Abstract
The incidence rate of tonsillar cancer is increasing worldwide. The current study identifies a parallel increase in the incidence of tonsillar cancer, human papilloma virus (HPV) and p16 expression among a population from northern Sweden, a sparsely populated area, confirming the strong association between p16 and HPV infection in tonsillar tissue. Data from the Swedish Cancer Registry was assessed to identify cases of tonsillar cancer in the northern territorial area of Sweden. HPV DNA was extracted from paraffin embedded diagnostic biopsies and detected by polymerase chain reaction using general primers Gp5+/6+ and CpI/IIG. Expression of p16 was identified by immunochemistry. Patients were grouped into urban or rural residence categories. A total of 214 cases were identified, comprising 155 (72.4%) men and 59 (27.6%) women, and 65 of these patients, who presented between 2000 and 2012, were analyzed. The overall median age for the analyzed patients was 58 years; 48 (74%) were males (median age, 57.5 years) and 17 (26%) were females (median age, 65 years). Of the 65 specimens, 59 (91%) were positive for HPV, and 62 (95%) expressed p16. The incidence of tonsillar cancer in the cohort demonstrated a 2-fold increase between 1990 and 2013; specifically, a 2.7-fold increase was observed in men whilst the female group exhibited only a small increase. These findings demonstrate a strong association between p16 expression and HPV infection in tonsillar malignancies. The incidence of HPV-positive tonsillar cancer has increased in recent years, even in sparsely populated regions, as demonstrated in northern Sweden.
Keywords: head and neck cancer, human papilloma virus, immunohistochemistry, incidence, p16, tonsillar cancer
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) is the sixth most common malignancy worldwide, with ~650,000 new cases diagnosed each year (1). These tumors may originate in the oral cavity, oropharynx, hypopharynx or larynx (2). The incidence of HNSCC in Sweden is ~1,200 new cases per year (2), which accounts for 3–4% of all new cancer diagnoses in the country. Worldwide, the incidence is two times higher in men than in women (3).
Oropharyngeal cancer develops in the base of the tongue and palatine tonsils, the posterior pharyngeal wall and the soft palate. Established predisposing factors include heavy tobacco smoking and alcohol consumption, which appear to act synergistically (4). Tonsillar cancer is the most common form of oropharyngeal cancer in Sweden (3). Typical symptoms include swallowing difficulties, unilateral pain in the throat and ear, and lumps in the neck. The incidence of tonsillar cancer appears to have been increasing during recent years, despite a decline in smoking and alcohol consumption. One possible factor contributing to this may be mucosal infection with human papilloma virus (HPV) (5–8).
It has been proposed that HPV-positive tonsillar carcinomas should be considered different tumor entities from HPV-negative tonsillar carcinomas, as HPV-positive tumors arise in the tonsillar crypts while the non-HPV-associated form originates from the tonsillar surface (9). The majority of HPV-positive tonsillar cancers are associated with the high-risk HPV type 16, and this HPV type may be present in ~95% of the HPV-positive cases, whilst other types, such as 18, 31, 33 and 35, are relatively infrequently associated with HNSCC (10).
Radiotherapy is the optimal and standard treatment option at present, and may be combined with chemotherapy in more advanced cases. To date, curative surgical monotherapy of tonsillar cancer has been unsuccessful. However, recent results using transoral robotic surgery as single modality treatment have demonstrated a potential therapeutic benefit (11,12). Certain reports have suggested that HPV-related malignancies have a more favorable prognosis, regardless of the chosen treatment strategy (13–15).
Currently, HPV is the most common sexually transmitted disease; however, even the most sensitive DNA tests may fail to detect infections caused by the virus (16). The recent introduction of vaccination against HPV 16-associated anogenital cancers in non-infected subjects has demonstrated a promising protective effect through the prevention of infection. This approach may have a role in the prevention of specific HPV-subtype-positive head and neck malignancies in the future (17). The use of HPV vaccines as preventive and therapeutic treatments has been discussed in the literature (18,19).
The aim of the current study was first to assess the incidence of tonsillar cancer in northern Sweden, one of western Europe's most sparsely populated regions. Secondly, the study aimed to address the recent incidence in this region compared with reported incidences in densely populated regions. It further aimed to assess the proportion of HPV-associated tonsillar cancers and its surrogate marker, p16, and finally, to determine whether there were variations in the manifestation of the disease associated with urban versus rural areas in the region.
Materials and methods
Patients
The Regional Ethical Review Board of Umeå University (Umeå, Sweden) approved the analysis of paraffin-embedded retrospective samples from the Biobank North (County Council of Västerbotten, Västerbotten, Sweden; approval nos. 2012-276-32M and 2010-277-31M). In addition, study was conducted in accordance with the Declaration of Helsinki (20).
