Abstract
One principal process driving fatty acid transport is vectorial acylation, where fatty acids traverse the membrane concomitant with activation to CoA thioesters. Current evidence is consistent with the proposal that specific fatty acid transport (FATP) isoforms alone or in concert with specific long chain acyl CoA synthetase (Acsl) isoforms function to drive this energy-dependent process. Understanding the details of vectorial acylation is of particular importance as disturbances in lipid metabolism many times leads to elevated levels of circulating free fatty acids, which in turn increases fatty acid internalization and ectopic accumulation of triglycerides. This is associated with changes in fatty acid oxidation rates, accumulation of reactive oxygen species, the synthesis of ceramide and ER stress. The correlation between chronically elevated plasma free fatty acids and triglycerides with the development of obesity, insulin resistance and cardiovascular disease has led to the hypothesis that decreases in pancreatic insulin production, cardiac failure, arrhythmias, and hypertrophy are due to aberrant accumulation of lipids in these tissues. To this end, a detailed understanding of how fatty acids traverse the plasma membrane, become activated and trafficked into downstream metabolic pools and the precise roles provided by the different FATP and Acsl isoforms are especially important questions. We review our current understanding of vectorial acylation and the contributions by specific FATP and Acsl isoforms and the identification of small molecule inhibitors from high throughput screens that inhibit this process and thus provide new insights into the underlying mechanistic basis of this process.
INTRODUCTION
Fatty acids are enigmatic molecules that on the one hand are essential for cellular structure, function and signaling and on the other must be contained or their detergent properties will prove lethal to cells. Mother nature has therefore developed ways to compartmentalize, sequester and regulate the movement of these molecules between and within cells. Within the blood stream free fatty acids (FFA) are buffered and moved by serum albumin and, as complex lipids, by the lipoproteins. Within cells, the fatty acid binding proteins serve a similar function for the free carboxylic acids (see review by Newberry and Davidson within this issue), while fatty acids esterified in highly hydrophobic complex lipid species are partitioned into membranes or sequestered in lipid droplets.
Understanding how free fatty acids move across membrane barriers has proven to be a challenging biophysical and biochemical problem, which after 30 years of research is still only poorly understood and remains somewhat controversial. Within the present article, we will review the arguments for protein mediated transport and will make the case that some members of the FATP family serve this function. The hypothesis that FATPs function in the transport of long chain fatty acids into cells was based on their identification though functional cloning of the first family member and has been supported by molecular and biochemical studies from our lab using a yeast model system, as well as studies using more complex animal cell and gene knockout approaches. However, the main hypothesis remains unproven, in part, because these proteins also function in the activation of certain lipophilic molecules by catalyzing the thioesterification of these substrates with coenzyme A. Thus, we will discuss the roles of these proteins in transport, activation and further metabolism of fatty acids.
FATTY ACID TRANSPORT IN HISTORICAL PERSPECTIVE
Upon presentation to the cell, fatty acids must be transported across the cell membrane and trafficked to sites of utilization. The free fatty acid concentration in the extracellular space is generally extremely low. Therefore the efficient transport of long-chain fatty acids is expected to require specific membrane-bound and membrane-associated transport systems to accumulate these compounds against a concentration gradient. Many different cell types contain a specific repertoire of membrane-bound and membrane-associated proteins, which are hypothesized to govern fatty acid transport in response to differentiation, hormonal stimulus, or environmental stimulus, including changes in nutritional state, temperature, or oxygen availability (1-6)).
The kinetics governing the transport of fatty acids into the cell is consistent with a protein-mediated process (7-13). In studies using model membranes, it has been demonstrated that uncharged fatty acids can flip between the two faces of the membrane, but remain membrane-bound (14). More recent studies have shown that as the radius of membrane curvature increases the flip of fatty acids between the two membrane faces becomes rate limiting (15). For fatty acids in the uncharged form, the flip of fatty acids between the two membrane leaflets in small unilammellar vesicles is very fast (t1/2 msec to sec). On the other hand, this step is slow for fatty acid anions (t1/2>2sec) (14). The movement of fatty acids out of the membrane is very slow unless there are specific enzymes or binding proteins, which in turn function to target the fatty acid into downstream metabolism and intracellular signaling. We hypothesize there is a cooperative interaction between factors involved in transmembrane movement and those involved in intracellular movement (e.g. FABP, Acsl, ACBP) that function to target the imported fatty acids to specific subcellular locations and metabolic fates. Based on our current understanding, proteins are likely to be involved in at least three aspects of this process: delivery of fatty acids to the membrane; in the transmembrane movement of the fatty acid from one leaflet to the other (particularly for fatty acid anions); and in the removal of fatty acids from the membrane.
Specific membrane-bound and membrane-associated proteins have been identified that appear to function in one or more of the steps detailed above in the transport of fatty acids across the membrane. To date, four different membrane-bound or membrane associated proteins have been defined in eukaryotic cells that participate in the transport of exogenous long-chain fatty acids: CD36/fatty acid translocase (CD36/FAT; see article by Nassir and Abumrad in this series) (1), fatty acid binding protein – plasma membrane-bound (FABPpm) (16), fatty acid transport protein (FATP) (17, 18), and long chain acyl CoA synthetase (Acsl) (2, 19). The roles of FAT/CD36 and FABPpm have been recently reviewed by others and will not be detailed further herein (6, 20, 21); rather this review will focus on the FATP isoforms with some discussion on their association with different Acsl isoforms.
