Abstract
Objectives
Obstructive respiratory events often terminate with an associated respiratory-related leg movement (RRLM). Such leg movements are not scored as periodic leg movements (PLMS), though the criteria for distinguishing RRLM from PLMS differ between American Academy of Sleep Medicine (AASM) and World Association of Sleep Medicine (WASM)/ International Restless Legs Syndrome Study Group (IRLSSG) scoring manuals. Such LMs may have clinical significance in patients with obstructive sleep apnea (OSA). We examined the prevalence and correlates of RRLM in men with OSA.
Methods
A case control sample of 575 men was selected from all men with an apnea-hypopnea index (AHI, ≥3% desaturation criteria) ≥ 10 and good data from piezoelectric leg movement sensors at the first home sleep study in the MrOS cohort (mean age=76.8 years). Sleep studies were rescored for RRLMs using five different RRLM definitions varying both latency of leg movement onset from respiratory event termination and duration of the leg movement. Quartile of RRLM% (the number of RRLM/the number of hypopneas+apneas) was derived.
Results
Nonparametric densities of RRLM% were most influenced by alterations in the latency rather than the duration of the LM. The most liberal RRLM definition (latency 0–5 sec, duration 0.5–10 sec) led to a median RRLM% of 23.4 (IQ range 12.41, 37.12) in this sample. The average AHI and arousal index increased as quartile of RRLM% increased, as well as prevalence of COPD. The prevalence of those with a history of hypertension decreased as RRLM% increased. Non-caucasian race was associated with lower RRLM%.
Conclusion
Within an elderly sample with moderate to severe OSA, piezoelectric-defined RRLM% is associated with a number of sleep-related and demographic factors. Further study of the optimal definition, predictors and consequences of RRLM is warranted.
Keywords: Leg Movement, obstructive sleep apnea, respiratory related events, cardiovascular disease, hypertension
1. Introduction
The termination of obstructive respiratory events (apneas, hypopneas and respiratory effort related arousals (RERA)) is typically associated with oxygen desaturation, arousal from sleep, and sympathetic activation [1–4]. Respiratory-related leg movements (RRLMs), recorded from the anterior tibialis, may also occur at the termination of obstructive respiratory events. In the original scoring rules of the American Sleep Disorders Association [5] such leg movements were scored and specifically counted as related to obstructive respiratory events. However, recent updates of the scoring rules [6, 7] leave leg movements unscored if they occur during a period from 0.5 seconds preceding, to 0.5 seconds following, an obstructive respiratory event [6] or within 0.5 seconds of the termination of the respiratory event [7]. While this definition does differentiate such RRLMs from those in a series of periodic limb movement during sleep (PLMS), it also leads to the disappearance of RRLMs from clinical reports, suggesting that they have no clinical significance.
Obstructive sleep apnea (OSA) is a demonstrated risk factor for incident hypertension and cardiovascular disease (CVD)[8, 9]. The relative contributions of oxygen desaturation and sleep-related arousal to this risk are unclear. There is a suggestion that RRLMs may also be a marker of, or contributor to, this cardiovascular risk [5, 10]. In a within-subjects study of patients with sleep apnea, obstructive respiratory events that terminated with a leg movement (LM) produced larger heart rate increases than those which did not have an associated LM, independent of the length of the respiratory event and its associated oxygen desaturation [10]. These findings may indicate that the autonomic nervous system is more activated when respiratory obstructive events terminate with a leg movement, and that such RRLMs may potentially be a marker for increased cardiovascular risk. Similarly, such RRLMs may be markers of more severe sleep disruption in the context of OSA and as such may be correlated with medical conditions which are associated with sleep disturbance.
Although RRLMs are frequently associated with apnea and hypopnea termination and may be markers of cardiovascular dysfunction, they have received little attention compared with other sleep-related leg movements, such as PLMS. Thus, we examined the prevalence and correlates of RRLMs in elderly males with moderate to severe OSA.
2. Materials and methods
2.1. Participants
During the Osteoporotic Fractures in Men Study (MrOS) baseline examination from 2000 to 2002, 5,994 community-dwelling men 65 years or older were enrolled at six clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California [11, 12]. In order to participate, men needed to be able to walk without assistance and must not have had a bilateral hip replacement.
The Outcomes of Sleep Disorders in Men (MrOS Sleep) Study, an ancillary study of the parent MrOS cohort, recruited 3,135 participants for a comprehensive sleep assessment between December 2003 and March 2005. The present study is a case-control study within the MrOS Sleep study (Figure 1).
Figure 1. Flowchart of participants.
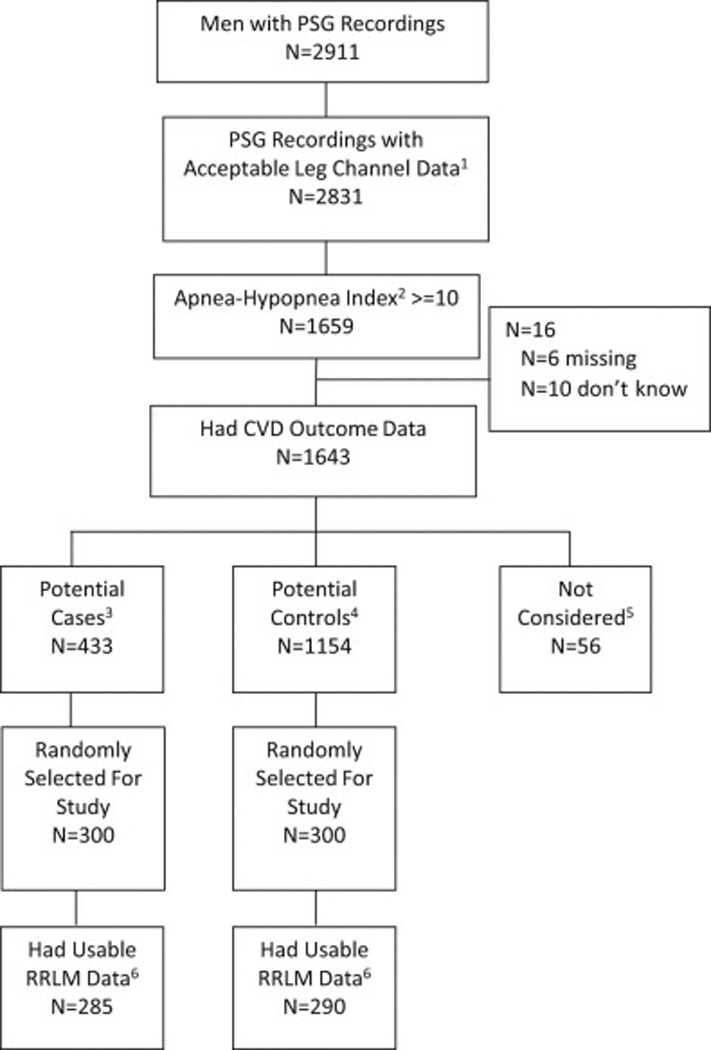
1Acceptable leg channel data was defined as those studies with acceptable signal quality on the leg sensors for >=75% of sleep time.
2Apnea Hypopnea Index at >=3% desaturation level.
