012). These macrophages have a largely anti-inflammatory, or Type-II, cytokine profile with high levels of expression of cytokines such as IL-10 and secretory leukocytes protease inhibitor (Antoniades et al., 2014). Moreover, these cells stimulate angiogenesis and while reducing the activation of status of neutrophils, which serves to regenerate new hepatic architecture and limit excess inflammatory damage (Zigmond et al., 2014). Thus, any potential Kupffer cell mediated injury during APAP overdose likely plays a secondary role to regenerative effects seen as the injury resolves.
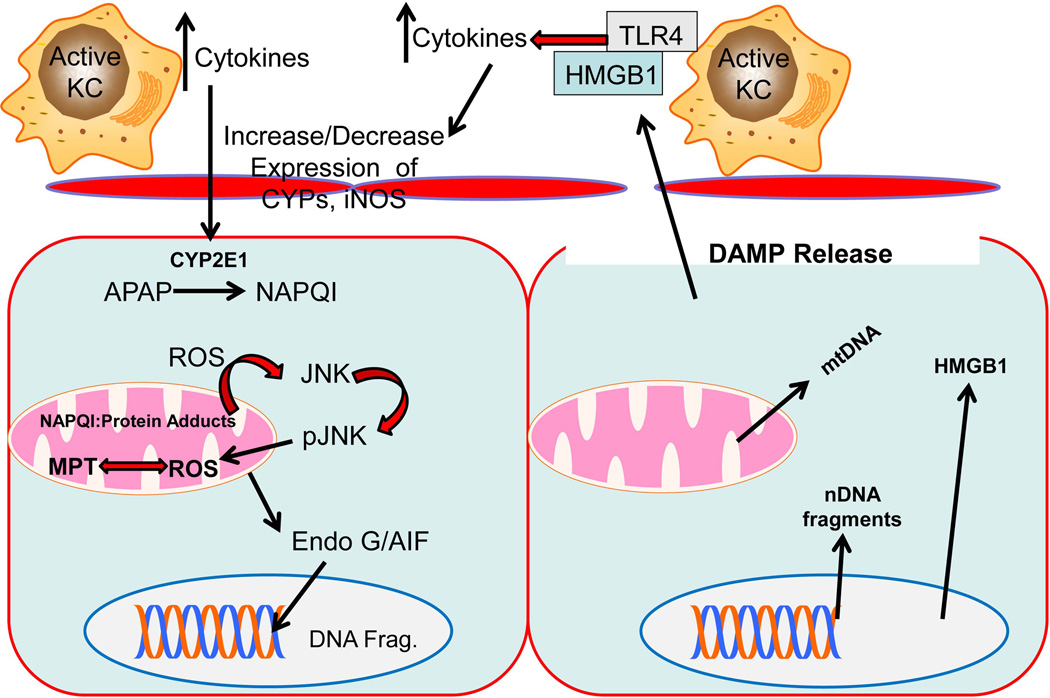
Another function of recruited monocytes and Kupffer cells during APAP overdose is the recruitment of other inflammatory cells. Both recruited monocytes and Kupffer cells are capable of producing multiple cytokines including TNF-α, IL-6, and more (Dixon et al., 2013). Neutrophils are known to accumulate at sites of injury after acetaminophen in murine models of APAP overdose (Figure 1). Initial reports indicated a lack of a role for neutrophils after APAP induced liver injury (Lawson et al., 2000); however, this was challenged when it was shown that pretreatment with a neutrophil depleting antibody (anti-Gr-1) was protective against APAP (Liu et al., 2006). However, the interpretation of these data has been questioned, as pretreatment with the Gr-1 antibody can cause adaptive changes with induction of protective genes (Jaeschke and Liu, 2007). In addition, ICAM-1−/− mice were not protected (Cover et al., 2006). This has led to significant controversy in the field regarding the role of neutrophils (Kubes and Mehal, 2012; Jaeschke et al., 2012b; Krenkel et al., 2014). While evidence in both human patients (Williams et al., 2014) and mice (Cover et al., 2006; Lawson et al., 2000; Williams et al., 2010a; Connolly et al., 2011) does not support the hypothesis of neutrophil mediated injury, a number of papers have shown a link between metabolism of APAP and inflammation that might explain why some models show modulation of injury with therapeutic regimens designed at mitigating inflammation. Short term treatment with IL-22 protected mice from APAP induced liver injury through a STAT-3 dependent mechanism, suggesting intracellular signaling mechanisms were present that attenuated the injury (Feng et al., 2014). On the other hand IL-22 transgenic mice that chronically overexpress IL-22 had increased expression of CY2E1, which lead to an exacerbation of the injury (Feng et al., 2014). Similarly, Jα-18−/− mice and CD1d−/− mice, which are largely depleted of iNKT and NKT cells, respectively, both had higher levels of injury after APAP overdose (Martin-Murphy et al., 2013). This was attributed to higher expression of CYP2E1, due to higher ketone body levels present in these mice (Martin-Murphy et al., 2013). This was in contrast to previous data using NK cell depleting antibodies that suggested NK cells might exacerbate APAP induced liver injury (Liu et al., 2004); although the involvement of NK/NKT cells was triggered by the use of DMSO as vehicle for APAP (Masson et al., 2008). Even so, further studies showed protection against APAP-induced liver injury in Jα-18−/− mice due to higher glutathione levels (Downs et al., 2012). The contrast in these studies points out two critical issues: first, assessments of metabolism are critical to the understanding of therapeutic potential, as even seemingly unrelated interventions can have profound effects on metabolism that might otherwise mask interpretation of data and second, studies performed in knockout mice must be carefully controlled and interpreted as contradictory data can occur in these models due to adaptive mechanisms. Moreover, chronic depletion of inflammatory cells can have completely different effects than immediate antibody based depletion schemes. Investigations using multiple models of depletion, as well as overexpression systems, if relevant, are advised due to potentially unforeseen alterations in basic metabolism. This section is summarized in Figure 3
Figure 3.