FATP, FATTY ACID TRANSPORT PROTEIN OR ACYL-COA SYNTHETASE?
Fatty acid transport protein (FATP1) was first identified in a functional cloning screen designed to identify proteins that resulted in elevated accumulation of the fluorescent fatty acid analogue C1-BODIPY-C12 (12). It was immediately recognized that FATP has a domain architecture similar to the acyl-CoA synthetases, which includes an ATP binding domain and a fatty acid binding domain (12, 22, 23). Thus began the controversy over whether or not FATP was a bona fide transporter or merely functioned in vectorial acylation as a fatty acid activating enzyme trapping fatty acid within the cell through metabolic utilization. Our lab exploited the molecular genetics of Saccharomyces cerevisiae to distinguish the functions of this protein in transport and activation (22, 24). Several clues indicated that the two functions might be separable. First, the fatty acid signature of the FATPs was divergent from the Acsl enzymes involved in activation of long chain fatty acids (24, 25). Indeed, several labs demonstrated that in yeast, deletion of the FATP homologue Fat1p did not affect long chain Acsl activity, but reduced activation of very long chain fatty acids (26, 27). These strains also accumulated very long chain fatty acids suggesting turnover of fatty acids >C20 in length was reduced. Deletion of the same gene rendered yeast strains unable to transport long chain fatty acids into the cells (22). Yeast do not import very long chain fatty acids so that function could not be tested. We further demonstrated through an extensive site-directed mutagenesis approach that variants could be identified that either affected activation or transport, separating the two functions (24).
In addition to the first FATP, five other related mammalian genes (called FATP2 through FATP6) have been cloned and their proteins characterized. The FATPs differ in expression pattern, tissue distribution and subcellular location. FATP1 is found in muscle and adipose tissue; FATP2 in liver and kidney; FATP3 in liver and testes; FATP4 is relatively ubiquitous in fat metabolizing tissues and skin; FATP5 is exclusive to liver; and FATP6 is exclusive to heart (28, 29). Depending on the specific FATP, each protein functions in thioesterification of a lipophilic substrate with coenzyme A. FATP1, FATP2 and FATP4 have both Acsvl activity and transport long chain fatty acids when examined in yeast and several mammalian cells lines (11, 22, 24). FATP 5 is a bile-CoA ligase (30, 31). FATP6 in yeast activates very long chain fatty acids but does not transport fatty acids of any chain length (32). In contrast, the sole activity assigned this protein in heart cells, where it was first identified, is fatty acid transport (33). Figure 1 illustrates an overview of how the FATPs are likely to function, alone or in concert with a long chain acyl CoA synthetase (Acsl) in fatty acid transport and activation. Studies that are presently underway are directed to address specificity, compartmentalization and selectivity in directing intracellular fatty acid trafficking. As detailed blow, targeted deletions in several of the FATP genes are consistent with roles in fatty acid transport and/or intracellular fatty acid trafficking.
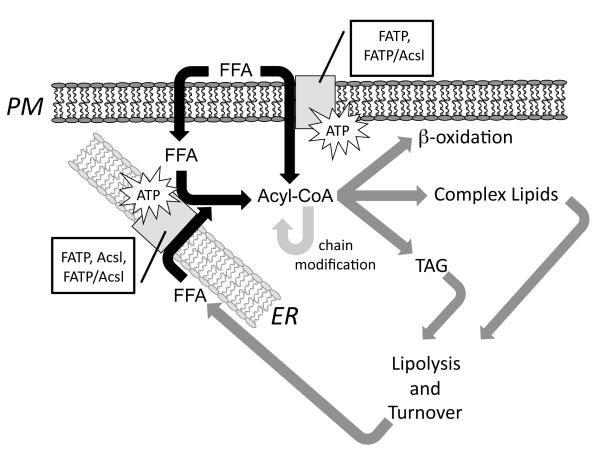
Figure 1.
Overview of FATP and Acsl functions in fatty acid transport and intracellular trafficking. The movement of fatty acids (FFA) across the plasma membrane (PM) through vectorial acylation requires FATP alone or in combination with an Acsl; the resulting product is acyl CoA. FFA may also move across the membrane by a diffusion process that may involve intracellular fatty acid binding proteins. Prior to any type of metabolic transformation, this fatty acid must also be activated to form acyl CoA. As illustrated this may proceed in the endoplasmic reticulum (ER) through specific FATPs or Acsls individually or through an FATP/Acsl complex. As illustrated acyl CoA is the crucial intermediate that is required for b-oxidation and synthesis of complex lipids and triglycerides (TAG). As part of normal lipid turnover or lipolysis of TAG is the formation of FFA, which must be reactivated to enter the metabolic pools. Acyl CoA is shown as a single pool, but in all likelihood these pools are compartmentalized, for example as is the case for those involved in acyl chain modification (elongation and desaturation). The role of the different FATP and Acsl isoforms is thought to promote both specificity and selectivity to these processes.