3Cases were selected from those with incident CVD over 6 years of follow-up, defined as coronary heart disease (CHD), peripheral vascular disease (PVD), or Cerebrovascular disease (CER): CHD: Acute myocardial infarction, coronary artery bypass surgery, ischemic congestive heart failure, mechanical coronary revascularization, non ST-elevation myocardial infarction, ST-elevation myocardial infarction, hospitalization for unstable angina, sudden CHD death, other CHD event. PVD: Acute arterial dissection, acute arterial occlusion, acute arterial rupture, vascular surgery. CER: Stroke, TIA.
4Controls were selected from those without incident CVD or incident deep vein thrombosis, pulmonary embolus, sudden death not specified, or other CVD event not classified above.
5Those not considered are men without incident CVD but had incident deep vein thrombosis, pulmonary embolus, sudden death not specified, or other CVD event not classified above.
6Twenty-five PSG recordings selected for the case-control study were unable to be read due to lost file, could not open file, no sleep staging scored, etc.
Of the 3,135 men recruited as participants in the MrOS sleep study, 2,911 underwent in-home single night polysommnography (PSG). Of these, 1,659 had an apnea-hypopnea index (AHI) ≥ 10 (≥3% desaturation criteria) and had acceptable leg movement recordings, achieved with piezoelectric sensors. Of these participants, 16 had missing cardiovascular disease (CVD) outcome data and thus 1,643 were available for further analysis. As this was part of a larger study of the predictive value of RRLM for CVD, the next phase of participant selection was based on CVD status. CVD was defined as coronary heart disease (CHD), peripheral vascular disease (PVD), or cerebrovascular disease (CER) (see definitions in Figure 1). Of the 1,643 participants, potential cases were selected (n=433) from those with incident CVD over 6 years of follow-up. Potential controls were selected (n=1,154) from those without incident CVD. From these two groups of participants, 600 participants (300 cases and 300 controls) were randomly selected for the study. Fifteen cases and 10 controls were excluded due to lost, inaccessible or unscored PSG files. Eventually, 575 participants (285 cases and 290 controls; mean age=76.8 years) were included in this analysis.
The study protocols were approved by the Institutional Review Board at each site, and participants provided signed informed consent.
2.2. Data measurement and PSG scoring
Data measurement was carried out in-home over a single night by unattended polysomnography (PSG) (Safiro, Compumedics, Inc®, Melbourne, Australia). The PSG recording montage consisted of the following: C3/A2 and C4/A1 electroencephalography (EEG), bilateral electrooculography, bipolar submental electromyography, thoracic/abdominal respiratory inductance plethysmography, naso-oral thermistry, nasal pressure transduction, oximetry, ECG, and bilateral anterior tibialis piezoelectric movement sensors. Centrally trained staff performed home visits for unit setup and impedance value verification for each channel as previously described [13]. Data were downloaded to a central server at the Central Sleep Reading Center (Cleveland, OH) and scored by certified research polysomnographic technologists using standard criteria[14, 15]. Apnea was defined as >90% decrement in thermistor amplitude for at least 10 seconds and was categorized as obstructive if effort persisted on thoraco-abdominal inductance channels or as central if there was no effort detected. Hypopnea was defined as at least 30% reduction in nasal pressure transduction or summed inductance bands for at least 10 seconds. This analysis included only apneas and hypopneas associated with ≥3% desaturation [16]. The apnea-hypopnea index (AHI) was calculated as the total number of apneas and hypopneas per hour of sleep. The central apnea index (CAI) and the obstructive apnea index (OAI) were calculated as the total number of apneas of that type at any desaturation level per hour of sleep. The oxygen desaturation index (ODI) was calculated as the number of desaturations of 3% or higher per hour of sleep. Nocturnal hypoxemia was expressed as the percent of time during overnight sleep in which arterial oxygen saturation (SaO2) was below 90% (% of sleep time with SaO2 <90%). Arousals were scored according to the American Academy of Sleep Medicine criteria [15]. The arousal index was calculated as the number of arousals per hour of sleep. Two additional measures of sleep fragmentation were calculated: wake after sleep onset (WASO) and sleep efficiency. WASO was defined as the minutes scored awake during the sleep period after sleep onset. Sleep efficiency was defined as the percent of time determined as sleep during the sleep period.
2.3. Scoring for RRLM
RRLM were scored based upon five different variations of the World Association of Sleep Medicine (WASM) criteria [7]: 1) an LM lasting 0.5–5 sec which had its onset within 2 seconds (before or after) of the respiratory event termination (02D1), 2) an LM lasting 0.5–10 sec which had its onset within 2 seconds (before or after) of the respiratory event termination (02D2), 3) an LM lasting 0.5–5 sec which had its onset within 2–5 seconds (before or after) of the respiratory event termination (25D1), 4) an LM lasting 0.5–10 sec which had its onset within 2–5 seconds (before or after) of the respiratory event termination (25D2), 5) an LM lasting 0.5–10 sec which had its onset within 5 seconds (before or after) of the respiratory event termination (05D2) (Figure 2). Respiratory event termination was defined as the nadir of the nasal pressure signal (for scoring hypopnea) or of the thermistor signal (for scoring apnea) immediately before the first breath which resumed normal respiration. In the case where the leg movement was part of a series of periodic leg movements, occurred at the end of a respiratory event, and met the criteria in the present study for a RRLM, the leg movement was counted as a RRLM.
Figure 2. Definitions of rescored RRLM.
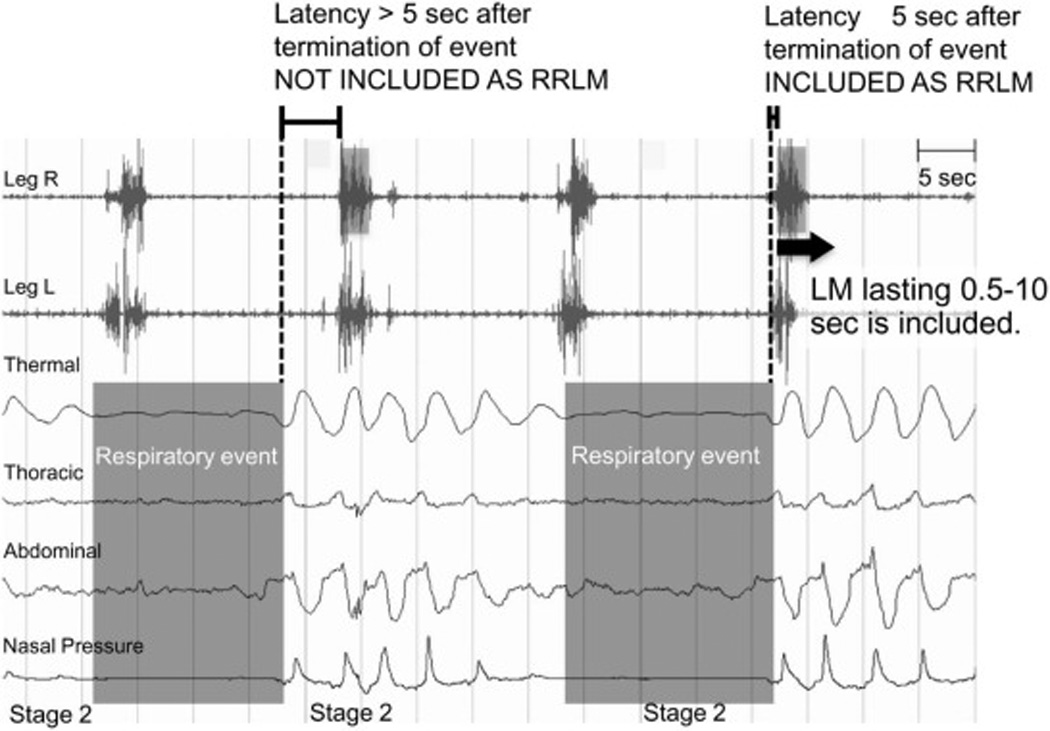
Respiratory-related leg movements (RRLMs) were scored based upon five different variations of the WASM criteria (Zucconi et al., 2006): Latency (from respiratory event at ≥3% desaturation): 0–2, 0–5, 2–5 sec and differing duration of leg movements: 0.5–5, 0.5–10 seconds. Respiratory event termination was defined as the nadir of the nasal pressure (for scoring hypopnea) or of the thermal sensor (for scoring apnea) signal immediately before the first breath which resumed normal respiration. In the case where the leg movement was part of a series of periodic leg movements, occurred at the end of a respiratory event, and met the criteria in the present study for a RRLM, the leg movement was rescored as a RRLM. The LM is not included as RRLM if the LM latency >5 seconds after the termination of event. The LM is included as RRLM if the LM latency ≤ 5 seconds after the termination of event.