This retrospective observational study included all consenting patients diagnosed with tonsillar cancer between 1990 and 2013 at the University Hospital of Umeå (Umeå, Sweden). In order to identify cases of tonsillar neoplasms, information was extracted from the Swedish Cancer Registry database (www.socialstyrelsen.se/register/halsodataregister/cancerregistret/inenglish) using the International Classification of Diseases (ICD)-7 code 145.0. The designation ‘northern Sweden’ was defined as the part of Sweden consisting of the counties Västerbotten, Norrbotten, Västernorrland and Jämtland, which include a total population of 882,563 and ~4 inhabitants per square kilometer. For comparative purposes, data from the Swedish Cancer Registry database regarding the whole Swedish population of ~9.5 million was used (2013).
Pre-treatment tumor samples were collected by biopsy or surgical resection. Paraffin-embedded tumor blocks were retrieved from the archive of the Department of Laboratory Medicine/Pathology at the University Hospital of Umeå. The clinical characteristics of the study population are summarized in Table I.
Table I.
Clinical characteristics of the patients included in the HPV DNA and p16 analysis.
| Specimen number | Year of diagnosis | % tumor cells in sample | p16 quickscore | HPV | Gender | Age at diagnosis, years |
|---|---|---|---|---|---|---|
| 1 | 2012 | 25a | 6 | 1 | F | 67 |
| 2 | 2012 | 70a | 12 | 1 | M | 64 |
| 3 | 2012 | 70a | 12 | 1 | M | 53 |
| 4 | 2012 | 50a | 12 | 1 | M | 51 |
| 6 | 2011 | 80a | 12 | 1 | M | 70 |
| 8 | 2012 | 40a | 12 | 1 | M | 53 |
| 9 | 2011 | 60a | 12 | 1 | M | 55 |
| 10 | 2011 | 30a | 12 | 1 | M | 54 |
| 12 | 2011 | 30a | 12 | 1 | M | 45 |
| 13 | 2011 | 70a | 12 | 1 | F | 65 |
| 14 | 2011 | 70a | 12 | 1 | M | 72 |
| 15 | 2012 | 80a | 12 | 1 | F | 76 |
| 16 | 2012 | 60a | 12 | 1 | F | 87 |
| 17 | 2012 | 95a | 6 | 1 | F | 65 |
| 18 | 2010 | 70a | 18 | 1 | M | 58 |
| 19 | 2010 | 20a | 12 | 1 | F | 75 |
| 20 | 2010 | 75a | 12 | 1 | M | 59 |
| 21 | 2010 | 80a | 12 | 1 | M | 70 |
| 23 | 2010 | 70a | 12 | 1 | M | 71 |
| 24 | 2010 | 50a | 12 | 1 | M | 49 |
| 25 | 2006 | 60a | 18 | 1 | M | 48 |
| 26 | 2006 | 80a | 12 | 1 | M | 55 |
| 27 | 2006 | 60a | 12 | 1 | F | 76 |
| 28 | 2007 | 60a | 0 | 0 | M | 51 |
| 29 | 2007 | 95a | 12 | 1 | M | 58 |
| 30 | 2007 | 5a/10b | 12 | 1 | M | 48 |
| 31 | 2007 | 60a | 12 | 1 | M | 64 |
| 33 | 2006 | 60a | 12 | 1 | M | 59 |
| 34 | 2006 | 75a | 12 | 1 | M | 60 |
| 35 | 2005 | 80a | 12 | 1 | M | 62 |
| 36 | 2005 | 80a | 12 | 1 | M | 47 |
| 37 | 2005 | 90a | 2 | 0 | F | 64 |
| 38 | 2005 | 75a | 12 | 1 | M | 58 |
| 39 | 2003 | 80a | 12 | 1 | M | 57 |
| 40 | 2002 | 20a | 18 | 1 | M | 61 |
| 41 | 2002 | 80a | 12 | 1 | F | 64 |
| 42 | 2001 | 50a | 0 | 0 | F | 53 |
| 43 | 2001 | 70a | 12 | 1 | F | 46 |
| 44 | 2001 | 80a | 5 | 1 | M | 58 |
| 45 | 2000 | 70a | 12 | 1 | F | 69 |
| 46 | 2000 | 85a | 12 | 1 | F | 63 |
| 47 | 2000 | 30a | 18 | 1 | M | 52 |
| 48 | 2001 | 60a | 12 | 1 | F | 68 |
| 49 | 2001 | 5a | 12 | 1 | F | 45 |
| 50 | 2004 | 60a | 2 | 0 | F | 54 |
| 51 | 2009 | 95a | 12 | 1 | M | 48 |
| 52 | 2009 | 80a | 12 | 1 | M | 53 |
| 54 | 2009 | 40a | 12 | 1 | M | 51 |
| 55 | 2001 | 30a | 12 | 1 | M | 74 |
| 56 | 2004 | 40a | 4 | 0 | M | 73 |
| 57 | 2008 | 80a | 12 | 1 | M | 60 |