TARGETED DELETIONS OF FATP GENES PROVIDE FEW CLUES AND MANY QUESTIONS REGARDING FUNCTION
Germline deletions in mice have been constructed for FATP1, FATP2, FATP4 and FATP5. Each, except the FATP4 deletion, was viable. Deletion of FATP1 did not alter fat metabolism or insulin sensitivity when the knockout mice were fed a standard chow diet (34). However, on a high fat diet the mice were protected from fat-induced insulin resistance and intramuscular accumulation of fatty acyl-CoA. Overexpression of FATP1 specifically in heart, resulted in lipotoxicity and cardiomyopathy without systemic disturbances in lipid metabolism or disturbances in insulin sensitivity (35). The cardiac dysfunction was attributed to excessive accumulation of lipids due to increased uptake and metabolism.
A deletion of FATP2/ACSVL1 in mice was engineered to specifically examine the effects of this protein in very long chain fatty acid metabolism since it was first identified as an Ascvl enzyme (36). It was hypothesized this protein was the peroxisomal Ascvl involved in the accumulation of very long chain fatty acids that are characteristic in patients affected with adrenoleukodystrophy. The FATP2 knockout mice were viable and had no obvious systemic or metabolic abnormalities apart from reduced peroxisomal Ascvl activity and VLCFA beta-oxidation. However, the knockout mice did not accumulate very long chain fatty acids or develop symptoms characteristic of adrenoleukodystrophy. Fatty acid uptake was not examined in tissues from these animals.
FATP4 knockouts cause neonatal lethality that was attributed to a restrictive dermopathy that prevented expansion of the diaphragm and the pups died within hours of birth by asphyxiation (37, 38). The epidermal ceramides had altered fatty acid composition with a decrease in the C26:0 and C26:0-OH fatty acid side chains, which was considered a major cause of the epidermal malfunction (38). Davidson and coworkers recently rescued the skin phenotype and embryonic lethality using an FATP4 transgene driven by a keratinocyte-specific promoter to evaluate the role of intestinal FATP4 in dietary lipid absorption (39). The growth and maturation of the mice was normal and they were examined for intestinal lipid absorption as adults. Fatp4−/−;Ivl-Fatp4tg/+ mice and wild type littermates displayed indistinguishable food consumption, growth and weight gain on either a low fat chow or high fat Western diet. There were no differences in intestinal triglyceride (TG) absorption or fecal fat loss, indicating loss of FATP4 did not reduce fatty acid uptake or metabolism across the GI track. Similarly, compounds identified as FATP4-specific inhibitors using a high throughput screening method did not reduce GI-specific fatty acid absorption in rodents (40).
Expression of FATP5 is restricted to liver where it is involved in bile acid recycling and fatty acid uptake as evidenced by studies in isolated cells and in the tissues of genetically ablated mice (4, 30, 31). FATP5 deletion significantly reduced LCFA uptake by hepatocytes isolated from FATP5 knockout animals. Livers of these mice had reduced triglycerides and free fatty acids concomitant with a redistribution of lipids to other LCFA-metabolizing tissues. In keeping with its function as a bile-CoA ligase, bile acid recycling was reduced in FATP5 knock out animals. Interestingly, FATP5 deletion mice failed to gain weight on a high-fat diet, had reduced food intake and increased metabolic rate. These varying phenotypes point to the complexity involved in dietary and endogenous lipid trafficking and metabolism.
Given the various tissue distributions it is not surprising that the different FATP deletion mice had unique and relatively non-overlapping phenotypes. As reviewed above, FATP4 is essential for functioning of skin, FATP5 for bile acid recycling, and FATP1 for fatty acid trafficking in adipose tissue and muscle. However, none of the FATP proteins (or any other putative fatty acid transporter, e.g. CD36, FABPpm) thus far has been demonstrated to be essential for lipid uptake in the GI track by studying mice with targeted deletions. (28, 29)
REGULATION OF FATP-DEPENDENT FATTY ACID TRANSPORT
The levels of long-chain fatty acids in blood decrease in response to insulin, but it is unclear if this is due to a combination of decreased release and increased uptake (41). Insulin induces adipocyte differentiation (42) and during that process FATP1 and Acsl1 are strongly induced (11, 41). The increased expression of these proteins correlates with an increase in fatty acid uptake under the same conditions (11, 43, 44). On the other hand, in mature adipocytes insulin acts as a negative regulator of FATP1 expression (45) and as a positive regulator of Acsl1 expression (46). Yet insulin treatment has been reported to promote the translocation of FATP1 to the plasma membrane where it facilitates fatty acid uptake (41). Murine FATP1 mRNA levels are also increased during starvation, which correlates with low levels of insulin (45). This contrasting information seems to indicate that insulin potentiates FATP1 protein function while, at the same time, depresses FATP1 gene expression.
