SUMMARY
Acinetobacter is a well-recognized nosocomial pathogen. Previous reports of community-associated Acinetobacter infections have lacked clear case definitions and assessment of healthcare-associated (HCA) risk factors. We identified Acinetobacter bacteraemia cases from blood cultures obtained <3 days after hospitalization in rural Thailand and performed medical record reviews to assess HCA risk factors in the previous year and compare clinical and microbiological characteristics between cases with and without HCA risk factors. Of 72 Acinetobacter cases, 32 (44%) had no HCA risk factors. Compared to HCA infections, non-HCA infections were more often caused by Acinetobacter species other than calcoaceticus–baumannii complex species and by antibiotic-susceptible organisms. Despite similar symptoms, the case-fatality proportion was lower in non-HCA than HCA cases (9% vs. 45%, P < 0·01). Clinicians should be aware of Acinetobacter as a potential cause of community-associated infections in Thailand; prospective studies are needed to improve understanding of associated risk factors and disease burden.
Key words: Community-acquired pneumonia, epidemiology, Gram-negative bacteria
INTRODUCTION
The genus Acinetobacter currently comprises 30 named species [1] and multiple DNA–DNA hybridization groups (genomic species) with provisional designations [2]. Several species, including members of the Acinetobacter calcoaceticus–A. baumannii (Acb) complex, which includes A. calcoaceticus and A. baumannii, among others, are well documented causes of nosocomial infections including pneumonia, meningitis and bacteraemia often with significant mortality [3]. Risk factors for nosocomial Acinetobacter infections include mechanical ventilation, central vascular catheterization, and prior antibiotic use [3, 4]. Acinetobacter species have acquired multiple drug-resistance mechanisms, making them increasingly difficult to treat and of particular public health concern [3]. In Thailand, marked increases in A. baumannii resistance to imipenem (from 1·0 to 48·4% of isolates taken from patients in non-ICU wards) and other drugs were observed from 2000 to 2005 [5].
Several reports of ‘community-acquired’ Acinetobacter infections have described cases with onset outside the healthcare setting with mortality estimates ranging from 40–64% [6–13]. However, descriptions have been limited by the absence of a standard case definition and lack of assessment of healthcare-associated (HCA) risk factors. Acinetobacter species can colonize indwelling medical devices [14], skin [15], and mucosal surfaces [13, 16], making it possible that patients could acquire Acinetobacter in the healthcare setting and then develop active infection after returning to the community, although this would remain as conjecture in the absence of strain-typing studies. Most reports of ‘community-acquired’ infection have not allowed for distinction between these HCA infections with onset in the community and community cases in patients without significant healthcare exposures. Descriptions have also been limited primarily to case reports or case series, mostly in adults, with very little data on infections in children.
The International Emerging Infections Program (IEIP), in collaboration with the Thailand Ministry of Public Health (MOPH), conducts active surveillance for bloodstream infections in two rural provinces in Thailand, Nakhon Phanom and Sa Kaeo. Early observations of unexpectedly high numbers of Acinetobacter bacteraemia cases in persons without known hospital exposures prompted further investigation. Using a rigorous case definition to minimize misclassification of HCA infections as community-associated, we describe clinical and microbiological characteristics of Acinetobacter bacteraemia cases in patients without apparent HCA exposures and compare these to cases with known HCA exposures.
METHODS
Setting
Sa Kaeo province is in eastern Thailand on the Cambodian border and has a population of 550 000. Nakhon Phanom borders Laos in northeastern Thailand and has 751 000 residents. Both provinces have primarily agricultural-based economies. The 2010 per capita GDP was US$2303 in Sa Kaeo province and US$1290 in Nakhon Phanom province, ranking 51st and 72nd of the 76 provinces in Thailand [17]. Thai citizens are entitled to universal healthcare which provides health services at a nominal or no charge.
Surveillance for pneumonia and bloodstream infections
Active surveillance for hospitalized cases of community-acquired pneumonia has been conducted in all district (n = 16) and military (n = 2) hospitals (10–140 inpatient beds) as well as both provincial hospitals (225–327 beds) in Sa Kaeo and Nakhon Phanom provinces since 2002–2003 [18, 19]. In 2005, automated blood culture systems (BacT/ALERT 3D®, bioMérieux, USA) were implemented in Sa Kaeo (May) and Nakhon Phanom (November). Blood cultures were performed at the clinicians' discretion.
Two blood culture bottles were inoculated for each patient, a standard bottle to support aerobic growth [BacT/ALERT FA® (FA) for ages ⩾5 years and BacT/ALERT PF® (PF) for ages <5 years] and a bottle to enhance growth of mycobacteria and other fastidious organisms [BacT/ALERT MB® (MB)]. If blood volume was insufficient to inoculate both bottles, only the FA/PF bottle was used and if collected at a district or military hospital, blood cultures were transported at 15–30°C to the provincial hospital for processing within 24 h. Bottles that indicated positive were subcultured by standard methods [20].