A simplified model of APAP toxicity. CYP2E1 converts APAP to NAPQI resulting in an intracellular oxidant stress. This results in phosphorylation of JNK and translocation of pJNK to the mitochondria. Translocation of pJNK amplifies the mitochondrial stress and causes the mitochondrial permeability transition pore. This results in release of nucleases such as endonuclease G and apoptosis inducing factor that cause DNA fragmentation. This results in cell death, causing release of DAMPs such as mitochondrial DNA, nuclear DNA fragments, and HMGB1. These DAMPs can then act on Kupffer cells, which secrete cytokines that can act on CYP2E1 by either reducing or increasing expression, resulting in magnification or reduction of the injury to subsequent hepatocytes. KC – Kupffer cell, APAP – acetaminophen, NAPQI – N-acetyl-p-benzoquinone imine, JNK – c-jun N-terminal kinase, Endo G - endonuclease G, DAMPs – damage associated molecular patterns, CYPs – cytochrome P450, AIF – apoptosis inducing factor, nDNA – nuclear DNA, mtDNA – mitochondrial DNA, HMGB1 – high mobility group box-1, TLR – toll like receptor, MPT – mitochondrial permeability transition pore, iNOS – inducible nitric oxide synthase, DNA frag – DNA fragments,
Halothane Hepatitis, Isoniazid, and Idiosyncratic Drug Induced Liver Injury
Halothane is a volatile anesthetic currently used predominantly in developing countries, due to its low cost (Habibollahi et al., 2011). Unlike a number of the other volatile anesthetics, halothane induces drug induced liver injury only in a sub-population of patients (Uetrecht, 2009). Halothane induced hepatitis is thought to be mediated by the conversion of halothane to trifluoroacetyl chloride (TFA) by CYPs, predominantly CYP2E1 (Bourdi et al., 2001). CYP2E1 has also been proposed as a cell surface autoantigen in murine models that exacerbates the inflammatory response (Eliasson and Kenna, 1996). Accumulation of TFA in tissue is thought to be partially responsible for the adaptive immune response that results in immune mediated liver injury (Bourdi et al., 2001); thus, CYP activity is a major determinant of halothane-induced liver injury. This immune response is strongly associated with previous exposure to halothane, leading to the “hapten hypothesis,” or the idea that haptens – protein-drug conjugates that are formed during halothane metabolism – can develop during repeated halothane exposure, and result in this immune response (reviewed in Ju, 2009). The mechanism of halothane-induced hepatitis has been somewhat controversial, both due to its unpredictability in animal models and the problems with identifying the inflammatory cells that are directly responsible for liver injury. Although some studies have proposed neutrophils as the main cytotoxic leukocyte in this model (You et al., 2006; Dugan et al., 2010), recent evidence suggested a limited role for neutrophils, with eosinophils being the likely contributor (Proctor et al., 2013). Eosinophil recruitment is driven by epithelial production of thymic stromal lymphopoietin (Proctor et al., 2014). More studies are required in this area, especially in regards to metabolism of halothane to its metabolites, to fully understand what drives halothane mediated hepatitis, especially in vulnerable sub-populations.
Halothane-induced hepatitis is a classic example of idiosyncratic drug-induced liver injury (IDILI), which is defined primarily by two different facets: one, that the injury is very rare and unpredictable (idiosyncratic) and two, that the injury usually only occurs on repeat exposure to the drug (Uetrecht, 2009). IDILI is especially troubling as a clinical manifestation of liver injury as the mechanisms are poorly defined, almost impossible to predict, and diagnosis can be difficult due to the lack of specific parameters. IDILI is largely considered to be an immune mediated pathophysiology rather than an injury caused by the parent drug, although substantial debate still occurs as to whether adaptive immunity fully explains the mechanism (Uetrecht, 2009). Evidence is favor of an immune response largely comes from clinical data. Even in patients that undergo mild increases in ALT after exposure to a drug, a majority of patients will undergo adaptation and ALT levels will return to normal (Watkins, 2005). What is unknown is why some patients undergo continued liver injury, with immune intolerance being the most common explanation (Eksteen et al., 2007). Upon rechallenge with a drug, these patients undergo a rapid adaptive immune response that results in DILI. Despite the current lack of understanding of the immune element, what is largely agreed upon is that metabolic activation of the drug by CYPs and other drug metabolizing enzymes probably plays a key role, and that autoantibodies against drug metabolizing enzymes are present during DILI in some cases (Mizutani et al., 2005). Although there is only limited evidence for the pathogenic role for these auto-antibodies in a majority of IDILI type injuries, their presence is an independent predictor of outcome in patients with numerous drug-mediated liver injuries (Sutti et al., 2014). A recent study using a novel model of drug (halothane)-induced allergic hepatitis suggests that auto-antibodies against protein adducts may be critical to liver injury in IDILI (Chakraborty et al., 2015). Further research in this area is needed, as both removal of pharmaceuticals from the market due to previously undescribed DILI, and clinical liver injury, can be attributed to IDILI and our current lack of understanding of this topic.
