Abstract
Importance
The overall incidence of colorectal cancer (CRC) has been decreasing since 1998, but there has been an apparent rise in the incidence of CRC in young adults.
Objective
To evaluate age-related disparities in secular trends in CRC incidence in the United States.
Design
A retrospective cohort study using the Surveillance, Epidemiology, and End Results (SEER) CRC registry. Age at diagnosis was analyzed in 15-year intervals starting at the age of 20. SEER*Stat was used to obtain the annual cancer incidence rates, annual percent change (APC), and corresponding p-values for the secular trends.
Setting
Data was obtained from the National Cancer Institute’s SEER registry.
Patients
All patients diagnosed with colon or rectal cancer from January 1, 1975 through December 31, 2010 (N = 393,241).
Main Outcome
Difference in CRC incidence by age.
Results
Overall age-adjusted CRC incidence rate decreased by 0.92% between 1975 and 2010. There has been a steady decline in the incidence of CRC in patients age 50 years or older, but the opposite trend has been observed for young adults. For patients 20 to 34 years of age, the incidence rate of localized, regional and distant colon and rectal cancer has increased. Increasing incidence rate was also observed for rectal cancer patients aged 35 to 49. Based on current trends, in 2030 the incidence rate for colon and rectal cancer will increase by 90.0% and 124.2% for patients 20 to 34 years of age and by 27.7% and 46.0% for patients 35 to 49 years of age.
Conclusion and Relevance
There has been a significant increase in the incidence of CRC diagnosed in young adults, with a decline in older patients. Further studies are needed to determine the cause for these trends and identify potential preventive and early detection strategies.
Keywords: Colorectal cancer incidence, young adults
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer among men and women with 142,820 estimated new cases and 50,830 estimated deaths in the United States in 2013 (1). Both the incidence and mortality rates of CRC have been decreasing in the United States (1). From 1998 through 2006, the incidence of CRC has decreased 3.0% per year in men and 2.4% per year in women (2). The observed decline in incidence is largely attributed to an increase in screening, specifically colonoscopy which is recommended for all adults 50 years of age or older (2-5). Screening reduces the incidence of CRC by detecting and removing adenomatous polyps (6, 7). Conversely, the incidence of CRC in adults younger than 50, for whom CRC screening is not recommended, appears to be increasing and these patients are more likely to present with advanced disease (8-10). Although no clear reasons have been identified, similar results have been observed in young women with breast cancer (11). Based on these observations, the aim of this study was to perform a population-based evaluation of age-related disparities in secular trends in CRC incidence in the United States.
METHODS
We performed a retrospective cohort study of patients with histologically confirmed colon or rectal cancer utilizing the Surveillance, Epidemiology and End Results (SEER) database. SEER is an authoritative source for cancer incidence, survival and prevalence, currently representing approximately 28% of the United States population. The SEER program collects demographic information (e.g. age, sex and race) and clinical information (e.g. primary tumor site, tumor histology, tumor grade, stage, treatment and survival) from 17 cancer registries across the United States. In order to evaluate secular trends beyond recent years, we utilized cases identified in SEER 9, which includes San Francisco-Oakland, Connecticut, Detroit (Metropolitan), Hawaii, Iowa, New Mexico, Seattle-Puget Sound, Utah and Atlanta (Metropolitan) (12). Because the Seattle-Puget Sound and Atlanta registries joined the SEER program in 1974 and 1975 respectively, we selected the 1975 through 2010 cohort for analysis. Cases reported from nursing homes, autopsies and death certificates were excluded.
Statistical Methods
The annual cancer incidence rates, annual percent change (APC), and corresponding p-values of trends were determined using SEER*Stat (version 8.04, National Cancer Institute). The APC indicates the cancer rate change at a constant percentage of the rate of the previous year and was calculated by fitting a weighted-least-squares regression line to the natural logarithm of the rates. The APC is commonly used to characterize trends in cancer rates over time. We utilized the widely accepted built in function of SEER*Stat, which precluded model diagnostics. To minimize the effect of a difference in age distributions over time, all rates were age-adjusted to the 2000 U.S. standard population for trend analysis. All p-values for significance testing of APC = 0 were two-sided and considered to be of statistical significance when p<0.05.
The predicted annual incidence rates were calculated based on the estimated APC by age and cancer site and assumed to change at a constant percentage of the rate of the previous year. For example, if the APC is 1%, and the rate is 10,000 per 100,000 in 2000, the predicted rate will be 10,000 × 1.01 = 10,100 per 100,000 in 2001 and 10,100 × 1.01 = 10,201 per 100,000 in 2002.
We analyzed age at diagnosis in 15-year intervals starting at the age of 20. We also used the SEER historic stage A definition for extent of disease at diagnosis. The SEER historic stage A variable is a simplified version of stage derived from Extent of Disease (EOD) from 1973-2003 and Collaborative Stage (CS) for 2004+. The historic staging variable has the advantage of being the only staging variable that has been recorded consistently over the study period. Localized disease refers to disease confined to the colon or rectum, regional disease refers to contiguous and adjacent organ spread (e.g. lymph nodes, kidney, pelvic wall, etc.), and distant disease refers to remote metastases.
