Abstract
The discovery of RNAi in the late 1990s unlocked a new realm of therapeutic possibilities by enabling potent and specific silencing of theoretically any desired genetic target. Better elucidation of the mechanism of action, the impact of chemical modifications that stabilize and reduce nonspecific effects of siRNA molecules, and the key design considerations for effective delivery systems has spurred progress toward developing clinically-successful siRNA therapies. A logical aim for initial siRNA translation is local therapies, as delivering siRNA directly to its site of action helps to ensure that a sufficient dose reaches the target tissue, lessens the potential for off-target side effects, and circumvents the substantial systemic delivery barriers. While topical siRNA delivery has progressed into numerous clinical trials, an enormous opportunity also exists to develop sustained-release, local delivery systems that enable both spatial and temporal control of gene silencing. This review focuses on material platforms that establish both localized and controlled gene silencing, with emphasis on the systems that show most promise for clinical translation.
Introduction
The discovery that double stranded RNA (dsRNA) can trigger catalytic degradation of messenger RNA (mRNA) has inspired more than two decades of research aimed at understanding and harnessing this mechanism. Because well-designed RNA interference (RNAi) therapeutics can potently and specifically suppress translation of any gene, including intracellular targets traditionally considered “undruggable”, they have been heavily studied as a potential new class of pharmaceutics that can modulate drug targets that are inaccessible by conventional small molecule inhibitors and antibody drugs. In particular, synthetic, double-stranded small interfering RNA (siRNA) has emerged as a leading candidate for the development of gene silencing therapeutics1, 2. siRNA is potentially advantageous in comparison to other RNAi approaches because it can directly load into the RNA induced silencing complex (RISC) machinery, simplifying dosing control and circumventing the requirement for delivery into the nucleus (e.g., as required with shRNA-encoding vectors)3, 4. However, emergence of clinically approved siRNA therapies has remained slow, with the primary challenge being the formidable anatomical and physiological barriers that must be overcome to deliver siRNA to its intracellular site of action in target cell types5.
To date, systemic delivery of siRNA therapeutics to targets in the liver has been most extensively tested in clinical trials; this approach is motivated by the ability to exploit the liver’s physiological function as a filtration and clearance system6–8. Through strategic targeting of relevant hepatic genes, multiple siRNA therapeutics have proven efficacious in preclinical and clinical trials7, 9, 10. One of the most advanced along the regulatory pathway is a therapeutic by Alnylam currently in Phase III trials that targets the transthyretin gene for treatment of transthyretin amyloidosis7, 11. However, development of systemically delivered siRNA therapeutics that target tissues other than the liver has proven more challenging8.
Local delivery systems offer a potentially more translatable alternative, as they confer the advantages of reducing off-target side effects and potentially achieving higher gene silencing at the target site8. For these reasons, many of the first therapeutic applications of siRNA tested clinically involved local delivery (primarily topical or injection-based). However, initial clinical trials involving local siRNA delivery were largely disappointing and did not meet the high expectations of the scientific and medical communities12, 13. These studies revealed unexpected concerns regarding siRNA safety (e.g., therapies based on naked siRNA triggered immune responses) and pharmacokinetics8, 12–15. The advancement of siRNA molecular design principles and improved delivery systems have increased the number of candidate siRNA therapeutics entering the clinical pipeline, but there is currently a dearth of locally delivered siRNA therapeutics in testing relative to systemically delivered formulations8, 12. This review will focus on recent technologies that leverage the significant advantages of local siRNA delivery and have made progress toward overcoming the barriers that have thus far limited these applications.
siRNA Mechanism
The molecular phenomenon of RNAi-based post-transcriptional gene silencing, first termed “reversible co-suppression”, was unraveled following the unexpected observation by Napoli et al. in 1990 that introduction of a transgene intended to overexpress chalcone synthase (CHS, a gene for flower pigmentation) yielded more white flowers and was associated with a 50-fold reduction of CHS mRNA16. The gene silencing capability of antisense oligodeoxynucleotides (ODNs) was first elucidated, but it was discovered soon thereafter that double-stranded RNA (dsRNA) are capable of achieving 100 to 1000-fold more potent gene suppression than ODNs17. The delivery of dsRNA of varying lengths, siRNA, short hairpin RNA (shRNA), and plasmids expressing shRNA can trigger gene-specific silencing, which is optimal when there is full complementarity between the guide strand and the target mRNA sequence2, 18. These synthetic dsRNA molecules are more effective than ODNs because they “hijack” the catalytically-active gene silencing machinery that is integral to endogenous, negative feedback pathways utilized by naturally expressed microRNA (miRNA)2, 19, 20. When larger dsRNA are delivered to the cellular cytoplasm, they are cleaved by the enzyme Dicer into siRNA, which are 19–21 nucleotides in length and characterized by 3′ nucleotide overhangs. The siRNA strands are then separated, and the antisense or guide strand, recognized by a less stable 5′ end, is incorporated into the RNA-induced silencing complex (RISC)21. The activated RISC loaded with the siRNA guide strand binds to complementary mRNA and initiates its degradation (Figure 1). Importantly, the activated RISC has enzymatic activity, enabling a single siRNA to elicit the degradation of multiple mRNAs22. In contrast, synthetic microRNA (miRNA) often modulate multiple mRNA targets with partial complementarity and thus can influence larger systems of genes23. While the coordinated control of multiple, related genes through miRNA therapeutics is a powerful strategy, properly designed siRNA-based therapeutics are desirable because they offer more predictable functional effects based on modulation of specific genes.
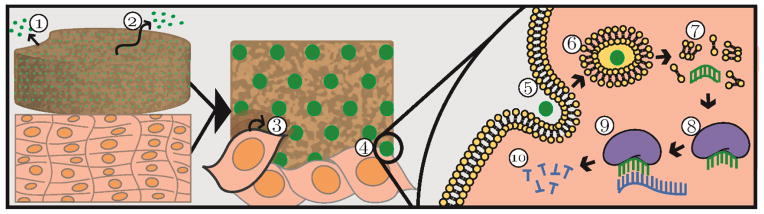
Figure 1.
Schematic of local delivery and mechanism of action of siRNA. The green circle represents siRNA packaged into a carrier. A local delivery reservoir releases siRNA via 1) degradation of the reservoir and/or 2) diffusion. The reservoir, shown in direct contact with host tissue, can also facilitate 3) cell migration into the reservoir and 4) substrate-mediated siRNA uptake through 5) endocytosis. If the siRNA avoids 6) degradation by the endo-lysosomal system and 7) reaches the cytoplasm, its guide strand can be 8) incorporated into the activated RISC complex, which 9) binds to complementary mRNA and 10) initiates its degradation.
siRNA Delivery Challenges
General Delivery Barriers
While discovery and development of small molecule drugs for clinical use remains an enormous challenge, the translation of siRNA therapeutics is fully unchartered. Thus, in addition to traditional drug development challenges, the “normal” pipeline for development of an siRNA drug for FDA clearance has yet to be established8, 12. The major difficulty faced when designing siRNA therapeutics is that of delivery to its site of action; synthetic dsRNA or siRNA molecules have relatively poor pharmacokinetic properties and thus face more formidable extracellular and intracellular delivery challenges relative to small molecule drugs. Oral bioavailability of siRNA molecules is very poor because they are relatively large, hydrophilic, and susceptible to degradation, and systemic, intravenous delivery of siRNA results in rapid renal filtration and clearance through the urine24. siRNA also has a short half-life in vivo and can be degraded by nucleases, especially if optimized chemical modifications are not incorporated onto the siRNA molecule25. Furthermore, siRNA does not readily translocate lipid bilayers, such as those that constitute the outer cellular membrane and the endo-lysosomal intracellular vesicles. The latter can cause siRNA that has been internalized by target cells to be degraded within lysosomes or exocytosed, rather than becoming bioavailable for interaction with the RISC machinery in the cytosol24, 26, 27. For example, in the absence of a mechanism for endosomal escape, only 1–2% of the siRNA delivered by lipid nanoparticles is believed to be released into the cytosol and to be bioavailable for RISC loading and target gene silencing. While it is not the focus of the current review, there are a variety of delivery systems under development for overcoming these systemic and general delivery barriers24.
Utilization of siRNA therapeutically is also complicated by the potential for toxicity and immunogenicity of both the siRNA molecules and the carriers used. siRNA molecules can activate Toll-like receptors (TLRs), which are a part of the innate immune system that recognizes and mounts an immune response against microbial invaders28–32. Additionally, siRNA can elicit off-target effects due to partial sequence complementarity to unintended genes or by saturating the cell’s RISC machinery, altering endogenous miRNA gene regulatory processes31, 33, 34. Furthermore, systems used to deliver siRNA can induce toxic and immunogenic consequences24. These inadvertent effects can override therapeutic benefits and confound interpretation of experiments designed to test the functional significance of siRNA therapeutics14.
Local Delivery Considerations
Localized siRNA delivery obviates many of the systemic delivery barriers but also raises unique challenges. In topical strategies, the skin, mucosal membranes, and epithelial cells can act as delivery barriers to target cells35. For example, siRNA delivery to cervicovaginal or gastrointestinal tissues requires transversing a superficial mucosal layer36–38. However, in most depot systems, direct contact exists between the local delivery reservoir and the target cells (Figure 1). In this scenario, a primary design concern is to control the kinetics of siRNA release such that duration of gene silencing can be temporally controlled and/or sustained without repeated treatments. Without a mechanism for controlled release from the delivery system, siRNA activity has a finite half-life and its activity will diminish over time. This is especially important in applications where the siRNA dose cannot be easily reapplied, including delivery from the surface of an implanted device, delivery to sites that are not easily accessible and/or would require assistance from a health care professional, or delivery from depots that also serve as biodegradable tissue engineering scaffolds39. At sites of tissue repair, there is the added challenge of rapid cellular turnover and proliferation as the different phases of regeneration proceed; when transfected cells undergo mitosis, the siRNA dose is diluted amongst the daughter cells. In this challenging environment, siRNA gene suppression has been shown to be maximal at approximately two days post-transfection and to disappear almost entirely after one week5, 40. By creating delivery platforms with tunable release profiles, gene silencing can be customized for specific therapeutic applications and for sustained effect in tissues that are remodeling and/or regenerating.
Local siRNA delivery systems and their degradation products must also be non-cytotoxic and should not interfere with the desired therapeutic response. Materials that degrade into biocompatible, resorbable byproducts eliminate the need for physical removal of the delivery system. For some systems, the rate at which the material degrades can be used to tune the temporal release profile of the siRNA41, 42. Altering the kinetics for diffusion-based release is also possible, for example by regulating the delivery system’s crosslinking density and/or porosity via synthesis techniques43–45. Of particular interest for regenerative medicine and tissue engineering are multifunctional, porous biomaterials that enable controlled siRNA release, support cellular ingrowth, and degrade at rates that match de novo tissue formation46–49. Therefore, many delivery systems are fabricated using materials with inherent in vivo degradation mechanisms; for example, scaffold and microparticle systems are commonly based on hydrolytically degradable polyesters such as poly(lactic-co-glycolic acid) (PLGA)43, 44, 48, 50. However, the degradation products of PLGA acidify the local environment and can result in inflammation, creating impetus for the exploration of other biodegradable systems51, 52. Environmentally-responsive systems that respond to cellular stimuli like proteases or reactive oxygen species (ROS) offer a promising alternative. For instance, biomaterials incorporating polythioketal crosslinkers confer ROS-dependent degradation that produces non-acidic, cytocompatible byproducts53, 54. The material choice for a local delivery depot determines not only the degradation rate but also the adherence of cells, their viability and their phenotype. In addition, the affinity of an siRNA therapeutic for the material influences release and cellular uptake characteristics42. All of these concerns should be considered, with the goal of designing a system in which the reservoir and siRNA therapeutic work synergistically to elicit the desired cellular response.
Another approach to engineering delivery systems for spatially confined, efficient cellular uptake is to leverage the phenomenon of substrate-mediated uptake49, 55. Substrate-mediated uptake or “reverse transfection” occurs when nucleic acids immobilized on a material surface are internalized by cells adherent to that surface rather than being internalized via solution phase endocytosis or pinocytosis (Figure 1). Substrate-mediated delivery concentrates the therapeutic at the cell-material interface and can enhance transfection efficiency by 10- to 100-fold42, 49, 55. This reduces diffusion of the siRNA away from the target site and is also especially relevant for tissue regenerative applications where cells adhere and grow within a biomaterial that possesses dual functions as a delivery depot and tissue template.
While local delivery presents novel challenges, it also has inherent advantages over systemic delivery that make it a logical focus for translation of siRNA therapies to a broader range of clinical applications. Systemic delivery systems elicit greater concerns regarding off-site toxicity, often cannot achieve sufficient doses within the tissue of interest, and must overcome the rapid renal clearance of siRNA which leads to low bioavailability35. Although many disease states will necessitate systemic delivery (e.g. metastatic carcinomas, systemic infections), local delivery may be a superior strategy for a subset of pathologies37.
siRNA Modifications and Carriers
Though local siRNA delivery avoids many of the challenges associated with systemic injection, it does require measures to prevent siRNA degradation and to overcome cell membrane/endo-lysosomal barriers following depot injection/implantation in vivo. A variety of chemical modifications, conjugation strategies, and lipid/polymer carriers have been identified to address the challenges inherent to siRNA therapies. As these strategies have previously been reviewed comprehensively24, this aspect of the literature will not be exhaustively overviewed herein.
A commonly-utilized chemical modification to siRNA molecules is replacement of phosphodiester linkages with phosphorothioate linkages at select locations on the backbone, which confers improved resistance of siRNA to nuclease degradation25, 56, 57. Further, modifications to the siRNA backbone at the 2′ position, such as 2′-O-methyl (2OME), endow siRNA molecules with greater stability and eliminate the TLR-driven immune response without impacting silencing efficacy25, 56, 58, 59. Careful siRNA sequence selection can also aid in avoiding modulation of non-targeted genes and enhance the activity at the targeted gene. Systematic, computer-aided optimization of siRNA sequence and design has become standard practice and accelerates identification of siRNA sequences likely to exhibit high target gene silencing with minimal off-target effects and immunogenicity25, 31.
Direct conjugation to molecules such as polymers, peptides, lipids, antibodies, and aptamers has also been explored for improving siRNA pharmacokinetic properties60–62. Lipid-like moieties such as cholesterol, α-tocopherol, and palmitic acid improve siRNA stability, cellular uptake, and gene silencing ability63–65. Alternatively, conjugation of targeting ligands to siRNA has shown promise as a means to facilitate receptor-mediated, cell-specific uptake. For example, Alnylam demonstrated the in vivo silencing efficacy of tri-antenna and trivalent N-acetylgalactosamine siRNA conjugates and showed hepatocyte-specific uptake when delivering the conjugates carrier-free66, 67. The conjugation of cell integrin-binding peptides such as RGD and cell penetrating peptides (CPPs) has also been explored for modifying siRNA molecules as well as carrier systems68, 69. CPPs have emerged as potent cellular membrane translocators, but they are also associated with concerns regarding cytotoxicity and immunogenicity70, 71.
While optimization of siRNA sequence and chemical modifications can improve upon safety and efficacy, siRNA activity benefits from delivery via a carrier system in most applications. These carriers typically improve stability against nucleases and enhance cellular penetration capacity and/or endosomal escape; thus, siRNA formulation into nanocarriers is often utilized synergistically with systems for localized, sustained delivery24. siRNA carriers vary widely but commonly consist of lipids/liposomes, polymers, or viral constructs24, 60, 62, 72, 73. Transfection with lipids/liposomes is the most broadly utilized technique, with a variety of commercial reagents available that can facilitate fusion with and transport across cellular membranes24, 60, 74. Similarly, cationic polymers and dendrimers are conventionally used for siRNA packaging, protection, and delivery. Synthetic polymers such as linear or branched polyethyleneimine (PEI), poly(L-lysine), poly(amidoamine) (PAMAM) dendrimers, poly(β-amino esters) (PBAEs), poly(dimethylaminoethylmethacrylate) (pDMAEMA), and histidine and/or imidazole containing copolymers, as well as natural polymers like atelocollagen and chitosan, are among the most extensively utilized24, 75–81. These cationic polymers electrostatically package/protect siRNA and in some cases, contain secondary and tertiary amines that enable endosomal escape via the proton sponge effect75. Other polymers have also been developed that have active membrane-disruptive behavior triggered by the slightly acidic pH of the endo-lysosomal pathway. These polymer-based systems have been utilized primarily to form siRNA nano-formulations with actively endosomolytic cores and have been combined with a variety of micelle/polyplex surface chemistries81–85. Several of these systems leverage a poly(DMAEMA-co-butyl methacrylate-co-propylacrylic acid (PAA) polymer block that is approximately charge-neutral and forms a stable micelle core at physiologic pH. When exposed to a more acidic pH, the DMAEMA and PAA monomers become concurrently more protonated, yielding a net cationic state that triggers micelle destabilization and endo-lysosomal membrane interaction/disruption81.
Cationic lipids and polymers have a number of shortcomings that are gradually being addressed through both rational design and high throughput synthesis/screening approaches. Traditional cationic transfection reagents generally cause cytotoxicity, especially at high concentrations, and are disposed to aggregation and loss of activity in serum and salt-containing environments. Additionally, while the nanocarrier should remain stable in the extracellular environment, the siRNA must be released from the packaging system intracellularly in order to ensure efficient incorporation into the RISC complex24, 86. Recent and ongoing research addresses these concerns; bioreducible, biodegradable, and environmentally-responsive polymers and PEGylation strategies can be utilized both to reduce cytotoxicity and to incorporate mechanisms of siRNA release24, 77, 85, 87, 88.