FATP1 has been linked directly to human dyslipidemia. A Swedish study examined FATP1 variants in over 1,000 healthy men and women (47). It was reported that a G/A substitution at position 48 in intron 8 of FATP1 was associated with increased postprandial lipemia, including elevated triglycerides, and smaller LDL particles in homozygous A/A individuals. These findings suggest that through regulation of non-esterified fatty acid trafficking FATP1 is involved in postprandial lipid metabolism and development of cardiovascular disease.
As with many fatty acid metabolic genes, expression of the FATP genes is controlled, in part, by PPAR transcription factor family members. In liver, treatment with a PPARα agonist induced the expression of a number of genes involved in fatty acid import, among them FATP1 (48, 49). PPARδ has been showed to induce lipid uptake and/or accumulation in adipose tissue, muscle, liver, macrophages and placental trophoblasts (50). Activation of PPARδ promotes adipocyte terminal differentiation, which correlates with an increase in FATP1 expression (48, 49, 51). In human placental trophoblast cells, PPARδ enhances fatty acid uptake and accumulation, and also increases the expression of FATP1 and FATP4 (52). Treatment of pregnant mice with a PPARδ agonist alters placental morphology and induces FATP1 and −4 expression while decreasing expression of FATP-2, -3, and -6 (53).
THE SEARCH FOR SMALL COMPOUND INHIBITORS
To further understand the roles of the FATP proteins in fatty acid uptake and intracellular trafficking, we devised a screening method for small compound inhibitors (P. N. Black and C. C. DiRusso, United States Patent 7,070,944) (9, 10). In designing this system we took advantage of a yeast strain that is deficient in fatty acid transport (Δfat1) and has reduced long chain acyl-CoA synthetase activity (Δfaa1). The strain was transformed with a plasmid encoding human FATP2, the intended target of the inhibitor screening. Using this procedure, a 2,080 compound library, SpectrumPlus (MicroSource), was screened to identify compounds that reduced the uptake of the fluorescent fatty acid analog 4,4-difluoro-5-methyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid (C1-BODIPY-C12). Primary and secondary screens were performed to eliminate false positives and a total of 28 compounds were selected as potential inhibitors of human FATP2-mediated fatty acid uptake (9). These compounds were further evaluated for specificity and efficacy in human intestine-derived Caco-2 cell line. Caco-2 cells express FATP2 and FATP4 and efficiently import fatty acids and incorporate them into higher lipids (11). Subsequently, these potential inhibitors were sorted into different groups according to structural features. As expected, one group was similar to fatty acids in having a long hydrocarbon chain. Two other groups were of particular interest because they included drugs used to treat schizophenia but that are also known to cause obesity, hypertriglycerdemia and type II diabetes (54-59). These included dibenazepines and phenothiazines such as clozapine and chlorpromazine, respectively.
The connection between antipsychotic medications and their unwanted metabolic side effects has been widely reported (reviewed in (58)), however the mechanism responsible for this close association has not been elucidated. Different mechanisms have been proposed including: binding of these drugs to serotonin, norepinephrine, dopamine, histamine and serotonin receptors; disruption of the hypothalamic regulation of glucose serum levels through hypothalamic dopamine antagonism; and unspecified direct effects on glucose metabolism. However, our identification of some of these drugs as fatty acid uptake inhibitors may indicate a more direct effect on fatty acid and complex lipid metabolism.
The correlations between antipsychotics and metabolic disorders vary, with the highest risks reported for chlorpromazine, clozapine and olanzapine (58-60). The association of the chronic use of the atypical antipsychotics with new-onset hyperlipidemia in adults with psychotic disorders has been established by considering the odds ratio between treated and untreated individuals as reference (61). The highest values correspond to clozapine at 1.82 (95% confidence limit (CL) 1.61–2.05) and olanzapine at 1.56 (CL, 1.47–1.67). Patients treated with either of these compounds had noticeable increases in weight and adiposity with both short- and long-term treatment. Several studies developed using a variety of methodologies support these observations (58). A mean increase of 4.45 kg with clozapine treatment and 4.15 kg with olanzapine treatment has been reported for a 10-week comparison study (62); longer-term treatment with clozapine was associated with additional weight increases up to 7.5 kg reached over a mean duration of 25 weeks (63).
The effect of these antipsychotic agents on serum lipid levels has also been examined. Statistically significant increases in mean plasma triglyceride levels from baseline were observed in diverse clinical studies with the most significant changes correlating with olanzapine and chlozapine (57, 58). On the other hand, perphenazine treatment was not associated with an increase in serum triglycerides and in at least one study, a significant decrease was recorded (64). The effects of clozapine treatment on total cholesterol levels are less clear and more difficult to interpret since there are discrepancies in studies reporting both statistically and non-statistically significant variations in cholesterol levels from baseline (65-68). In follow-up studies to our HTS screening trials we found that perphenazine, chlorpromazine and clozapine inhibited the uptake of the fluorescent fatty acid analog C1-BODIPY-C12 in Caco-2 cells with Kis in the high micromolar range (Table 1).
Table 1.