The primary predictor variable was the RRLM%, calculated as the number of RRLM divided by the number of hypopneas+apneas. The RRLM% was divided into quartiles for the most inclusive RRLM definition (an LM lasting 0.5–10 sec which had its onset within 5 seconds (before or after) of the respiratory event termination). We also examined RRLMI, which is the number of RRLM per hour of sleep.
2.4. Other Measures
Participants completed questionnaires on demographics, medical history, physical activity, smoking, and alcohol use. History of coronary heart disease was defined as self-reported prior diagnosis of myocardial infarction, angina, bypass surgery, congestive heart failure, pacemaker placement or angioplasty. History of peripheral vascular disease was defined as prior diagnosis of intermittent claudication, aortic aneurysm repair, angioplasty, or bypass procedure of lower-extremity arteries or carotid endarterectomy. Prescription and nonprescription medications were inventoried, verified by pill bottle examination, and matched to ingredient(s) with the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City)[17]. The Geriatric Depression Scale was used to assess the number of depressive symptoms [18]. The Physical Activity Scale for the Elderly measured the level of physical activity [19]. The Epworth Sleepiness Scale (ESS), a self-administered questionnaire, was used to classify subjective daytime sleepiness. Scores on the ESS range from 0–24, with a score >10 indicating excessive daytime sleepiness [20, 21]. Participants completed the Pittsburgh Sleep Qulaity Index (PSQI), a validated measure of subjective sleep quality and sleep disturbances over a one-month time period. Global PSQI scores range from 0–21 and a score >5 is indicative of poor sleep [22]. Resting blood pressure, weight and height were measured. Body mass index (BMI) was calculated.
2.5. Statistical Analysis
The different definitions of RRLM% were summarized as median (interquartile range) by case and control status, and the 2 groups were compared using a Wilcoxon rank-sum test. Nonparametric kernel estimation densities of RRLM% were plotted for each of the five definitions. Participants’ characteristics were compared across case-control status with a t-test for normally distributed continuous variables, a Wilcoxon rank-sum test for skewed continuous data, and a chi-square test for categorical data. Sampling weights were calculated to account for unequal selection probabilities (CVD cases: 1/(285/433); controls: 1/(290/1154)). These weights were used in all analyses to produce estimates that were representative of the MrOS Sleep cohort. Participants’ characteristics were compared across quartile of RRLM% categories with ANOVA for continuous variables, and a Rao-Scott chi-square test for categorical variables. P-values testing linear trend were performed using the quartile variable as a continuous variable using logistic regression for dichotomous covariates and linear regression for continuous covariates. Models were further adjusted by AHI to examine if statistically significant relationships between RRLM% and the characteristic were independent of AHI. Skewed continuous variables were transformed to meet normality assumptions for the p-value estimations. All significance levels reported were 2-sided. Analyses were conducted with SAS version 9.2 (SAS Institute Inc, Cary, NC).
3. Results
Table 1 indicates demographic characteristics by case-control status. Compared to controls, the cases are on average one year older, have more depressive symptoms, and have higher systolic blood pressure. The cases were more likely to have an existing medical condition, to take beta blockers, and to have higher levels of nocturnal hypoxemia.
Table 1.
Characteristics by Case-Control Status
| All | Control | Case | ||
|---|---|---|---|---|
| Characteristic | (N= 575) | (N= 290) | (N= 285) | P-value1 |
| Age, y | 76.81 ± 5.38 | 76.27 ± 5.35 | 77.36 ± 5.36 | 0.01 |
| Not of Caucasian race | 38 (6.61) | 20 (6.9) | 18 (6.32) | 0.78 |
| Body mass index, kg/m2 | 28.01 ± 3.89 | 27.93 ± 3.89 | 28.08 ± 3.88 | 0.64 |
| Depression, GDS score (range 0 to 15) | 1.94 ± 2.19 | 1.83 ± 2.26 | 2.05 ± 2.12 | 0.03 |
| History of self-reported medical conditions: | ||||
| Diabetes | 79 (13.76) | 30 (10.34) | 49 (17.25) | 0.02 |
| Stroke or TIA | 70 (12.2) | 23 (7.93) | 47 (16.55) | 0.002 |
| Coronary heart disease2 | 218 (38.11) | 87 (30.1) | 131 (46.29) | <.0001 |
| COPD | 32 (5.57) | 12 (4.14) | 20 (7.04) | 0.13 |
| Hypertension | 308 (53.66) | 135 (46.55) | 173 (60.92) | 0.0006 |
| PVD | 79 (14.18) | 26 (9.22) | 53 (19.27) | 0.0007 |
| Diastolic blood pressure, mmHg | 68.08 ± 9.35 | 68.3 ± 9.32 | 67.84 ± 9.39 | 0.56 |
| Systolic blood pressure, mmHg | 127.77 ± 16.83 | 126.36 ± 14.6 | 129.22 ± 18.75 | 0.04 |
| Current antidepressant use | 56 (9.74) | 29 (10) | 27 (9.47) | 0.83 |
| Current prescription sleep medication use | 11 (1.91) | 5 (1.72) | 6 (2.11) | 0.74 |
| Current use of beta blockers | 184 (32) | 71 (24.48) | 113 (39.65) | 0.0001 |
| Smoking | ||||
| Never | 229 (39.9) | 121 (41.72) | 108 (38.03) | 0.66 |
| Past | 333 (58.01) | 163 (56.21) | 170 (59.86) | |
| Current | 12 (2.09) | 6 (2.07) | 6 (2.11) | |
| Alcohol intake (drinks/week) | ||||
| 0–2 | 349 (61.01) | 169 (58.48) | 180 (63.6) | 0.26 |
| 3–13 | 184 (32.17) | 96 (33.22) | 88 (31.1) | |
| 14+ | 39 (6.82) | 24 (8.3) | 15 (5.3) | |
| PASE physical activity score | 144.19 ± 71.68 | 142.6 ± 66.38 | 145.81 ± 76.8 | 0.59 |
| Apnea-hypopnea index | 25.71 ± 14.67 | 25.19 ± 14.18 | 26.24 ± 15.16 | 0.47 |
| Obstructive apnea index | 8.25 ± 10.00 | 8.00 ± 10.12 | 8.51 ± 9.90 | 0.27 |
| Central apnea index | 2.52 ±6.31 | 2.69 ± 6.42 | 2.34 ± 6.20 | 0.86 |
| Oxygen desaturation index | 30.09 ±15.53 | 29.46 ± 15.05 | 30.74 ± 16.01 | 0.35 |
| Percent of sleep time with SaO2<90% ≥10% | 107 (18.61) | 44 (15.17) | 63 (22.11) | 0.03 |
| Sleep efficiency, % | 75.05 ± 11.77 | 75.91 ± 11.39 | 74.16 ± 12.09 | 0.09 |
| Arousal Index | 26.96 ± 12.28 | 26.55 ± 11.36 | 27.38 ± 13.15 | 0.42 |
| WASO, min | 120.96 ± 66.38 | 118.32 ± 66.71 | 123.65 ± 66.05 | 0.34 |
| Pittsburgh sleep quality index (range 0–21) | 5.87 ± 3.35 | 5.62 ± 3.11 | 6.11 ± 3.57 | 0.08 |
| Pittsburgh sleep quality index >5 | 273 (47.56) | 133 (45.86) | 140 (49.3) | 0.41 |
| Epworth sleepiness scale (range 0–24) | 6.5 ± 3.95 | 6.19 ± 3.7 | 6.82 ± 4.17 | 0.05 |
| Epworth sleepiness scale>10 | 89 (15.48) | 41 (14.14) | 48 (16.84) | 0.37 |
GDS, Geriatric Depression Scale; TIA, Transient ischemic attack; COPD, Chronic obstructive pulmonary disease; PVD, peripheral vascular disease; PASE score, physical activity score of elderly; SaO2,oxygen saturation; WASO, wake after sleep onset.