Laboratory methods
Suspected Acinetobacter isolates were identified in the hospital laboratories by phenotypic testing and confirmed at the Thailand National Institute of Health (NIH) using conventional biochemical testing [21–23]; any equivocal results by biochemical testing were confirmed by polymerase chain reaction at the NIH [24–26]. Isolates were grouped into two categories: (a) Acb complex which included A. baumannii, A. calcoaceticus, A. pittii (genospecies 3), A. nosocomialis (genospecies TU13), Acinetobacter genospecies close to TU13 and Acinetobacter genospecies between 1 and 3, and (b) non-Acb complex which included all other species. Antibiotic susceptibility testing was done using the disk diffusion method according to the Clinical and Laboratory Standards Institute's guidelines [27, 28]. Isolates resistant to cefotaxime, amikacin, and imipenem were classified as multi-drug resistant (MDR) [13].
Data collection
We abstracted data on demographics, clinical characteristics, comorbidities, recent hospitalizations, and alcohol use and smoking from medical records of patients with Acinetobacter bacteraemia using a structured form. Trained research nurses and a study physician (S.T.) independently reviewed the charts and about half of the charts were audited by the study physician. When available, chest radiographs were reviewed and interpreted by a panel of radiologists to determine whether they were consistent with pneumonia [29].
Case definitions for Acinetobacter
We identified patients with Acinetobacter isolated from blood cultures between May 2005 and December 2008. To minimize the possibility of including Acinetobacter-positive cultures that represented contamination, patients whose cultures became positive after 24 h of incubation or grew additional organisms were excluded. We then limited our analyses to patients with objective signs of active infection (temperature ⩾38·0°C or abnormal white blood cell count).
We considered patients with Acinetobacter bacteraemia who had their blood cultured ⩾3 days after admission to have hospital-onset infections and patients whose blood was cultured <3 days after admission were considered to have community-onset infections [30]. Within the group with community-onset infections, patients were further differentiated between those with and without known HCA risk factors. Owing to the lack of standard definitions for community-associated Acinetobacter infections, criteria used to define community-associated methicillin-resistant Staphylococcus aureus (MRSA) [31] were adapted to develop a list of HCA risk factors. These included hospitalization within the past year, residence in a nursing home, or any of the following within the previous 6 months: dialysis, renal disease, a surgical procedure, an indwelling urinary catheter, intermittent urinary catheterization, or a vascular access cannula.
Statistical methods
Two groups were compared: (1) patients with community-onset infections and no known HCA risk factors (henceforth referred to as non-HCA infections), and (2) patients with either hospital-onset infections or community-onset infections with known HCA risk factors (HCA infections). Using SAS v. 9.2 software (SAS Institute Inc., USA), we compared demographic, clinical, and microbiological characteristics between HCA and non-HCA cases; Pearson's χ2 test was used to assess statistical significance and an exact test was used when expected cell sizes were <5.
Ethical considerations
Medical record reviews were conducted under a protocol approved by a CDC Institutional Review Board (protocol no. 5694) and the Ethical Review Committee of the Thailand MOPH.
RESULTS
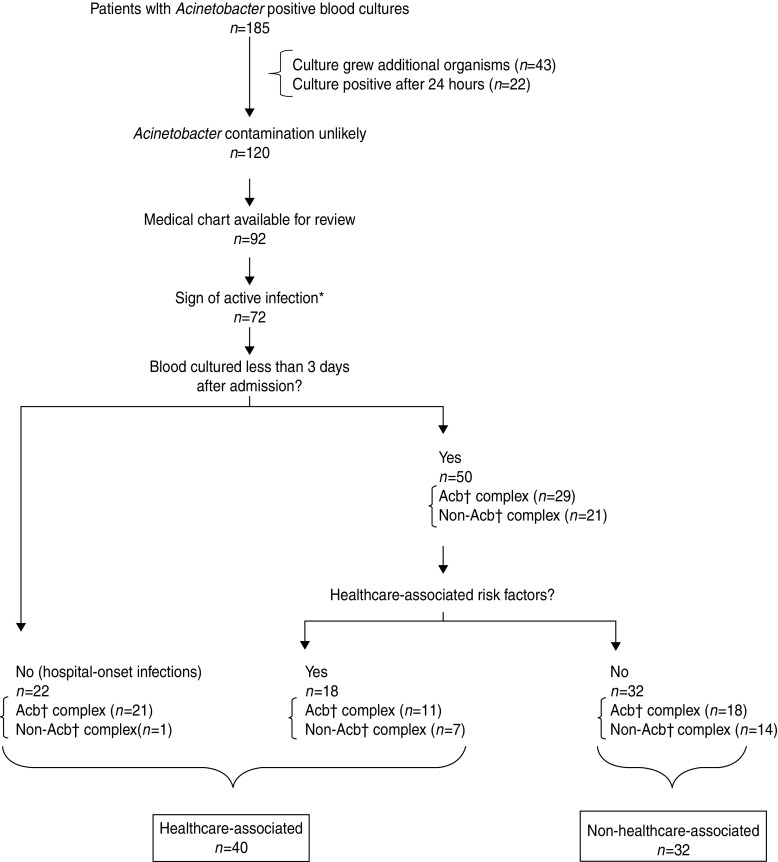
We identified 185 patients with Acinetobacter-positive blood cultures in Nakhon Phanom and Sa Kaeo provinces from May 2005 to December 2008. We excluded 65 patients because the Acinetobacter isolate was a possible contaminant (22 became positive after 24 h incubation and 43 cultures grew an additional organism), 28 patients without charts available for review, and 20 others with no objective evidence of active infection (Fig. 1). Of the remaining 72 patients with Acinetobacter bacteraemia, 52 had blood inoculated into both FA/FP and MB bottles; 22 (42%) of those were positive in both bottles. Acinetobacter was the seventh most common pathogen identified in patients with community-onset bacteraemia (i.e. blood cultured <3 days after admission, n = 50) and the second most common in those with hospital-onset infection (n = 22) (data not shown).