Local Delivery Strategies
Local delivery of siRNA has been pursued to direct cellular responses in applications ranging from tissue engineering and regenerative medicine to treatment of carcinomas and infections. This section reviews some of the most promising local, reservoir-based siRNA delivery strategies reported to date (Table 1).
Table 1.
Summary of material and carrier choices and release and gene silencing outcomes for local depot-based siRNA delivery.
| Local Delivery Strategy | Intracellular Delivery Strategy | Release Kinetics | siRNA Gene Target/Silencing | Model | ||
|---|---|---|---|---|---|---|
| Natural Materials | Agarose | Topical matrix | Liposomal complex | Burst over 6 hours | MAPK1, Lamin A/C/>50% at 21 days127 | Murine wound model |
| Not measured | PHD2/Potent silencing (evaluated by protein expression) at 7, 21 days128 | |||||
| p53/>75% 2 days after final treatment129 | ||||||
| SMAD3/No silencing shown but functional effect130 | Murine skin irradiation model | |||||
| Alginate | Hydrogel | PEI, chitosan, or naked110 | On the order of days tunable by crosslinking and carrier | deGFP/>90% at 6 days | HEK293s | |
| Scaffold | Liposomal complex131 | On the order of hours tunable by alginate content | Lamin A/C/>85% at 48 hours | Murine, vaginal application | ||
| Dextran | Hydrogel | PEI or naked111 | Tunable by dextran content (9–17 days) and carrier | deGFP/>90% at 3, 7, 14 days | HEK293s | |
| Dextran sulfate/laponite silicate clay, protamine sulfate | LbL film | Calcium phosphate nanoparticles126 | Sustained over 7–10 days | GFP/>50% sustained for 7 days | NIH3T3s | |
| Collagen | Scaffold | PAMAM dendrimers100 | Burst in hours | Snail1/>50% at 7 days | NIH3T3s | |
| Polyplex complex132 | Not measured | COL1A1, HtrA1/>50% from 2–10 days | HACs | |||
| Hydrogel | PEI, chitosan, or naked110 | Minimal release over 15 days | deGFP/>90% with siRNA released at 6 days | HEK293s | ||
| Chitosan | Hydrogel | Polymer/lipid complexes133 | On the order of hours | TG2/>80% at 4 days | Murine tumor model | |
| Naked134 | On the order of days (varies in vitro vs. in vivo) | RANK/>50% at 9 days | RAW264.7s | |||
| Chitosan, HA | LbL film | Chitosan imidazole135 | Sustained over 14 days | RhoA/>50% at 48 hours | PC12s | |
| Chitosan136 | Burst in 2 days | Not measured | N/A | |||
| PEI41 | Minimal, degradation-based | HCV (antiviral)/>50% at 7 days | Huh7.5s | |||
| Acellular dermal matrix | Coating | Naked137 | Burst; 70% in 1 hour | PHD2/>90% at 14 days | Murine cutaneous wound model | |
| Combination | Dextran and PEG-based components | Hydrogel | PLGA microparticles loaded with siRNA-PEI complexes97 | Burst in hours | IL-10/>80% at 5 days | Primary mouse APCs |
| Alginate, HA, hydroxyethyl cellulose, PCL | Hydrogel | Naked89 | Not measured | GFP/>40% at 72 hours | hMSCs | |
| PAMAM, dextran aldehyde | Hydrogel | PBAE nanoparticles138 | 30% after 24 hours, then sustained to 90% at 6 days | Luciferase/70% at 6 days | Murine tumor model | |
| Synthetic Materials | PLA, dioxanone PEG, | Hydrogel | Naked139 | Not measured | Noggin/>70% at maximum, sustained over 7 days | Murine dorsal muscle pouch |
| PCL, ethyl ethylene phosphate | Nano-fibers | Polymer/lipid complex or naked109 | Sustained over 10–15 days | GAPDH/>30% at 96 hours | NIH3T3s in vitro | |
| Polymer/lipid or CPP complex140 | On the order of days (carrier-dependent) | COL1A1/maximum >80% in vitro at 3 days, fibrosis reduction in vivo | HDFs in vitro; rat subcutaneous | |||
| PCL | Nano-fibers | Polymer/lipid complex or naked90 | Sustained but incomplete release for 28 days | GAPDH/>60% over 30 days | HEK293s in vitro | |
| Polydopamine141 | Burst over 3 days | REST/~30% sustained over 7 days | Mouse NPCs | |||
| Scaffold | Polymer/lipid complex142 | Release not measured | BCL2L2, TRIB2/~50% at 72 hours | hMSCs | ||
| PLGA, PLA | Scaffold | Lipidoid complexes143 | Release not measured | KDR receptor/~90% at 2, 3 days | hESCs | |
| Polyurethane | Scaffold | Stimuli-sensitive polymers39, 108 | Sustained release for 35 days controllable by fabrication | PPIB, PHD2/>90% at maximum (day 12), potent silencing for 21 days | Murine subcutaneous | |
| PLGA | Microparticles | PEI | Not measured96 | IL-10/~50% at 2 days in vitro, increased survival time in vivo | BMDCs, murine lymphoma model | |
| Over 30 days dependent on formulation, carrier93 | TNFα/20–50% protein-level at 9 days, reduced appetite changes and cytokine production98 | Rat joint inflammation model | ||||
| PLGA | Nanofibers | Chitosan43 | Sustained for 30–35 days | eGFP/~50% at 2 days | H1299s | |
| PEI and poly(organophos-phazene) | Hydrogel | PEI121 | Sustained release for ~24 days in vivo | VEGF/>90% at 2 days | PC-3s | |
| NIPAAM, double hydroxides | Hydrogel | Naked118 | Not measured | GAPDH/>80% at 6 days | Osteoarthritic chondrocytes | |
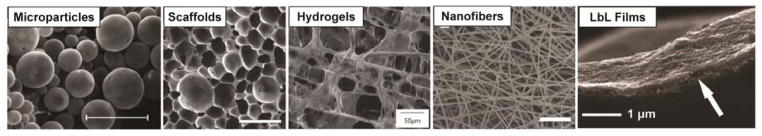
Microparticles
Microparticles were the earliest explored delivery strategy for localized, sustained delivery of siRNA. Microparticles are generally formed using a double emulsion technique (water/oil/water) followed by solvent extraction/evaporation. The initial aqueous phase, containing the oligonucleotide (often pre-packaged into a carrier), is added to the oil phase, containing the polymer that will comprise the matrix of the microparticles. This emulsion is added to a final aqueous phase containing a surfactant, allowing stable microparticle formation. The particles are then isolated through evaporative removal of the volatile, nonpolar solvent. Microparticles can be tuned to possess optimal size for a combination of injectability and tissue retention and have proven effective at protecting loaded nucleic acids from degradation44, 92–95.
Exploration into the loading and release characteristics of microparticles has elucidated the effects of dose of nucleic acid loaded, polymer molecular weight, and particle size. Early reports revealed that smaller PLGA microspheres (1–2 μm) released loaded oligonucleotide more rapidly than larger microspheres (10–20 μm)94. While the small microspheres exhibited a typical burst release profile over several days, followed by a slower rate of release over two months, larger microspheres released ODN in a triphasic profile, with a less substantial initial stage of burst release followed by sustained release for about a month, at which point the remaining ODN was quickly released. This final phase of release was due to degradation of the microspheres and could therefore be modulated by modifying the molecular weight of PLGA used to form the microparticles; for example, slower release after day 25 was seen in microspheres of 30,000 MW PLGA than in microspheres of 3,000 MW PLGA94. It was additionally shown that loading efficiency using the double emulsion, solvent evaporation technique was approximately doubled with the large microspheres in comparison to the small miscropheres (60–70% vs. 30% encapsulation efficiency)94, 95.
Another key concern when designing microparticle systems is the release characteristics of the loaded nucleic acid. Changes in pH, for example that occur in ischemic environments, endosomal compartments, or due to release of degradation products, can significantly impact release kinetics and particle behavior. The impact of pH extremes on PLGA microparticle release of loaded ODN was investigated in a study comparing PLGA microparticles with 0%, 5%, and 10% PEG composition92. At physiological pH, all microparticle compositions exhibited >85% release of the total loaded ODN in a sustained, triphasic manner over 28 days. However, at a pH of 5 and 10, PLGA microparticles with no PEG content achieved <50% total ODN release due to microparticle aggregation. Inhibiting this aggregation through the incorporation of PEG accomplished greater total ODN release at pH extremes while maintaining a similar release profile to 100% PLGA microparticles at physiological pH. However, in all cases a higher degree of burst release was still observed at the pH extremes92. This study also investigated the impact of quantity of oligonucleotide loaded on release rate, revealing that higher loading was associated with a greater burst release. The capacity to modulate release kinetics by varying composition, loading density, and size is central to microparticle utility in nucleic acid delivery applications93, 96.
The application of PLGA microparticles for sustained nucleic acid delivery was first demonstrated with encapsulated ODN. In in vitro studies with RAW 264.7 macrophage-like cells, a 4 hour exposure to microparticles loaded with an ODN designed to silence the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NFκ-β) reduced protein expression of NFκ-β by approximately half and reduced downstream inflammatory cytokine production. Sustained release of ODN from the microspheres was shown for approximately one month in vitro, but the capacity of the microparticles to elicit long-term silencing was not investigated50. These ODN-loaded microparticles established a precedent for using microparticles to deliver siRNA, an application that is discussed in more detail in the following sections93, 96–98.
Scaffolds
The use of polymer scaffolds to facilitate sustained release of siRNA is a potentially transformative strategy to influence local cellular behavior in tissue regenerative applications. An extensive array of polymers and polymer combinations has been explored for scaffold fabrication. Naturally-derived polymers, such as physiological extracellular matrix (ECM) components and polysaccharides (commonly anionic alginate, agarose, dextran, and hyaluronic acid (HA) and cationic chitosan), are often utilized because they have inherent cell-adhesion and degradation mechanisms. Collagen is a particularly popular ECM biomaterial because it is a fibrous extracellular matrix component that is enzymatically degradable99. One of the earliest studies that investigated scaffold-mediated delivery as a means to control siRNA release kinetics utilized collagen scaffolds loaded with siRNA formulated with PAMAM dendrimers100. These scaffolds achieved greater than 50% gene silencing at 7 days (using a 200 nM siRNA dose) in in vitro studies with fibroblasts, demonstrating the potential for sustained silencing via scaffold-based local delivery.
While natural polymers offer advantages in terms of host cell recognition, using synthetic materials enables greater control over a range of scaffold properties, including the pore structure, degradation mechanism/rate, and mechanical stiffness/strength. PLGA offers the distinct advantage of highly tunable degradation rates, and PLGA scaffolds have been shown to promote cellular ingrowth and produce localized and long-term nucleic acid transfection in numerous applications49, 101. Poly(ester urethane) (PEUR) scaffolds formed from non-toxic isocyanates, such as lysine triisocyanate (LTI), have also been successfully adapted for the sustained delivery of a variety of biomacromolecules102–104.
For all scaffold-based delivery applications, it is vital that the siRNA intracellular delivery technology can be readily incorporated into the bulk delivery depot without significant loss of gene silencing activity. Lyophilization of siRNA-loaded poly- and lipo-plexes can reduce their activity, primarily because extensive particle aggregation occurs at high concentrations. Several stabilization strategies have been developed to maintain gene silencing efficacy post-fabrication into local delivery systems39, 105–107. For example, siRNA nanoparticles were shown to have a 50% activity loss when directly incorporated into PEUR scaffolds. However, when the natural sugar trehalose was used as a stabilizing agent, nanoparticle size and activity were retained relative to fresh siRNA formulations39, 108. Additionally, siRNA complexed with either chitosan or Transit TKO transfection reagent and lyophilized onto the surface of tissue culture plates maintained transfection efficiency only when sucrose was utilized as a lyoprotectant106. Sucrose stabilization showed similar efficacy as a lyoprotectant of polyplexes of DNA and PEI107. These results demonstrate the necessity of carefully optimizing the integration of the siRNA intracellular delivery system with the bulk scaffold/depot fabrication method and chemistry.
Electrospun Fibers
Electrospun fibers are another strategy for localized, reservoir-based delivery. Electrospinning enables the formation of nanoscale fibrous structures (biomimetic of those present in natural ECM) using polymers48. These fibers are often used in scaffold-like coatings or dressings but merit discussion independent of the generalized scaffold discussion above. While adsorption of siRNA onto electrospun fibers can mediate gene silencing, a more powerful strategy for controlled release is encapsulation of siRNA into these fibers. For example, siRNA complexed with chitosan and encapsulated into PLGA nanofibers demonstrated sustained siRNA release dictated by PLGA degradation for more than 30 days43. It was also shown that the fibers mediated transfection superior to that of the standard forward transfection method. The silencing efficacy was investigated 48 hours after seeding green fluorescent protein (GFP)-expressing human lung carcinoma cells onto the PLGA fibers in vitro, with a maximum silencing of about 60% observed using the nanofibers. Given the controlled release profile shown, there is a strong impetus to further explore the capacity of this system to achieve sustained silencing.
Electrospun fibers formed from polycaprolactone (PCL), a synthetic polymer with a slower hydrolytic degradation mechanism than PLGA, have also been investigated as a means to control the release of siRNA48. The Chew group loaded naked siRNA into PCL nanofibers but showed minimal (3%) release of siRNA over 28 days90. The incorporation of poly(ethylene glycol) (PEG), a hydrophilic polymer, increased the release of the encapsulated siRNA (up to 30%) by acting as a porogen. While this modification showed promise as a way to tune siRNA release kinetics from the fibers, a full and sustained release profile was not achieved. Additionally, the combination PCL-PEG nanofiber scaffolds proved less cytocompatible than PCL scaffolds when cells were seeded onto them. However, this system produced in vitro silencing in cells seeded upon the scaffolds; there was approximately 20% silencing efficacy when naked siRNA was encapsulated and higher (about 80%) silencing when siRNA-TKO complexes were incorporated. Surprisingly, there was no effect of scaffold composition (0%, 20% and 60% PEG were investigated) on gene silencing90. The Chew group subsequently improved upon the siRNA release through copolymerization of ethyl ethylene phosphate (EEP) with PCL109. siRNA-TKO complexes or naked siRNA loaded into these nanofibers demonstrated scaffold-mediated silencing of fibroblasts in vitro (approximately 40% with the siRNA-TKO complexes and 20% with naked siRNA). In all cases, the most effective nanofiber-mediated silencing was achieved when siRNA was packaged into commercial transfection reagents prior to nanofiber incorporation. However, these agents were also associated with significant cytotoxicity. Nevertheless, these nanofiber-based delivery systems establish silencing efficacy in model gene targets and motivate further development of matrix biomimetic electrospun constructs for controlled siRNA release.
Hydrogels
Hydrogels are a subclass of swellable, highly hydrated scaffolds that can also be composed of natural or synthetic polymers. Strong proof-of concept work from the Alsberg group has proven siRNA-based silencing of model genes from a variety of natural hydrogel systems. In particular, the group investigated collagen, alginate, and dextran injectable hydrogels using calcium- and photo-crosslinking techniques, with the goal of identifying differences in siRNA release rates and silencing efficacy from these systems. A study comparing calcium-crosslinked alginate, photo-crosslinked alginate, and collagen revealed that each of these hydrogels, when loaded with 13.3 μg of naked siRNA per 100 μL gel, achieved greater than 90% gene silencing of encapsulated HEK293 cells in vitro110. More recently, this group demonstrated tunable and sustained siRNA release using photocrosslinked hydrogels comprising dextran (8% or 12% w/w) and linear PEI incorporated at varying concentrations (0, 5, or 10 μg/100 μL gel)111. Both a higher concentration of PEI and a higher mass percentage dextran composition corresponded to slower release kinetics and less burst release, with sustained release achieved out to 8 days.
Hybrid delivery systems comprising combinations of natural and synthetic polymers can leverage the advantages of each (e.g. cytocompatibility of natural materials and tunability of synthetic materials). In a recent study, hydrogels synthesized with a combination of alginate, HA, and hydroxyethylcellulose were 3-dimensionally co-printed with PCL and loaded with Lipofectamine-complexed siRNAs in defined spatial patterns112. This system allowed gene silencing of green fluorescent protein (GFP)-expressing mesenchymal stem cells seeded upon the hydrogels, with the strongest silencing effects localized to the areas patterned with siRNA against the GFP gene. This proof-of-concept work could be modified for long-term gene silencing by optimizing the selection of siRNA carrier and/or hydrogel composition to allow for controlled release. The authors’ application of a novel fabrication technique to achieve spatial and temporal control of gene silencing activity is particularly exciting for engineering of multicomponent or gradient tissue architectures42.
Another subset of synthetic hydrogels that is promising for use in local delivery is environmentally-responsive hydrogels that undergo stimuli-dependent gelation. For instance, poly(N-isopropylacrylamide) (pNIPAAM) is a well-characterized thermoresponsive polymer with an ideal transition temperature from sol to gel that occurs between room and physiologic temperature. pNIPAAM-based hydrogels have been proven to support cellular growth and infiltration, and the random or block copolymerization with other monomers can be used to tune its transition temperature, degradability, and other characteristics113–116. Hydrogels based upon pNIPAAM and other thermoresponsive polymers have been pursued as injectable systems that form a reservoir confining siRNA to the site of injection. For example, a copolymer of NIPAAM and acrylamide (AAM) has been investigated for application as an injectable depot when loaded with gold nanoshell-encapsulated DNA117. Additional work has described an injectable hydrogel composed of NIPAAM and layered double hydroxides (LDHs; either MgAL or MgFe), where the LDHs were utilized to electrostatically incorporate siRNA118. The combination pNIPAAM-LDH gel elicited >80% silencing in osteoarthritic chondrocytes in vitro at 6 days in a model gene. This promising result provides strong motivation for exploration of this system and other pNIPAAM-based injectable options conjunction with functional gene targets and in vivo models.