Results shown as mean ± SD or n(percent).
P-values for continuous variables are from a t-test for normally distributed variables, a Wilcoxon rank-sum test for skewed data GDS score, apnea-hypopnea index, central apnea index, obstructive apnea index, oxygen desaturation index. P-values for categorical data from a chi-square test.
Self reported history of coronary heart disease includes myocardial infarction, angina, congestive heart failure, bypass surgery, angioplasty or pacemaker placement.
The RRLM % for each definition for all participants, those who developed CVD over 6 years of follow-up and controls, is presented in Table 2. There was no difference in RRLM%, RRLM or RRLMI between cases and controls for any of the five definitions. The RRLM% was significantly correlated with RRLMI (latency 0–5, duration 0.5 to 10) (r=0.97, p<0.0001).
Table 2.
Differing Definitions of RRLM by Case and Control Status, Median (Interquartile range)
| RRLM | Control | Case | |||
|---|---|---|---|---|---|
| Measure | Definition | Overall | No Incident CVD | Had Incident CVD | P-value1 |
| RRLM% | Latency 0–5 sec, duration 0.5–10 sec | 23.42 (12.41, 37.12) | 22.06 (12.63, 38.14) | 24.1 (12.39, 36.47) | 0.85 |
| Latency 0–2 sec, duration 0.5–5 sec | 10.96 (5.59, 18.95) | 11.03 (5.75, 19.81) | 10.96 (5.59, 18.22) | 0.59 | |
| Latency 0–2 sec, duration 0.5–10 sec | 16 (8.08, 25.81) | 16 (8.16, 26.09) | 16 (8.08, 25.27) | 0.61 | |
| Latency 2–5 sec, duration 0.5–5 sec | 4.55 (2.4, 7.74) | 4.47 (2.41, 7.46) | 4.65 (2.37, 7.84) | 0.46 | |
| Latency 2–5 sec, duration 0.5–10 sec | 6.34 (3.47, 11.11) | 6.35 (3.39, 10.68) | 6.33 (3.61, 11.24) | 0.46 | |
| #RRLM | Latency 0–5 sec, duration 0.5–10 sec | 28 (13, 57) | 27 (13, 59) | 28 (12, 55) | 0.82 |
| Latency 0–2 sec, duration 0.5–5 sec | 13 (6, 28) | 12.5 (6, 29) | 14 (5, 27) | 0.71 | |
| Latency 0–2 sec, duration 0.5–10 sec | 19 (8, 39) | 19 (9, 40) | 19 (8, 38) | 0.66 | |
| Latency 2–5 sec, duration 0.5–5 sec | 6 (3, 12) | 5.5 (2, 11) | 6 (3, 12) | 0.56 | |
| Latency 2–5 sec, duration 0.5–10 sec | 8 (4, 17) | 8 (4, 17) | 8 (4, 17) | 0.62 | |
| RRLMI | Latency 0–5 sec, duration 0.5–10 sec | 4.69 (2.26, 9.55) | 4.38 (2.33, 9.47) | 5.04 (2.16, 9.61) | 0.78 |
| Latency 0–2 sec, duration 0.5–5 sec | 2.24 (1.01, 4.76) | 2.11 (1.06, 4.75) | 2.47 (0.95, 4.76) | 0.87 | |
| Latency 0–2 sec, duration 0.5–10 sec | 3.26 (1.45, 6.49) | 3.16 (1.49, 6.42) | 3.37 (1.4, 6.6) | 0.97 | |
| Latency 2–5 sec, duration 0.5–5 sec | 0.97 (0.45, 1.98) | 0.95 (0.4, 1.95) | 1.02 (0.49, 2.07) | 0.30 | |
| Latency 2–5 sec, duration 0.5–10 sec | 1.31 (0.66, 2.82) | 1.28 (0.66, 2.76) | 1.41 (0.68, 2.83) | 0.38 | |
p-value from a Wilcoxon sign-rank test
Figure 3 shows the nonparametric densities of RRLM% using the various RRLM definitions. One plot is shown combining both cases and controls because the RRLM% measures were similar between the case and control groups. The most liberal RRLM% definition (05D2: latency 0–5 sec, duration 0.5–10 sec) produced the highest median RRLM% (05D2: 23.4%, 02D1: 11.0%, 02D2: 16.0%, 25D1: 4.6%, 25D2: 6.3%). In general, RRLM% density was most influenced by changes in latency (from respiratory event termination) rather than duration of the LM itself. For example, the median difference between RRLM% calculation when latency was held constant and the duration varied was −5.06 and −2.22 for latencies 0–2 sec and 2–5 sec, respectively. The median difference between RRLM% calculation when duration is held constant and latencies vary was 10.45 and 7.61 for duration 0.5–10 sec and latency 0–2 sec vs. 2–5 sec, duration 0.5–5 and latency 0–2 sec vs. 2–5 sec respectively. Specifically, latencies limited to 2–5 seconds led to the majority of participants having much lower RRLM% (duration 0.5–5 sec: 15.48% had RRLM >10%; duration 0.5–10 sec: 29.57% had RRLM >10%) whereas definitions that included shorter latencies (0–2 seconds) had a higher percentage of cases with RRLM% greater than 10% (duration 0.5–5 sec: 54.26% had RRLM >10%; duration 0.5–10 sec: 69.57% had RRLM >10%). Additionally, Figure 4 shows a scatterplot of RRLM% at 0–2 sec latency and 0–5 sec latency for duration 0.5 to 10 sec. As the scatterplot indicates, there was a regular association between 0–5 sec latency and 0–2 sec latency (when 0–5 sec latency is at 30%, 0–2 sec latency is 20%), suggesting that the 0–5 sec latency detects more RRLM% than the 0–2 sec latency.