Fig. 1.
Defining healthcare- vs. non-healthcare-associated Acinetobacter bacteraemia cases in hospitalized patients with Acinetobacter-positive blood culture bacteraemia, Nakhon Phanom and Sa Kaeo provinces, Thailand, May 2005–December 2008. * Temperature ⩾38°C, age-specific elevated white blood cell count (WBC) [32]: <7 days old >30 000; 7 days–5 years >15 000; 6–17 years >13 500; ⩾18 years >11 000 or WBC <5000. † Acb=Acinetobacter calcoaceticus–baumannii complex.
Thirty-two (44%) of the 72 patients had non-HCA infections and 40 (56%) had HCA infections. Of the 40 patients with HCA infections, 22 (55%) had their blood cultured ⩾3 days after admission; the remaining were considered HCA because the patient had at least one HCA risk factor. The most common HCA risk factor was a hospital admission within the past year. Two newborns that developed infection within 10 days after birth were considered HCA cases but were excluded from further analysis because the implications of hospital exposure during birth were unclear.
Non-HCA cases tended to be younger than HCA cases (Table 1). Twenty-five Acinetobacter cases occurred in children aged ⩽15 years (15 had non-HCA infections and 10 had HCA infections). Thirteen (87%) of 15 children aged ⩽15 years with non-HCA infections and 8/10 (80%) with HCA infections were from Nakhon Phanom. Six (40%) of the 15 children with non-HCA infections were diagnosed with pneumonia. One child with a non-HCA infection had been hospitalized with a second-degree scald burn and the following day developed high fever and had a seizure during surgical debridement. It is unclear whether this surgery led to introduction of a community pathogen that had contaminated his skin. Demographic and clinical characteristics of the children with non-HCA infections are detailed in Table 2.
Table 1.
Characteristics of hospitalized patients with Acinetobacter bacteraemia with and without healthcare-associated (HCA) risk factors, Nakhon Phanom and Sa Kaeo provinces, Thailand, May 2005–December 2008
| Non-HCA* (n = 32) | HCA (n = 38)† | P value‡ | |
|---|---|---|---|
| Age, n (%) | |||
| <1 year | 3 (9·4) | 3 (7·9) | |
| 1–<5 years | 6 (18·8) | 6 (15·8) | |
| 5–15 years | 6 (18·8) | 1 (2·6) | |
| 16–64 years | 12 (37·5) | 12 (31·6) | |
| ⩾65 years | 5 (15·6) | 16 (42·1) | 0·04 |
| Male, n (%) | 20 (62·5) | 20 (52·6) | 0·47 |
| Species | |||
| Acb complex | 18 (56·3) | 30 (79·0) | |
| Non-Acb complex§ | 14 (43·8) | 8 (21·0) | 0·04 |
Acb, Acinetobacter calcoaceticus–baumannii.
Non-HCA indicates that the blood culture was collected <3 days after admission and the patient had no known HCA risk factors.
There were 40 patients with HCA infections; two newborns were excluded from analyses.
Pearson's χ2 test.
Table 2.