| 58 | 2008 | 70a | 12 | 1 | M | 49 |
| 59 | 2008 | 80a | 12 | 1 | F | 53 |
| 60 | 2009 | 70a | 18 | 1 | M | 63 |
| 61 | 2008 | 10a/50b | 12 | 1 | M | 56 |
| 62 | 2009 | 40a | 12 | 1 | M | 63 |
| 63 | 2009 | 5a/15b | 12 | 1 | M | 60 |
| 64 | 2009 | 50a | 12 | 1 | M | 49 |
| 66 | 2009 | 60a | 12 | 0 | M | 63 |
| 68 | 2008 | 60a | 18 | 1 | M | 53 |
| 69 | 2008 | 70a | 12 | 1 | M | 51 |
| 70 | 2008 | 60a | 18 | 1 | M | 53 |
| 71 | 2010 | 60a | 0 | 1 | M | 66 |
| 73 | 2012 | 70a | 12 | 1 | M | 62 |
| 74 | 2002 | 20a | 12 | 1 | M | 56 |
Before
after sectioning. p16 quickscore = intensity score [0 (negative), 1 (weak), 2 (intermediate) or 3 (strong)] × proportion score [1 (0–4%), 2 (5–19%), 3 (20–39%), 4 (40–59%), 5 (60–79%) or 6 (80–100%)]. HPV: 0, negative; 1, positive. M, male; F, female; HPV, human papilloma virus.
HPV detection by polymerase chain reaction (PCR)
DNA was extracted from paraffin-embedded diagnostic biopsies using a QIAamp DNA FFPE Tissue Kit or QIAamp Mini Kit (Qiagen, Inc., Valencia, CA, USA), according to the manufacturer's instructions. A general HPV PCR analysis was run using 100 ng of extracted DNA from each patient and the general primers GP5+/6+, as previously described (21). The primers were as follows: 5′-TTT GTT ACT GTG GTA GAT ACT AC-3′ for GP5+ and 5′-GAA AAA TAA ACT GTA AAT CAT ATT C-3′ for GP6+. The 50 µl PCR mixture consisted of 5 µl GeneAmp 10X PCR Gold Buffer, 200 µM of each dNTP (GeneAmp dNTP mix), 3.5 mM MgCl2 (all from Applied Biosystems Life Technologies, Foster City, CA, USA), 25 pmol of each primer and 1 unit of AmpliTaq Gold DNA Polymerase (Applied Biosystems Life Technologies).
Amplification was performed in a Biometra Professional Thermocycler (Thermo Fisher Scientific, Waltham, MA, USA) and was initiated with denaturation for 4 min at 94°C, followed by 40 amplification cycles of denaturation at 94°C for 1 min, annealing at 44°C for 1 min and elongation at 72°C for 2 min. The final cycle ended with a prolonged elongation step at 72°C for 10 min. PCR products were run on a 2.5% agarose gel (SeaKem® LE Agarose; Lonza, Rockland, ME, USA) in 0.5X Tris/Borate/EDTA-buffer (TBE; 1 L of 10X TBE: 121.1 g tris base, 46 g boric acid and 7.44 g EDTA made up to 1 L with distilled water), stained with 0.5X GelRed (Biotium, Hayward, CA, USA), and visualized under UV-light. Fragments of 130–150 bp were considered positive.
To avoid false negative results due to a disrupted L1 gene, negative samples were run with the general primers CpI/IIG, as described in Smits et al (22). The primers were as follows: 5′-TTA TCW TAT GCC CAY TGT ACC AT-3′ for CpI and 5′-ATG TTA ATW SAG CCW CCA AAA TT-3′ for CpIIG. Briefly, the PCR mixture consisted of 5 µl GeneAmp 10X PCR Gold Buffer, 200 µM of each dNTP (GeneAmp dNTP mix), 3 mM MgCl2, 17 pmol CpI, 26 pmol CpIIG, 0.5 µl bovine serum albumin and 1 unit of AmpliTaq Gold DNA Polymerase. The amplification consisted of denaturation for 5 min at 94°C, followed by 40 amplification cycles of denaturation at 95°C for 1 min, annealing at 55°C for 1 min and elongation at 72°C for 2 min. The final cycle ended with a prolonged elongation step at 72°C for 4 min. Fragments were analyzed as described for the previous PCR analysis, and products of 188 bp were considered positive.