The other most commonly observed form of IDILI is due to isoniazid toxicity (Metushi et al., 2011). Isoniazid is a first line drug for treatment of tuberculosis, and is commonly administered in combination with rifampin to enhance efficacy. Isoniazid carries a number of commonalities with halothane, including delayed increases in serum transaminases, delayed liver injury, a small subportion (~20%) of susceptible individuals in the population, the idea that the major toxicity is due to a reactive metabolite (either acetylhydrazine or hydrazine in the case of isoniazid) generated by P450s, and the idea of liver “adapation” to the drug (Metushi et al., 2011). While the exact mechanism of toxicity of isoniazid is very poorly understood, it is thought that one of the reactive metabolites results in activation of the immune system and immune-mediated liver injury (Metushi et al., 2011). Isoniazed administration results in auto-antibodies against both isoniazid and CYP2E1 in human patients and is associated with increases in Th17 and IL-10 cytokines (Metushi et al., 2014a; 2014b), all indicating a complex inflammatory environment after administration of isoniazid. It remains to be determined how metabolism of isoniazid by P450s results in formation of reactive metabolites that stimulate the immune system and block immune tolerance. Additionally, a greater understanding of which immune populations mediate the injury is required for further progress in the area.
Cross-Talk between Inflammation and Liver CYPs by Endogenous Macromolecules
Cholestasis
While the cytochrome P450 family is well characterized in its role in drug metabolism, a substantial amount of P450 activity is involved in metabolism of endogenous macromolecules. With the recent discovery of the farnesoid X receptor (FXR) as a nuclear receptor for bile acids, increased focus has been placed on bile acids as signaling molecules (Makishima et al., 1999; reviewed in Li and Chiang, 2015). Generation of bile acids is dependent upon multiple CYP mediated processes, with the rate limiting step being the conversion of cholesterol to 7α hydroxyl-cholesterol by CYP7A1 (reviewed in Li and Chiang, 2015). A number of different events can result in the regulation of CYP7A1, including activation of FXR by bile acids (Chiang, 2009), activation of FGFR4 by its ligand FGF15/FGF19 (Inagaki et al., 2005), and downregulation by increased exposure of hepatocytes to cytokines (Feingold et al., 1996). This interaction between cytokine levels and CYP7A1 was first noted in macrophages (Feingold et al., 1996) although it has subsequently been identified to occur in human hepatocytes as well (Li et al., 2006). This might be species dependent though, as some cytokines such as TGF-β increased CYP7A1 levels in rats (Li et al., 2008). As inflammation is a noted aspect of cholestasis (Saito and Maher, 2000; Gujral et al., 2003; Woolbright et al., 2013, 2014), and in vivo models of cholestatic liver injury such as the bile duct ligation (BDL) model have increased expression of numerous cytokines during the initial injury period (Gujral et al., 2004c), it is probable that cytokines help to protect the liver from excess accumulation bile acids in hepatocytes during cholestasis. Inflammation is a critical regulator of cholestatic liver injury in multiple murine models (Gujral et al., 2003; Kodali et al., 2006) and is present even in models where it does not directly mediate the injury (Woolbright et al., 2014), where it is accompanied by significant cytokine production (Woolbright et al., 2014). BDL causes a dramatic increase in multiple serum cytokine levels between 3 and 72 h after BDL (Saito and Maher, 2000; Gujral et al., 2004c). These increases occur concurrently with retention of biliary constituents including bile acids (Zhang et al., 2012). Thus, both the retention of bile acids during the actual cholestasis, and the subsequent production of cytokines by local inflammatory cells downregulate CYP7A1 and other CYPs such as CYP27A1 in a redundant fashion. Recently, it was noted that administration of bile acids to hepatocytes can increase the expression of chemokines including mouse keratinocyte factor (mKC) and macrophage inhibitory protein-2 (MIP-2) in an FXR independent fashion (Allen et al., 2011). This might serve as a further redundancy through paracrine actions of cytokines produced directly by hepatocytes. Thus, while established mechanisms of FXR signaling during cholestasis are likely critical to the compensatory responses associated with cholestatic hepatocytes, the induction of cytokines and their subsequent paracrine response on hepatocytes serve as an additional mechanism of regulation of bile acid levels through modulation of CYP expression.