IC50 values and 95% Confidence Intervals (95% CI) for C1-BODIPY-C12 uptake in the yeast strain expressing hsFATP2 (“humanized yeast”) and in Caco-2 cells.
| Yeast expressing hsFATP2P | Caco-2 cells | |
|---|---|---|
| Perphenazine | 23.4μM (95% CI, 19.5μM - 27.9μM) |
79.0μM (95% CI, 72.8-μM – 85.6μM) |
| Clozapine | 98.8μM (95% CI, 70.4μM – 138.5μM) |
537.1μM (95% CI,174.8μM – 1652.2 μM) |
| Clomipramine | 74.6μM (95% CI, 46.1μM – 120.8μM) |
231.4μM (95% CI, 212.4μM – 250.2μM) |
| Chlorpromazine | 37.8μM (95% CI, 30.5μM – 46.9μM) |
258.7μM (95% CI, 194.3μM – 340.1μM) |
So the question becomes, is there a direct correlation between fatty acid uptake inhibition and the drug induced metabolic side effects including hypertriglyceridemia and obesity? This is a difficult question to answer with the limited information at hand. Our studies were done using an immortalized human intestinal cell line while the clinical studies report effects on whole body metabolism. We do know that there is not a direct correlation between Ki of uptake inhibition and relative severity of obesity and hypertriglyceridemia. For example, while we targeted perphenazine, chlozapine and chlorpromazine in humanized yeast, they were less effective in Caco-2 cells. We found the inhibition of transport was perphenazine> chlozapine or chlomipirmine while clinical studies did not demonstrate a correlation between perphenzine treatment and elevated serum triglycerides.
For the compounds that both inhibit fatty acid uptake in isolated cell systems and increase triglycerides in patients, we question why inhibition of fatty acid import would result in weight gain and obesity, processes that would require fatty acid accumulation in adipocytes. The answer might lie in the affinity and specificity of the drug for particular fatty acid transport proteins that govern fatty acid distribution between various tissues. If adipocytes, for example, were relatively insensitive to a specific compound then they might accumulate fat that is not being oxidized by muscle resulting in weight gain; while sensitivity of myocytes to fatty acid uptake inhibition by the same compounds might increase demand for glucose as an energy source by these cells, thus disrupting insulin regulation. Our investigations on both the FATP proteins and the inhibitory compounds are ongoing with the goal of providing a mechanistic understanding of these processes. We plan to define structure-activity relationships (SAR) between these compounds and the FATPs with the goal of generating rationally designed molecules that interact specifically with either neuroreceptors or a fatty acid transport protein. The results of these studies may lead to drugs useful to treat psychotic disorders without causing metabolic disorders as well as to other drugs useful to treat obesity-related disorders.
CONCLUSIONS
Members of the FATP family are multifunctional proteins that have overlapping as well as distinct functions in lipid metabolism. There is substantial evidence supporting a role for FATP1, FATP2 and FATP4 in both fatty acid transport and very long chain fatty acid activation. It is unclear at present whether or not these proteins will be beneficial targets as pharmacological inhibitors. However, small compound inhibitors hold great promise as tools to uncover mechanisms of fatty acid transport and activation dependent upon these proteins. FATP5 is probably a minor player in fatty acid transport but plays a major role in bile acid recycling. Much work remains to be done on FATP3 and the cardiac-specific FATP6 to further define their roles in fatty acid metabolism.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (GM56850 and DK07076) to PNB and CCD.
References Cited
- 1.Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim Biophys Acta. 1999;1441:4–13. doi: 10.1016/s1388-1981(99)00137-7. [DOI] [PubMed] [Google Scholar]
- 2.Black PN, DiRusso CC. Vectorial acylation: linking fatty acid transport and activation to metabolic trafficking. Novartis Found Symp. 2007;286:127–138. doi: 10.1002/9780470985571.ch11. discussion 138-141, 162-123, 196-203. [DOI] [PubMed] [Google Scholar]
- 3.Bonen A, Miskovic D, Kiens B. Fatty acid transporters (FABPpm, FAT, FATP) in human muscle. Can J Appl Physiol. 1999;24:515–523. doi: 10.1139/h99-033. [DOI] [PubMed] [Google Scholar]