Demographic and clinical characteristics of children aged ⩽15 years hospitalized with non-healthcare-associated (non-HCA)* Acinetobacter bacteraemia, Nakhon Phanom and Sa Kaeo provinces, Thailand, May 2005–December 2008
| Patient | Age (years) | Discharge diagnosis | Acinetobacter species | Maximum temp. (°C)† | WBC‡ at admission | Antibiotic Therapy | Susceptibility of isolate to antibiotic used for treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | <1 | Burn and surgical debridement§ | Acb complex | 41·0 | 14 520 | Cloxacillin | Resistant | Survived |
| 2 | <1 | Pneumonia | Acb complex | 39·1 | 15 100 | Ampicillin | Resistant | Survived |
| 3 | <1 | Pneumonia | Acb complex | 38·9 | – | Amoxicillin | Susceptible to amoxicilin | Survived |
| Ceftriaxone | Susceptible to cefotaxime | |||||||
| 4 | 1–<5 | Pneumonia | Acb complex | 40·0 | – | Ampicillin | Resistant | Survived |
| Ceftriaxone | Resistant to cefotaxime | |||||||
| 5 | 1–<5 | Diarrhoea | Non-Acb complex | 38·0 | – | None noted | – | Survived |
| 6 | 1–<5 | Pneumonia | Non-Acb complex | 38·4 | – | Amoxicillin/clavulanate | Susceptible to amoxicillin | Survived |
| Ampicillin | Susceptible to ampicillin | |||||||
| 7 | 1–<5 | Pneumonia | Non-Acb complex | 38·8 | 20 700 | Amoxicillin | Susceptible to amoxicillin | Survived |
| Ceftriaxone | Susceptible to cefotaxime | |||||||
| 8 | 1–<5 | Pneumonitis | Acb complex | 39·9 | 11 100 | Amoxicillin | Susceptibility unknown | Survived |
| Ceftriaxone | ||||||||
| 9 | 1–<5 | Fever | Non-Acb complex | 40·0 | 27 090 | Amoxicillin | Susceptible to amoxicillin | Survived |
| Ceftazadime | Ceftazadime unknown | |||||||
| Ceftriaxone | Susceptible to cefotaxime | |||||||
| 10 | 5–15 | Acute bronchitis | Non-Acb complex | 40·3 | 2100 | Ampicillin | Resistant to ampicillin | Survived |
| Cephalexin | Susceptibility to cephalexin unknown | |||||||
| 11 | 5–15 | Septicaemia | Acb complex | 39·6 | 6100 | Ampicillin | Resistant to ampicillin | Survived |
| Ciprofloxacin | Ciprofloxacin unknown | |||||||
| Gentamicin | Susceptible to gentamicin | |||||||
| 12 | 5–15 | Fever | Non-Acb complex | 39·0 | 8400 | None noted | – | Survived |
| 13 | 5–15 | Viral infection | Acb complex | 38·1 | 3400 | None noted | – | Survived |
| 14 | 5–15 | Cellulitis | Non-Acb complex | 39·9 | 18 000 | Ceftazidime | Resistant to cefotaxime | Survived |
| Ceftriaxone | Ceftriaxone unknown | |||||||
| Clindamycin | Clindamycin unknown | |||||||
| Roxithromycin | Roxithromycin unknown | |||||||
| Co-trimoxazole | Resistant to trimethoprim | |||||||
| 15 | 5–15 | Acute pharyngitis | Non-Acb complex | 38·7 | 6600 | Amoxicillin | Susceptible | Survived |
Acb, Acinetobacter calcoaceticus–baumannii.
Non-HCA indicates that the blood culture was collected <3 days after admission and the patient had no known HCA risk factors.
Maximum temperature during first 24 h of admission.
White blood cell count/mm3.
This child was hospitalized with second-degree scald burn of right forearm, the following day he developed high fever and had a seizure during surgical debridement.
Although patients with non-HCA infections were slightly more likely than patients with HCA infections to have a temperature ⩾38°C or a fever history, generally the two groups had similar signs and symptoms of disease. HCA patients were more likely to have underlying illnesses such as diabetes, chronic obstructive pulmonary disease, or heart disease (Table 3). The case-fatality proportion was lower in the non-HCA group [9% (3/32) vs. 45% (17/38), P < 0·01]. The three patients with non-HCA infections who died were a 38-year-old with no recorded history of an underlying condition, admitted for alcohol withdrawal with delirium; a 59-year-old with a history of heart disease, hypertension, and dyspepsia, admitted with septicaemia and malnutrition; and a 61-year-old with no recorded history of an underlying condition, admitted for gastroenteritis of presumed infectious origin. Of these three patients, two (67%) had non-Acb complex isolates. Seventy-one per cent (12/17) of the deaths in patients with HCA infections occurred in patients who were aged ⩾65 years. Of the 17 deaths in patients with HCA infections, one (6%) was a patient with a non-Acb complex isolate. The majority of admission diagnoses for patients with both non-HCA and HCA infections were infectious conditions, most commonly pneumonia (Table 3).
Table 3.
Clinical characteristics and potential risk factors of hospitalized patients with Acinetobacter bacteraemia with and without healthcare-associated (HCA) risk factors, Nakhon Phanom and Sa Kaeo provinces, Thailand, May 2005–December 2008
| Non-HCA* (n = 32) n (%)‡ | HCA (n = 38)† n (%)‡ | P value§ | |
|---|---|---|---|
| Signs/symptoms at admission: | |||
| Temperature ⩾38°C | 28 (87·5) | 28 (73·7) | 0·15 |
| Fever history | 26 (81·3) | 25 (65·8) | 0·15 |
| Elevated WBC∥ | 11 (44·0) | 22 (61·1) | 0·19 |
| Cough | 12 (41·4) | 16 (42·1) | 0·95 |
| Dyspnea | 9 (29·0) | 10 (28·6) | 0·97 |
| Rales or crepitations | 6 (18·8) | 4 (10·8) | 0·50 |
| Sputum production | 2 (6·9) | 6 (17·7) | 0·27 |
| Any indication of respiratory illness | 16 (51·6) | 20 (57·1) | 0·65 |
| Low haemoglobin¶ | 14 (77·8) | 23 (69·7) | 0·74 |
| Admission diagnoses | |||
| Pneumonia | 9 (28·1) | 11 (28·9) | 0·94 |
| Other respiratory illness | 3 (9·4) | 6 (15·8) | 0·49 |
| Septicaemia | 4 (12·5) | 3 (7·9) | 0·69 |
| Fever, unspecified | 3 (9·4) | 2 (5·3) | 0·65 |
| Other infectious condition | 5 (15·6) | 5 (13·2) | 0·77 |
| Other | 7 (22·6) | 8 (22·9) | >0·99 |
| Underlying illness | |||
| Diabetes | 4 (16·0) | 11 (33·3) | 0·14 |
| Chronic obstructive pulmonary disease | 2 (8·7) | 4 (12·5) | 0·70 |
| Renal failure | 0 (0·0) | 5 (13·2) | 0·06 |
| Heart disease | 1 (4·4) | 4 (12·5) | 0·39 |
| HIV | 1 (3·1) | 2 (5·3) | >0·99 |
| None identified | 23 (71·9) | 8 (21·1) | <0·01 |
| Other potential risk factors: | |||
| Immunosuppressive therapy | 0 (0·0) | 4 (12·5) | 0·13 |
| Intubation | 0 (0·0) | 3 (23·1) | 0·04 |
| Smoking# | 5 (33·3) | 8 (30·8) | >0·99 |
| Alcohol use# | 5 (33·3) | 5 (19·2) | 0·45 |
| Death | 3 (9·4) | 17 (44·7) | <0·01 |
Non-HCA indicates that the blood culture was collected <3 days after admission and the patient had no known HCA risk factors.