While pNIPAAM remains a strong option for injectable systems, many other approaches have shown promise. Recent work by Ishii et. al. produced a redox-active injectable gel comprised of polyion complexes of a triblock cationic polymer PMNT-PEG-PMNT (PMNT = poly[4-(2,2,6,6-tetramethylpiperidine-N-oxyl)aminomethylstyrene]) and poly acrylic acid (PAAc)119. Sustained release of PAAc from the gel was shown over 28 days, with negligible burst characteristics and a release of about 30% at last measure. This system would be of interest particularly in treatment of inflammatory conditions, as PMNT contains a TEMPO nitroxide radical, known for its capacity to scavenge reactive oxygen species. While this gel has not been investigated for nucleic acid delivery, it may be amenable to incorporation of siRNA (in replacement of PAAc) and merits further investigation in therapeutic applications. Another injectable system that has demonstrated promise is a liquid crystalline formulation composed of monoglycerides, PEI, propylene glycol, and Tris buffer120. PEI functions to package siRNA and in vitro studies showed 44% of total loaded siRNA-PEI complexes were released over 72 hours in vitro. Subcutaneous implantation of the gels in mice revealed gel degradation over 30 days but also indicated an immune response upon implantation. However, despite this drawback, the capacity to load and release siRNA complexes motivates investigation of gene silencing capabilities. While the injectable strategies discussed above have not yet progressed to functional gene targets, similar systems incorporating pre-packaged oligonucleotide have been investigated in cancer therapies, and these applications are discussed further below117, 121.
Surface Coatings
Coatings onto devices or other biomaterials, such as wound dressings, have also been pursued for local siRNA delivery; coating types include hydrogels as well as single or multilayer surface coatings. The simplest version of a coating for local delivery is a single layer adsorbed or deposited onto a surface. In these adsorbed coatings, control over the release kinetics is limited, but this approach has helped to elucidate the benefit of substrate-mediated transfection48. For example, complexes of plasmid DNA and PEI adsorbed onto nano-graphene oxide substrates exhibited sustained release (over 7 days) and resulted in both a higher transfection efficacy and lower cytotoxicity than complexes delivered in solution122. This approach capitalized on efficient, substrate-mediated transfection and also facilitated gene delivery localized to patterns on the nano-graphene oxide, enabling spatial control of therapeutic delivery.
Multilayer coatings, many of which are assembled through layer-by-layer (LbL) processes, have been one of the most promising approaches to surface-mediated siRNA release. LbL films are typically formed by alternating deposition of materials of opposing charge, with the layers bound together by electrostatic forces. Relative to other systems, LbL coatings offer a high degree of control over release kinetics of a loaded therapeutic. By altering the number of layers and the composition of the layers, it is possible to tune the release rate of loaded molecules and to achieve sustained release123–125. The Hammond group has extensively characterized layer-by-layer assembly, utilizing this approach to coat a variety of devices and biomaterials. They investigated several LbL systems for coating siRNA onto a woven nylon surgical dressing. Each LbL system used protamine sulfate (PrS) as the cationic layering agent, while the anionic layers comprised calcium phosphate-siRNA nanoparticles (CaPh), dextran sulfate, and/or laponite silicate clay (LaP) 126. The most promising candidate was composed of PrS/CaP/PrS/LaP; these films demonstrated a siRNA loading capacity of 19 μg/cm2 and an approximately linear release profile for about 6 days (release leveled off at 10 days with a third of the siRNA unreleased). These films achieved target gene silencing in fibroblasts seeded upon them, with peak silencing of 64% observed at 7 days. The highly tunable release from LbL systems makes them among the most exciting options under investigation for local siRNA delivery, and there is a strong need to investigate these systems in treatment of disease states.
Therapeutic Applications
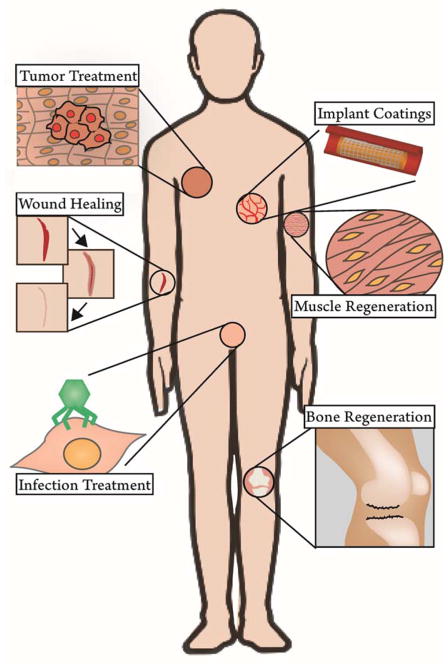
Due to the flexibility to design siRNA against any gene target and the tunability of delivery reservoirs, localized RNAi is a broadly applicable therapeutic approach with potential applications in tissue engineering, regenerative medicine, medical device implants, cancer, infections, and immunomodulation. The prospective applications (Figure 3) amenable to local reservoir-based delivery will be discussed briefly in general and specifically as they relate to promising therapeutic targets that have been most extensively explored (Table 2).
Figure 3.
Schematic demonstrating the breadth of potential applications for local, reservoir-based delivery of siRNA therapeutics. Pictured: tumor treatment, wound healing (e.g. concerning fibrosis and angiogenesis), infection treatment (e.g. sexually transmitted diseases), implant coatings (e.g. stents), muscle/tendon regeneration, and bone repair.
Table 2.
Summary of gene targets for local siRNA-based silencing, categorized by the processes they modulate
| Pathology | Gene | |
|---|---|---|
| Inflammation | TNFα131, 170, 171; CD1698, IgG cell surface receptor98 | |
| Autoimmune Diseases | Tbox21183; CD86184 | |
| Fibrosis and Scarring | CTGF185; mTOR169; Col1α1140; Smad3130, 164; ERK2168; TGFβ165, 166; RhoA135 | |
| Cell Cycle Control | p53129; p21186 | |
| Bone Repair/Regeneration | Noggin139; NFκβ2; Src152; osteopontin, osteocalcin146 | |
| Muscle Regeneration | Myostatin147, 148 | |
| Tendon Regeneration | Col5α1149 | |
| Cellular Differentiation | BCL2L2187; TRIB2187; KDR Receptor143; REST141; Col1α1/HtrA1132 | |
| Angiogenesis | Promotion | PHD239 |
| Inhibition | VEGF188, 189 | |
| Intimal Hyperplasia | TSP2177; MMP2174 | |
| Infections | Viral Genome Components38, 41, 178, 190; RANK134 | |
| Immune Response | IL-1096, 97 | |
| Carcinoma Growth/Survival | TG2133, VEGF121, 191; Cyclin B1180 | |
Tissue Regeneration
A variety of scaffold and hydrogel chemistries have shown promise in tissue engineering applications as a means to modulate cellular ingrowth, proliferation, and phenotype. By modification of mechanical and chemical characteristics of the construct and/or by incorporation of bioactive factors, tissue engineering depots aim to direct cellular behavior and fate48. For in situ tissue engineering (delivery of tissue scaffolds without the incorporation of exogenous cells), the ability to modulate cellular infiltration and phenotype are primary concerns. Growth factors and chemokines are commonly utilized to stimulate desired cell phenotypes, promote infiltration, or encourage specific cell functions, but the capacity of siRNA to be directed at any desired gene target (and to modify intracellular molecules and “undruggable” targets such as transcription factors) makes it a particularly powerful approach to guide cellular behavior.
Bone repair is one tissue engineering application in which local RNAi has been effectively applied. The Noggin protein is an endogenous inhibitor of bone morphogenetic proteins (BMPs) that stimulate osteogenesis, and silencing the Noggin gene can potentiate BMP activity and enhance bone formation144. This helps to override the physiological negative feedback mechanism in which BMPs induce an upregulation of Noggin, limiting the osteogenic response. Elucidation of this mechanism motivated co-delivery of BMP-2 and Noggin siRNA144. One particularly promising study tested PLA-polydioxanone-PEG copolymer hydrogels loaded with 1 nmol Noggin siRNA and 2.5 μg BMP-2 and implanted into mouse dorsal pouches139. Maximum observed silencing of Noggin was 75% at day 1, and a significant silencing effect was achieved for 7 days. Importantly, Noggin silencing stimulated ectopic bone formation, with higher bone mineral content and greater bone amount observed than that triggered by delivery of an equivalent dose of BMP-2 alone. While the degradation profile and release kinetics were not characterized, this system shows potential for bone repair applications, particularly if the hydrogel system can be modified to produce a more prolonged silencing effect. Another possible genetic target to prompt bone formation is gremlin, also a BMP antagonist145. Targeting these and alternative, relevant genes with RNAi is a promising strategy to assist bone regeneration and enable repair of critically-sized bone defects.
Application of siRNA against other osteogenesis-related genes such as osteopontin and osteocalcin has also been explored as a means to regulate bone mineralization. An LbL film composed of alternating layers of calcium phosphate-shRNA nanoparticles and PLL achieved a sustained silencing effect (at 21 days) in human osteoblasts grown on the films, superior to that of the nanoparticles delivered in solution146. The strategy of alternately depositing nucleic acid nanoparticles and polymer layers, coupled with the long-term silencing effect shown, suggests the potential for LbL to achieve timed release of siRNA against multiple targets by loading of different siRNAs at different “depths” within the LbL film. However, the gene silencing effect was only assessed at 21 days, and the release characteristics of the system have not yet been elucidated.
Regeneration of muscle and connective tissue is also a primary interest in tissue engineering, especially as a treatment for genetic diseases such as muscular dystrophy. siRNA against myostatin, a gene that impedes muscle growth, delivered at a dose of 0.5 nmols from atelocollagen nanoparticles was shown to increase muscle growth, size, and activity in a mouse model of muscular dystrophy147, 148. These results were achieved via a local injection into mouse masseter muscle; however, the proven impact of myostatin siRNA on muscle growth motivates its further exploration in controlled delivery depots to regenerate weak or otherwise compromised muscle tissue. The use of siRNA to promote functional tendon regeneration has also been examined using siRNA against the collagen V α1 (Col5α1) chain. The ratio of collagen V to collagen I is important for tendon fibrilogenesis, and overexpression of Col5α1 negatively impacts fibril diameter. By silencing Col5α1 mRNA in cultured tenocytes using Lipofectamine-complexed siRNA and thereafter combining them with normal tenocytes at an optimized ratio, fibrils of a larger diameter (closer to that of a healthy tendon) were generated in vitro149. This study and those involving bone and muscle restoration establish the utility of siRNA therapeutics as a means to stimulate functional musculoskeletal tissue regeneration at sites of pathology or injury.
Directing Cellular Differentiation
The therapies described above demonstrate the potential value of RNAi in functional tissue regeneration by modulating cellular behavior. A related strategy involves using siRNA to drive cellular differentiation to that of a desired tissue type.
Human mesenchymal stem cells (hMSCs) are capable of differentiating into adipocytes, chondrocytes, and osteocytes. To optimize performance of tissue engineering strategies using hMSCs, there is a strong need to develop potent and effective methods to direct their differentiation. Work by Andersen et. al. showed that hMSCs seeded onto PCL scaffolds coated with BCL2L2 or TRIB2 siRNA exhibited enhanced expression of osteogenic or adipogenic markers, respectively, after 7 days142. In this study, the PCL scaffolds were soaked post-fabrication with an approximately 500 nM solution of siRNA/TKO complexes and frozen and lyophilized prior to cell seeding. This approach yielded a 40–50% reduction in the target proteins at 72 hours. While the release was not quantified, microscopy showed substantial retention of nanoparticles in the scaffold at 24 hours, suggesting that the silencing effect could be localized to the coated areas. This was supported by data showing spatially specific gene silencing from a scaffold coated with BCL2L2 siRNA/TKO complexes on one half and TRIB2 siRNA/TKO complexes on the other. A subsequent in vivo study, performed using scaffolds pre-seeded with hMSCs and implanted in diabetic/immunodeficient mice for 8 weeks, led to the conclusion that the scaffolds could prompt early differentiation of hMSCs but could not direct terminal differentiation142. Still, this pioneering work was one of the first to report that a scaffold coated in siRNA nanoparticles can direct differentiation of stem cells. It is anticipated that further characterization and control of the release kinetics and/or exploration of new gene targets may enhance the functional significance of this approach.
In another study aiming to modulate cellular differentiation, embryonic stem cells (ESCs) were seeded onto PLGA/PLA scaffolds loaded with siRNA/lipidoid complexes against a type III tyrosine kinase receptor (KDR)143. The goal was to drive differentiation of embryonic stem cells (ESCs) toward a particular germ layer. For tissue engineering and regenerative medicine applications, it is often desirable to work with cells of a specific lineage; however, ESCs naturally differentiate into a mixture of the three germ layers (endoderm, mesoderm and ectoderm) and techniques to induce differentiation toward a single layer are complicated and incompletely effective. KDR expression occurs early in embryonic development; due to its role as a receptor for VEGF, KDR is critical in development of the cardiovascular system and is associated with development of the endoderm germ layer. Therefore, it was hypothesized that KDR silencing would block endodermic differentiation of ESCs. 70% knockdown of KDR was achieved in hESCs seeded on scaffolds loaded with 0.01 ng/cell siRNA, leading to a pronounced influence on hESC differentiation; a 60–90% downregulation of genes characteristic of the endoderm and a corresponding upregulation of genes characteristic of the mesoderm was observed143. The long-term silencing capacity of the system as well as its capacity to fully direct embryogenesis remains to be investigated.
Other proof-of concept work has shown the suitability of local delivery systems for tissue regeneration without identifying a functional gene target for RNAi. In the previous sections, we reviewed a number of cytocompatible, three-dimensional constructs, many of which have demonstrated general applicability for tissue regeneration applications2, 53, 142.
Bone Pathologies
Several strategies to promote bone regeneration are discussed above; however, distinct from these approaches is the application of RNAi to treat bone and cartilage pathologies (e.g. osteoarthritis and osteonecrosis). One genetic target explored for treatment of osteoarthritis is NF-κβ. NF-κβ is a transcription factor that impacts myriad cellular processes; one pathological implication of NF-κβ is that its upregulation contributes to local inflammation and tissue damage associated with osteoarthritis. Adenoviral-mediated delivery of siRNA against NF-κβp65, a major subunit of the NF-κβ complex, has been shown to reduce production of inflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) in a rat osteoarthritis model2, 150. Thus, local treatments designed to ameliorate and/or reverse bone resorption in osteoarthritis could logically focus on NF-κβ as a target for RNAi. Direct silencing of TNF-α has also been investigated in the context of clinical indications involving bone resorption and deterioration. An antisense ODN targeting TNF-α and delivered from a gelatin/chitosan hydrogel (5 μg ODN per 500 mg hydrogel film) inhibited TNF-α by 80% in an endotoxin-induced bone resorption mouse model151. TNF-α gene inhibition functionally inhibited production of related inflammatory stimuli macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κβ (RANK) by >70% and diminished bone resorption by ~95%. This study provides impetus for investigation of anti-TNF-α siRNA in other clinical conditions exacerbated by local hyper-inflammatory environments.
Additionally relevant to treatment of osteoarthritis is modulation of joint repair. Both type I collagen and/or HtrA1 serine protease are overexpressed in dedifferentiated chondrocytes in comparison to differentiated, healthy chondrocytes. Exposure to hypoxia and BMP2 in combination with siRNA against these genes (delivered via a commercial transfection reagent at a dose of 50 nM) was shown to promote a chondrocyte phenotype appropriate for cartilage formation at a site of joint degeneration132. However, the phenotypic differences were observed in cells pre-seeded in collagen scaffolds, pre-treated with BMP2, hypoxia, and siRNA and subsequently implanted subcutaneously in mice. Development of a system compatible with an in situ tissue engineering approach would enhance the utility of this therapeutic combination.
Local delivery of siRNA has also been pursued for preventing bone loss in periodontitis. RANK, mentioned above and known to modulate osteoclast differentiation and activation, was the genetic target. Chitosan hydrogels were loaded with siRNA-TKO complexes that produced a maximum silencing effect at 9 days of approximately 60% in RAW macrophages134. Characterization of the release kinetics showed sustained but incomplete release in vitro over 14 days with a much faster release profile in vivo (about 70% of the loaded siRNA released in 24 hours). Based upon the successful silencing of RANK in vitro, this system warrants further evaluation in vivo in a model of periodontitis.
Often, siRNA-based treatments of bone pathologies (i.e. osteoarthritis or steroid-associated osteonecrosis) are based upon repeated local injections at the site of bone repair and lack a mechanism for sustained release and prolonged silencing143, 150, 152–154. While these can be useful options, controlling the temporal gene silencing profile would enable greater convenience and, potentially, efficacy. Testing of these known therapeutically-validated targets with technologies for local, sustained release is justified and may enable development of superior, translatable treatments for bone disorders.