Figure 3. Nonparametric density of RRLM%.
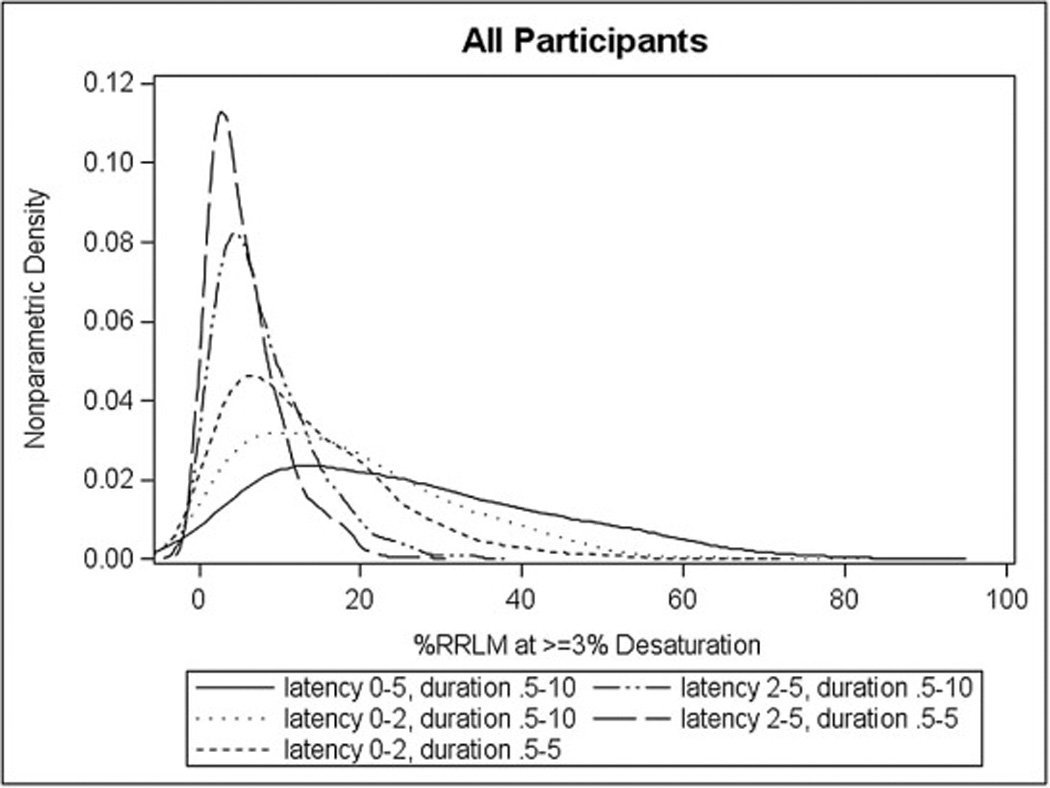
The density plots for the %RRLM variables at all the different timeframe/durations: an overall plot.
Figure 4. Scatter plot of RRLM% at 0–2 latency and 0–5 latency for duration .5 to 10 sec.
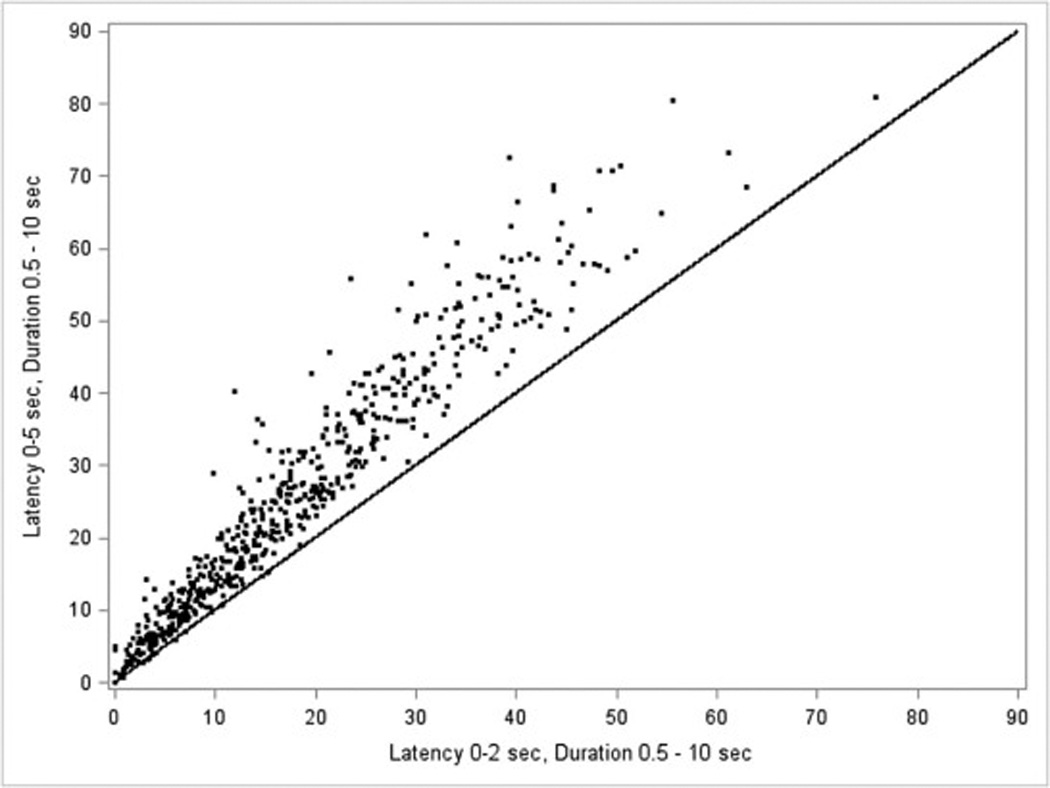
The scatterplot of RRLM% at 0–2 sec latency and 0–5 sec latency for duration 0.5 to 10 sec. The line is where the values are equal
Table 3 shows the demographic, sleep-related and medical correlates of RRLM% (by quartile) for the most inclusive RRLM definition (latency from respiratory event termination 0–5 sec and duration 0.5–10 sec). The average AHI, OAI, ODI, and arousal index all increased as the quartile of RRLM% increased (p-trend <0.001, respectively). History of COPD (p=0.04) was associated with higher RRLM% (Table 3). The prevalence of self-reported history of hypertension decreased as RRLM% increased (p-trend=0.01)(Table 3). Those in the higher quartiles of RRLMI% had higher rates of excessive daytime sleepiness (p-trend=0.03). No associations were seen between RRLM% and age or BMI. Results from the other definitions of RRLM% were largely similar (data not shown). The prevalence of greater hypoxemia (≥10% percent of sleep time with SaO2<90%) decreased as RRLM% increased (p=0.007). The correlation of RRLM% with AHI was 0.19 (p=ns). The correlation of RRLM% with arousal index was 0.34. After further adjustment for AHI, all results remained significant (p<0.05) except the association of RRLM% with excessive daytime sleepiness (p=0.08) and ODI (p=0.42).
Table 3.