There were 40 patients with HCA infections, two newborns were excluded from analyses.
Denominators exclude individuals with missing data.
P value from Pearson's χ2 test.
White blood cell count: <5 years, >1000/mm3; 6–17 years, >13 500/mm3; >17 years, >11 000/mm3 [34].
Under 5 years, <11 g/dl; 5–11 years, <11·5 g/dl; 12–14 years, <12 gm/dl; males ⩾15 years, <13 g/dl; females ⩾15 years, <12 g/dl (based on WHO classification of anaemia).
Among individuals aged ⩾18 years.
The proportion of Acinetobacter bacteraemia cases that were non-HCA was higher in Nakhon Phanom (66%, 27/41) than in Sa Kaeo (17%, 5/29). We did not observe marked seasonality or case clustering indicative of an outbreak in either province (Fig. 2).
Fig. 2.
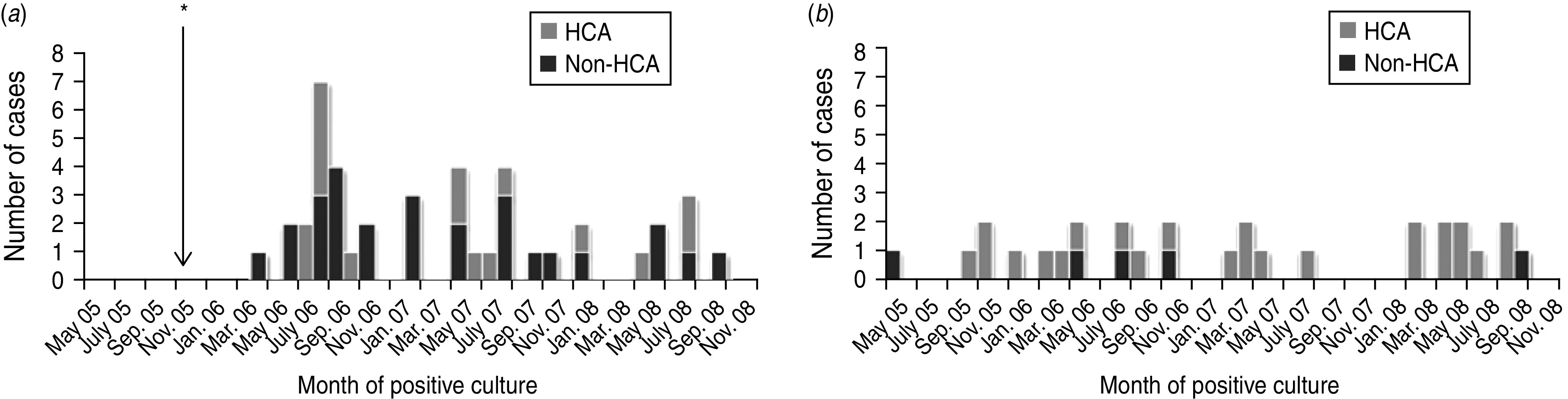
Hospitalized patients with Acinetobacter bacteraemia by month for (a) Nakhon Phanom and (b) Sa Kaeo provinces, Thailand, May 2005–December 2008. * Surveillance started in November 2005. Non-healthcare-associated (HCA) indicates that the blood culture was collected <3 days after admission and the patient had no known HCA risk factors.
Microbiological characteristics
Compared to HCA infections, a greater proportion of non-HCA infections were due to non-Acb complex species (44% vs. 21%, P = 0·04) (Table 1).
Antimicrobial resistance was common, especially in Acb complex isolates and isolates from HCA infections (Table 4). Imipenem resistance was common in Acb complex isolates: 53·9% for HCA infections vs. 23·5% for non-HCA infections (P = 0·05). Acb complex isolates from non-HCA infections were also less likely to be resistant to amoxicillin/clavulanic acid and co-trimoxazole than those from HCA infections. These differences were not observed in isolates of non-Acb species. Twelve (30%) of the 40 Acb isolates tested for drug resistance were MDR compared to one (5%) of the 22 non-Acb isolates tested.