Angiogenesis and Wound Healing
The process of angiogenesis is closely tied to tissue regeneration. In tissue engineering constructs, the promotion of angiogenesis is a primary focus, as the development of stable, functional blood vessels is essential to delivering nutrients to cells and facilitating long-term tissue viability99. The formation of functional vessel networks is also essential to the healing process following injury, and dysfunctional wound healing is often characterized by delayed or absent angiogenesis155. Plasmids encoding platelet-derived growth factor (PDGF) or VEGF can promote angiogenesis when delivered from PLGA scaffolds, but there is evidence that the upregulation of a single growth factor will not be sufficient to stimulate both sprouting and maturation of vessels49, 156–158. While delivery of combinations of multiple growth factors can more closely recapitulate an environment that stimulates functional vessel formation, these strategies are typically complex and expensive159. siRNA provides an attractive alternative because by silencing one gene it can influence activity of multiple downstream targets.
The Saadeh group demonstrated proof-of concept work for localized gene silencing in a wound model via an agarose matrix system containing 20 pmols of liposomal siRNA transfection complexes127. In this study, therapeutically relevant siRNA were not investigated but rather the ubiquitously expressed, essential mitogen-activated protein kinase 1 and lamin A/C genes. Targeting these genes facilitated demonstration of the localized nature of the matrix-based silencing and established that the delivery system in itself had no adverse effects on the wound healing process. siRNA complexes distributed in the agarose matrix were applied to a mouse wound and allowed to gel, then removed at 5 days and replaced at 7 days. At day 14, 50–60% silencing of model genes was observed specifically at the wound site, with protein-level knockdown observed via immunological staining and Western blots at day 14 and 21. However, the necessity for repeated dosing suggests that further optimization of the release kinetics could improve this system’s utility. This work demonstrated the feasibility of scaffold systems to regulate gene expression locally at wound sites as well as other targets127. The Saadeh group leveraged this proof-of-concept work in subsequent studies, discussed in detail in later sections, that target relevant genes for therapeutic applications in wound healing and related pathologies128–130, 137.
Several functional targets for stimulation of angiogenesis via genetic repression have been identified, among them prolyl hydroxylase domain 2 (PHD2). PHD2 is an endogenous negative regulator of the transcription factor hypoxia-inducible factor-1α (HIF-1α), and its inhibition promotes expression of genes controlled by HIF-1α, including VEGF, stromal cell-derived factor 1 (SDF-1), and additional pro-angiogenic factors160–162. PHD2 deficiency stimulates both formation and maturation of blood vessels, and silencing its expression is therefore of interest both in broad tissue engineering applications and in chronic, ischemic skin wounds163.
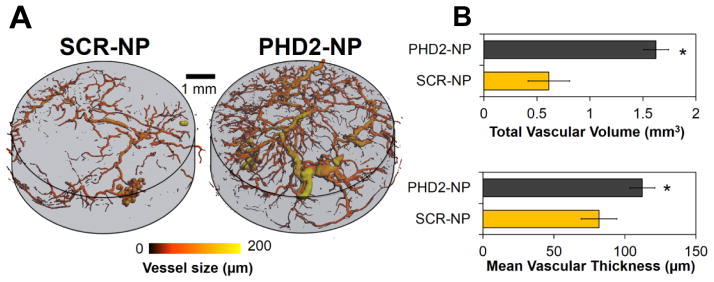
Nelson et. al. used a PEUR scaffold loaded with siRNA/polymer nanoparticle complexes to investigate the impact of silencing PHD2 in a subcutaneous model of wound healing39, 108. The system has proven promising due to its capacity for tunable, sustained release and prolonged gene silencing. Tuning the release rate was possible by adjusting the type of isocyanate used and the amount of excipient added during scaffold formulation. Lysine triisocyanate (LTI) contributed to faster release than hexamethylene diisocyanate trimer (HDIt), and increasing the percentage of the excipient trehalose (0–5% incorporated trehalose was investigated) also promoted faster release39. A broad range of release rates could thus be achieved, with in vitro results showing scaffolds incorporating LTI and 5% trehalose released all siRNA nanoparticles in 5 days while scaffolds incorporating HDIt and 0% trehalose released only ~5% siRNA nanoparticles over 20 days. Modulating the release rate elicited corresponding changes in the in vivo silencing profile. Silencing of a model gene in a mouse subcutaneous implant model (with 0.5 nmols siRNA/nanoparticle complexes per scaffold) peaked at >90%; scaffolds incorporating LTI and 0% trehalose exhibited silencing at this level at 35 days but scaffolds incorporating LTI and 5% trehalose resulted in a silencing peak at 5 days and <50% silencing at 35 days. To prove the therapeutic utility of this system, siRNA against PHD2 was investigated in scaffolds utilizing LTI and 5% trehalose. At 14 days, 80% silencing of PHD2 was observed, resulting in greater than 2-fold upregulation of downstream pro-angiogenic markers VEGF and fibroblast growth factor (FGF). Silencing PHD2 also increased vascular volume within the scaffolds by more than 2-fold and increased mean vascular thickness, suggesting that PHD2 silencing may support both angiogenesis and vessel enlargement and maturation (Figure 4)39. This approach to local PHD2 siRNA delivery shows promise as a means of promoting angiogenesis in wound healing and tissue regeneration applications. Additionally, the sustained and controllable release from the PEUR scaffolds provides motivation to investigate this delivery system in other localized pathologies.
Figure 4.
Sustained silencing of PHD2 increases angiogenesis within PEUR tissue scaffolds. A) Micro-CT images visually demonstrate the increased vasculature within the PHD2-NP scaffolds. B) Quantitative analysis of 3D micro-CT vessel images reveals a significant increase in vascular volume within PHD2-NP-loaded scaffolds relative to control scaffolds containing scrambled (SCR) siRNA. *p<0.05.
Vandegrift et. al. subsequently investigated PHD2 silencing as a means to promote angiogenesis137. They aimed to improve upon the success rate of acellular dermal matrix (ADM) implantation for dermal replacement and reconstruction by promoting incorporation of the ADM into the host tissue via vascularization. ADM was loaded with 20 pmol siRNA by soaking in siRNA solution. In vitro release of siRNA from ADM occurred almost immediately, with 70% released after 1 hour and a maximum of 80% release achieved. However, in a mouse model of a cutaneous dorsum wound, siRNA-loaded ADM silenced PHD2 by about 70% at 7 days and 93% at 14 days. While the sustained silencing contrasted with the burst release profile, further validation of PHD2 silencing was observed based on upregulation of VEGF and FGF mRNA expression by 2.3-fold and 4.7-fold, respectively, at 7 days, and VEGF and SDF-1 protein expression by 4-fold and 2-fold, respectively, at 14 days. The ADM integration was improved but not significant; however, this local delivery system further validates the promise of PHD2 silencing with siRNA as a pro-angiogenic therapy for wound healing.
Another genetic target of interest for RNAi treatment of chronic wounds is p53, a cell cycle inhibitor that is upregulated at sites of chronic wounds. Topical delivery of Lipofectamine-complexed p53 siRNA from an agarose matrix was shown to increase SDF-1 expression and to lead to faster wound closure in a diabetic mouse model129. These effects were observed after application of 20 pmols of siRNA to the wound bed at day 1 and day 8 post-wounding, which resulted in >75% silencing of p53 at the wound sites when tissue was harvested at day 10. However, silencing of p53 and similar cell cycle regulators has raised concerns due to potential carcinogenic effects. When considering these genetic targets, it is of particular import to establish local and finely tuned temporal silencing profiles, ensuring safe application of RNAi.
Fibrosis
The modulation of the immune response, typically to reduce inflammation and/or fibrosis, is another area particularly suited for local therapeutic delivery from reservoir systems. Upon tissue injury, an inflammatory response is activated and normally leads to either tissue regeneration or fibrosis and eventual scar formation. Fibrosis, while characteristic of the natural healing process, becomes problematic when the deposition of connective tissue impedes functionality of damaged organs or medical implants. Excessive scar formation can also cause aesthetic issues (e.g., keloids)160. RNAi shows particular promise to decrease the fibrotic response and improve the quality of regenerated tissue.
Transforming growth factor β (TGFβ) is a key effector of the inflammatory and fibrotic response to injury, as it recruits inflammatory cells and stimulates extracellular matrix deposition. While it is an important regulator of normophysiological healing processes, TGFβ overexpression has been linked to pathological/undesirable scar formation2. The Smad3 gene is a regulator of TGFβ, and topical application of Smad3 siRNA modulated the fibrotic response in a mouse dermal irradiation model130. 500 pmol of siRNA, applied weekly over 4 weeks, silenced Smad3 (near-total silencing was observed at 1 and 4 weeks via histological staining) and led to reductions in collagen deposition and epidermal thickness and producing mechanical properties more characteristic of healthy skin. Additionally, an in vitro study using keloid fibroblasts revealed a decrease in procollagen expression upon silencing Smad3164. Smad3 inhibition thus has potential to reduce radiation-induced fibrosis (common following cancer treatments) and in other pathological fibrosis-related conditions, such as keloid disease.
Direct silencing of TGFβ (rather than it’s regulator Smad3) has also been investigated, motivated by prior results showing that synthetic TGFβ antagonists accelerate wound healing by reducing scarring and fibrosis165. This approach is especially relevant for corneal scarring, which can cause vision problems after ocular interventions. For this application, TGF-β type II receptor (TGFβR2) has been investigated as an RNAi target. Nakamura et. al. showed that a 200 nM dose of siRNA/TKO complexes led to >70% TGFβR2 silencing and inhibited fibronectin assembly and cell migration in human corneal fibroblasts166. The authors subsequently demonstrated in vivo efficacy; a subconjunctival injection of 6 pmol siRNA/TKO complexes reduced matrix deposition in a mouse model of ocular inflammation. Another study by Sniram et. al. using rabbit corneal fibroblasts in vitro revealed that dual and triple delivery of siRNA/TKO complexes (at 30 nM for each siRNA) against TGFβ, TGFβR2, and connective tissue growth factor (CTGF) reduced the fibrotic phenotype of the cells167. Triple delivery of a combination of siRNAs against these genes downregulated >80% of collagen I and smooth muscle α actin expression and decreased cell migration. While these investigations focused on the reduction of ocular fibrosis, the genetic targets explored remain relevant to pathological fibrosis in general and could be of interest for application in conjunction with local delivery depots in other therapeutic indications.
An additional genetic target of interest in modulation of fibrosis is extracellular signal-regulated kinase 2 (ERK2), which, when downregulated via lentiviral-based siRNA transfection, decreased TGFβ and IL-6 expression >50% and reduced inflammation (as evaluated by histological differences in fibroblast and inflammatory cells) in a rat laminectomy model168. Although the motivation of this work was to reduce epidural fibrosis following back surgery, this, along with the previously discussed work, further emphasizes the potential impact of silencing TGFβ or its regulators/effectors.
In addition to the issues related to excessive scar deposition, fibrosis can also contribute to encapsulation of medical implants and tissue engineering constructs, damaging their functionality by creating a relatively acellular capsule at the cell-material interface. Fibrous capsules are characterized by acellular tissue, primarily collagen, and form due to the physiological foreign body reaction. Fibrosis can impede implant performance by obstructing input/output of devices (relevant to neural electrodes or glucose sensors) and by blocking vascularization and surrounding tissue integration of the implant (relevant to tissue engineering scaffolds). In an attempt to ameliorate the fibrotic response, siRNA designed against mammalian target of rapamycin (mTOR), an effector of cellular proliferation, was complexed with branched PEI and encapsulated by PEG hydrogel coatings on model polymer implants169. mTOR inhibition limited fibroblast proliferation as well as decreased fibroblast expression of type I collagen in vitro, and sustained release spanning 2 weeks in vitro was achieved from the PEG hydrogel system. However, no effect on mTOR expression or fibrous capsule thickness was observed in vivo in a rat subcutaneous implant model. The lack of in vivo efficacy was attributed to the inability to load the hydrogel with a dose corresponding to more than 0.4–0.5 mg/kg (siRNA dose per animal weight). It is also possible that the release characteristics in vivo did not match those observed in vitro. While an in vivo effect was not observed using this system, mTOR is worthy of further exploration as a target for anti-fibrotic applications.
The Chew group directly investigated silencing of collagen type I, a major component of fibrotic capsules and scar tissue, using PCL-co-EEP nanofibers encapsulating siRNA-TKO or siRNA-CPP (either the CPP CADY or MPG) complexes140. Nanofibers loaded with the siRNA-TKO complexes showed sustained release over 28 days, while siRNA-CPP complexes exhibited faster release (maximal release for siRNA-CPP complexes occurred at approximately 10 days). In vitro studies of human fibroblasts seeded on all fibers showed prolonged silencing, specifically from 7 to 14 days, with maximal observed silencing of about 50% at 14 days. Additionally, in a rat subcutaneous implant model at 4 weeks post-implantation, the siRNA-loaded nanofibers decreased fibrous capsule formation by greater than 50% for the two leading groups (PCL-co-EEP nanofibers encapsulated with siRNA-CADY or siRNA-TKO complexes, using 0.75 nmols siRNA per nanofiber scaffold).
Fibrous encapsulation can be a particularly detrimental occurrence in neuronal implants. SiRNA-loaded coatings specific to this application have been explored, with the aim of silencing Ras homolog gene family, member A (RhoA). This particular genetic target is highly relevant to neuronal implant applications, as expression of RhoA inhibits neuronal repair. Chitosan imidazole/siRNA nanoparticles were used as the cationic component in HA/chitosan polyelectrolyte multilayer films135. These films were coated onto polydioxanone filaments (used as a model for a neuronal implant intended to facilitate nerve regeneration). Release of siRNA from the films was sustained for approximately 14 days from the filaments in vitro, and localized uptake of fluorescently labeled siRNA was observed via microscopy at 2 and 6 days in studies of neuronal rat cells seeded onto the coated filaments. Gene silencing from the film-coated filaments was not measured, but cells seeded upon film-coated glass slides showed >50% silencing of RhoA at 48 hours135. Based on the release characteristics of the system, it would be relevant to investigate uptake and silencing from the coated filaments at later time points to evaluate whether the sustained release corresponds to prolonged gene silencing.
Inflammation
Controlled delivery of siRNA is also promising for pathologies characterized by excessive, local inflammation. In a temporomandibular joint inflammation model, siRNA silencing of IgG cell surface Fc receptor (an antibody-binding receptor functional in the immune response) reduced production of inflammatory cytokines IL-6 and IL1-β by ~50% and ~20% respectively98. PLGA microparticles were loaded with siRNA-PEI polyplexes and demonstrated sustained release of approximately 150 nm polyplexes (for 28 days). However, the PEI and siRNA were not released at the same rates, potentially contributing to minimal silencing of the target gene. While the lowered expression of IL-6 and IL1β was significant at 9 days, better stabilization of the PEI-siRNA formulation such that the polyplexes are released intact may be an important target for improving the performance of this system.
RNAi against TNF-α, discussed above in relation to treatment of osteoarthritis, has also been investigated to reduce inflammation in skin applications. In a xenograft transplantation model of psoriasis in mice, lentiviral-mediated transfection of TNF-α shRNA decreased TNF-a expression by ~30% (though the reduction was not statistically significant), increased epidermal thickness by a significant 25% and restored healthier skin morphology (as visualized by histological staining) when injected intradermally170. Additionally, mucosal application of siRNA targeting TNF-α (via direct application of a solution of 20 nmol of Lipofectamine-complexed siRNA to the cervicovaginal and rectal regions) was demonstrated to reduce inflammation in a mouse model of colitis171. While neither of these studies investigated techniques for sustained delivery, they reveal the utility of RNAi in clinical indications characterized by chronic pain and inflammation and suited to local application.
A unique strategy has been developed for local delivery from a perfused device to effect gene knockdown in specific blood-circulating cells (i.e., circulating inflammatory cells). The authors utilized PEGylated, targeted siRNA liposomes adsorbed onto microrenathane tubing. P-selectin was used as the targeting moiety due to its capacity to recruit and attach circulating cells in physiological flow conditions. Physiological flow of a cell solution through the coated tubes showed cellular attachment, siRNA uptake, and gene silencing (up to 77%) in neutrophils perfused through the tubes in vitro172. There is potential to incorporate the device into the in vivo circulatory system to enable gene knockdown within specific types of circulating cells. This is an intriguing concept, and this result merits further exploration as a cell-specific substrate mediated siRNA delivery approach.
Vascular inflammation is also implicated in atherosclerosis and the clinical problem of intimal hyperplasia following vascular grafting and stent placement. Intimal hyperplasia is characterized by invasion of the vessel lumen by smooth muscle cells and is a primary cause of graft/stented vessel stenosis and failure. Therapeutic-eluting stent coatings to modulate the inflammatory response and cell proliferation within the stent can reduce rates of stent failure, and siRNA strategies merit investigation in this application173. Matrix metalloproteinase 2 (MMP2) is upregulated in coronary stents that undergo restenosis, and MMP was explored as a genetic target for siRNA loaded into hydrogel coatings on coronary stents. The natural polysaccharide pullulan was cationically modified and used to form MMP siRNA-loaded hydrogels; siRNA loading capacity was modulated by the content and nature of the cationic modifications174. It would be expected that varying the cationic modifications would also allow for modulation of the release kinetics, but this was not investigated. In the investigated hydrogel formulation, 20% release was shown after 1 hour and minimal additional release occurred between 24 hours and 4 days. A modest decrease in pro-MMP2 protein level was observed in vivo in a rabbit carotid artery model, but this decrease was minimal and no significant change was observed for protein level of the active form of MMP2. It is possible that the protein-level effect was not yet observable at 24 hours post-implantation of the stent, but no later time points were investigated. Delivery of drugs to vascular grafts during explant and controlled release from stent surfaces are promising but very underdeveloped approaches for reducing vascular inflammation175, 176.