Characteristics of RRLM% (duration of 0–10 seconds, latency of 0–5 seconds from respiratory event termination)
| Q1: < 12.41 | Q2: 12.41 to < 23.42 | Q3: 23.42 to < 37.12 | Q4: >= 37.12 | |||
|---|---|---|---|---|---|---|
| Characteristic | (N= 143) | (N= 144) | (N= 144) | (N= 144) | P-value1 | P-trend2 |
| Age, y | 76.83 (75.91, 77.75) | 76.76 (75.84, 77.68) | 75.57 (74.59, 76.55) | 77.02 (76.03, 78) | 0.10 | 0.82 |
| Not of Caucasian race | 15 (10.21) | 11 (8.12) | 6 (3.83) | 6 (4.62) | 0.17 | 0.05 |
| Body mass index, kg/m2 | 28 (27.31, 28.69) | 28.13 (27.43, 28.84) | 27.97 (27.25, 28.69) | 27.79 (27.11, 28.46) | 0.90 | 0.60 |
| Depression, GDS score (range 0 to 15) | 1.54 (1.24, 1.83) | 1.99 (1.54, 2.44) | 2.03 (1.59, 2.47) | 1.99 (1.6, 2.39) | 0.24 | 0.08 |
| History of self-reported medical conditions: | ||||||
| Diabetes | 21 (13.79) | 19 (12.25) | 20 (11.65) | 19 (11.22) | 0.93 | 0.53 |
| Stroke or TIA | 19 (11.13) | 17 (9.74) | 15 (8.91) | 19 (11.22) | 0.91 | 0.98 |
| Coronary heart disease3 | 59 (35.43) | 49 (33.35) | 55 (34.22) | 55 (35.06) | 0.99 | 1.00 |
| COPD | 7 (3.34) | 6 (2.78) | 7 (4.25) | 12 (9.23) | 0.04 | 0.03 |
| Hypertension | 83 (57.91) | 85 (55.81) | 75 (44.53) | 65 (43.22) | 0.04 | 0.01 |
| PVD | 19 (10.46) | 22 (13.57) | 21 (14.87) | 17 (8.98) | 0.42 | 0.74 |
| Diastolic blood pressure, mmHg | 67.79 (66.05, 69.53) | 69.47 (67.87, 71.06) | 68.05 (66.29, 69.81) | 67.35 (65.75, 68.94) | 0.23 | 0.45 |
| Systolic blood pressure, mmHg | 125.89 (122.78, 128.99) | 128.83 (126.4, 131.26) | 126.93 (124.45, 129.42) | 126.79 (123.98, 129.6) | 0.44 | 0.92 |
| Current antidepressant use | 15 (10.21) | 9 (5.62) | 19 (13.92) | 13 (10.2) | 0.20 | 0.51 |
| Current prescription sleep medication use | 2 (0.78) | 5 (3.58) | 3 (2.59) | 1 (0.37) | 0.08 | 0.41 |
| Current use of beta blockers | 57 (35.91) | 36 (21.32) | 45 (30.75) | 46 (27.2) | 0.07 | 0.38 |
| Smoking | ||||||
| Never | 50 (35.7) | 59 (41.6) | 61 (43.42) | 59 (42.2) | 0.39 | |
| Past | 91 (62.89) | 82 (57.08) | 77 (52.07) | 83 (56.47) | ||
| Current | 2 (1.4) | 2 (1.32) | 6 (4.5) | 2 (1.34) | ||
| Alcohol intake (drinks/week) | ||||||
| 1: 0–2 | 87 (58.17) | 86 (59.13) | 89 (61.75) | 87 (60.57) | 0.64 | |
| 2: 3–13 | 43 (32.69) | 50 (35.96) | 48 (32.66) | 43 (29.2) | ||
| 3: 14+ | 12 (9.14) | 7 (4.92) | 7 (5.59) | 13 (10.24) | ||
| PASE physical activity score | 130.37 (119.19, 141.55) | 151.6 (137.78, 165.42) | 148.51 (137.87, 159.15) | 143.24 (131.31, 155.17) | 0.05 | 0.21 |
| Apnea-hypopnea Index | 23.56 (21.05, 26.07) | 22.52 (20.23, 24.81) | 26.68 (24, 29.35) | 29.24 (26.62, 31.87) | <.0001 | 0.0001 |
| Obstructive apnea index | 5.67 (4.49, 6.85) | 6.75 (5.14, 8.36) | 8.22 (6.57, 9.88) | 11.82 (9.5, 14.14) | .0001 | <0.0001 |
| Central apnea index | 3.02 (1.62, 4.41) | 2.4 (1.34, 3.45) | 2.19 (1.22, 3.17) | 2.75 (1.65, 3.85) | 0.96 | 0.75 |
| Oxygen desaturation index | 27.69 (25.13, 30.26) | 26.83 (24.52, 29.13) | 31.99 (28.69, 35.3) | 32.91 (30.33, 35.48) | .0004 | .0003 |
| Percent of sleep time with SaO2<90% | 0.01 | 0.049 | ||||
| ≥10% | 35 (23.61) | 22 (15.06) | 28 (16.98) | 22 (12.93) | ||
| Sleep efficiency, % | 74.2 (72.12, 76.29) | 74.92 (72.86, 76.98) | 76.44 (74.56, 78.33) | 76.23 (74.1, 78.37) | 0.31 | 0.12 |
| Arousal Index | 21.97 (20.37, 23.58) | 25.02 (23.06, 26.98) | 26.9 (24.86, 28.94) | 33.07 (30.86, 35.27) | <.0001 | <.0001 |
| WASO, min | 127.62 (115.12, 140.12) | 119.59 (108.57, 130.61) | 117.48 (105.52, 129.45) | 114.51 (102.32, 126.71) | 0.38 | 0.14 |
| Pittsburgh sleep quality index (range 0–21) | 5.56 (4.92, 6.19) | 5.78 (5.27, 6.29) | 5.63 (5.09, 6.17) | 6.04 (5.47, 6.61) | 0.60 | 0.33 |
| Pittsburgh sleep quality index >5 | 66 (45.04) | 64 (44.61) | 72 (48.66) | 71 (49.03) | 0.85 | 0.43 |
| Epworth sleepiness scale (range 0–24) | 5.94 (5.31, 6.57) | 6.24 (5.54, 6.93) | 7.02 (6.32, 7.71) | 6.31 (5.64, 6.98) | 0.12 | 0.23 |
| Epworth sleepiness scale>10 | 13 (8.81) | 20 (14.92) | 28 (18.33) | 28 (17.55) | 0.15 | 0.03 |
GDS, Geriatric Depression Scale; TIA, Transient ischemic attack; COPD, Chronic obstructive pulmonary disease; PVD, peripheral vascular disease; PASE score, physical activity score of elderly; SaO2,oxygen saturation; WASO, wake after sleep onset.
Results shown as mean (95% CI) or percent.
P-values for continuous variables are from a ANOVA, for categorical variables a Rao-Scott chi-square test.
P-values for trend are from a linear regression for continuous variables, logistic regression for 0/1 variables, using the quartile variable as a continuous variable for the p-trend.
Skewed continuous variables were transformed for normality for the p-value estimation (GDS score, apnea-hypopnea index, central apnea index, obstructive apnea index, oxygen desaturation index).
Self reported history of coronary heart disease includes myocardial infarction, angina, congestive heart failure, bypass surgery, angioplasty or pacemaker placement.