In addition to the response from hepatocytes, Kupffer cells may help to control bile acid homeostasis during cholestasis as well. Kupffer cells express the bile acid receptor TGR5, which can activate anti-inflammatory cascades when activated by bile acids (Keitel et al., 2008; Wang et al., 2011). The presence of these anti-inflammatory cytokines may help limit excessive inflammation, and normalize CYP7A1 levels. TGR5 may also help to directly limit increases in intrahepatic bile acids levels as whole body TGR5 knockout mice had increased liver and plasma bile acid levels after partial hepatectomy, although a direct interaction between TGR5 and CYP7A1 was not shown (Pean et al., 2013). The role of TGR5 on macrophages, hepatocytes, and sinusoidal endothelial cells in the liver and their regulation of inflammation and the CYP system is an ongoing area of research.
Lipopolysaccharide
Lipopolysaccharide (LPS) or endotoxin is a component of cell walls of gram-negative bacteria that increases in concentration in serum during alcoholic liver injury, and in septic shock. LPS activates TLR4 present on inflammatory cells to initiate a potent inflammatory response and induction of multiple cytokine expression cascades (Chow et al., 1999). LPS administration reduces both CYP7A1 and CYP27A1 levels in syrian hamsters (Feingold et al., 1996; Memon et al., 2001). This may be through the previously mentioned effects of cytokines on CYP7A1 and CYP27A1 expression, or may be due to direct effects on FXR (Kim et al., 2003). LPS also reduces levels of hepatobiliary transporters such as the sodium taurocholate cotransporting polypeptide (NTCP) indicating the FXR response as a transcriptional regulator is likely a part of the effect (Green et al., 1996). LPS also affects hepatic levels of specific drug metabolizing enzymes. Administration of even very low quantities LPS to human patients results in decreases in hepatic CYP activity (Shedlofsky et al., 1994). This has recently been modeled in human hepatocyte cell culture, as HepaRG cells, a bipotential line of hepatoma cells that express drug metabolizing enzymes including CYPs, are responsive to LPS, and treatment with LPS results in downregulation of CYPs including CYP1A2, CYP2B6 and CYP3A4 (Rubin et al., 2015). Administration of IL-6 had similar effects, indicating cytokines are also likely capable of affecting drug metabolism (Rubin et al., 2015). LPS also protects against the aforementioned APAP induced liver injury (Lui et al., 2000). Administration of LPS before APAP reduces CYP expression through cytokines such as IL-1α that in turn blocks drug metabolism and protects the mouse against injury (Liu et al., 2000). Thus, chronic inflammation related to septic infections can result in alterations in CYP levels that can affect normal metabolic processes and drug metabolism.
CYPs in Immune Cells and Their Role in Inflammation
While a majority of the CYP activity is present in epithelial tissue, with hepatocytes expressing and maintaining significant activity, small amounts of CYP activity is present in immune cells themselves. While this expression is quite modest in most cases, there are a few examples of CYPs in immune cells potentially affecting inflammation. Most notably, immune cells express the aryl hydrocarbon receptor (AhR) and respond to prototypical AhR ligands such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Germolec et al., 1995) or polyaromatic hydrocarbons (Houser et al., 1992) with an increase in CYP1A1 expression (Germolec et al., 1995). This induction is selective for specific lymphoid tissues and only occurs in those that express high concentrations of AhR (Germolec et al., 1995). TCDD is a noted immunosuppressive agent with numerous toxic actions in mice (Kerkvliet, 2002). Recently it was shown that not all of the actions of AhR might be immunosuppressive though, as activation of AhR regulates Treg and Th17 cell formation in a ligand dependent manner (Quintana et al., 2008; Velhoen et al., 2008). While activation of CYP1A1 was observed in all mice with functional AhR in these experiments, the role of CYP1A1 in this process has not been directly investigated. Assessment of the role of CYPs in the induction of T cell differentiation caused by TCDD and other AhR ligands may provide fruitful information on the relatively untested idea that CYPs commonly recognized for drug metabolism might also have a role in endogenous metabolic activity.
Mitochondria Specific P450s and Their Role in Xenobiotic and Endobiotic Metabolism and Inflammation
Mitochondria in liver express a number of CYP families, including 24A and 27A involved in steroidal biogenesis (Omura, 2006). While a majority of xenobiotic metabolism occurs through microsomal P450s, mitochondria express small quantities of xenobiotic metabolizing P450s including CYP2E1, 2D6, 1A1 and more (Anandatheerthavarada et al., 1997; Avadhani et al., 2011). Mitochondrial CYP2E1 is likely generated in the same fashion as microsomal CYP2E1 and instead targeted to the mitochondrial post-translationally and it appears that like microsomal CYP2E1, the mitochondrial form is inducible (Avadhani et al., 2011). Mitochondrial CYP2E1 may be important to both alcoholic liver injury and acetaminophen-induced liver injury, as a model where mitochondrial CYP2E1 is induced exclusively in vitro resulted in both acetaminophen and ethanol-induced hepatotoxicity (Knockaert et al., 2011). Given the importance of CYPs such as CYP2E1 to drug-induced toxicity and subsequent inflammation, a role for mitochondrial 2E1 may develop in both of these toxicities.