Another approach that has been tested for the delivery of siRNA formulations from vascular stents was via adsorption of siRNA complexes onto electrospun polyethylene terephthalate (PET) 177. This study showed effective (>50%) silencing in aortic smooth muscle cells when 2–6 μg of PEI-complexed siRNA was adsorbed onto PET grafts. In this application, the target was thrombospondin 2 (TSP2), which is a matricellular protein upregulated at sites of implantation and therefore believed to be linked to the physiological response of intimal hyperplasia. Though no functional impact was observed for either MMP2 or TSP2 silencing, these studies provide proof of concept for successful stent coating with siRNA therapeutics. Both utilized a method of adsorption of siRNA complexes onto a stent via dip coating, which could translate well to potential applications in the medical field due to its relative simplicity. However, the loading and release characteristics were not described and the adsorption method typically leads to burst release and does not enable controlled or sustained release. Mechanisms to modulate release could lead to a more therapeutically beneficial delivery system that can be integrated with stent implants. Other work has revealed the feasibility of layer-by-layer coating of stents as an siRNA therapeutic delivery system, although gene silencing efficacy from the coated stents was not established136. Gene therapy is a potentially transformative way to address the high-impact area of vascular graft patency, and further development of these and other local delivery systems will aid in identifying a translatable system.
Microbial Infections
The use of local siRNA depots to treat infections or their negative consequences is another area of ongoing research. A broadly applicable strategy for treating infections is through the delivery of siRNA targeting the viral genome. siRNA targeting the hepatitis C virus (used as a model infection) and delivered from multilayer films was shown to inhibit viral replication and infection in in vitro experiments41. siRNA complexed to PEI was adsorbed onto films of PLL and PGA, and, building from this starter layer, PEI-siRNA, HA, and chitosan were used to form the successive layers of the film. Increasing the number of layers (from 1 to 3 to 5) was shown to improve transfection efficacy, and siRNA delivered from films exhibited prolonged inhibition of Hepatitis C viral replication (over 12 days) in vitro. These films are promising for reducing viral infections and merit future investigation in an in vivo model.
Additionally, siRNA therapies have been tested as a prophylactic in viral sexually transmitted infections (STIs) such as herpes simplex virus 2 (HSV2). SiRNA against genes crucial to HSV2, at a dose of 500 pmol complexed with Oligofectamine, protected mice from developing HSV2 when applied as a topical microbicide intravaginally within hours of HSV2 infection178. This system, while lacking a mechanism for sustained efficacy, provides powerful proof-of-concept for antiviral siRNA treatments against STIs. In work applied to HIV, siRNA aptamers designed to bind to HIV receptors and silence critical viral demonstrated promise as a prophylactic against HIV infection in a mouse model32. The siRNA aptamer treatment was applied 4 hours post-infection at a <0.2 mg/kg dose in a hydroxyethyl cellulose gel to the vaginal tract mucosa. Though the complete preclusion of HIV infection was only accomplished in 2 of 4 mice, this study further establishes the promise of siRNA therapeutics in STI prevention and motivates development of a controlled-release system for sustained antiviral protection. Such a system was explored by Woodrow et. al. using siRNA-loaded PLGA nanoparticles in topical intravaginal administration179. This strategy was the first proven to penetrate and distribute into the vaginal tract and achieve sustained knockdown in mice. A delivered dose of 0.1 mg/kg produced silencing out to 14 days, with a maximum of >50% silencing in the vaginal tract observed at 10 days. By prolonging the silencing profile further, antiviral prophylactic treatments for STIs could gain therapeutic relevancy.
In an attempt to accomplish a further sustained silencing effect, a solid system for intravaginal application was investigated131. This system comprises an alginate scaffold loaded with lipoplex-encapsulated siRNA. The release could be tuned by alginate:lipoplex ratio, with slower release at higher alginate, but the uptake of lipoplexes decreased at higher alginate ratios. PEGylation conferred muco-penetrative abilities to the lipoplexes, resulting in a 6-fold greater uptake in vivo in comparison to non-PEGylated lipoplexes. In a mouse model and after two daily doses of a scaffold loaded with 600 pmol siRNA, 85% silencing of the model gene lamin A/C was observed (at 24 hours after the second dose) 131. A prolonged effect could be possible with optimization of the degradation (shown to be complete in 3 hours in salt buffer) and release (40% release seen after only 1 hour) of the system. The promising results of this solid system, as well as the topically applied systems, to elicit silencing intravaginally, provides motivation for further exploration of these therapies to prevent STI transmission.
Cancer
While RNAi therapeutics have been investigated extensively for the treatment of cancers via systemic delivery, some carcinomas are well-suited to local delivery strategies. siRNA injected intratumorally has been shown to inhibit tumor growth, providing motivation for approaches using injectable depot-like systems, especially in cases where primary tumors may be inoperable79. A widely investigated strategy involves the injection of a hydrogel or a hydrogel precursor at the local tumor site. In work by Sood and authors, a chitosan hydrogel was loaded with chitosan-siRNA complexes133. siRNA was targeted to tissue transglutaminase, a gene critical to carcinoma growth and chemoresistance. The hydrogels were injected intratumorally and shown to inhibit tumor growth in both melanoma and breast tumor models in mice. The system shows promise to localize cancer treatment at a tumor site; however, as described, it required multiple injections over several weeks and was not designed for sustained release.
Another study investigated local, hydrogel-based delivery of siRNA for treatment of breast cancer. The hydrogel, comprised of PAMAM dendrimers crosslinked with dextran aldehyde, was loaded with siRNA encapsulated by PBAE nanoparticles138. The study investigated the degradation characteristics of the nanoparticles alone in PBS (60% degraded at 7 hours and complete degradation at 24 hours) as well as nanoparticles loaded into the hydrogel. The gel was shown to protect the nanoparticles from rapid degradation. The hydrogel-loaded nanoparticles exhibited a phase of rapid degradation (about 50%) over 24 hours followed by a second and sustained phase of degradation reaching completion at 12 days. siRNA-PBAE complexes loaded into the hydrogels exhibited sustained release over 6 days (30% of the siRNA was released after 24 hours, followed by slower release) and was associated with hydrogel degradation. When 10 μg of siRNA was loaded into this system and injected intratumorally into a mouse tumor model, 70% silencing of the model gene luciferase was achieved at 6 days (as opposed to a maximum of 20% knockdown achieved using an injection of siRNA-PBAE complexes without the hydrogel). The first phase of degradation was found to correspond to burst release of physically entrapped nanoparticles, with the remaining sustained release being due to release of nanoparticles chemically linked to the hydrogel (via a covalent bond between aldehydes on the dextran component and amines on the surface of the PBAE nanoparticles)138. The comprehensive investigation of the release kinetics of this system as well as the sustained silencing shown provides impetus for application of this and similar systems to functional genetic targets.
Hydrogels comprised of NIPAAM-co-AAM have also been investigated for application in intratumoral injections. This polymer exhibits thermoresponsive gelation at 40°C and was loaded with silica gold nanoshells encapsulating an anti-cancer drug and DNA duplexes117. The release of therapeutic (e.g. DNA, used as a model for siRNA) from the nanoshells was shown to be optically triggered by irradiation with a specific wavelength of light; this was evaluated at room temperature with nanoshells in polymer solution. Controlled, optically-triggered release of DNA from the nanoshells was established, with delivery localized to the site of tumors by the hydrogel system. However, the cellular uptake of the released DNA has yet to be investigated. PEI-conjugated poly(organophosphazene) is another thermally responsive polymer used in injectable hydrogel applications; it was shown to encapsulate siRNA and gel upon transition to body temperature. siRNA against cyclin B1 (critical to cell division) and VEGF (significant in tumor angiogenesis) were investigated in separate applications of this hydrogel delivery system121, 180. This system has the potential to translate to various local, long-term gene delivery applications, as it exhibited sustained release for a week in vivo and >60% protein expression inhibition in a mouse tumor model at 30 days (using a 50 ug dose of cyclin B1 siRNA) (Figure 5)180. The in vitro transfection of the system was improved upon by conjugating a CPP to poly(organophosphazene) via a degradable linker (>90% VEGF protein-level inhibition), but the in vivo tumor inhibition remained similar to the previous hydrogel system lacking a CPP121, 180.
Figure 5.
Cyclin B1 siRNA delivered via PEI-poly(organophosphazene) hydrogels inhibits tumor growth in a mouse tumor xenograft model. Conjugate 4 corresponds to poly(organophosphazene) conjugated to linear PEI of MW 800. *p<0.001 vs. PBS-injected group.
Local delivery of siRNA can also be useful as an adjuvant for cancer vaccines. In this application, it is desirable to enhance the Th1 immune response while minimizing the Th2 immune response in dendritic cells. Delivering CpG oligonucleotides can potently initiate the Th1 response; however, this results in simultaneous upregulation of Th2-promoting cytokine IL-10. Roy et al. found that co-delivery of IL-10 siRNA and CpG oligonucleotides via PEI-conjugated PLGA microparticles prophylactically prior to a lethal B cell lymphoma in mice extended survival times significantly96. However, repeated immunizations (of 50 μg siRNA each) were necessary, and the release kinetics of siRNA from the microparticles was not investigated. The same group also explored a hydrogel system for delivery of a DNA antigen and IL-10 siRNA, similar to the microparticle system, with the addition of chemokines to recruit dendritic cells into the hydrogels97. The injectable hydrogels comprised a combination of dextran and PEG components (precise formulation was varied) and loaded with PLGA microparticles core-loaded with siRNA and functionalized with PEI for surface loading of plasmid DNA. This hydrogel allowed primary dendritic cell infiltration in vitro and achieved a maximum of 80% silencing of IL-10 for 5 days in vitro, but in vivo efficacy has yet to be established. Additionally, microparticles delivered via standard transfection in the cell culture media outperformed all hydrogel formulations in vitro, raising the question of whether the hydrogel construct is a necessary addition to the microparticle delivery system. Although a benefit could be expected in vivo due to localization of microparticles at the site of interest via the hydrogel, more work is required to better elucidate the functional contribution of the hydrogel.
Clinical Prospects
The only siRNA delivery depot in the clinical pipeline is the siG12D LODER therapeutic, developed by Silenseed Ltd., to combat non-resectable pancreatic ductal adenocarcinoma (PDA)181. A mutation in the KRAS oncogene is a typical characteristic of PDA, and a particularly common mutation is a substitution of the glycine at codon 12 with aspartate (G12D). This mutation causes perpetual activation of the KRAS signaling pathway which results in heightened proliferation and migration as well as other cancerous indicators182. Silenseed Ltd. has developed an siRNA that specifically targets this mutant version (siG12D) and encapsulated it within a PLGA polymer matrix (LODER, or LOcal Drug EluteR). The siG12D LODER system exhibited iimpressively prolonged release of naked siRNA; 10 μg of siG12D loaded into LODER sustained a consistent rate of release over 2 months in PBS at 37°C, and the released siRNA was shown to be intact at 97 days in PBS (and at 70 days at the site of subcutaneous implant in vivo). In in vitro silencing studies in the Panc1 human pancreatic cancer cell line, siG12D released from LODER specifically silenced mutant KRAS, decreasing downstream targets phospho-Erk and phospho-Akt (protein kinases) at the protein level, and inhibited Panc1 growth and migration. Interestingly, the in vitro silencing was achieved via naked siG12D released from LODER in the presence of transfection agent Lipofectamine, which aided in siG12D’s intracellular delivery. However, in in vivo studies, siG12D LODER achieved effective silencing without the aid of a nanocarrier or other transfection reagent.
In in vivo studies, the siG12D LODER matrix (loaded such that the final siRNA dose was 0.32 mg/kg) was stitched to tumors to ensure localized release over time. Testing was done in immunocompetent mice with Panc02 mouse pancreatic cancer cell subcutaneous tumors as well as in immunocompromised mice with Panc1 tumors. siG12D LODER implantation onto the tumors resulted in development of a large necrotic area (>60% of total tumor area for the Panc02-derived tumors). In the Panc1-derived tumors, the treatment also caused a reduction in tumor volume and increased mouse survival time. siG12D LODER was additionally evaluated in an orthotopic tumor model using Panc1 cells, and the therapy was also effective in this setting, inhibiting tumor growth and lengthening survival time (Figure 6). These promising results in vivo in a relevant disease model set siG12D LODER apart in the realm of local, controlled-release siRNA therapeutics. To date, siG12D LODER has successfully undergone phase I clinical trials; although the journey to clinical application is by no means complete, this therapeutic exhibits great potential. Importantly, clinical translation of siG12D LODER could also help to spur more broad interest in translational development of systems for sustained, localized, siRNA delivery.
Figure 6.
Treatment with siG12D LODER significantly improves mean survival time of mice with orthotopic Panc1 tumors.
Future Outlook
The ability of siRNA to orchestrate coordinated cellular responses via potent and specific gene silencing makes it a unique and powerful therapeutic option. The potential to tailor siRNA to silence any gene of interest endows this strategy with broad applicability. The promise of therapeutic RNAi in local delivery has already been adapted for clinical trials in a variety of indications, including respiratory syncytial virus infection, macular degeneration, pancreatic ductal adenocarcinoma, and pachyonychia congenita24, 192–196. A comprehensive list of local siRNA therapeutics in varying stages of clinical trials is provided in Table 3. Of note is that only one of these technologies incorporates a mechanism for controlled siRNA release, the siG12D LODER system from Silenseed Ltd (discussed in greater detail previously).
Table 3.
Clinical trials for local siRNA delivery systems, from earliest to most recent; highlighted in gray is the only sustained release system.
| Name | Genetic Target |
Delivery System |
Delivery Route |
Control Release? |
Company | Pathology Addressed | Ph. | Dose | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Cand5, Bevasiranib198, 199 | VEGF | In solution, no carrier | Intravitreal injection | No | OPKO Health, Inc. | Macular degeneration (MD) | I | 0.1–3 mg/eye200 | Complete |
| II | 0.2–3 mg/eye201 | Complete | |||||||
| Diabetic macular edema | II | 0.2–3 mg/eye202 | Complete | ||||||
| Age-related MD | III | 2.5 mg/eye every 8 wks203 | Terminated (company decision) | ||||||
| N/A | Withdrawn | ||||||||
| AGN211745, siRNA-027 | VEGFR1 | In solution, no carrier | Intravitreal injection | No | Allergan siRNA Therapeutics, Inc. | Age-related MD, Choroidal neovascularization | I/II | 0.1–1.6 mg/eye193, 204 | Complete |
| II | 0.1–1 mg/eye205 | Terminated (company decision) | |||||||
| PF- 04523655, PF-655 | RTP801 | In solution, no carrier | Intravitreal injection | No | Quark Pharma | Age-related MD | I | 0.05–3 mg206, 207 | Complete |
| Diabetes complications, Diabetic retinopathy | II | 0.4–3 mg208 | Terminated (goal not achieved) | ||||||
| Choroidal neovascularization, Diabetic retinopathy | II | 1, 3 mg every 4 wks209 | Complete | ||||||
| Diabetic macular edema | II | Not listed210 | Complete | ||||||
| ALN-RSV01 | RSV-N gene | In solution, no carrier | Nasal, nebulized | No | Alnylam Pharma | Respiratory syncytial virus infections | II | 1.5–150 mg/day for 5 days211, 212 | Complete |
| II | 0.6 mg/kg/day for 3 days192, 213 | Complete | |||||||
| IIb | Not listed; One dose/day, 5 days214 | Complete | |||||||
| TD101 | K6A N171K | In solution, no carrier | Intra- lesional injection | No | Pachyonychia Congenital Project | Pachyonychia congenita | I | Escalation; Once per week, 17 mg over 17 weeks194, 215 | Complete |
| Bamosiran, SYL040012 | β2-AR | In solution, no carrier | Ophthalmic installation | No | Sylentis, S.A. | Ocular hypertension, Open angle glaucoma | I | 0.6–0.9 mg/eye/day for 7 days216, 217 | Complete |
| I/II | Not listed218 | Complete | |||||||
| II | 0.08–0.9 mg/eye/day for 14 days219 | Complete | |||||||
| II | N/A220 | Recruiting | |||||||
| QPI-1007221 | Caspase -2 | In solution, no carrier | Intravitreal injection | No | Quark Pharma | Optic atrophy, Anterior ischemic optic neuropathy | I | Escalation; Not listed222 | Complete |
| Acute primary angle- closure, glaucoma | II | 1.5 mg/eye single injection223 | Recruiting | ||||||
| siG12D LODER181 | KRAS G12D | LODER polymer | EUS biopsy needle | Yes | Silenseed Ltd. | Pancreatic ductal adenocarcinoma | I | Escalation; not listed224 | Complete |
| II | N/A225 | Not yet recruiting | |||||||
| SYL1001 | TRPV1 | In solution, no carrier | Ophthalmic installation | No | Silentis, S.A. | Ocular pain | I | Not listed; One dose/day, 11 days226 | Complete |
| Dry eye syndrome | I/II | Not listed; One dose/day, 10 days227 | Recruiting |
Despite the pioneering progress of siG12D LODER in the clinical pipeline, there remains a strong need to further translate in vitro silencing from local delivery depots to functional improvements in clinical indications in vivo. Many systems showing promising silencing in vitro have not been explored in medically relevant animal disease models. In addition, of the multitude of pathologically relevant genes identified in in vitro RNAi studies, only a small portion have been examined in conjunction with established local delivery systems. Further investigation of potential synergism between the impact of the local constructs themselves and the silencing of genetic targets is an exciting avenue to explore.