PCR with the GP5+/6+ primers was run at least twice. In cases with weak or divergent results, the PCR was repeated. Additionally, in random cases, a new DNA preparation was used to validate the method.
p16 immunohistochemistry and scoring system
For the detection of p16, staining was performed in a Ventana staining machine (BenchMark ULTRA; Ventana Medical Systems, Tuscon, AZ, USA) according to the supplier's recommendations. An antibody against p16 (monoclonal mouse anti-human; cat. no. sc-56330) from Santa Cruz Biotechnology (Dallas, TX, USA) was used at a dilution of 1:200. Prior to staining, slides were pretreated in Tris-EDTA (10 mM Tris-HCl, 1 mM disodium EDTA; pH 8.0). The antibody was visualized using the Ultraview Universal DAB Detection Kit (Ventana Medical Systems) and staining was observed using a light microscope (BX51; Olympus Corporation, Tokyo, Japan).
The quickscore system was used for scoring the percentage of tumor cells expressing p16 and intensity of staining (23). The proportion of tumor cells expressing p16 was graded from 1–6: 1, 0–4%; 2, 5–19%; 3, 20–39%; 4, 40–59%; 5, 60–79%; or 6, 80–100%. Staining intensity in turn was divided into 4 grades: 0, negative; 1, weak; 2, intermediate; and 3, strong. By multiplying the score for percentage of tumor cells expressing the protein by the intensity score, a quickscore ranging from 0–18 was determined.
Classification according to place of residence
The code numbers of the biopsy samples were used to identify the personal identification number of each patient and, subsequently, the residence address was extracted through the patients' electronic records system (Systeam Cross; Eskilstuna, Sweden). Subjects were placed into either urban or rural groups depending on the location of their private residence. A Swedish urban area was defined as a residential area with ≥200 inhabitants where the distance between buildings is <200 meters (24), whilst a rural address was one where the number of local inhabitants was <200 and the distance between houses was ≥200 meters (25).
Analysis and statistics
The total age-standardized incidence of tonsillar cancer over the period from 1990–2013 was determined using northern Sweden's standard population (2000) and data obtained from the Swedish Cancer Registry. Data regarding the cases of head and neck cancer was extracted from the Swedish Cancer Registry for comparative purposes (2). The Mann-Whitney U test was used to compare patient ages between different genders and HPV-positive and HPV-negative patients. The χ2 test was used to investigate any differences in HPV-status between genders. Statistical analysis was performed using SPSS software version 22.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Incidence of HNSCC
From the period between 1990 and 2012, a total of 22,640 cases of head and neck cancer were identified in the Swedish Cancer Registry database, including tumors located in the hypopharynx, larynx, oropharynx, oral cavity, tongue, lips, nasopharynx, nose, paranasal sinuses and middle ear. Of these, 15,563 (68.7%) were men and 7,077 (31.3%) women. Cases from 2013 were not yet registered. The total number of cases of head and neck cancer in northern Sweden during the same period was 1,978 (9% of all cases in Sweden). Of these patients, 1,297 (65.6%) were men and 681 (34.4%) were women.
The number of cases of tonsillar cancer (ICD-7 code 145.0) in northern Sweden between 1990 and 2013 according to the Swedish Cancer Registry was 214, comprising 155 (72.4%) men and 59 (27.6%) women.
The total age-standardized incidence of tonsillar cancer in northern Sweden doubled between 1990 and 2013, from 0.69–1.38 per 100,000 individuals. The increase in incidence in men was 2.7-fold (0.83–2.25 per 100,000), while the female incidence increased from 0.46 (1990) to 0.48 (2013) per 100,000 (Table II; Fig. 1).
Table II.
Incidence of tonsillar cancer in northern Sweden per 100,000 individuals, according to the population of northern Sweden in 2000.
| Period | ||||||
|---|---|---|---|---|---|---|
| Group | 1990–94 | 1995–99 | 2000-04 | 2005-04 | 2010-13 | |
| Male | 0.826 | 0.968 | 1.13 | 1.88 | 2.25 | |
| Female | 0.46 | 0.488 | 0.564 | 0.494 | 0.482 | |
| Total | 0.692 | 0.724 | 0.83 | 0.998 | 1.379 | |
Figure 1.
Incidence of tonsillar cancer in northern Sweden, 1990–2013. A 2-fold overall increase was observed; a 2.7-fold increase was observed in males while the female group demonstrated only a small increase.
HPV analysis
A total of 74 biopsy specimens from patients with tonsillar cancer between 2000 and 2012 were identified and obtained from the archives of the Department of Pathology, University Hospital of Umeå. Of these, 4 specimens were excluded as they contained too little or a complete lack of tumor tissue. Additionally, 5 samples were excluded as they were duplicates of patients already included in the database.