- 4.Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, Hirsch D, Watson N, Gimeno RE, Stahl A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Dutta-Roy AK. Cellular uptake of long-chain fatty acids: role of membrane-associated fatty-acid-binding/transport proteins. Cell Mol Life Sci. 2000;57:1360–1372. doi: 10.1007/PL00000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg IG, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2008 doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abumrad NA, Forest CC, Regen DM, Sanders S. Increase in membrane uptake of long-chain fatty acids early during preadipocyte differentiation. Proc Natl Acad Sci U S A. 1991;88:6008–6012. doi: 10.1073/pnas.88.14.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirusso CC, Connell EJ, Faergeman NJ, Knudsen J, Hansen JK, Black PN. Murine FATP alleviates growth and biochemical deficiencies of yeast fat1Delta strains. Eur J Biochem. 2000;267:4422–4433. doi: 10.1046/j.1432-1327.2000.01489.x. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Black PN, Chokshi A, Sandoval-Alvarez A, Vatsyayan R, Sealls W, DiRusso CC. High-throughput screening for fatty acid uptake inhibitors in humanized yeast identifies atypical antipsychotic drugs that cause dyslipidemias. J Lipid Res. 2008;49:230–244. doi: 10.1194/jlr.D700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Black PN, DiRusso CC. A live-cell high-throughput screening assay for identification of fatty acid uptake inhibitors. Anal Biochem. 2005;336:11–19. doi: 10.1016/j.ab.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Sandoval A, Fraisl P, Arias-Barrau E, Dirusso CC, Singer D, Sealls W, Black PN. Fatty acid transport and activation and the expression patterns of genes involved in fatty acid trafficking. Arch Biochem Biophys. 2008;477:363–371. doi: 10.1016/j.abb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 13.Stremmel W, Strohmeyer G, Berk PD. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986;83:3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton JA. Transport of fatty acids across membranes by the diffusion mechanism. Prostaglandins Leukot Essent Fatty Acids. 1999;60:291–297. doi: 10.1016/s0952-3278(99)80002-7. [DOI] [PubMed] [Google Scholar]
- 15.Kampf JP, Cupp D, Kleinfeld AM. Different mechanisms of free fatty acid flip-flop and dissociation revealed by temperature and molecular species dependence of transport across lipid vesicles. J Biol Chem. 2006;281:21566–21574. doi: 10.1074/jbc.M602067200. [DOI] [PubMed] [Google Scholar]
- 16.Berk PD, Potter BJ, Sorrentino D, Stump D, Kiang CL, Zhou SL, Horio Y, Wada H. Hepatocellular fatty acid uptake is mediated by a plasma membrane fatty acid binding protein closely related to mitochondrial glutamic oxaloacetic transaminase. Ann N Y Acad Sci. 1990;585:379–385. doi: 10.1111/j.1749-6632.1990.tb28070.x. [DOI] [PubMed] [Google Scholar]
- 17.Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda) 2006;21:259–268. doi: 10.1152/physiol.00014.2006. [DOI] [PubMed] [Google Scholar]
- 18.Stahl A. A current review of fatty acid transport proteins (SLC27) Pflugers Arch. 2004;447:722–727. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- 19.Black PN, DiRusso CC. Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta. 2007;1771:286–298. doi: 10.1016/j.bbalip.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res. 2009;48:52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Pelsers MM, Stellingwerff T, van Loon LJ. The role of membrane fatty-acid transporters in regulating skeletal muscle substrate use during exercise. Sports Med. 2008;38:387–399. doi: 10.2165/00007256-200838050-00003. [DOI] [PubMed] [Google Scholar]
- 22.Faergeman NJ, DiRusso CC, Elberger A, Knudsen J, Black PN. Disruption of the Saccharomyces cerevisiae homologue to the murine fatty acid transport protein impairs uptake and growth on long-chain fatty acids. J Biol Chem. 1997;272:8531–8538. doi: 10.1074/jbc.272.13.8531. [DOI] [PubMed] [Google Scholar]
- 23.Coe NR, Smith AJ, Frohnert BI, Watkins PA, Bernlohr DA. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J Biol Chem. 1999;274:36300–36304. doi: 10.1074/jbc.274.51.36300. [DOI] [PubMed] [Google Scholar]
- 24.Zou Z, DiRusso CC, Ctrnacta V, Black PN. Fatty acid transport in Saccharomyces cerevisiae. Directed mutagenesis of FAT1 distinguishes the biochemical activities associated with Fat1p. J Biol Chem. 2002;277:31062–31071. doi: 10.1074/jbc.M205034200. [DOI] [PubMed] [Google Scholar]
- 25.Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acylcoenzyme A synthetase genes in the human genome. J Lipid Res. 2007;48:2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Watkins PA, Lu JF, Steinberg SJ, Gould SJ, Smith KD, Braiterman LT. Disruption of the Saccharomyces cerevisiae FAT1 gene decreases very long-chain fatty acyl-CoA synthetase activity and elevates intracellular very long-chain fatty acid concentrations. J Biol Chem. 1998;273:18210–18219. doi: 10.1074/jbc.273.29.18210. [DOI] [PubMed] [Google Scholar]