4. Discussion
This is the first study examining the prevalence of respiratory related leg movements in a population sample with OSA≥10 and has the advantage that we used several possible definitions of such leg movements. We found that in a large group of elderly men with moderate to severe OSA, the median prevalence of respiratory events with an associated respiratory–related leg movement was 23.4% (defined as lasting 0.5–10.0 seconds and having its onset within (before or after) 5 seconds of respiratory event termination). As expected, the definition of an RRLM strongly influenced the frequency of such events. In particular, latency from respiratory event termination to leg movement onset was the most important predictor of the presence of an RRLM. As expected, the most liberal definition (latency 0–5 sec and duration 0.5–10 sec) was associated with the highest RRLM%. AHI, arousal index, sleep-related hypoxemia and history of COPD all were associated with higher RRLM%, though age and BMI were not.
4.1 RRLM scoring criteria
Differences between definitions in latency of LM onset from respiratory event termination rather than in duration of the LM had the most influence on nonparametric densities of RRLM%. It is these latency differences that distinguish the AASM from WASM criteria for RRLM. The AASM updated scoring criteria does not count leg movements as PLMs if they occur at any time from 0.5 seconds proceeding the beginning of a respiratory event to 0.5 seconds following the termination of an respiratory event [6]. The WASM/IRLSSG task force excludes leg movements when they precede or follow respiratory event termination by less than 0.5 seconds [7]. An alternate definition has recently been proposed by Manconi et al [23] based on back-averaging leg movement onset with respect to respiratory events and change point analysis. That study, in a clinically referred population with OSA, revealed that the vast majority of RRLMs had their onset during a period extending from 3.5 seconds before the end of respiratory events to 8 seconds after respiratory events. The peak onset was 2.5 s after the end of the respiratory event. Our study used a number of different RRLMs definitions by varying the latency of leg movement onset from respiratory event termination. Although our definition is closer to that of Manconi’s analysis than to the AASM definition, we found substantially fewer RRLM in our population sample (median=23.4%) than they did in their clinical sample (45.5%). A number of possible methodological differences between the studies could account for this difference: our temporal window (0–5 seconds after respiratory event termination) was less extensive than theirs; our study was a population based study of elderly (mean age of 76.8 years) men while theirs was a clinically referred population (predominantly male) with mean age of 56. Their study participants had an AHI>20 required for entry (mean AHI of 57), whereas our entry cutoff was AHI>10 (mean AHI of 26). As our data shows, AHI is a significant, but weak, predictor of RRLM%. If we include only those 299 men with an AHI> 20 to be consistent with the Manconi inclusion requirement, the median RRLM% increases to 28.50%. Importantly, the Manconi study required greater than 15 leg movements per hour of sleep for entry into the analysis whereas we did not limit our study group on this parameter. If we did limit our population to those n=170 with AHI>20 and PLMI>15 the median RRLM% increases to 34.19%. Finally, we used piezoelectric sensors to measure leg movement activity and the Manconi study used surface EMG recordings. The latter is certainly the gold standard for measuring leg movements and this difference may have influenced our analyses.
4.2 RRLM predictors
Our population based sample of elderly male participants was used to assess the predictors of RRLM%. Neither age or BMI predicted RRLM%. On the other hand, RRLMs were more common in Caucasians than non-Caucasians (p=0.05). Previous studies have documented a similar finding for PLMS in adults [24, 25] and children [26]. One potential explanation for this finding is that what we defined as RRLMs in this study were scored as PLMS in those previous reports. Alternately, RRLMs may, like PLMS, be more common in Caucasians, as a result of either differences in iron indices [27] or genetic influences [28].
We were not able to examine the relationship of RRLM% to PLMI and PLMAI due to limitations in our data analysis. Such analyses will be of value in the future to assess whether they in fact are related, potentially based on overactivity of the motor system during sleep [29]. Nevertheless, as Manconi’s data suggests, some leg movements previously scored as PLMS in individuals with coexisting OSA and PLMS were probably time locked to respiratory event termination, and under their revised scoring rules would be considered RRLMs rather than PLMS. An important consequence of such a change, as those authors emphasize, would be that a reexamination of studies investigating the effects of PLMS on sleep, sleepiness and medical illness may be necessary.
RRLM% was also related to both AHI and arousal index. More severe OSA was associated with a higher percentage of respiratory events which terminated with a RRLM. However, this association was not strong, and the overall correlation (r=0.19) was not significant. The mechanism behind this association is unclear. A potential relationship between OSA severity and PLMS [30, 31] has been previously suggested. However, the relationship of AHI with PLMI in previous studies is confounded by the exclusion of leg movements during respiratory events according to AASM scoring criteria. The association of AHI and RRLM% raises the possibility that RRLMs are an unmeasured confounder in studies investigating the association of OSA to various short and long-term outcomes. Further, as they are only weakly related, it suggests that investigation of RRLM may provide additional risk information for such outcome studies. The relationship of RRLM% to arousal index may be a proxy for that of AHI and RRLM% as termination of a respiratory event is usually associated with EEG arousal. As the association of RRLM% with the arousal index was stronger than that with AHI, the presence of a leg movement at respiratory event termination may signal a more “arousing” event. However, in our previous work [10] we did not find a relationship between the presence of RRLM and the duration of respiratory events or of EEG arousals following respiratory events.
Our data demonstrate an increased prevalence of COPD as well as nocturnal hypoxemia in those with higher RRLM%. Again, the mechanism underlying these associations is unknown, though it is possible that hypoxemia related to COPD [32] might predispose to the occurrence of respiratory leg movements. One previous study demonstrated an association of self-reported PLMS and COPD [33].
RRLM% was not associated with central sleep apnea (CSA), suggesting that there is a different appearance of RRLM in CSA compared with that in OSA or other respiratory events. Previous studies reported that the timing of arousal following termination of central apneas is later than observed with obstructive respiratory events (8 seconds versus 1 second, particularly in heart disease patients) [34, 35]. Thus, it would be anticipated that RRLM would also appear at 8 seconds following respiratory event termination. As the longest of our RRLM definitions had a latency from event termination to RRLM onset of 5 seconds it makes sense that we would not have counted leg movements associated with central apnea.
It is also noteworthy that RRLM% was not associated with prevalent diabetes, stroke/TIA, peripheral vascular disease or coronary heart disease or with the use of antidepressants or hypnotics. The lack of association of RRLM% with antidepressants is surprising given the tendency of these medications to provoke PLMS [36– 38]. On the other hand, higher RRLM% was associated with a significantly lower probability of self-reported hypertension. However, current blood pressure was no different in the four quartiles of RRLM%, suggesting that the use of antihypertensives may have corrected this disorder in those with the lowest RRLM%. This is partially confirmed by the near significant association of RRLM% with the use of beta blockers in our population, with the highest use in the lowest quartile of RRLM%. It is also possible that beta blockers suppress RRLM. However, the relationship of the use of this medication and RRLM% was not linear and was primarily driven by their high use in the group with the lowest RRLM% quartile. Similarly, although depression has been associated with lower rates of hypertension [39], there was only a weak association of depression scores with RRLM% in our data.
Higher RRLM% was associated with greater likelihood of excessive daytime sleepiness (measured as an Epworth sleepiness scales score > 10) though there was no association when the ESS was calculated as a continuous variable, and this relationship was further diminished when AHI was added as a covariate. There were no associations between RRLM% and the PSQI.