Of the 65 remaining specimens (median age, 58 years; mean age, 59.3 years; range, 45–87 years), 48 (74%) were males (median age, 57.5 years; mean, 57.6 years; range, 45–74 years) and 17 (26%) were females (median age, 65 years; mean, 64.1 years; range, 45–87 years). Age was significantly higher among female subjects compared with male subjects (U test, P=0.016).
HPV DNA was detected in 59 (91%) of the 65 biopsy specimens, while the remaining 6 samples (9%) were HPV-negative. There was no difference when comparing the variable of age between the HPV-positive and HPV-negative samples (P=0.856, U test). A χ2 test identified no association between HPV-status and gender (P=0.179). In the HPV-positive cohort, there was a male dominance [3:1; 45 males (76%) vs. 14 females (24%)], whilst an equal proportion of males and females (1:1) was observed in the HPV-negative cohort. When comparing gender and age in the HPV-positive cohort, age was significantly higher in females compared with in males (U test, P=0.006).
p16 expression
Expression of p16 was scored in all 65 samples, with 62 samples (95%) positive for the p16 marker and 3 (5%) negative (Fig 2). Of the p16-positive samples, 7 (11%) received the highest score of 18 points, 49 (79%) a score of 12 (intermediate intensity) and 6 samples (10%) received 2–6 points.
Figure 2.
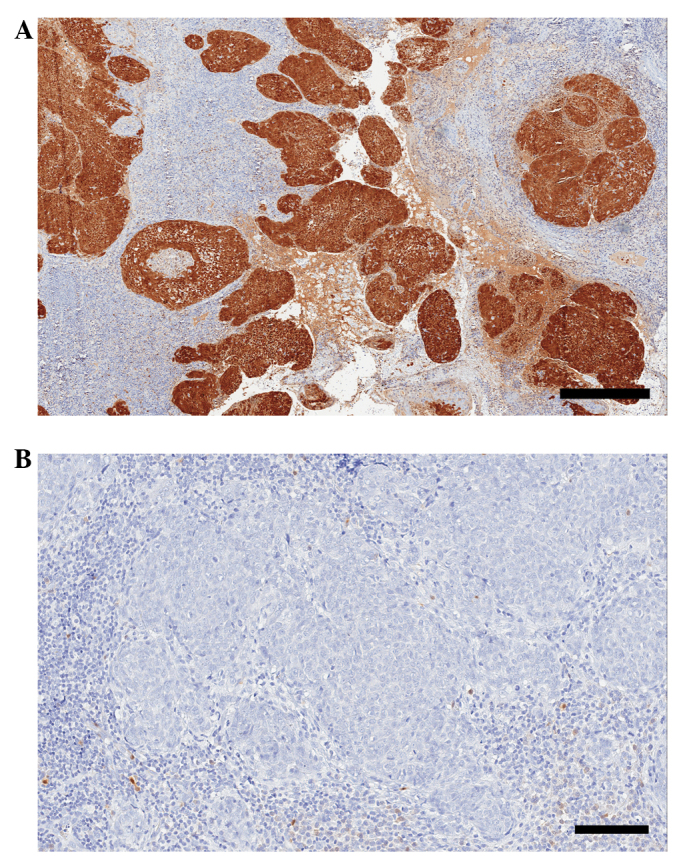
(A) Positive and (B) negative p16 staining in tonsillar squamous cell carcinoma. Scale bar, A, 400 µm; B, 100 µm. Stain, p16. (Quickscore, A, 12; B; 0).
Classification according to residence
The patients' residence area at the time of diagnosis indicated that the vast majority (64 patients, 98.5%) were living in urban areas, while only 1 patient (1.5%) resided in a rural area. This difference is due to the overall populations of these areas.
Discussion
The aims of the current study were to investigate the proportion of p16-positive cases of HPV-related tonsillar cancer, to study the incidence of tonsillar cancer in the sparsely populated region of northern Sweden, and to examine whether this increasing trend is parallel to the incidence observed in other, more densely populated areas.
A 2-fold increase in cases of HPV-positive tonsillar cancer was observed during the analyzed period of 23-years (1990–2013), preferentially in male patients. Hammarstedt et al (5) reported a fold increase in tonsillar cancer incidence in the urban region of Stockholm of 2.8 (2.6 in men and 3.5 in women), while smoking had decreased in Sweden during the same period (1970–2002) (26). In the current findings, the increased incidence of tonsillar cancer was observed only in the male group. The reason for this disparity is unclear, although the present study included sampling from a more recent period of 11 years. Another factor may be the relatively recent knowledge and treatment patterns related to HPV 16 as a causative agent of cervical cancer.