Conclusions
In summary, interactions occur between immune cells, cytokine production and the cytochrome P450 system that mediate a number of different aspects of liver disease and immunology. While a considerable amount of effort has gone into the delineation of these mechanisms, there is a great deal of work left to be done in this field. Experimental models of alcoholic liver injury and acetaminophen hepatotoxicity provide excellent, clinically relevant models to better understand how specific CYPs such as CYP2E1 can interact with immunity. At the same time, cytokine production and bile acid retention during cholestasis has obvious implications for feedback mediated effects of endogenous metabolism through CYP7A1. Continued research in these areas focusing on novel aspects of how CYPs interact with different inflammatory states will yield novel information that can improve patient safety and therapeutic efficacy of drugs.
Acknowledgments
Work in the authors’ laboratory was supported in part by the National Institutes of Health grants R01 DK070195 and DK102142, and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. Dr. Woolbright was supported by the “Training Program in Environmental Toxicology” T32 ES007079-26A2 from the National Institute of Environmental Health Sciences.
Abbreviations
- APAP
acetaminophen
- CYP
cytochrome P450
- MIP-2
macrophage inhibitor protein-2
- mKC
mouse keratinocyte factor
- ICAM-1
intercellular adhesion molecule-1
- NAPDH oxidase
nicotinamide adenine dinucleotide phosphate oxidase
- HMGB1
high mobility group box-1
- DAMP
damage associated molecular pattern
- PAMP
pathogen associated molecular pattern
- STAT3
signal transducer and activator of transcription
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, et al. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Addya S, Dwivedi RS, Biswas G, Mullick J, Avadhani NG. Localization of multiple forms of inducible cytochromes P450 in rat liver mitochondria: immunological characteristics and patterns of xenobiotic substrate metabolism. Arch Biochem Biophys. 1997;339:136–150. doi: 10.1006/abbi.1996.9855. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoniades CG, Berry PA, Davies ET, Hussain M, Bernal W, Vergani D, et al. Reduced monocyte HLA-DR expression: a novel biomarker of disease severity and outcome inacetaminophen-induced acute liver failure. Hepatology. 2006;44:34–43. doi: 10.1002/hep.21240. [DOI] [PubMed] [Google Scholar]
- Antoniades CG, Khamri W, Abeles RD, Taams LS, Triantafyllou E, Possamai LA, et al. Secretory leukocyte protease inhibitor: a pivotal mediator of anti-inflammatory responses in acetaminophen-induced acute liver failure. Hepatology. 2014;59:1564–1576. doi: 10.1002/hep.26933. [DOI] [PubMed] [Google Scholar]
- Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–746. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- Avadhani NG, Sangar MC, Bansal S, Bajpai P. Bimodal targeting of cytochrome P450s to endoplasmic reticulum and mitochondria: the concept of chimeric signals. FEBS J. 2011;278:4218–4229. doi: 10.1111/j.1742-4658.2011.08356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar TR, Lysz TW, Curran RD, Bentz BG, Machiedo GW, Simmons RL. Hepatocyte modulation of Kupffer cell prostaglandin E2 production in vitro. J Leukoc Biol. 1990;47:305–311. [PubMed] [Google Scholar]
- Bourdi M, Amouzadeh HR, Rushmore TH, Martin JL, Pohl LR. Halothane-induced liver injury in outbred guinea pigs: role of trifluoroacetylated protein adducts in animal susceptibility. Chem Res Toxicol. 2001;14:362–370. doi: 10.1021/tx000244x. [DOI] [PubMed] [Google Scholar]
- Chakraborty M, Fullerton AM, Semple K, Chea LS, Proctor WR, Bourdi M, et al. Drug-induced allergic hepatitis developed in mice when myeloid-derived suppressor cells were depleted prior to halothane treatment. Hepatology. 2015 doi: 10.1002/hep.27764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;31:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DY, Ban JO, Kim SC, Hong JT. CCR5 knockout mice with C57BL6 background are resistant to acetaminophen-mediated hepatotoxicity due to decreased macrophages migration into the liver. Arch Toxicol. 2015;89:211–220. doi: 10.1007/s00204-014-1253-3. [DOI] [PubMed] [Google Scholar]
- Chosay JG, Essani NA, Dunn CJ, Jaeschke H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am J Physiol. 1997;272:G1195–G1200. doi: 10.1152/ajpgi.1997.272.5.G1195. [DOI] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Clot P, Albano E, Eliasson E, Tabone M, Aricò S, Israel Y, et al. Cytochrome P4502E1 hydroxyethyl radical adducts as the major antigen in autoantibody formation among alcoholics. Gastroenterology. 1996;111:206–216. doi: 10.1053/gast.1996.v111.pm8698201. [DOI] [PubMed] [Google Scholar]
- Connolly MK, Ayo D, Malhotra A, Hackman M, Bedrosian AS, Ibrahim J, et al. Dendritic cell depletion exacerbates acetaminophen hepatotoxicity. Hepatology. 2011;54:959–68. doi: 10.1002/hep.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, et al. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785–797. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs I, Aw TY, Liu J, Adegboyega P, Ajuebor MN. Vα14iNKT cell deficiency prevents acetaminophen-induced acute liver failure by enhancing hepatic glutathione and altering APAP metabolism. Biochem Biophys Res Commun. 2012;428:245–251. doi: 10.1016/j.bbrc.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Dugan CM, MacDonald AE, Roth RA, Ganey PE. A mouse model of severe halothane hepatitis based on human risk factors. J Pharmacol Exp Ther. 2010;333:364–372. doi: 10.1124/jpet.109.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksteen B, Afford SC, Wigmore SJ, Holt AP, Adams DH. Immune-mediated liver injury. Semin Liver Dis. 2007;27:351–366. doi: 10.1055/s-2007-991512. [DOI] [PubMed] [Google Scholar]
- Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. 1996;50:573–582. [PubMed] [Google Scholar]
- Feingold KR, Spady DK, Pollock AS, Moser AH, Grunfeld C. Endotoxin, TNF, and IL-1 decrease cholesterol 7 alpha-hydroxylase mRNA levels and activity. J Lipid Res. 1996;37:223–228. [PubMed] [Google Scholar]
- Feng D, Wang Y, Wang H, Weng H, Kong X, Martin-Murphy BV, et al. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 2014;193:2512–2518. doi: 10.4049/jimmunol.1400588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germolec DR, Adams NH, Luster MI. Comparative assessment of metabolic enzyme levels in macrophage populations of the F344 rat. Biochem Pharmacol. 1995;50:1495–1504. doi: 10.1016/0006-2952(95)02062-4. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- Green RM, Beier D, Gollan JL. Regulation of hepatocyte bile salt transporters by endotoxin and inflammatory cytokines in rodents. Gastroenterology. 1996;111:193–198. doi: 10.1053/gast.1996.v111.pm8698199. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004a;287:G243–G252. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Hinson JA, Jaeschke H. Chlorotyrosine protein adducts are reliable biomarkers of neutrophil-induced cytotoxicity in vivo. Comp Hepatol. 2004b;14(Suppl 1):S48. doi: 10.1186/1476-5926-2-S1-S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004c;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Habibollahi P, Mahboobi N, Esmaeili S, Safari S, Dabbagh A, Alavian SM. Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis. Hepat Mon. 2011;11:3–6. [PMC free article] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkak R, Korourian S, Ronis MJ, Ingelman-Sundberg M, Badger TM. Effects of diet and ethanol on the expression and localization of cytochromes P450 2E1 and P450 2C7 in the colon of male rats. Biochem Pharmacol. 1996;51:61–69. doi: 10.1016/0006-2952(95)02154-x. [DOI] [PubMed] [Google Scholar]
- Harley IT, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59:1830–1839. doi: 10.1002/hep.26746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289:G760–G767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- Hoque R, Sohail MA, Salhanick S, Malik AF, Ghani A, Robson SC, et al. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1171–G1179. doi: 10.1152/ajpgi.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser WH, Raha A, Vickers M. Induction of CYP1A1 gene expression in H4-II-E rat hepatoma cells by benzo[e]pyrene. Mol Carcinog. 1992;5:232–237. doi: 10.1002/mc.2940050310. [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Isayama F, Froh M, Bradford BU, McKim SE, Kadiiska MB, Connor HD, et al. The CYP inhibitor 1-aminobenzotriazole does not prevent oxidative stress associated with alcohol-induced liver injury in rats and mice. Free Radic Biol Med. 2003;35:1568–1581. doi: 10.1016/j.freeradbiomed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Ito Y, Abril ER, Bethea NW, McCuskey RS. Role of nitric oxide in hepatic microvascular injury elicited by acetaminophen in mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G60–G67. doi: 10.1152/ajpgi.00217.2003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Innate immunity and acetaminophen-induced liver injury: why so many controversies? Hepatology. 2008;48:699–701. doi: 10.1002/hep.22556. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CW. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am J Physiol. 1991;261:G1051–G1056. doi: 10.1152/ajpgi.1991.261.6.G1051. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology. 2007;45:1588–1588. doi: 10.1002/hep.21549. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012a;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity--a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;25:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Schini VB, Farhood A. Role of nitric oxide in the oxidant stress during ischemia/reperfusion injury of the liver. Life Sci. 1992;50:1797–1804. doi: 10.1016/0024-3205(92)90064-v. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012b;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev. 2012;26:103–114. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 2003;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541–3554. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- Ju C. The role of haptic macrophages in regulation of idiosyncratic drug reactions. Toxicol Pathol. 2009;37:12–17. doi: 10.1177/0192623308329475. [DOI] [PubMed] [Google Scholar]
- Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, et al. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI, Shepherd DM, Baecher-Steppan L. T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol Appl Pharmacol. 2002;185:146–152. doi: 10.1006/taap.2002.9537. [DOI] [PubMed] [Google Scholar]
- Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C. Repression of farnesoid X receptor during the acute phase response. J Biol Chem. 2003;278:8988–8995. doi: 10.1074/jbc.M212633200. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46646. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- Knockaert L, Fromenty B, Robin MA. Mechanisms of mitochondrial targeting of cytochrome P450 2E1: physiopathological role in liver injury and obesity. FEBS J. 2011;278:4252–4260. doi: 10.1111/j.1742-4658.2011.08357.x. [DOI] [PubMed] [Google Scholar]
- Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol. 2006;291:G355–G363. doi: 10.1152/ajpgi.00458.2005. [DOI] [PubMed] [Google Scholar]
- Koivisto T, Mishin VM, Mak KM, Cohen PA, Lieber CS. Induction of cytochrome P-4502E1 by ethanol in rat Kupffer cells. Alcohol Clin Exp Res. 1996;20:207–212. doi: 10.1111/j.1530-0277.1996.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, et al. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–G1012. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- Kono H, Bradford BU, Yin M, Sulik KK, Koop DR, Peters JM, et al. CYP2E1 is not involved in early alcohol-induced liver injury. Am J Physiol. 1999;277:G1259–G1267. doi: 10.1152/ajpgi.1999.277.6.G1259. [DOI] [PubMed] [Google Scholar]
- Krenkel O, Mossanen JC, Tacke F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg Nutr. 2014;3:331–343. doi: 10.3978/j.issn.2304-3881.2014.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- Leung CH, Caldarone CA, Wang F, Venkateswaran S, Ailenberg M, Vadasz B, et al. Remote Ischemic Conditioning Prevents Lung and Liver Injury After Hemorrhagic Shock/Resuscitation: Potential Role of a Humoral Plasma Factor. Ann Surg. 2014 Sep 1; doi: 10.1097/SLA.0000000000000877. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Li T, Ma H, Chiang JY. TGFbeta1, TNFalpha, and insulin signaling crosstalk in regulation of the rat cholesterol 7alpha-hydroxylase gene expression. J Lipid Res. 2008;49:1981–1989. doi: 10.1194/jlr.M800140-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Jahan A, Chiang JY. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology. 2006;43:1202–1210. doi: 10.1002/hep.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31:159–165. doi: 10.1097/MOG.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Metabolism of alcohol. Clin Liver Dis. 2005;9:1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Liu J, Sendelbach LE, Parkinson A, Klaassen CD. Endotoxin pretreatment protects against the hepatotoxicity of acetaminophen and carbon tetrachloride: role of cytochrome P450 suppression. Toxicology. 2000;147:167–176. doi: 10.1016/s0300-483x(00)00193-1. [DOI] [PubMed] [Google Scholar]
- Liu P, McGuire GM, Fisher MA, Farhood A, Smith CW, Jaeschke H. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock. 1995;3:56–62. [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP andcytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–74. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakova-Devine T, O'Farrelly C. Tissue-specific NK cell populations and their origin. J Leukoc Biol. 2014;96:981–990. doi: 10.1189/jlb.1RU0514-241R. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Martin-Murphy BV, Kominsky DJ, Orlicky DJ, Donohue TM, Jr, Ju C. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology. 2013;57:1575–1584. doi: 10.1002/hep.26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson MJ, Carpenter LD, Graf ML, Pohl LR. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology. 2008;48:889–897. doi: 10.1002/hep.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S, Xu M, Wang H, Bertola A, Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:G819–G823. doi: 10.1152/ajpgi.00041.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim SE, Gäbele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Memon RA, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. In vivo and in vitro regulation of sterol 27-hydroxylase in the liver during the acute phase response. Potential role of hepatocyte nuclear factor-1. J Biol Chem. 2001;276:30118–30126. doi: 10.1074/jbc.M102516200. [DOI] [PubMed] [Google Scholar]
- Metushi IG, Cai P, Vega L, Grant DM, Uetrecht J. Paradoxical attenuation of autoimmune hepatitis by oral isoniazid in wild-type and N-acetyltransferase-deficient mice. Drug Metab Dispos. 2014a;42:963–973. doi: 10.1124/dmd.113.056622. [DOI] [PubMed] [Google Scholar]
- Metushi IG, Cai P, Zhu X, Nakagawa T, Uetrecht JP. A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin Pharmacol Ther. 2011;89:911–914. doi: 10.1038/clpt.2010.355. [DOI] [PubMed] [Google Scholar]
- Metushi IG, Sanders C, Acute Liver Study Group Lee WM, Uetrecht J. Detection of anti-isoniazid and anti-cytochrome P450 antibodies in patients with isoniazid-induced liver failure. Hepatology. 2014b;59:1084–1093. doi: 10.1002/hep.26564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–195. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Shinoda M, Tanaka Y, Kuno T, Hattori A, Usui T, et al. Autoantibodies against CYP2D6 and other drug-metabolizing enzymes in autoimmune hepatitis type 2. Drug Metab Rev. 2005;37:235–252. doi: 10.1081/dmr-200028798. [DOI] [PubMed] [Google Scholar]
- Omura T. Mitochondrial P450s. Chem Biol Interact. 2006;163:86–93. doi: 10.1016/j.cbi.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Pålsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péan N, Doignon I, Garcin I, Besnard A, Julien B, Liu B. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58:1451–1460. doi: 10.1002/hep.26463. [DOI] [PubMed] [Google Scholar]
- Plunk AD, Syed-Mohammed H, Cavazos-Rehg P, Bierut LJ, Grucza RA. Alcohol consumption, heavy drinking, and mortality: rethinking the j-shaped curve. Alcohol Clin Exp Res. 2014;38:471–478. doi: 10.1111/acer.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter WZ, Davis DC, Mitchell JR, Jollow DJ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther. 187:203–210. [PubMed] [Google Scholar]