Table 4.
Antimicrobial resistance in Acinetobacter calcoaceticus–baumannii complex isolated from blood cultures of hospitalized patients with and without healthcare-associated (HCA) risk factors, Nakhon Phanom and Sa Kaeo provinces, Thailand, May 2005–December 2008
| Antimicrobial agent | Non-HCA* (n = 18) | HCA (n = 30) | P value‡ |
|---|---|---|---|
| Resistant n (%)† | Resistant n (%)† | ||
| Ampicillin | 17 (100·0) | 25 (96·2) | >0·99 |
| Amoxicillin/clavulanic acid | 9 (52·9) | 21§ (80·8) | 0·05 |
| Cefotaxime | 12 (70·6) | 22 (84·6) | 0·44 |
| Co-trimoxazole | 5 (29·4) | 18 (69·2) | 0·01 |
| Amikacin | 10 (58·8) | 11 (42·3) | 0·29 |
| Gentamicin | 5 (29·4) | 14 (53·9) | 0·11 |
| Imipenem | 4 (23·5) | 14 (53·9) | 0·05 |
| MDR∥ | 4 (23·5) | 8 (30·8) | 0·73 |
Non-HCA indicates that the blood culture was collected <3 days after admission and the patient had no known HCA risk factors.
Denominators exclude individuals with missing data.
P value from Pearson's χ2 test.
One isolate was intermediately susceptible.
Multi-drug resistant (resistant to cefotaxime, amikacin, and imipenem).
DISCUSSION
We identified 72 Acinetobacter bacteraemia cases identified through bloodstream infection surveillance in two rural Thailand provinces from May 2005 to December 2008. Using a restrictive case definition to minimize misclassification, 32 cases occurred in patients with no identifiable HCA risk factors. The relatively high frequency of these infections, the high case-fatality proportion, and the high levels of resistance to antibiotics frequently used for empirical treatment of sepsis and pneumonia underscore the need for clinician awareness of Acinetobacter as a potential cause of community-associated bacteraemia in Thailand.
Differences in microbiological characteristics of infecting isolates suggest that non-HCA Acinetobacter infections were epidemiologically distinct from HCA cases. Compared to HCA infections, non-HCA infections were more likely to be caused by non-Acb complex species and by isolates that were less commonly resistant to antibiotics. These differences support the a priori hypothesis that Acinetobacter acquisition occurred in different settings for patients with non-HCA vs. HCA infections (i.e. community vs. hospital acquisition). Although signs and symptoms of infection were similar, patients with HCA infections had a significantly higher case-fatality proportion and were, as expected, older and more likely to have comorbidities.
Non-HCA infections were more common in Nakhon Phanom and almost all of the non-HCA infections in children aged ⩽15 years were from Nakhon Phanom. This may be a surveillance artifact, because blood cultures were more commonly performed in Nakhon Phanom, especially on children [18]. However, Acinetobacter infections may truly be more common in the Northeast compared to other regions of Thailand. It is well documented that Burkholderia pseudomallei, the cause of meliodosis and another soil organism, occurs at much higher rates in northeastern Thailand [32, 33]. Although the clinical relevance of environmental Acinetobacter exposures remains uncertain, the geographical distribution of Acinetobacter infections should be explored further to identify bacterial reservoirs and potential risk factors for infection. Carriage or environmental surveys may also help characterize community-associated risk.
We identified 15 children aged ⩽15 years with community-onset Acinetobacter infections and no known HCA risk factors. These children had high fevers, elevated white blood cell counts, and clinical presentations consistent with systemic Acinetobacter infection, suggesting true infections and not contaminants. Children are virtually absent from the literature on community-onset Acinetobacter infections. One study identified a subset of children with infection onset in the community, but 96% of these had indwelling central venous catheters [9], a clear HCA risk factor, which was not present in the children described here.
Despite the fact that several of the 15 children with non-HCA infections were treated with antibiotics to which the infecting Acinetobacter species was not susceptible, all children recovered. This suggests that in vitro antibiotic resistance does not necessarily predict clinical failure or that some non-HCA Acinetobacter infections may resolve with sub-optimal antibiotic therapy. However, given the limitations of this analysis, these data should not be taken to support alternative treatment approaches to Acinetobacter infections. Instead, our results support the view that Acinetobacter should be considered in the evaluation of children with sepsis in rural Thailand and that antibiotic therapy should be guided by prevailing antibiotic resistance patterns.
Our results differed from previous reports of ‘community-acquired’ Acinetobacter infections. Our observed case-fatality proportion was lower (9%) in patients with non-HCA infections than the 58% 30-day mortality reported for patients with ‘community-acquired’ Acinetobacter pneumonia in Hong Kong [12] and an overall mortality of 44% in a review of ‘community-acquired’ cases [11]. Indeed, the case-fatality proportion in previously reported ‘community-acquired’ cases is more consistent with the case-fatality proportion observed for HCA infections (45%) in our analysis. Previous reports may have misclassified Acinetobacter cases because they defined community-acquired infection as any infection with community onset. However, we have demonstrated the importance of assessing HCA risk factors as 18/50 patients (36%) with community-onset infections had clear healthcare exposures that may have put them at risk for acquisition of Acinetobacter in a healthcare setting. An additional dissimilar result was that, although alcohol abuse was common in those with community-acquired disease in previous reports [11], alcohol use was only noted in 24% of adults in our series. In our study population, a relatively high proportion (72%) of non-HCA cases occurred in individuals without documented comorbid conditions or previously described risk factors, suggesting that additional work to identify risk factors is warranted.