Another complicating factor relevant to the clinical adoption of local siRNA therapeutics is the plethora of polymers, siRNA carriers, and construct types pursued in basic research. Among local siRNA therapeutics in the clinical pipeline, systems achieving sustained delivery are notably absent. Instead, simple topical treatments or local injection strategies predominate due to the far greater ease with which these treatments proceed through the regulatory pathway. In order to reap the therapeutic benefits of controlled-release delivery systems that maintain strong gene silencing activity without repeated doses, a balance must be struck between complicated fabrication processes and therapeutic efficacy.
Additionally, obfuscating comparison of strategies to therapeutic delivery of siRNA is the growing use of complex and costly modified siRNA chemistries. Emerging clinical studies have departed from use of unmodified siRNA due to recognized immunogenic effects, and recent industry-sponsored strategies employ heavily modified siRNA molecules in conjunction with the company’s patent-protected delivery strategy195, 197. Due to the variety of modified siRNA used and because widespread use of the highly modified siRNA is often not financially feasible in early-stage or academic research applications, variation in silencing efficacy due to potency of the siRNA molecules must be carefully assessed when evaluating and comparing the published results of different delivery technologies.
Despite these challenges, the ability to localize the siRNA therapeutic to a site of interest, minimizing physiological clearance and off-site effects, endows local delivery methods with an enormous advantage over systemic delivery techniques. Recent advancements in wound healing applications have been particularly promising. As research and understanding of the variety of delivery systems and genetic targets continues to expand, functional improvements following sustained, localized siRNA silencing will increase rapidly. With properly designed material delivery platforms and new molecular targets identified, powerful RNAi-based local therapies will soon be clinically realized.
Figure 2.
Scanning electron microscopy images of commonly employed constructs for local siRNA delivery39, 50, 89–91.
References
- 1.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao Y, Wang C, Varshney RR, Wang DA. Antisense makes sense in engineered regenerative medicine. Pharmaceut Res. 2009;26:263–275. doi: 10.1007/s11095-008-9772-3. [DOI] [PubMed] [Google Scholar]
- 3.Sliva K, Schnierle BS. Selective gene silencing by viral delivery of short hairpin RNA. Virology. 2010;7:248. doi: 10.1186/1743-422X-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett DW, Davis ME. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006;34:322–333. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: SiRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- 6.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nature Rev Gen. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 7.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. New Engl J Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 8.Haussecker D. Current issues of RNAi therapeutics delivery and development. J Control Release. 2014;195:49–54. doi: 10.1016/j.jconrel.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Zuckerman JE, Hsueh T, Koya RC, Davis ME, Ribas A. SiRNA knockdown of ribonucleotide reductase inhibits melanoma cell line proliferation alone or synergistically with temozolomide. J Invest Dermat. 2011;131:453–460. doi: 10.1038/jid.2010.310. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda M, Gan L, Chen B, Kadirvel S, Yu C, Phillips JD, New MI, Liebow A, Fitzgerald K, Querbes W, Desnick RJ. RNAi-mediated silencing of hepatic ALAS1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. P Natl Acad Sci USA. 2014;111:7777–7782. doi: 10.1073/pnas.1406228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alnylam RNAi roundtable: Conjugate delivery. 2012
- 12.Haussecker D. The business of RNAi therapeutics in 2012. Mol Ther Nucleic Acids. 2012;1:e8. doi: 10.1038/mtna.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue HY, Liu S, Wong HL. Nanotoxicity: A key obstacle to clinical translation of siRNA-based nanomedicine. Nanomed. 2014;9:295–312. doi: 10.2217/nnm.13.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue T, Sugimoto M, Sakurai T, Saito R, Futaki N, Hashimoto Y, Honma Y, Arai I, Nakaike S. Modulation of scratching behavior by silencing an endogenous cyclooxygenase-1 gene in the skin through the administration of siRNA. J Gene Med. 2007;9:994–1001. doi: 10.1002/jgm.1091. [DOI] [PubMed] [Google Scholar]
- 16.Napoli C, Lemieux C, Jorgensen R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes ín trans. Plant Cell. 1990;2 doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertrand J, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C. Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochem Biophys Res Commun. 2002;296 doi: 10.1016/s0006-291x(02)02013-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. 2005;23:222–226. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]
- 19.Baker BF. 2′-O-(2-methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J Biol Chem. 1997;272:11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- 20.Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in drosophila cells. Nature. 2000;404 doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409 doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 22.Haley B, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nat Struct Biol. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- 23.Beavers KR, Nelson CE, Duvall CL. MiRNA inhibition in tissue engineering and regenerative medicine. Adv Drug Deliver Rev. 2014 doi: 10.1016/j.addr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: Advances in siRNA delivery. Nature Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18:305–319. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- 26.Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2004;110 doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 27.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, Stoter M, Epstein-Barash H, Zhang L, Koteliansky V, Fitzgerald K, Fava E, Bickle M, Kalaidzidis Y, Akinc A, Maier M, Zerial M. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nature Biotech. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 28.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411 doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 29.Kariko K, Bhuyan P, Capodici J, Weissman D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 30.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303 doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 31.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nature Rev Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 32.Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R, Chmielowski B, Ribas A, Davis ME, Yen Y. Correlating animal and human phase IA/IB clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. P Natl Acad Sci USA. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi R, Doehle BP, Qin Y, Macara IG, Cullen BR. Overexpression of exportin 5 enhances RNA interference mediated by short hairpin RNAs and microRNAs. RNA. 2005;11:220–226. doi: 10.1261/rna.7233305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003 doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 35.Vicentini FT, Borgheti-Cardoso LN, Depieri LV, de Macedo Mano D, Abelha TF, Petrilli R, Bentley MV. Delivery systems and local administration routes for therapeutic siRNA. Pharmaceutic Res. 2013;30:915–931. doi: 10.1007/s11095-013-0971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadio A, Gustafsson JK, Pereira B, Gomes CP, Hansson GC, David L, Pego AP, Almeida R. Modified-chitosan/siRNA nanoparticles downregulate cellular cdx2 expression and cross the gastric mucus barrier. PloS One. 2014;9 doi: 10.1371/journal.pone.0099449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiser JR, Saltzman WM. Controlled release for local delivery of drugs: Barriers and models. J Control Release. 2014;190:664–673. doi: 10.1016/j.jconrel.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheeler LA, Vrbanac V, Trifonova R, Brehm MA, Gilboa-Geffen A, Tanno S, Greiner DL, Luster AD, Tager AM, Lieberman J. Durable knockdown and protection from HIV transmission in humanized mice treated with gel-formulated CD4 aptamer-siRNA chimeras. Mol Ther. 2013;21:1378–1389. doi: 10.1038/mt.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson CE, Kim AJ, Adolph EJ, Gupta MK, Yu F, Hocking KM, Davidson JM, Guelcher SA, Duvall CL. Tunable delivery of siRNA from a biodegradable scaffold to promote angiogenesis in vivo. Adv Mat. 2014;26:607–614. 506. doi: 10.1002/adma.201303520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Layzer JM. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–771. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimitrova M, Affolter C, Meyer F, Nguyen I, Richard DG, Schuster C, Bartenschlager R, Voegel JC, Ogier J, Baumert TF. Sustained delivery of siRNAs targeting viral infection by cell-degradable multilayered polyelectrolyte films. P Natl Acad Sci USA. 2008;105:16320–16325. doi: 10.1073/pnas.0800156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laporte LD, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliver Rev. 2007;59 doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M, Gao S, Dong M, Song J, Yang C, Howard KA, Kjems J, Besenbacher F. Chitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for siRNA delivery. ACS Nano. 2012;6 doi: 10.1021/nn300106t. [DOI] [PubMed] [Google Scholar]
- 44.Klose D, Siepmann F, Elkharraz K, Krenzlin S, Siepmann J. How porosity and size affect the drug release mechanisms from PLGA-based microparticles. Int J Pharm. 2006;314:198–206. doi: 10.1016/j.ijpharm.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Tai HHD, Takae S, Wang W, Vermonden T, Hennink EW, Stayton PS, Hoffman AS, Endruweit A, Alexander C, Howdle SM, Shakesheff KM. Photo-cross-linked hydrogels from thermoresponsive PEGMEMA-PPGMA-EGDMA copolymers containing multiple methacrylate groups: Mechanical property, swelling, protein release, cytotoxicity. Biomacromolecules. 2009;10:2895–2903. doi: 10.1021/bm900712j. [DOI] [PubMed] [Google Scholar]
- 46.Mann BKGA, Tsai AT, Schmelen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: Synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22 doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 47.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32 [Google Scholar]
- 48.Ma PX. Biomimetic m aterials for tissue engineering. Adv Drug Deliver Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nature Biotech. 1999;17 doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 50.De Rosa G, Maiuri MC, Ungaro F, De Stefano D, Quaglia F, La Rotonda MI, Carnuccio R. Enhanced intracellular uptake and inhibition of NF-κβ activation by decoy oligonucleotide released from PLGA microspheres. J Gene Med. 2005;7 doi: 10.1002/jgm.724. [DOI] [PubMed] [Google Scholar]
- 51.Estey T, Kang J, Schwendeman SP, Carpenter JF. BSA degradation under acidic conditions: A model for protein instability during release from PLGA delivery systems. J Pharm Sci. 2006;95:1626–1639. doi: 10.1002/jps.20625. [DOI] [PubMed] [Google Scholar]
- 52.Felix Lanao RP, Leeuwenburgh SC, Wolke JG, Jansen JA. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta biomater. 2011;7:3459–3468. doi: 10.1016/j.actbio.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 53.Martin JR, Gupta MK, Page JM, Yu F, Davidson JM, Guelcher SA, Duvall CL. A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials. 2014;35:3766–3776. doi: 10.1016/j.biomaterials.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nature Mater. 2010;9 doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segura T, Shea LD. Surface-tethered DNA complexes for enhanced gene delivery. Bioconjugate Chem. 2002;13 doi: 10.1021/bc015575f. [DOI] [PubMed] [Google Scholar]
- 56.Amarzguioui M, Holen T, Babaie E, Prydz H. Tolerance for mutations and chemical modications in a siRNA. Nucleic Acids Res. 2003;31 doi: 10.1093/nar/gkg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR. RNA interference in mammalian cells by chemically-modified RNA. Biochemistry. 2003;42 doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- 58.Judge A, MacLachlan I. Overcoming the innate immune response to small interfering RNA. Human Gene Ther. 2008;19:111–124. doi: 10.1089/hum.2007.179. [DOI] [PubMed] [Google Scholar]
- 59.Chiu YL. SiRNA function in RNAi: A chemical modification analysis. RNA. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong JH, Mok H, Oh Y, Park TG. SiRNA conjugate delivery systems. Bioconjugate Chem. 2009;20:5–14. doi: 10.1021/bc800278e. [DOI] [PubMed] [Google Scholar]
- 62.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12 doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 63.Kubo T, Takei Y, Mihara K, Yanagihara K, Seyama T. Amino-modified and lipid-conjugated dicer-substrate siRNA enhances RNAi efficacy. Bioconjugate Chem. 2012;23:164–173. doi: 10.1021/bc200333w. [DOI] [PubMed] [Google Scholar]
- 64.Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H, Yokota T. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol Ther. 2008;16:734–740. doi: 10.1038/mt.2008.14. [DOI] [PubMed] [Google Scholar]
- 65.Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009;11:639–652. doi: 10.1208/s12248-009-9140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajeev KG, Nair JK, Jayaraman M, Charisse K, Taneja N, O’Shea J, Willoughby JL, Yucius K, Nguyen T, Shulga-Morskaya S, Milstein S, Liebow A, Querbes W, Borodovsky A, Fitzgerald K, Maier MA, Manoharan M. Hepatocyte-specific delivery of siRNAs conjugated to novel non-nucleosidic trivalent N-acetylgalactosamine elicits robust gene silencing in vivo. Chembiochem. 2015;16:903–908. doi: 10.1002/cbic.201500023. [DOI] [PubMed] [Google Scholar]
- 67.Matsuda S, Keiser K, Nair JK, Charisse K, Manoharan RM, Kretschmer P, Peng CG, VKi A, Kandasamy P, Willoughby JL, Liebow A, Querbes W, Yucius K, Nguyen T, Milstein S, Maier MA, Rajeev KG, Manoharan M. SiRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem Biol. 2015;10:1181–1187. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 68.Alam MR, Ming X, Fisher M, Lackey JG, Rajeev KG, Manoharan M, Juliano RL. Multivalent cyclic RGD conjugates for targeted delivery of small interfering RNA. Bioconjugate Chem. 2011;22:1673–1681. doi: 10.1021/bc200235q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: Intracellular pathways and pharmaceutical perspectives. Pharmaceut Res. 2007;24:1977–1992. doi: 10.1007/s11095-007-9303-7. [DOI] [PubMed] [Google Scholar]
- 70.De Coupade C, Fittipaldi A, Chagnas V, Michel M, Carlier S, Tasciotti E, Darmon A, Ravel D, Kearsey J, Giacca M, Cailler F. Novel human-derived cell-penetrating peptides for specific subcellular delivery of therapeutic biomolecules. Biochem J. 2005;390:407–418. doi: 10.1042/BJ20050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holm T, Raagel H, Andaloussi SE, Hein M, Mae M, Pooga M, Langel U. Retro-inversion of certain cell-penetrating peptides causes severe cellular toxicity. Biochim Biophys Acta. 2011;1808:1544–1551. doi: 10.1016/j.bbamem.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 72.Lee HL-JA, Chen Y, Love KT, Park AI, Karagiannis ED, Sehgal A, Querbes W, Zurenko CS, Jayaraman M, Peng CG, Charisse K, Borodovsky A, Manoharan M, Donahoe JS, Truelove J, Nahrendorf M, Langer R, Anderson DG. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotech. 2012;7 doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen C, Buck AK, Liu X, Winkler M, Reske SN. Gene silencing by adenovirus-delivered siRNA. FEBS Letters. 2003;539:111–114. doi: 10.1016/s0014-5793(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 74.de Fougerolles A, Vornlocher H, Maraganore J, Lieberman J. Interfering with disease: A progress report on siRNA therapeutics. Nat Rev Drug Discov. 2007;6 doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 76.Pichon C, Goncalves C, Midoux P. Histidine-rich peptides and polymers for nucleic acids delivery. Adv Drug Deliv Rev. 2001;53 doi: 10.1016/s0169-409x(01)00221-6. [DOI] [PubMed] [Google Scholar]
- 77.Vandenbroucke RE, De Geest BG, Bonne S, Vinken M, Van Haecke T, Heimberg H, Wagner E, Rogiers V, De Smedt SC, Demeester J, Sanders NN. Prolonged gene silencing in hepatoma cells and primary hepatocytes after small interfering RNA delivery with biodegradable poly(beta-amino esters) J Gene Med. 2008;10:783–794. doi: 10.1002/jgm.1202. [DOI] [PubMed] [Google Scholar]
- 78.Zauner W, Ogris M, Wagner E. Polylysine-based transfection systems utilizing receptor-mediated delivery. Advanced drug delivery reviews. 1998;30 doi: 10.1016/s0169-409x(97)00110-5. [DOI] [PubMed] [Google Scholar]