- 27.Watkins PA, Lu JF, Braiterman LT, Steinberg SJ, Smith KD. Disruption of a yeast very-long-chain acyl-CoA synthetase gene simulates the cellular phenotype of X-linked adrenoleukodystrophy. Cell Biochem Biophys. 2000 Spring;32:333–337. doi: 10.1385/cbb:32:1-3:333. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci U S A. 1998;95:8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol. 2006;26:3455–3467. doi: 10.1128/MCB.26.9.3455-3467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubbard B, Doege H, Punreddy S, Wu H, Huang X, Kaushik VK, Mozell RL, Byrnes JJ, Stricker-Krongrad A, Chou CJ, Tartaglia LA, Lodish HF, Stahl A, Gimeno RE. Mice deleted for fatty acid transport protein 5 have defective bile acid conjugation and are protected from obesity. Gastroenterology. 2006;130:1259–1269. doi: 10.1053/j.gastro.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Mihalik SJ, Steinberg SJ, Pei Z, Park J, Kim DG, Heinzer AK, Dacremont G, Wanders RJ, Cuebas DA, Smith KD, Watkins PA. Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J Biol Chem. 2002;277:24771–24779. doi: 10.1074/jbc.M203295200. [DOI] [PubMed] [Google Scholar]
- 32.DiRusso CC, Li H, Darwis D, Watkins PA, Berger J, Black PN. Comparative biochemical studies of the murine fatty acid transport proteins (FATP) expressed in yeast. J Biol Chem. 2005;280:16829–16837. doi: 10.1074/jbc.M409598200. [DOI] [PubMed] [Google Scholar]
- 33.Gimeno RE, Ortegon AM, Patel S, Punreddy S, Ge P, Sun Y, Lodish HF, Stahl A. Characterization of a heart-specific fatty acid transport protein. J Biol Chem. 2003;278:16039–16044. doi: 10.1074/jbc.M211412200. [DOI] [PubMed] [Google Scholar]
- 34.Kim JK, Gimeno RE, Higashimori T, Kim HJ, Choi H, Punreddy S, Mozell RL, Tan G, Stricker-Krongrad A, Hirsch DJ, Fillmore JJ, Liu ZX, Dong J, Cline G, Stahl A, Lodish HF, Shulman GI. Inactivation of fatty acid transport protein 1 prevents fat-induced insulin resistance in skeletal muscle. J Clin Invest. 2004;113:756–763. doi: 10.1172/JCI18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 36.Heinzer AK, Watkins PA, Lu JF, Kemp S, Moser AB, Li YY, Mihalik S, Powers JM, Smith KD. A very long-chain acyl-CoA synthetase-deficient mouse and its relevance to X-linked adrenoleukodystrophy. Hum Mol Genet. 2003;12:1145–1154. doi: 10.1093/hmg/ddg126. [DOI] [PubMed] [Google Scholar]
- 37.Gimeno RE, Hirsch DJ, Punreddy S, Sun Y, Ortegon AM, Wu H, Daniels T, Stricker-Krongrad A, Lodish HF, Stahl A. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J Biol Chem. 2003;278:49512–49516. doi: 10.1074/jbc.M309759200. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann T, van der Hoeven F, Grone HJ, Stewart AF, Langbein L, Kaiser I, Liebisch G, Gosch I, Buchkremer F, Drobnik W, Schmitz G, Stremmel W. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol. 2003;161:1105–1115. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shim J, Moulson CL, Newberry EP, Lin MH, Xie Y, Kennedy SM, Miner JH, Davidson NO. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J Lipid Res. 2008 doi: 10.1194/jlr.M800400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackburn C, Guan B, Brown J, Cullis C, Condon SM, Jenkins TJ, Peluso S, Ye Y, Gimeno RE, Punreddy S, Sun Y, Wu H, Hubbard B, Kaushik V, Tummino P, Sanchetti P, Yu Sun D, Daniels T, Tozzo E, Balani SK, Raman P. Identification and characterization of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones as inhibitors of the fatty acid transporter FATP4. Bioorg Med Chem Lett. 2006;16:3504–3509. doi: 10.1016/j.bmcl.2006.03.102. [DOI] [PubMed] [Google Scholar]
- 41.Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell. 2002;2:477–488. doi: 10.1016/s1534-5807(02)00143-0. [DOI] [PubMed] [Google Scholar]
- 42.Hwang CS, L. T, Mandrup S, Lane MD. Adipocyte differentiation and leptin expression. Annu Rev Cell Dev Biol. 1997;13:231–259. doi: 10.1146/annurev.cellbio.13.1.231. [DOI] [PubMed] [Google Scholar]
- 43.Abumrad NA, F. C, Regen DM, Sanders S. Increase in membrane uptake of long-chain fatty acids early during preadipocyte differentiation. Proc Natl Acad Sci U S A. 1991;88:6008–6012. doi: 10.1073/pnas.88.14.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caserta F, T. T, Civelek VN, Prentki M, Brown NF, McGarry JD, Forse RA, Corkey BE, Hamilton JA, Kirkland JL. Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. Am J Physiol Endocrinol Metab. 2001;280:238–247. doi: 10.1152/ajpendo.2001.280.2.E238. [DOI] [PubMed] [Google Scholar]
- 45.Man MZ, Hui TY, Schaffer JE, Lodish HF, Bernlohr DA. Regulation of the murine adipocyte fatty acid transporter gene by insulin. Mol Endocrinol. 1996;10:1021–1028. doi: 10.1210/mend.10.8.8843418. [DOI] [PubMed] [Google Scholar]
- 46.Kansara MS, M. A, Von Hagen J, Kabotyansky E, Smith PJ. Physiological concentrations of insulin and T3 stimulate 3T3-L1 adipocyte acyl-CoA synthetase gene transcription. Am J Physiol. 1996;270:873–881. doi: 10.1152/ajpendo.1996.270.5.E873. [DOI] [PubMed] [Google Scholar]