The findings demonstrating no recent change in the incidence of tonsillar cancer in women in this region may be an indication of the differences in HPV exposure and susceptibility between sparsely populated and densely populated areas. This issue has not been examined to date. The increased incidence of HPV-related tonsillar cancer in males has been described in a study examining ethnic perspectives (27), in which a recent decline in incidence rates of tonsillar cancer was also observed for both white and black females aged 40–64 years in the USA. Hence, HPV infection in the oropharynx may be an explanation for at least part of the increase in the incidence of tonsillar cancer in men. Another possible explanation may be the traditional risk factors of alcohol and smoking found in the male population in northern Sweden, which could have a synergistic effect with the HPV virus. At present, <20% of Swedish men smoke tobacco. The significantly lower mean age of the males in the current sample and, in particular, the lower mean age of the male HPV-related cases, may be an indicator of an altered sexual behavior and consequent frequent HPV contamination among men in northern Sweden.
Hammarstedt et al (28) reported that patients with HPV-positive tonsillar malignancies were younger (median age, 55 years) compared with patients with HPV-negative disease, and no similar difference regarding other oral cancers could be identified. This finding could not be confirmed in the current data due to the limited number of cases of HPV-negative tonsillar cancer.
The present results revealed that 91% of the cases of tonsillar cancer were HPV-positive. A similarly high proportion of HPV-positive cases of tonsillar cancer in Sweden has been reported by Näsman et al (29), who identified an increase from 68% (2000–2002) to 93% (2006–2007) (29). In general, HPV DNA has been reported to be detected in 45–100% of tonsillar cancer cases (30). It is possible that the cohorts with a lower proportion of HPV-positive cases are less recent, and this could indicate that the numbers of HPV-related tonsillar cancer are increased in more current published data due to the more reliable diagnostic methods available.
Differences in HPV-related tonsillar malignancies have been observed not only between urban and rural areas in Sweden, but also in other countries. Blomberg et al (7) described an increased incidence rate of HPV related tonsillar cancer between 1978 and 2007 in Denmark, while the incidence of non-HPV-associated HNSCC decreased in men, but remained unchanged in the female group. Another study of patterns of tonsillar cancer in South-eastern England, comparing the years 1987 and 2006, revealed an incidence of tonsillar cancer increasing from 0.600–1.45 per 100,000 among men (40–59 years), a lower age at diagnosis and an increase in median survival time (8). Similar studies have been performed in the USA and Finland, demonstrating a rising incidence of tonsillar cancer during the last decades (27,31).
Overexpression of p16 was observed in the present tumor specimens, confirming the results of a number of previous studies (4,32–36), as well as demonstrating the strong association between p16 and HPV-infection in tonsillar carcinomas. The majority of these specimens exhibited p16 expression of medium intensity, while a further 11% showed strong p16 expression.
In the last decade, a number of studies have reported the prognostic value of HPV and p16 analysis in HNSCC in general and in tonsillar cancer in particular. It has been confirmed that patients with HPV-associated tumors in the tonsillar region have a better prognosis compared with patients with HPV-negative tumors in terms of locoregional control and overall survival, regardless of treatment modality (4,14,15). Nichols et al (13) reported that patients with HPV-related tumors are younger and have smaller primary-site tumors and a more favorable survival rates compared with those with HPV-negative tumors. The overexpression of p16 is highly associated with HPV infection, and this may be used clinically as a surrogate marker and a prognostic guide of the disease. Large and often cystic lymph node involvement, along with clinically advanced stage (III–IV), are also associated with HPV-positive tumors (35,37).
The favorable prognosis associated with HPV positivity in tonsillar cancers has been studied by Lindquist et al (35), who reported an increased disease-specific survival rate in patients with HPV-associated tonsillar cancer (81%) compared with patients with HPV-negative tumors (36%), regardless of age, gender or tumor stage. These findings have been further supported by Fischer et al (36), who reported that the 5-year survival rate of patients with p16-positive oropharyngeal cancer of stages III–IV was nearly as good as for those with tumors of stages I–II. The prognostic value of p16 in the outcome of HPV-positive tonsillar neoplasms was also addressed. Cases with p16-positive tumors in the advanced stages (III–IV) had a 5-year survival of 54.1%, compared to 18% for those with p16-negative tumors. Regardless of tumor stage, p16-positive cases had a 5-year survival rate of 59.3% compared to 24.5% for the p16-negative cases (36). Results from the Danish DAHANCA 5 study revealed that p16-positive HNSCCs were associated with improved locoregional tumor control (58% vs. 28%) and increased disease-specific (72% vs. 34%) and overall (62% vs. 26%) survival rates compared with p16-negative tumors (38). The detection of p16 and HPV may therefore be an important tool as part of a comprehensive strategy to develop personalized treatment for tonsillar cancer.