- 79.Noh SM, Han SE, Shim G, Lee KE, Kim CW, Han SS, Choi Y, Kim YK, Kim WK, Oh YK. Tocopheryl oligochitosan-based self assembling oligomersomes for siRNA delivery. Biomaterials. 2011;32:849–857. doi: 10.1016/j.biomaterials.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 80.Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K, Teratani T, Namatame N, Yamamoto Y, Hanai K, Kato T, Sano A, Ochiya T. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. P Natl Acad Sci USA. 2005;102:12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Convertine AJ, Benoit DS, Duvall CL, Hoffman AS, Stayton PS. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J Control Release. 2009;133:221–229. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H, Yu SS, Miteva M, Nelson CE, Werfel T, Giorgio TD, Duvall CL. Matrix metalloproteinase responsive, proximity-activated polymeric nanoparticles for siRNA delivery. Adv Funct Mater. 2013;23:3040–3052. doi: 10.1002/adfm.201202215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson CE, Kintzing RJ, Hanna A, Shannon JM, Gupta MK, Duvall CL. Balancing cationic and hydrophobic content of pegylated siRNA polyplexes enhances endosome escape, stability, blood circulation time, and bioactivity in vivo. ACS Nano. 2013;7:8870–8880. doi: 10.1021/nn403325f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H, Miteva M, Kirkbride KC, Cheng MJ, Nelson CE, Simpson EM, Gupta MK, Duvall CL, Giorgio TD. Dual mmp7-proximity-activated and folate receptor-targeted nanoparticles for siRNA delivery. Biomacromolecules. 2015;16:192–201. doi: 10.1021/bm501394m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miteva M, Kirkbride KC, Kilchrist KV, Werfel TA, Li H, Nelson CE, Gupta MK, Giorgio TD, Duvall CL. Tuning pegylation of mixed micelles to overcome intracellular and systemic siRNA delivery barriers. Biomaterials. 2015;38:97–107. doi: 10.1016/j.biomaterials.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–116. doi: 10.1016/j.addr.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Green MD, Foster AA, Greco CT, Roy R, Lehr RM, Epps TH, III, Sullivan MO. Catch and release: Photocleavable cationic diblock copolymers as a potential platform for nucleic acid delivery. Polym Chem. 2014;5 doi: 10.1039/C4PY00638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kozielski KL, Tzeng SY, Hurtado De Mendoza BA, Green JJ. Bioreducible cationic polymer-based nanoparticles for efficient and environmentally triggered cytoplasmic siRNA delivery to primary human brain cancer cells. ACS Nano. 2015;8 doi: 10.1021/nn500704t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andersen MØ, Le DQS, Chen M, Nygaard JV, Kassem M, Bünger C, Kjems J. Spatially controlled delivery of siRNAs to stem cells in implants generated by multi-component additive manufacturing. Adv Funct Mater. 2013;23:5599–5607. [Google Scholar]
- 90.Cao H, Jiang X, Chai C, Chew SY. RNA interference by nanofiber-based siRNA delivery system. J Control Release. 2010;144:203–212. doi: 10.1016/j.jconrel.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Lee SWKB, Chen S, Shao-Horn Y, Hammond PT. Layer-by-layer assembly of all carbon nanotube ultrathin films for electrochemical applications. J Am Chem Soc. 2008;131:671–679. doi: 10.1021/ja807059k. [DOI] [PubMed] [Google Scholar]
- 92.Zhu X, Lu L, Currier BL, Windebank AJ, Yaszemski MJ. Controlled release of NFκβ decoy oligonucleotides from biodegradable polymer microparticles. Biomaterials. 2002;23 doi: 10.1016/s0142-9612(01)00409-4. [DOI] [PubMed] [Google Scholar]
- 93.Mountziaris PM, Sing DC, Chew SA, Tzouanas SN, Lehman ED, Kasper FK, Mikos AG. Controlled release of anti-inflammatory siRNA from biodegradable polymeric microparticles intended for intra-articular delivery to the temporomandibular joint. Pharmaceut Res. 2011;28:1370–1384. doi: 10.1007/s11095-010-0354-9. [DOI] [PubMed] [Google Scholar]
- 94.Lewis SJ, Irwin WJ, Akhtar S. Development of a sustained-release biodegradable polymer delivery system for site-specific delivery of oligonucleotides: Characterization of p(LA-GA) copolymer microspheres in vitro. J Drug Target. 1998;5 doi: 10.3109/10611869808995882. [DOI] [PubMed] [Google Scholar]
- 95.Akhtar S, Lewis KJ. Antisense oligonucleotide delivery to cultured macrophages is improved by incorporation into sustained-release biodegradablepolymer microspheres. Int J Pharm. 1997;151 [Google Scholar]
- 96.Pradhan P, Qin H, Leleux JA, Gwak D, Sakamaki I, Kwak LW, Roy K. The effect of combined IL10 siRNA and CPG ODN as pathogen-mimicking microparticles on TH1/TH2 cytokine balance in dendritic cells and protective immunity against B cell lymphoma. Biomaterials. 2014;35:5491–5504. doi: 10.1016/j.biomaterials.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh A, Suri S, Roy K. In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA-DNA carrying microparticles to dendritic cells. Biomaterials. 2009;30:5187–5200. doi: 10.1016/j.biomaterials.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mountziaris PM, Tzouanas SN, Sing DC, Kramer PR, Kasper FK, Mikos AG. Intra-articular controlled release of anti-inflammatory siRNA with biodegradable polymer microparticles ameliorates temporomandibular joint inflammation. Acta Biomater. 2012;8:3552–3560. doi: 10.1016/j.actbio.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8 doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 100.Vinas-Castells R, Holladay C, di Luca A, Diaz VM, Pandit A. Snail1 down-regulation using small interfering RNA complexes delivered through collagen scaffolds. Bioconj Chem. 2009;20 doi: 10.1021/bc900241w. [DOI] [PubMed] [Google Scholar]
- 101.Salvay DM, Zelivyanskaya M, Shea LD. Gene delivery by surface immobilization of plasmid to tissue-engineering scaffolds. Gene Ther. 2010;17:1134–1141. doi: 10.1038/gt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guelcher SA. Biodegradable polyurethanes: Synthesis and applications in regenerative medicine. Tiss Eng Pt B-Rev. 2008;14:3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 103.Hafeman AE, Li B, Yoshii T, Zienkiewicz K, Davidson JM, Guelcher SA. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. Pharmaceut Res. 2008;25:2387–2399. doi: 10.1007/s11095-008-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li B, Brown KV, Wenke JC, Guelcher SA. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Control Release. 2010;145:221–230. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 105.Lei Y, Rahim M, Ng Q, Segura T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/pei polyplexes for local gene delivery. J Control Release. 2011;153:255–261. doi: 10.1016/j.jconrel.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andersen MO, Howard KA, Paludan SR, Besenbacher F, Kjems J. Delivery of siRNA from lyophilized polymeric surfaces. Biomaterials. 2008;29:506–512. doi: 10.1016/j.biomaterials.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 107.Lei Y, Huang S, Sharif-Kashani P, Chen Y, Kavehpour P, Segura T. Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds. Biomaterials. 2010;31:9106–9116. doi: 10.1016/j.biomaterials.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nelson CE, Gupta MK, Adolph EJ, Shannon JM, Guelcher SA, Duvall CL. Sustained local delivery of siRNA from an injectable scaffold. Biomaterials. 2012;33:1154–1161. doi: 10.1016/j.biomaterials.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rujitanaroj PO, Wang YC, Wang J, Chew SY. Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials. 2011;32:5915–5923. doi: 10.1016/j.biomaterials.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 110.Krebs MD, Jeon O, Alsberg E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J Am Chem Soc. 2009;131 doi: 10.1021/ja9037615. [DOI] [PubMed] [Google Scholar]
- 111.Nguyen K, Dang PN, Alsberg E. Functionalized, biodegradable hydrogels for control over sustained and localized siRNA delivery to incorporated and surrounding cells. Acta Biomater. 2013;9:4487–4495. doi: 10.1016/j.actbio.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andersen MØ, Le DQS, Chen M, Nygaard JV, Kassem M, Bünger C, Kjems J. Spatially controlled delivery of siRNAs to stem cells in implants generated by multi-component additive manufacturing. Adv Funct Mater. 2013;23:5599–5607. [Google Scholar]
- 113.Galperin A, Long TJ, Garty S, Ratner BD. Synthesis and fabrication of a degradable poly(n-isopropyl acrylamide) scaffold for tissue engineering applications. J Biomed Mater Res A. 2013;101:775–786. doi: 10.1002/jbm.a.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gupta MK, Martin JR, Werfel TA, Shen T, Page JM, Duvall CL. Cell protective, ABC triblock polymer-based thermoresponsive hydrogels with ROS-triggered degradation and drug release. J Am Chem Soc. 2014;136:14896–14902. doi: 10.1021/ja507626y. [DOI] [PubMed] [Google Scholar]
- 115.Joshi RV, Nelson CE, Poole KM, Skala MC, Duvall CL. Dual pH- and temperature-responsive microparticles for protein delivery to ischemic tissues. Acta Biomater. 2013;9:6526–6534. doi: 10.1016/j.actbio.2013.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma Z, Nelson DM, Hong Y, Wagner WR. Thermally responsive injectable hydrogel incorporating methacrylate-polylactide for hydrolytic lability. Biomacromolecules. 2010;11:1873–1881. doi: 10.1021/bm1004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Strong LE, Dahotre SN, West JL. Hydrogel-nanoparticle composites for optically modulated cancer therapeutic delivery. J Control Release. 2014;178:63–68. doi: 10.1016/j.jconrel.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang HY, van Ee RJ, Timmer K, Craenmehr EG, Huang JH, Oner FC, Dhert WJ, Kragten AH, Willems N, Grinwis GC, Tryfonidou MA, Papen-Botterhuis NE, Creemers LB. A novel injectable thermoresponsive and cytocompatible gel of poly(n-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3d culture. Acta Biomater. 2015 doi: 10.1016/j.actbio.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 119.Ishii S, Kaneko J, Nagasaki Y. Dual stimuli-responsive redox-active injectable gel by polyion complex based flower micelles for biomedical applications. Macromolecules. 2015;48:3088–3094. [Google Scholar]
- 120.Borgheti-Cardoso LN, Depieri LV, Kooijmans SA, Diniz H, Calzzani RA, Vicentini FT, van der Meel R, Fantini MC, Iyomasa MM, Schiffelers RM, Bentley MV. An in situ gelling liquid crystalline system based on monoglycerides and polyethylenimine for local delivery of siRNAs. Eur J Pharm Sci. 2015;74:103–117. doi: 10.1016/j.ejps.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 121.Kim YM, Park MR, Song SC. An injectable cell penetrable nano-polyplex hydrogel for localized siRNA delivery. Biomaterials. 2013;34:4493–4500. doi: 10.1016/j.biomaterials.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 122.Li K, Feng L, Shen J, Zhang Q, Liu Z, Lee ST, Liu J. Patterned substrates of nano-graphene oxide mediating highly localized and efficient gene delivery. ACS Appl Mater Inter. 2014;6:5900–5907. doi: 10.1021/am5008134. [DOI] [PubMed] [Google Scholar]
- 123.Min J, Braatz RD, Hammond PT. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials. 2014;35:2507–2517. doi: 10.1016/j.biomaterials.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim BS, Smith RC, Poon Z, Hammond PT. MAD (multiagent delivery) nanolayer: Delivering multiple therapeutics from hierarchically assembled surface coatings. Langmuir. 2009;25:14086–14092. doi: 10.1021/la9017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hong JSN, Drake AC, DeMuth PC, Lee JB, Chen J, Hammond PT. Graphene multilayers as gates for multi-week sequential release of proteins from surfaces. ACS Nano. 2012;6 doi: 10.1021/nn202607r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Castleberry S, Wang M, Hammond PT. Nanolayered siRNA dressing for sustained localized knockdown. ACS Nano. 2013;7 doi: 10.1021/nn401011n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thanik VD, Greives MR, Lerman OZ, Seiser N, Dec W, Chang CC, Warren SM, Levine JP, Saadeh PB. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14 doi: 10.1038/sj.gt.3302986. [DOI] [PubMed] [Google Scholar]
- 128.Wetterau M, George F, Weinstein A, Nguyen PD, Tutela JP, Knobel D, Cohen BO, Warren SM, Saadeh PB. Topical prolyl hydroxylase domain-2 silencing improves diabetic murine wound closure. Wound Repair Regen. 2011;19:481–486. doi: 10.1111/j.1524-475X.2011.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nguyen PD, Tutela JP, Thanik VD, Knobel D, Allen RJ, Jr, Chang CC, Levine JP, Warren SM, Saadeh PB. Improved diabetic wound healing through topical silencing of p53 is associated with augmented vasculogenic mediators. Wound Repair Regen. 2010;18:553–559. doi: 10.1111/j.1524-475X.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee JW, Tutela JP, Zoumalan RA, Thanik VD, Nguyen PD, Varjabedian L, Warren SM, Saadeh PB. Inhibition of SMAD3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch Otolaryngol Head Neck Surg. 2010;136 doi: 10.1001/archoto.2010.107. [DOI] [PubMed] [Google Scholar]
- 131.Wu SY, Chang HI, Burgess M, McMillan NA. Vaginal delivery of siRNA using a novel PEGylated lipoplex-entrapped alginate scaffold system. J Control Release. 2011;155:418–426. doi: 10.1016/j.jconrel.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 132.Ollitrault D, Legendre F, Drougard C, Briand M, Benateau H, Goux D, Chajra H, Poulain L, Hartmann D, Vivien D, Shridhar V, Baldi A, Mallein-Gerin F, Boumediene K, Demoor M, Galera P. BMP-2, hypoxia, and COL1a1/HTRA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes. Tiss Eng Pt C-Meth. 2014 doi: 10.1089/ten.tec.2013.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han HD, Mora EM, Roh JW, Nishimura M, Lee SJ, Stone RL, Bar-Eli M, Lopez-Berestein G, Sood AK. Chitosan hydrogel for localized gene silencing. Cancer Biol Ther. 2011;11:839–845. doi: 10.4161/cbt.11.9.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma Z, Yang C, Song W, Wang Q, Kjems J, Gao S. Chitosan hydrogel as siRNA vector for prolonged gene silencing. J Nanobiotechnology. 2014;12 doi: 10.1186/1477-3155-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hartmann H, Hossfeld S, Schlosshauer B, Mittnacht U, Pego AP, Dauner M, Doser M, Stoll D, Krastev R. Hyaluronic acid/chitosan multilayer coatings on neuronal implants for localized delivery of siRNA nanoplexes. J Control Release. 2013;168:289–297. doi: 10.1016/j.jconrel.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 136.Hossfeld S, Nolte A, Hartmann H, Recke M, Schaller M, Walker T, Kjems J, Schlosshauer B, Stoll D, Wendel HP, Krastev R. Bioactive coronary stent coating based on layer-by-layer technology for siRNA release. Acta Biomater. 2013;9:6741–6752. doi: 10.1016/j.actbio.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 137.Vandegrift MT, Szpalski C, Knobel D, Weinstein A, Ham M, Ezeamuzie O, Warren SM, Saadeh PB. Acellular dermal matrix-based gene therapy augments graft incorporation. J Surg Res. 2015;195:360–367. doi: 10.1016/j.jss.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Segovia N, Pont M, Oliva N, Ramos V, Borros S, Artzi N. Hydrogel doped with nanoparticles for local sustained release of siRNA in breast cancer. Adv Healthc Mater. 2015;4:271–280. doi: 10.1002/adhm.201400235. [DOI] [PubMed] [Google Scholar]
- 139.Manaka T, Suzuki A, Takayama K, Imai Y, Nakamura H, Takaoka K. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials. 2011;32:9642–9648. doi: 10.1016/j.biomaterials.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 140.Rujitanaroj PO, Jao B, Yang J, Wang F, Anderson JM, Wang J, Chew SY. Controlling fibrous capsule formation through long-term down-regulation of collagen type I (COL1a1) expression by nanofiber-mediated siRNA gene silencing. Acta Biomater. 2013;9:4513–4524. doi: 10.1016/j.actbio.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Low WC, Rujitanaroj PO, Lee DK, Messersmith PB, Stanton LW, Goh E, Chew SY. Nanofibrous scaffold-mediated REST knockdown to enhance neuronal differentiation of stem cells. Biomaterials. 2013;34:3581–3590. doi: 10.1016/j.biomaterials.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 142.Andersen MO, Nygaard JV, Burns JS, Raarup MK, Nyengaard JR, Bunger C, Besenbacher F, Howard KA, Kassem M, Kjems J. SiRNA nanoparticle functionalization of nanostructured scaffolds enables controlled multilineage differentiation of stem cells. Mol Ther. 2010;18:2018–2027. doi: 10.1038/mt.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zoldan J, Lytton-Jean AK, Karagiannis ED, Deiorio-Haggar K, Bellan LM, Langer R, Anderson DG. Directing human embryonic stem cell differentiation by non-viral delivery of siRNA in 3D culture. Biomaterials. 2011;32:7793–7800. doi: 10.1016/j.biomaterials.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takayama K, Suzuki A, Manaka T, Taguchi S, Hashimoto Y, Imai Y, Wakitani S, Takaoka K. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J Bone Miner Metab. 2009;27:402–411. doi: 10.1007/s00774-009-0054-x. [DOI] [PubMed] [Google Scholar]
- 145.Gazzerro E, Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Economides AN, Canalis E. Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J Biol Chem. 2007;282:31549–31557. doi: 10.1074/jbc.M701317200. [DOI] [PubMed] [Google Scholar]
- 146.Zhang X, Kovtun A, Mendoza-Palomares C, Oulad-Abdelghani M, Fioretti F, Rinckenbach S, Mainard D, Epple M, Benkirane-Jessel N. SiRNA-loaded multi-shell nanoparticles incorporated into a multilayered film as a reservoir for gene silencing. Biomaterials. 2010;31:6013–6018. doi: 10.1016/j.biomaterials.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 147.Kinouchi N, Ohsawa Y, Ishimaru N, Ohuchi H, Sunada Y, Hayashi Y, Tanimoto Y, Moriyama K, Noji S. Atelocollagen-mediated local and systemic applications of myostatin-targeting siRNA increase skeletal muscle mass. Gene Ther. 2008;15:1126–1130. doi: 10.1038/gt.2008.24. [DOI] [PubMed] [Google Scholar]
- 148.Kawakami E, Kawai N, Kinouchi N, Mori H, Ohsawa Y, Ishimaru N, Sunada Y, Noji S, Tanaka E. Local applications of myostatin-siRNA with atelocollagen increase skeletal muscle mass and recovery of muscle function. PloS one. 2013;8:e64719. doi: 10.1371/journal.pone.0064719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lu P, Zhang GR, Song XH, Zou XH, Wang LL, Ouyang HW. Col v siRNA engineered tenocytes for tendon tissue engineering. PloS One. 2011;6 doi: 10.1371/journal.pone.0021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen LX, Lin L, Wang HJ, Wei XL, Fu X, Zhang JY, Yu CL. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated nf-kappabp65-specific siRNA. Osteoarthr Cartilage. 2008;16:174–184. doi: 10.1016/j.joca.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 151.Dong L, Huang Z, Cai X, Xiang J, Zhu YA, Wang R, Chen J, Zhang J. Localized delivery of antisense oligonucleotides by cationic hydrogel suppresses TNF-alpha expression and endotoxin-induced osteolysis. Pharmaceut Res. 2011;28:1349–1356. doi: 10.1007/s11095-010-0334-0. [DOI] [PubMed] [Google Scholar]