- 47.Gertow K, Skoglund-Andersson C, Eriksson P, Boquist S, Orth-Gomer K, Schenck-Gustafsson K, Hamsten A, Fisher RM. A common polymorphism in the fatty acid transport protein-1 gene associated with elevated post-prandial lipaemia and alterations in LDL particle size distribution. Atherosclerosis. 2003;167:265–273. doi: 10.1016/s0021-9150(02)00454-9. [DOI] [PubMed] [Google Scholar]
- 48.Martin G, Poirier H, Hennuyer N, Crombie D, Fruchart JC, Heyman RA, Besnard P, Auwerx J. Induction of the fatty acid transport protein 1 and acyl-CoA synthase genes by dimer-selective rexinoids suggests that the peroxisome proliferator-activated receptor-retinoid X receptor heterodimer is their molecular target. J Biol Chem. 2000;275:12612–12618. doi: 10.1074/jbc.275.17.12612. [DOI] [PubMed] [Google Scholar]
- 49.Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- 50.Norris AW, H. M, Yao J, Jessen N, Musi N, Chen L, Sivitz WI, Goodyear LJ, Kahn CR. Endogenous peroxisome proliferator-activated receptor-gamma augments fatty acid uptake in oxidative muscle. Endocrinology. 2008;149:5374–5383. doi: 10.1210/en.2008-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin G, Nemoto M, Gelman L, Geffroy S, Najib J, Fruchart JC, Roevens P, de Martinville B, Deeb S, Auwerx J. The human fatty acid transport protein-1 (SLC27A1; FATP-1) cDNA and gene: organization, chromosomal localization, and expression. Genomics. 2000;66:296–304. doi: 10.1006/geno.2000.6191. [DOI] [PubMed] [Google Scholar]
- 52.Schaiff WT, B. I, Cheong M, Chern PL, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab. 2005;90:4267–4275. doi: 10.1210/jc.2004-2265. [DOI] [PubMed] [Google Scholar]
- 53.Schaiff WT, K. FJ, Barak Y, Biron-Shental T, Nelson DM, Sadovsky Y. Ligand-activated peroxisome proliferator activated receptor gamma alters placental morphology and placental fatty acid uptake in mice. Endocrinology. 2007;148:3625–3634. doi: 10.1210/en.2007-0211. [DOI] [PubMed] [Google Scholar]
- 54.Kane JM, Barrett EJ, Casey DE, Correll CU, Gelenberg AJ, Klein S, Newcomer JW. Metabolic effects of treatment with atypical antipsychotics. J Clin Psychiatry. 2004;65:1447–1455. doi: 10.4088/jcp.v65n1102. [DOI] [PubMed] [Google Scholar]
- 55.Melkersson K, Dahl ML. Adverse metabolic effects associated with atypical antipsychotics: literature review and clinical implications. Drugs. 2004;64:701–723. doi: 10.2165/00003495-200464070-00003. [DOI] [PubMed] [Google Scholar]
- 56.Meyer JM. Novel antipsychotics and severe hyperlipidemia. J Clin Psychopharmacol. 2001;21:369–374. doi: 10.1097/00004714-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70:1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literarure review. CNS Drugs. 2005;19:1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 59.Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51:480–491. doi: 10.1177/070674370605100803. [DOI] [PubMed] [Google Scholar]
- 60.L’Italien GJ, Casey DE, Kan HJ, Carson WH, Marcus RN. Comparison of metabolic syndrome incidence among schizophrenia patients treated with aripiprazole versus olanzapine or placebo. J Clin Psychiatry. 2007;68:1510–1516. doi: 10.4088/jcp.v68n1006. [DOI] [PubMed] [Google Scholar]
- 61.Olfson M, Marcus SC, Corey-Lisle P, Tuomari AV, Hines P, L’Italien GJ. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163:1821–1825. doi: 10.1176/ajp.2006.163.10.1821. [DOI] [PubMed] [Google Scholar]
- 62.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 63.Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry. 1999;60:358–363. [PubMed] [Google Scholar]
- 64.Meyer JM, Davis VG, McEvoy JP, Goff DC, Nasrallah HA, Davis SM, Daumit GL, Hsiao J, Swartz MS, Stroup TS, Lieberman JA. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE Schizophrenia Trial phase 1. Schizophr Res. 2008;103:104–109. doi: 10.1016/j.schres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baymiller SP, Ball P, McMahon RP, Buchanan RW. Serum glucose and lipid changes during the course of clozapine treatment: the effect of concurrent beta-adrenergic antagonist treatment. Schizophr Res. 2003;59:49–57. doi: 10.1016/s0920-9964(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 66.Gaulin BD, Markowitz JS, Caley CF, Nesbitt LA, Dufresne RL. Clozapine-associated elevation in serum triglycerides. Am J Psychiatry. 1999;158:1270–1272. doi: 10.1176/ajp.156.8.1270. [DOI] [PubMed] [Google Scholar]
- 67.Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160:290–296. doi: 10.1176/appi.ajp.160.2.290. [DOI] [PubMed] [Google Scholar]
- 68.Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder SR, Wirshing WC. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002;63:856–865. doi: 10.4088/jcp.v63n1002. [DOI] [PubMed] [Google Scholar]