Our study has several limitations. First, this study was part of the MrOS community-based cohort of elderly men so our results may not be generalizable to women or younger men. Second, measurement of RRLM at a single PSG study does not account for potential night-to-night variability, as is observed with PLMS [40, 41]. Third, we scored leg movements with piezoelectric sensors rather than anterior tibialis EMG electrodes, which may have influenced our sensitivity to leg movements. The latter is certainly the gold standard for PLM recording and was employed in the Manconi study which describes potential new rules for the definition of RRLM. As a means to assess the relationship of these two methods of leg movement scoring in the MrOS data, a recent study found that the correlation of PLM scored by the identical MrOS piezoelectrodes and scoring methodology with simultaneous recording by anterior tibialis EMG was 0.81 [42]. Other recent data demonstrated a very similar correlation (0.78) between piezoelectric monitoring and surface EMG recording [43]. However, as these are two distinct methods of leg movement detection it is not clear that our conclusions can be generalized to PLMS recorded with surface EMG. Fourth, we included only apneas and hypopneas with at least 3% desaturation and thus did not include respiratory event related arousals (RERAs) in our sample. Finally, we used self-reports of medical and psychiatric illness rather than information from medical records.
In the present study, we investigated for the first time the prevalence of RRLMs in a community sample and their associations with medical conditions, sleep-related complaints and medication use of the participants. Increased RRLM are associated with increasing AHI, arousal index, and history of COPD. Further study is needed to establish accepted RRLM definitions and to determine their potential clinical significance.
Supplementary Material
Highlights.
Respiratory events often terminate with a respiratory-related leg movement (RRLM).
RRLMs may have clinical significance in patients with obstructive sleep apnea (OSA).
Sleep studies were rescored for RRLMs using five different RRLM definitions.
The most liberal RRLM definition led to a median RRLM% of 23.4 in this sample.
Increased RRLM% is associated increases in AHI, ArI and is decreased in non-Caucasians in this sample.
Acknowledgements
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hardy JC, Gray K, Whisler S, et al. Sympathetic and blood pressure responses to voluntary apnea are augmented by hypoxemia. J Appl Physiol. 1994;77:2360–2365. doi: 10.1152/jappl.1994.77.5.2360. [DOI] [PubMed] [Google Scholar]
- 2.AASM American Academy of Sleep Medicine. International classification of sleep disorders. 2nd ed. Diagnostic and coding manual; 2005. [Google Scholar]
- 3.Guilleminault C, Poyares D, Rosa A, et al. Heart rate variability, sympathetic and vagal balance and EEG arousals in upper airway resistance and mild obstructive sleep apnea syndromes. Sleep Med. 2005;6:451–457. doi: 10.1016/j.sleep.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Phillips CL, O’Driscoll DM. Hypertension and obstructive sleep apnea. Nat Sci Sleep. 2013;5:43–52. doi: 10.2147/NSS.S34841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ASDA American Sleep Disorders Association. Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–759. [PubMed] [Google Scholar]
- 6.AASM American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification, ed. American Academy of Sleep Medicine; 2007. [Google Scholar]
- 7.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–183. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis. 2011;219:963–968. doi: 10.1016/j.atherosclerosis.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang CK, Jordan AS, White DP, et al. Heart rate response to respiratory events with or without leg movements. Sleep. 2006;29:553–556. doi: 10.1093/sleep/29.4.553. [DOI] [PubMed] [Google Scholar]
- 11.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining analyzing unattended polysomnography data for a multicenter study Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 15.ASDA American Sleep Disorders Association, editor. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. 1992/04/01 ed. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 16.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 17.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 18.Sheikh J, Geriatric YJ. Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 19.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Manconi M, Zavalko I, Fanfulla F, et al. An Evidence-Based Recommendation for a New Definition of Respiratory-Related Leg Movements. Sleep. 2015;38:295–304. doi: 10.5665/sleep.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claman F, Miller T. Premenstrual syndrome and premenstrual dysphoric disorder in adolescence. J Pediatr Health Care. 2006;20:329–333. doi: 10.1016/j.pedhc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Scofield H, Roth T, Drake C. Periodic limb movements during sleep: population prevalence, clinical correlates, and racial differences. Sleep. 2008;31:1221–1227. [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien LM, Holbrook CR, Faye Jones V, et al. Ethnic difference in periodic limb movements in children. Sleep Med. 2007;8:240–246. doi: 10.1016/j.sleep.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- 28.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–647. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 29.Ferrillo F, Beelke M, Canovaro P, et al. Changes in cerebral and autonomic activity heralding periodic limb movements in sleep. Sleep Med. 2004;5:407–412. doi: 10.1016/j.sleep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Al-Alawi A, Mulgrew A, Tench E, et al. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:281–287. [PubMed] [Google Scholar]
- 31.Chervin RD. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med. 2001;164:1454–1458. doi: 10.1164/ajrccm.164.8.2011062. [DOI] [PubMed] [Google Scholar]
- 32.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valipour A, Lavie P, Lothaller H, et al. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12:367–372. doi: 10.1016/j.sleep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Kohnlein T, Welte T, Tan LB, et al. Central sleep apnoea syndrome in patients with chronic heart disease: a critical review of the current literature. Thorax. 2002;57:547–554. doi: 10.1136/thorax.57.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simms T, Brijbassi M, Montemurro LT, et al. Differential timing of arousals in obstructive and central sleep apnea in patients with heart failure. J Clin Sleep Med. 2013;9:773–779. doi: 10.5664/jcsm.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58:510–514. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Hao Y, Jia F, et al. Sertraline and periodic limb movements during sleep: an 8-week open-label study in depressed patients with insomnia. Sleep Med. 2013;14:1405–1412. doi: 10.1016/j.sleep.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Goerke M, Rodenbeck A, Cohrs S, et al. The influence of the tricyclic antidepressant amitriptyline on periodic limb movements during sleep. Pharmacopsychiatry. 2013;46:108–113. doi: 10.1055/s-0032-1331702. [DOI] [PubMed] [Google Scholar]
- 39.Licht CM, de Geus EJ, Seldenrijk A, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631–638. doi: 10.1161/HYPERTENSIONAHA.108.126698. [DOI] [PubMed] [Google Scholar]
- 40.Edinger JD, McCall WV, Marsh GR, et al. Periodic limb movement variability in older DIMS patients across consecutive nights of home monitoring. Sleep. 1992;15:156–161. [PubMed] [Google Scholar]
- 41.Sforza E, Haba-Rubio J. Night-to-night variability in periodic leg movements in patients with restless legs syndrome. Sleep Med. 2005;6:259–267. doi: 10.1016/j.sleep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Claman DM, Ewing SK, Redline S, et al. Periodic leg movements are associated with reduced sleep quality in older men: the MrOS Sleep Study. J Clin Sleep Med. 2013;9:1109–1117. doi: 10.5664/jcsm.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi M, Namba K, Ito E, et al. The validity of the PAM-RL device for evaluating periodic limb movements in sleep and an investigation on night-to-night variability of periodic limb movements during sleep in patients with restless legs syndrome or periodic limb movement disorder using this system. Sleep Med. 2014;15:138–143. doi: 10.1016/j.sleep.2013.08.790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