- 152.Zheng LCH, Chen S, Tang T, Fu W, Huang L, Ho D, Chow K, Wang Y, Griffith JF, He W, Zhou H, Zhao D, Zhang G, Wang X, Qin L. Blockage of Src by specific siRNA as a novel therapeutic strategy to prevent destructive repair in steroid-associated osteonecrosis in rabbits. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2542. [DOI] [PubMed] [Google Scholar]
- 153.Chu X, You H, Yuan X, Zhao W, Li W, Guo X. Protective effect of lentivirus-mediated siRNA targeting ADAMTS-5 on cartilage degradation in a rat model of osteoarthritis. Int J Molec Med. 2013;31:1222–1228. doi: 10.3892/ijmm.2013.1318. [DOI] [PubMed] [Google Scholar]
- 154.Pi Y, Zhang X, Shao Z, Zhao F, Hu X, Ao Y. Intra-articular delivery of anti-HIF-2alpha siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 2015;22:439–448. doi: 10.1038/gt.2015.16. [DOI] [PubMed] [Google Scholar]
- 155.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tokatlian T, Cam C, Segura T. Non-viral DNA delivery from porous hyaluronic acid hydrogels in mice. Biomaterials. 2014;35:825–835. doi: 10.1016/j.biomaterials.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: Transgene expression and cellular transfection. Mol Ther. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alfranca A. VEGF therapy: A timely retreat. Cardiovasc Res. 2009;83:611–612. doi: 10.1093/cvr/cvp228. [DOI] [PubMed] [Google Scholar]
- 159.Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials. 2013;34:9201–9209. doi: 10.1016/j.biomaterials.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cheema SK, Chen E, Shea LD, Mathur AB. Regulation and guidance of cell behavior for tissue regeneration via the siRNA mechanism. Wound Repair Regen. 2007;15:286–295. doi: 10.1111/j.1524-475X.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- 161.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 162.Agis H, Watzek G, Gruber R. Prolyl hydroxylase inhibitors increase the production of vascular endothelial growth factor by periodontal fibroblasts. J Periodontal Res. 2012;47:165–173. doi: 10.1111/j.1600-0765.2011.01415.x. [DOI] [PubMed] [Google Scholar]
- 163.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of phd2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Z, Gao Z, Shi Y, Sun Y, Lin Z, Jiang H, Hou T, Wang Q, Yuan X, Zhu X, Wu H, Jin Y. Inhibition of smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J Plast Reconstr Aes. 2007;60:1193–1199. doi: 10.1016/j.bjps.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 165.Huang JS, Wang Y, Ling T, Chuang S, Johnson FE, Huang SS. Synthetic TGF-β antagonist accelerates wound healing and reduces scarring. The FASEB Journal. 2002;16 doi: 10.1096/fj.02-0103fje. [DOI] [PubMed] [Google Scholar]
- 166.Nakamura H, Siddiqui SS, Shen X, Malik AB, Pulido JS, Kumar NM, Yue BY. RNA interference targeting transforming growth factor-β type II receptor suppresses ocular inflammation and fibrosis. Mol Vis. 2004;10 [PubMed] [Google Scholar]
- 167.Sriram S, Robinson P, Pi L, Lewin AS, Schultz G. Triple combination of siRNAs targeting TGFbeta1, TGFbetaR2, and CTGF enhances reduction of collagen I and smooth muscle actin in corneal fibroblasts. Invest Ophth Vis Sci. 2013;54:8214–8223. doi: 10.1167/iovs.13-12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang C, Kong X, Liu C, Liang Z, Zhao H, Tong W, Ning G, Shen W, Yao L, Feng S. Erk2 small interfering RNAs prevent epidural fibrosis via the efficient inhibition of collagen expression and inflammation in laminectomy rats. Biochem Bioph Res Com. 2014;444:395–400. doi: 10.1016/j.bbrc.2014.01.070. [DOI] [PubMed] [Google Scholar]
- 169.Takahashi H, Wang Y, Grainger DW. Device-based local delivery of siRNA against mammalian target of rapamycin (mTOR) in a murine subcutaneous implant model to inhibit fibrous encapsulation. J Control Release. 2010;147:400–407. doi: 10.1016/j.jconrel.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jakobsen M, Stenderup K, Rosada C, Moldt B, Kamp S, Dam TN, Jensen TG, Mikkelsen JG. Amelioration of psoriasis by anti-TNF-alpha RNAi in the xenograft transplantation model. Mol Ther. 2009;17:1743–1753. doi: 10.1038/mt.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang Y, Cristofaro P, Silbermann R, Pusch O, Boden D, Konkin T, Hovanesian V, Monfils PR, Resnick M, Moss SF, Ramratnam B. Engineering mucosal RNA interference in vivo. Mol Ther. 2006;14:336–342. doi: 10.1016/j.ymthe.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 172.Huang Z, King MR. An immobilized nanoparticle-based platform for efficient gene knockdown of targeted cells in the circulation. Gene Ther. 2009;16:1271–1282. doi: 10.1038/gt.2009.76. [DOI] [PubMed] [Google Scholar]
- 173.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W, Group VACS. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a department of veterans affairs cooperative study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 174.San Juan A, Bala M, Hlawaty H, Portes P, Vranckx R, Feldman LJ, Letourneur D. Development of a functionalized polymer for stent coating in the arterial delivery of small interfering RNA. Biomacromolecules. 2009;10 doi: 10.1021/bm900740g. [DOI] [PubMed] [Google Scholar]
- 175.Evans BC, Hocking KM, Osgood MJ, Voskresensky I, Dmowska J, Kilchrist KV, Brophy CM, Duvall CL. MK2 inhibitory peptide delivered in nanopolyplexes prevents vascular graft intimal hyperplasia. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dzau VJRC, Sedding DG. Vascular proliferation and atherosclerosis: New perspectives and therapeutic strategies. Nat Med. 2002;8 doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 177.Nabzdyk CS, Chun MC, Oliver-Allen HS, Pathan SG, Phaneuf MD, You JO, Pradhan-Nabzdyk LK, LoGerfo FW. Gene silencing in human aortic smooth muscle cells induced by pei-siRNA complexes released from dip-coated electrospun poly(ethylene terephthalate) grafts. Biomaterials. 2014;35:3071–3079. doi: 10.1016/j.biomaterials.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 179.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8 doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim Y, Park M, Song S. Injectable polyplex hydrogel for localized and long-term delivery of siRNA. ACS Nano. 2012;6 doi: 10.1021/nn300842a. [DOI] [PubMed] [Google Scholar]
- 181.Khvalevsky EZ, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, Shemi A, Golan T, Domb AJ, Yavin E, Giladi H, Rivkin L, Simerzin A, Eliakim R, Khalaileh A, Hubert A, Lahav M, Kopelman Y, Goldin E, Dancour A, Hants Y, Arbel-Alon S, Abramovitch R, Shemi A, Galun E. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20723–20728. doi: 10.1073/pnas.1314307110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Eser S, Schnieke A, Schneider G, Saur D. Oncogenic kras signalling in pancreatic cancer. Br J Cancer. 2014;111:817–822. doi: 10.1038/bjc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nakamura M, Jo J, Tabata Y, Ishikawa O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (alopecia areata) in a C3H/HEJ mouse model. Am J Pathol. 2008;172:650–658. doi: 10.2353/ajpath.2008.061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ritprajak P, Hashiguchi M, Azuma M. Topical application of cream-emulsified CD86 siRNA ameliorates allergic skin disease by targeting cutaneous dendritic cells. Mol Ther. 2008;16:1323–1330. doi: 10.1038/mt.2008.91. [DOI] [PubMed] [Google Scholar]
- 185.Abraham D. Connective tissue growth factor: Growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSC? Rheumatology. 2008;47(Suppl 5):v8–9. doi: 10.1093/rheumatology/ken278. [DOI] [PubMed] [Google Scholar]
- 186.Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, Cheverud JM, Lieberman P, Heber-Katz E. Lack of p21 expression links cell cycle control and appendage regeneration in mice. P Natl Acad Sci USA. 2010;107:5845–5850. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou Y, Guan X, Yu M, Wang X, Zhu W, Wang C, Yu M, Wang H. Angiogenic/osteogenic response of bmmscs on bone-derived scaffold: Effect of hypoxia and role of PI3K/AKT-mediated VEGF-VEGFR pathway. Biotechnol J. 2014;9:944–953. doi: 10.1002/biot.201300310. [DOI] [PubMed] [Google Scholar]
- 188.Kim YM, Song SC. Targetable micelleplex hydrogel for long-term, effective, and systemic siRNA delivery. Biomaterials. 2014;35:7970–7977. doi: 10.1016/j.biomaterials.2014.05.070. [DOI] [PubMed] [Google Scholar]
- 189.Patil SP, Kim SH, Jadhav JR, Lee JH, Jeon EM, Kim KT, Kim BH. Cancer-specific gene silencing through therapeutic siRNA delivery with B vitamin-based nanoassembled low-molecular-weight hydrogelators. Bioconj Chem. 2014 doi: 10.1021/bc500249g. [DOI] [PubMed] [Google Scholar]
- 190.Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, Smith DD, Swiderski P, Rossi JJ, Akkina R. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4+ t cell decline in humanized mice. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim HA, Nam K, Kim SW. Tumor targeting RGD conjugated bio-reducible polymer for VEGF siRNA expressing plasmid delivery. Biomaterials. 2014;35:7543–7552. doi: 10.1016/j.biomaterials.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zamora MR, Budev M, Rolfe M, Gottlieb J, Humar A, Devincenzo J, Vaishnaw A, Cehelsky J, Albert G, Nochur S, Gollob JA, Glanville AR. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Resp Crit Care. 2011;183:531–538. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]
- 193.Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV, Reisen G, Lockridge JA, Short B, Guerciolini R, Nguyen QD SiRNA-027 Study I. RNAi-based treatment for neovascular age-related macular degeneration by siRNA-027. Am J Opthalmol. 2010;150:33–39. e32. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 194.Leachman SA, Hickerson RP, Schwartz ME, Bullough EE, Hutcherson SL, Boucher KM, Hansen CD, Eliason MJ, Srivatsa GS, Kornbrust DJ, Smith FJ, McLean WI, Milstone LM, Kaspar RL. First-in-human mutation-targeted siRNA phase ib trial of an inherited skin disorder. Mol Ther. 2010;18:442–446. doi: 10.1038/mt.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thompson JD. Clinical development of synthetic siRNA therapeutics. Drug Discov Today. 2013;10:e133–e138. [Google Scholar]
- 196.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 197.Snead NM, Rossi JJ. RNA interference trigger variants: Getting the most out of RNA for RNA interference-based therapeutics. Nucleic Acid Ther. 2012;22:139–146. doi: 10.1089/nat.2012.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tolentino MJ, Brucker AJ, Fosnot J, Ying GS, Wu IH, Malik G, Wan S, Reich SJ. Intravitreal injection of vascular endothelial growth factor small interfering RNA inhibits growth and leakage in a nonhuman primate, laser-induced model of choroidal neovascularization. Retina-J Ret Vit Dis. 2004;24 doi: 10.1097/00006982-200408000-00039. [DOI] [PubMed] [Google Scholar]
- 199.Dejneka NS, Wan S, Bond OS, Kornbrust DJ, Reich SJ. Ocular biodistribution of bevasiranib following a single intravitreal injection to rabbit eyes. Mol Vis. 2008;14 [PMC free article] [PubMed] [Google Scholar]
- 200.OPKO Health, Inc; 2008. Open Label Study for the Evaluation of Tolerability of Five Dose Levels of Cand5. Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00722384) [Google Scholar]
- 201.OPKO Health, Inc; 2008. Safety and Efficacy Study of Small Interfering Ribonucleic Acid (RNA) Molecule (Cand5) to Treat Wet Age-Related Macular Degeneration. Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00259753) [Google Scholar]
- 202.OPKO Health, Inc; 2008. Safety and Efficacy Study of Small Interfering RNA Molecule (Cand5) to Treat Diabetic Macular Edema. Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00306904) [Google Scholar]
- 203.OPKO Health, Inc; 2014. Safety & Efficacy Study Evaluating the Combination of Bevasiranib & Lucentis Therapy in Wet AMD (COBALT) Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00499590) [Google Scholar]
- 204.A Dose Escalation Trial of an Intravitreal Injection of Sirna-027 in Patients With Subfoveal Choroidal Neovascularization (CNV) Secondary to Age-Related Macular Degeneration (AMD) (Allergan) 2008 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00363714)
- 205.A Study Using Intravitreal Injections of a Small Interfering RNA in Patients With Age-Related Macular Degeneration (Allergan) 2015 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00395057)
- 206.Study of PF-04523655 (REDD14NP) In Subjects With Choroidal Neovascularization (CNV) Secondary to Age-Related Macular Degeneration (Wet AMD) (Quark Pharmaceuticals) 2012 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00725686)
- 207.Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, Chi-Burris K, Yan E, Paggiarino DA, Rosenblatt I, Khan A, Aitchison R, Erlich SS, Group PFS. Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye. 2012;26:1099–1105. doi: 10.1038/eye.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Study Evaluating Efficacy and Safety of PF-04523655 Versus Laser in Subjects With Diabetic Macular Edema (DEGAS) (Quark Pharmaceuticals) 2012 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00701181)
- 209.Quark Pharmaceuticals; 2012. Phase II Open Label Multicenter Study For Age Related Macular Degeneration Comparing PF-04523655 Versus Lucentis In The Treatment Of Subjects With CNV (MONET Study) Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00713518) [Google Scholar]
- 210.PF-04523655 Dose Escalation Study, and Evaluation of PF-04523655 With/Without Ranibizumab in Diabetic Macular Edema (DME) (MATISSE) (Quark Pharmaceuticals) 2015 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01445899)
- 211.Intranasal ALN-RSV01 Administered to Adult Volunteers Experimentally Inoculated With Respiratory Syncytial Virus (Alnylam Pharmaceuticals) 2007 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00496821)
- 212.DeVincenzo J, Cehelsky JE, Alvarez R, Elbashir S, Harborth J, Toudjarska I, Nechev L, Murugaiah V, Van Vliet A, Vaishnaw AK, Meyers R. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antiviral Res. 2008;77:225–231. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 213.Alnylam Pharmaceuticals; 2012. Phase 2b Study of ALN-RSV01 in Lung Transplant Patients Infected With Respiratory Syncytial Virus (RSV) Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01065935) [Google Scholar]
- 214.Alnylam Pharmaceuticals; 2009. Phase 2 Study of ALN-RSV01 in Lung Transplant Patients Infected With Respiratory Syncytial Virus (RSV) Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00658086) [Google Scholar]
- 215.Study of TD101, a Small Interfering RNA (siRNA) Designed for Treatment of Pachyonychia Congenita (Pachyonychia Congenita Project) 2008 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00716014)
- 216.Tolerance and Effect on Intraocular Pressure After Administration of SYL040012 (Sylentis, S.A.) 2010 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT00990743)
- 217.Moreno-Montanes J, Sadaba B, Ruz V, Gomez-Guiu A, Zarranz J, Gonzalez MV, Paneda C, Jimenez AI. Phase I clinical trial of SYL040012, a small interfering RNA targeting beta-adrenergic receptor 2, for lowering intraocular pressure. Mol Ther. 2014;22:226–232. doi: 10.1038/mt.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.SYL040012. Tolerance and Effect on Intraocular Pressure in Subjects With Intraocular Pressure (IOP) >= 21 mm Hg (Sylentis, S.A.) 2012 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01227291)
- 219.Dose Finding Clinical Trial With SYL040012 to Evaluate the Tolerability and Effect on Intraocular Pressure in Subjects With Ocular Hypertension or Open Angle Glaucoma (Sylentis, S.A.) 2013 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01739244)
- 220.SYL040012, Treatment for Open Angle Glaucoma (SYLTAG) (Sylentis, S.A.) 2015 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT02250612)
- 221.Ahmed Z, Kalinski H, Berry M, Almasieh M, Ashush H, Slager N, Brafman A, Spivak I, Prasad N, Mett I, Shalom E, Alpert E, Di Polo A, Feinstein E, Logan A. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011;2:e173. doi: 10.1038/cddis.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Safety Study of a Single IVT Injection of QPI-1007 in Chronic Optic Nerve Atrophy and Recent Onset NAION Patients (Quark Pharmaceuticals) 2013 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01064505)
- 223.Quark Pharmaceuticals; 2015. Phase IIA Double-Masked Randomized Sham-Controlled Trial of QPI-1007 Delivered by a Single Intravitreal Injection to Subjects With Acute Primary Angle-Closure Glaucoma (APACG) Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01965106) [Google Scholar]
- 224.Phase I - Escalating Dose Study of siG12D LODER (Local Drug EluteR) in Patients With Locally Advanced Adenocarcinoma of the Pancreas, and a Single Dose Study of siG12D LODER (Local Drug EluteR) in Patients With Non-operable Adenocarcinoma of the Pancreas (Silenseed Ltd) 2014 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01188785)
- 225.Silenseed Ltd; 2015. A Phase II Study of siG12D LODER in Combination With Chemotherapy in Patients With Unresectable Locally Advanced Pancreatic Cancer. Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01676259) [Google Scholar]
- 226.Study to Evaluate the Ocular Tolerance of SYL1001 in Healthy Volunteers (Sylentis, S.A.) 2012 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01438281)
- 227.Pilot Study to Evaluate SYL1001 Safety and Effect in Patients With Ocular Pain (Sylentis, S.A.) 2015 Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT01776658)