Antimicrobial resistance was common, especially in Acb complex isolates, for which the proportion of resistant isolates exceeded that for non-Acb species for all antibiotics except co-trimoxazole. Particularly marked was the high proportion of resistance to imipenem (42%) in all Acb complex isolates and 54% of Acb complex isolates from HCA cases. The high proportion of resistant isolates is consistent with data from the National Antimicrobial Resistance Surveillance of Thailand (NARST), which has identified marked increases in imipenem resistance (62% of Acb complex isolates in 2010) [5]. These data highlight both the importance of local antimicrobial resistance data to guide treatment and the need for alternative treatment options. National and regional antimicrobial resistance surveillance should continue and efforts to reduce antimicrobial resistance (e.g. judicious antibiotic use education) should be strengthened.
Although not the focus of this analysis, our results demonstrate the importance of Acinetobacter as a cause of HCA infections, which were highly resistant to antibiotics and associated with a very high case-fatality proportion. The substantial public health impact of HCA Acinetobacter infections underscores the importance of vigilant infection control practices and surveillance to detect breakdowns in prevention measures.
Our findings must be interpreted in light of certain limitations. One of our primary objectives was to define a group of patients with clear evidence of community-associated disease. Although our strict case definition reduced the possibility of an HCA infection being misclassified as non-HCA, it probably resulted in an underestimation of the true number of non-HCA infections. The presence of an HCA risk factor does not preclude the possibility that the infection was acquired outside a healthcare facility. Another limitation was that only HCA risk factors recorded in the patient's chart were captured. Additionally, we cannot rule out the possibility that some of these cases may have represented contamination rather than true infection. However, we tried to limit the inclusion of contaminated cultures by excluding those that grew additional organisms or became positive after 24 h of incubation.
Our study benefited from the availability from both enhanced microbiology capacity at hospital laboratories and confirmatory testing at the national reference laboratory. Although many isolates were identified as Acinetobacter at hospital laboratories, 12/70 isolates (17%) were only identified as Acinetobacter at the reference laboratory. Our findings highlight the value of strengthening laboratory capacity and the potential impact on clinical care.
Thorough assessment of HCA risk factors allowed us to identify likely community-associated Acinetobacter infections with confidence and compare clinical and microbiological features of infections of individuals with and without known HCA exposures in a way that has not previously been done. More recent data from 2009 and 2010 indicate that Acinetobacter species continue to be a cause of bacteraemia; 29 (2009) and 36 (2010) patients had positive cultures <3 days after hospitalization that were not likely to be due to contamination. Our results indicate that Acinetobacter species may be an important cause of community-associated sepsis and pneumonia in rural Thailand and add to previous reports, which have not explored healthcare exposures. Prospective studies are needed to confirm and better describe the epidemiology and risk factors of community-associated infections.
ACKNOWLEDGEMENTS
We thank the Provincial Health Offices in Nakhon Phanom and Sa Kaeo for their leadership and support; Toni Whistler, Prasong Srisaengchai and Thantapat Akarachotpong of the U.S. Centers for Disease Control and Prevention's (CDC) International Emerging Infections Program in Thailand for laboratory guidance and data management expertise; and Alexander Kallen, Brandi Limbago, David Lonsway, and Kamile Rasheed of CDC, Atlanta, Georgia for their technical consultation and review. K. A. Porter received support from a National Research Service Award from the National Institute of Allergy and Infectious Diseases (grant no. 1-T32-AI070114-01A1).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Bacterio.net. List of prokaryotic names with standing in nomenclature (http://www.bacterio.net/a/acinetobacter.html). Accessed 16 July 2013.