Despite these findings, many current recommendations for the treatment of HPV-positive tonsillar tumors do not differ from those for HPV-negative tumors (39). Certain investigators have questioned the need for aggressive treatments for patients with HPV-positive tonsillar cancer, which has now been found to be associated with a better prognosis and is often observed in younger and healthier groups (4,39). The risks and benefits of aggressive chemoradiotherapy and surgery must be considered carefully. Current research is now focused on the de-escalation of treatment modalities for the HPV-related cases and emphasizes the introduction of a number of prognostic markers in order to identify such patients in the pre-treatment stage (4,38).
At present, there is a lack of clear evidence regarding the biological basis behind the differences in survival and prognosis between HPV-associated and HPV-negative malignancies. A possible explanation may be that the different molecular profile in patients with HPV- and p16-positive tumors constitutes a distinct subset of tonsillar malignancies (34). In addition, it is also reasonable to speculate that a younger, non-smoking cohort is associated with a reduced rate of co-morbidity and, therefore, improved non-cancer-related outcomes.
The route of transmission of HPV infection in oral carcinomas remains unclear. Much speculation has occurred regarding possible sexual transmission. The increasing evidence of tonsillar cancer during the last decades has been associated with a significant change in sexual habits, and HPV is currently considered to be the most common sexually transmitted infection (16). This issue has been studied by Hemminki et al (40), with results indicating that the husbands of patients with cervical cancer have a higher risk of tonsillar and tongue-based malignancies. Hence, the prophylactic vaccination of women may also lead to decreased rates of oral HPV infection in the male population. The numbers of HPV infections in the oral cavity and, subsequently, HPV-associated tonsillar tumors are expected to surpass the rate of cervical malignancies by 2020, and this fact highlights the importance of vaccinating both genders in the future as a preventive measure against tonsillar cancer (41).
In summary, the present study identified incidences of HPV-positive and p16-expressing tonsillar cancers in men from northern Sweden that were similar to those already reported for Swedish urban populations, as well as populations outside of Sweden. This study may provide a basis for future projects investigating the prognosis of tonsillar carcinomas. Further investigation into the biological mechanisms of the improved survival and prognosis associated with the HPV-positive tonsillar cancers is warranted. Furthermore, it may be beneficial to evaluate the role and effect of HPV vaccination as a prophylactic measure against tonsillar and oral cancer, and the necessity for introducing vaccination strategies to the male population.
Acknowledgements
This study was supported by grants from Lion's Cancer Research Foundation (13-1998), at Umeå University Sweden.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.National Board of Health and Welfare (SocialStyrelsen) Stockholm, Sweden: 2011. Center for Epidemiology: Cancer incidence in Sweden 2011. [Google Scholar]
- 3.Lind M, Lewin F, Lundgren J, Nathanson ALF, Strander H, editors. Karolinska University Hospital. Stockholm: Oncological Centrum; 2001. [Jul 11;2014 ]. Head, neck and oesophageal cancer: Diagnosis, treatment and follow-up of the Stockholm-Gotland region. (In Swedish) Available from. Accessed. [Google Scholar]
- 4.Panwar A, Batra R, Lydiatt WM, Ganti AK. Human papilloma virus positive oropharyngeal squamous cell carcinoma: A growing epidemic. Cancer Treat Rev. 2014;40:215–219. doi: 10.1016/j.ctrv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, Creson N, Lindholm J, Ye W, Dalianis T, Munck-Wikland E. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, Spencer S, Harris J, Chung CH, Ang KK. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978-2007: Focus on human papillomavirus associated sites. Int J Cancer. 2011;129:733–741. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 8.Olaleye O, Moorthy R, Lyne O, Black M, Mitchell D, Wiseberg J. A 20-year retrospective study of tonsil cancer incidence and survival trends in South East England: 1987-2006. Clin Otolaryngol. 2011;36:325–335. doi: 10.1111/j.1749-4486.2011.02361.x. [DOI] [PubMed] [Google Scholar]
- 9.Klussmann JP, Weissenborn SJ, Wieland U, Dries V, Eckel HE, Pfister HJ, Fuchs PG. Human papillomavirus-positive tonsillar carcinomas: A different tumor entity? Med Microbiol Immunol. 2003;192:129–132. doi: 10.1007/s00430-002-0126-1. (In Swedish) [DOI] [PubMed] [Google Scholar]
- 10.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 11.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: A United States multicenter study. Head Neck. 2011;33:1683–1694. doi: 10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 12.Richmon JD, Quon H, Gourin CG. The effect of transoral robotic surgery on short-term outcomes and cost of care after oropharyngeal cancer surgery. Laryngoscope. 2014;124:165–171. doi: 10.1002/lary.24358. [DOI] [PubMed] [Google Scholar]