- Proctor WR, Chakraborty M, Chea LS, Morrison JC, Berkson JD, Semple K, et al. Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2013;57:2026–2036. doi: 10.1002/hep.26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor WR, Chakraborty M, Fullerton AM, Korrapati MC, Ryan PM, Semple K, et al. Thymic stromal lymphopoietin and interleukin-4 mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2014;60:1741–1752. doi: 10.1002/hep.27169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Renton KW, Dickson G. The prevention of acetaminophen-induced hepatotoxicity by the interferon inducer poly(rI.rC) Toxicol Appl Pharmacol. 1984;72:40–45. doi: 10.1016/0041-008x(84)90247-3. [DOI] [PubMed] [Google Scholar]
- Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Rubin K, Janefeldt A, Andersson L, Berke Z, Grime K, Andersson TB. HepaRG cells as human-relevant in vitro model to study the effects of inflammatory stimuli on cytochrome P450 isoenzymes. Drug Metab Dispos. 2015;43:119–125. doi: 10.1124/dmd.114.059246. [DOI] [PubMed] [Google Scholar]
- Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology. 2000;118:1157–1168. doi: 10.1016/s0016-5085(00)70369-6. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Schüngel S, Buitrago-Molina LE, Nalapareddy Pd, Lebofsky M, Manns MP, Jaeschke H, Gross A, Vogel A. The strength of the Fas ligand signal determines whether hepatocytes act as type 1 or type 2 cells in murine livers. Hepatology. 2009;50:1558–66. doi: 10.1002/hep.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlofsky SI, Israel BC, McClain CJ, Hill DB, Blouin RA. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J Clin Invest. 1994;94:2209–2214. doi: 10.1172/JCI117582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutti S, Rigamonti C, Vidali M, Albano E. CYP2E1 autoantibodies in liver diseases. Redox Biol. 2014;3:72–78. doi: 10.1016/j.redox.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, et al. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654–1662. doi: 10.1002/hep.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H. Cyp2e1 and ALD. Hepatology. 2000;32:154–156. doi: 10.1002/hep.510320125. [DOI] [PubMed] [Google Scholar]
- Uetrecht J. Immunoallergic drug-induced liver injury in humans. Semin Liver Dis. 2009;29:383–392. doi: 10.1055/s-0029-1240007. [DOI] [PubMed] [Google Scholar]
- Üllen A, Singewald E, Konya V, Fauler G, Reicher H, Nusshold C, et al. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo. PLoS One. 2013;14:e64034. doi: 10.1371/journal.pone.0064034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vermijlen D, Luo D, Robaye B, Seynaeve C, Baekeland M, Wisse E. Pit cells (Hepatic natural killer cells) of the rat induce apoptosis in colon carcinoma cells by the perforin/granzyme pathway. Hepatology. 1999;29:51–56. doi: 10.1002/hep.510290143. [DOI] [PubMed] [Google Scholar]
- Wang H, Feng D, Park O, Yin S, Gao B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-γ. Hepatology. 2013;58:1474–1485. doi: 10.1002/hep.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mathews WR, Guido DM, Farhood A, Jaeschke H. Inhibition of nitric oxide synthesis aggravates reperfusion injury after hepatic ischemia and endotoxemia. Shock. 1995;4:282–288. doi: 10.1097/00024382-199510000-00009. [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PB. Idiosyncratic liver injury: challenges and approaches. Toxicol Pathol. 2005;33:1–5. doi: 10.1080/01926230590888306. [DOI] [PubMed] [Google Scholar]
- Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol. 2011;252:289–297. doi: 10.1016/j.taap.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010a;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275:122–133. doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol. 2010b;247:169–178. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18:4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Li F, Xie Y, Farhood A, Fickert P, Trauner M, et al. Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol Lett. 2014;228:56–66. doi: 10.1016/j.toxlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273:524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wang X, Zhou R, Yang L, Cederbaum AI. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic Biol Med. 2012;53:1346–1357. doi: 10.1016/j.freeradbiomed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Williams CD, McGill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation. Toxicol Sci. 2013;131:325–335. doi: 10.1093/toxsci/kfs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, et al. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224:186–95. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Gores GJ. Death receptor-mediated apoptosis and the liver. J Hepatol. 2002;37:400–410. doi: 10.1016/s0168-8278(02)00209-x. [DOI] [PubMed] [Google Scholar]
- You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- You Q, Holt M, Yin H, Li G, Hu CJ, Ju C. Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem Pharmacol. 2013;86:836–843. doi: 10.1016/j.bcp.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JW, Son MJ, Abdelmegeed MA, Banerjee A, Morgan TR, Yoo SH, et al. Binge alcohol promotes hypoxic liver injury through a CYP2E1-HIF-1α-dependent apoptosis pathway in mice and humans. Free Radic Biol Med. 2014;77:183–194. doi: 10.1016/j.freeradbiomed.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, et al. Infiltrating monocyte-derived macrophages and resident Kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–353. doi: 10.4049/jimmunol.1400574. [DOI] [PubMed] [Google Scholar]