- 2.Dijkshoorn L. Acinetobacter baumannii. In: de Filippis I, McKee M, eds. Molecular Typing in Bacterial Infections. New York: Springer, 2012, pp. 434. [Google Scholar]
- 3.Munoz-Price LS, Weinstein RA. Acinetobacter infection. New England Journal of Medicine 2008; 358: 1271–1281. [DOI] [PubMed] [Google Scholar]
- 4.Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Current Opinion in Infectious Diseases 2010; 23: 332–339. [DOI] [PubMed] [Google Scholar]
- 5.Dejsirilert S, et al. Antimicrobial resistance of Acinetobacter baumannii: six years of National Antimicrobial Resistance Surveillance Thailand (NARST) surveillance. Journal of the Medical Association of Thailand 2009; 92 (Suppl. 4): S34–45. [PubMed] [Google Scholar]
- 6.Barnes DJ, Naraqi S, Igo JD. Community-acquired acinetobacter pneumonia in adults in Papua New Guinea. Reviews of Infectious Diseases 1988; 10: 636–639. [DOI] [PubMed] [Google Scholar]
- 7.Chen MZ, et al. Severe community-acquired pneumonia due to Acinetobacter baumannii. Chest 2001; 120: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 8.Wang JT, et al. Community-acquired Acinetobacter baumannii bacteremia in adult patients in Taiwan. Journal of Clinical Microbiology 2002; 40: 1526–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segal SC, et al. Epidemiology of and risk factors for Acinetobacter species bloodstream infection in children. Pediatric Infectious Disease Journal 2007; 26: 920–926. [DOI] [PubMed] [Google Scholar]
- 10.Anstey NM, Currie BJ, Withnall KM. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clinical Infectious Diseases 1992; 14: 83–91. [DOI] [PubMed] [Google Scholar]
- 11.Falagas ME, et al. Community-acquired Acinetobacter infections. European Journal of Clinical Microbiology and Infectious Diseases 2007; 26: 857–868. [DOI] [PubMed] [Google Scholar]
- 12.Leung WS, et al. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest 2006; 129: 102–109. [DOI] [PubMed] [Google Scholar]
- 13.Anstey NM, et al. Community-acquired bacteremic Acinetobacter pneumonia in tropical Australia is caused by diverse strains of Acinetobacter baumannii, with carriage in the throat in at-risk groups. Journal of Clinical Microbiology 2002; 40: 685–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ku SC, et al. Clinical and microbiological characteristics of bacteremia caused by Acinetobacter lwoffii. European Journal of Clinical Microbiology and Infectious Diseases 2000; 19: 501–505. [DOI] [PubMed] [Google Scholar]
- 15.Zeana C, et al. The epidemiology of multidrug-resistant Acinetobacter baumannii: does the community represent a reservoir? Infection Control and Hospital Epidemiology 2003; 24: 275–279. [DOI] [PubMed] [Google Scholar]
- 16.Wolf B, et al. Carriage of gram-negative bacilli in young Brazilian children with community-acquired pneumonia. International Journal of Infectious Diseases 2001; 5: 155–159. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Information and Communication Technology of Thailand. National Statistical Office (http://web.nso.go.th/index.htm).
- 18.Baggett HC, et al. Incidence of pneumococcal bacteremia requiring hospitalization in rural Thailand. Clinical Infectious Diseases 2009; 48 (Suppl. 2): S65–74. [DOI] [PubMed] [Google Scholar]
- 19.Olsen SJ, et al. The incidence of pneumonia in rural Thailand. International Journal of Infectious Diseases 2006; 10: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perilla MJ, et al. Manual for the Laboratory Identification and Antimicrobial Susceptibility Testing of Bacterial Pathogens of Public Health Importance in the Developing World. Centers for Disease Control and Prevention and the World Health Organization, Department of Communicable Disease, 2003. [Google Scholar]
- 21.Murray PR (eds). Manual of Clinical Microbiology, 9th edn. Washington, DC: American Society for Microbiology Press, 2007. [Google Scholar]
- 22.Versalovic J (eds). Manual of Clinical Microbiology, 10th edn. Washington, DC: American Society for Microbiology Press, 2011. [Google Scholar]
- 23.Gerner-Smidt P, Tjernberg I, Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. Journal of Clinical Microbiology 1991; 29: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang MC, et al. Polymerase chain reaction assay for the detection of Acinetobacter baumannii in endotracheal aspirates from patients in the intensive care unit. Journal of Microbiology Immunology and Infection 2011; 44: 106–110. [DOI] [PubMed] [Google Scholar]
- 25.Higgins PG, et al. gyrB multiplex PCR to differentiate between Acinetobacter calcoaceticus and Acinetobacter genomic species 3. Journal of Clinical Microbiology 2010; 48: 4592–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karah N, et al. Species identification and molecular characterization of Acinetobacter spp. blood culture isolates from Norway. Journal of Antimicrobial Chemotherapy 2011; 66: 738–744. [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk susceptibility tests; approved standard – 11th edn. Wayne, PA, 2012. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. Wayne, PA, 2012. [Google Scholar]
- 29.Javadi M, et al. Diagnosing pneumonia in rural Thailand: digital cameras versus film digitizers for chest radiograph teleradiology. International Journal of Infectious Diseases 2006; 10: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen AL, et al. Recommendations for metrics for multidrug-resistant organisms in healthcare settings: SHEA/HICPAC Position paper. Infection Control and Hospital Epidemiology 2008; 29: 901–913. [DOI] [PubMed] [Google Scholar]
- 31.Fridkin SK, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. New England Journal of Medicine 2005; 352: 1436–1444. [DOI] [PubMed] [Google Scholar]
- 32.Limmathurotsakul D, et al. Increasing incidence of human melioidosis in Northeast Thailand. American Journal of Tropical Medicine and Hygiene 2010; 82: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhengsri S, et al. Incidence of bacteremic melioidosis in eastern and northeastern Thailand. American Journal of Tropical Medicine and Hygiene 2011; 85: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunn VL, Nechyba C (eds). The Harriett Lane Handbook, 16th edn. Philadelphia: Mosby, Inc., 2002. [Google Scholar]