- 13.Nichols AC, Faquin WC, Westra WH, Mroz EA, Begum S, Clark JR, Rocco JW. HPV-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;140:228–234. doi: 10.1016/j.otohns.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 14.O'Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012;48:1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse and survival. Int J Cancer. 2000;89:300–304. doi: 10.1002/1097-0215(20000520)89:3<300::AID-IJC14>3.3.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman M, Castle PE. Human papillomavirus: Epidemiology and public health. Arch Pathol Lab Med. 2003;127:930–934. doi: 10.5858/2003-127-930-HPEAPH. [DOI] [PubMed] [Google Scholar]
- 17.D'souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011;55(Suppl 1):S5–S11. doi: 10.1016/j.ypmed.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinau M, Saraiya M, Goodman MT, Peters ES, Watson M, Cleveland JL, Lynch CF, Wilkinson EJ, Hernandez BY, Copeland G. Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction, United States. Emerg Infect Dis. 2014;20:822–828. doi: 10.3201/eid2005.131311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osazuwa-Peters N. Human papillomavirus (HPV), HPV-associated oropharyngeal cancer and HPV vaccine in the United States-do we need a broader vaccine policy? Vaccine. 2013;31:5500–5505. doi: 10.1016/j.vaccine.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Williams JR. The Declaration of Helsinki and public health. Bull World Health Organ. 2008;86:650–652. doi: 10.2471/BLT.08.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 22.Smits HL, Tieben LM, Tjong-A-Hung SP, Jebbink MF, Minnaar RP, Jansen CL, ter Schegget J. Detection and typing of human papillomaviruses present in fixed and stained archival cervical smears by a consensus polymerase chain reaction and direct sequence analysis allow the identification of a broad spectrum of human papillomavirus types. J Gen Virol. 1992;73:3263–3268. doi: 10.1099/0022-1317-73-12-3263. [DOI] [PubMed] [Google Scholar]
- 23.Detre S, Saclani Jotti G, Dowsett M. A ‘quickscore’ method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Statistical Central Office. Stockholm, Sweden: 2012. Sweden Official Statistics: Urban areas 2010. (In Swedish) [Google Scholar]
- 25.Statistical Central Office. Stockholm, Sweden: 2012. Sweden Official Statistics: Smaller localities 2010. (In Swedish) [Google Scholar]
- 26.Nordlund LA. Trends in smoking habits and lung cancer in Sweden. Eur J Cancer Prev. 1998;7:109–116. [PubMed] [Google Scholar]
- 27.Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35:98–108. doi: 10.1111/j.1600-0528.2007.00299.x. [DOI] [PubMed] [Google Scholar]
- 28.Hammarstedt L, Dahlstrand H, Lindquist D, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127:988–992. doi: 10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 29.Näsman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 30.Dahlstrand HM, Dalianis T. Presence and influence of human papillomaviruses (HPV) in Tonsillar cancer. Adv Cancer Res. 2005;93:59–89. doi: 10.1016/S0065-230X(05)93002-9. [DOI] [PubMed] [Google Scholar]
- 31.Syrjänen S. HPV infections and tonsillar carcinoma. J Clin Pathol. 2004;57:449–455. doi: 10.1136/jcp.2003.008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oguejiofor KK, Hall JS, Mani N, et al. The Prognostic Significance of the Biomarker p16 in Oropharyngeal Squamous Cell Carcinoma. Clin Oncol (R Coll Radiol) 2013;25:630–638. doi: 10.1016/j.clon.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Mellin Dahlstrand H, Lindquist D, Björnestål L, et al. P16(INK4a) correlates to human papillomavirus presence, response to radiotherapy and clinical outcome in tonsillar carcinoma. Anticancer Res. 2005;25:4375–4383. [PubMed] [Google Scholar]
- 34.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 35.Lindquist D, Romanitan M, Hammarstedt L, Näsman A, Dahlstrand H, Lindholm J, Onelöv L, Ramqvist T, Ye W, Munck-Wikland E, Dalianis T. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol. 2007;1:350–355. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer CA, Kampmann M, Zlobec I, Green E, Tornillo L, Lugli A, Wolfensberger M, Terracciano LM. p16 expression in oropharyngeal cancer: Its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann Oncol. 2010;21:1961–1966. doi: 10.1093/annonc/mdq210. [DOI] [PubMed] [Google Scholar]
- 37.Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, Califano JA, Tufano RP, Koch WM. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 38.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 39.Marklund L, Hammarstedt L. Impact of HPV in Oropharyngeal Cancer. J Oncol. 2011;2011:509036. doi: 10.1155/2011/509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemminki K, Dong C, Frisch M. Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur J Cancer Prev. 2000;9:433–437. doi: 10.1097/00008469-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, Graubard BI, Chaturvedi AK. Prevalence of oral HPV infection in the United States, 2009-2010. Jama. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]