RESULTS
There were 393,241 patients with histologically confirmed colon or rectal cancer who met inclusion criteria and were analyzed. A total of 281,463 (71.6%) patients had colon cancer of which 38.8% presented with right (including transverse) colon cancer, 30.9% presented with left colon cancer, and 1.9% presented with disease not otherwise specified. The remaining patients (N=111,779) presented with rectosigmoid and rectal cancer. Overall, the age-adjusted CRC incidence rates significantly decreased between 1975 and 2010 (APC −0.92; 95% CI −1.14 to −0.70, P<0.001). Results by gender, race, age, stage at diagnosis, site of disease and geographic region are shown in Table 1. CRC incidence rates declined overall by 1.03% in males and 0.91% in females. Rates for whites, black and other decreased by less than 1%. The most pronounced decline was observed in patients 75 years of age or older (APC −1.15; 95% CI −1.47 to –0.84, P<0.001). However, the CRC incidence rates increased for patients 20 to 49 years of age with the most pronounced increase observed in patients aged 20 to 34 years of age (APC 1.99; 95% CI 1.45 to 2.51, P<0.001). Other factors associated with decreasing CRC incidence rates include stage, site of disease and geographic region.
Table 1.
Number of Cases and Annual Percent Change (APC) in the Age-Adjusted Incidence Rates of Colon and Rectal Cancer
| Characteristics | Cases | APC | 95%CI |
|---|---|---|---|
| Overall (US) | 393,241 | −0.92* | −1.14, −0.70 |
| Sex | |||
| Male | 198,259 (50.4) | −1.03 | −1.30, −0.76 |
| Female | 194,982 (49.6) | −0.91 | −1.09, −0.73 |
| Race | |||
| White | 331,761 (84.4) | −1.00* | −1.23, −0.77 |
| Black | 33,507 (8.5) | −0.17 | −0.43, 0.09 |
| Other/Unknown | 27,973 (7.1) | −0.53* | −0.75, −0.31 |
| Age at Diagnosis | |||
| 20-34 | 3,815 (1.0) | 1.99* | 1.48, 2.51 |
| 35-49 | 26,893 (6.8) | 0.41* | 0.14, 0.69 |
| 50-74 | 219,059 (55.7) | −0.97* | −1.17, −0.76 |
| 75+ | 143,474 (36.5) | −1.15* | −1.47, −0.84 |
| Stage at Diagnosis | |||
| Localized | 160,903 (40.9) | −0.39* | −0.65, −0.13 |
| Regional | 154,711 (39.4) | −1.32* | −1.60, −1.04 |
| Distant | 77,627 (19.7) | −1.24* | −1.35,−1.14 |
| Site of Disease | |||
| Right colon | 152,408 (38.8) | −0.26 | −0.49, −0.04 |
| Left colon | 121,766 (30.9) | −1.78* | −2.10, −1.46 |
| Rectosigmoid/rectum | 111,779 (28.4) | −1.00* | −1.16,−0.84 |
| NOS | 7,288 (1.9) | 0.71* | 0.31, 1.13 |
| Geographic region | |||
| San Francisco/Oakland | 61,981 (15.8) | −1.08* | −1.24, −0.92 |
| Connecticut | 66,243 (16.8) | −1.28* | −1.53, −1.03 |
| Detroit | 67,993 (17.3) | −0.59* | −0.86, −0.31 |
| Hawaii | 18,366 (4.7) | −0.69* | −0.99, −0.39 |
| Iowa | 62,053 (15.8) | −0.60* | −0.88, −0.32 |
| New Mexico | 19,114 (4.9) | −0.26 | −0.56, 0.04 |
| Seattle (Puget Sound) | 52,872 (13.4) | −1.19* | −1.43, −0.94 |
| Utah | 17,875 (4.5) | −0.50* | −0.79, −0.21 |
| Atlanta (Metropolitan) | 26,744 (6.8) | −0.57* | −0.82, −0.31 |
P<0.05
Abbreviations: NOS, not otherwise specified; CI, confidence interval
Colon Cancer Trends by Stage and Age
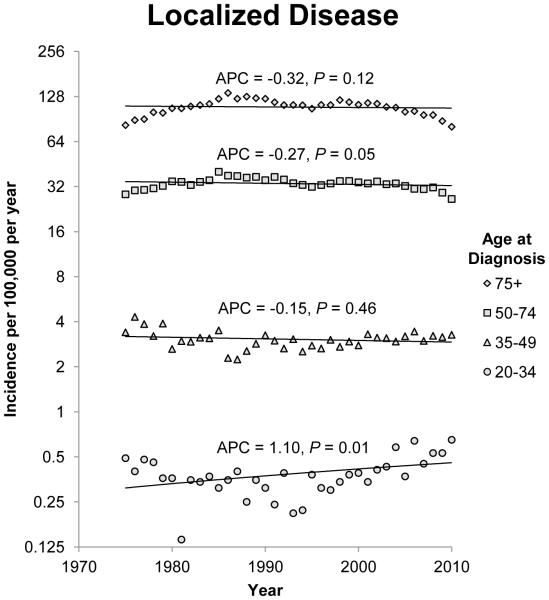
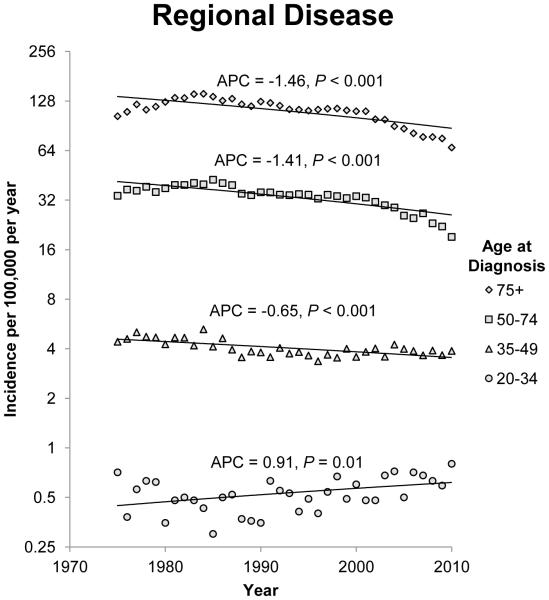
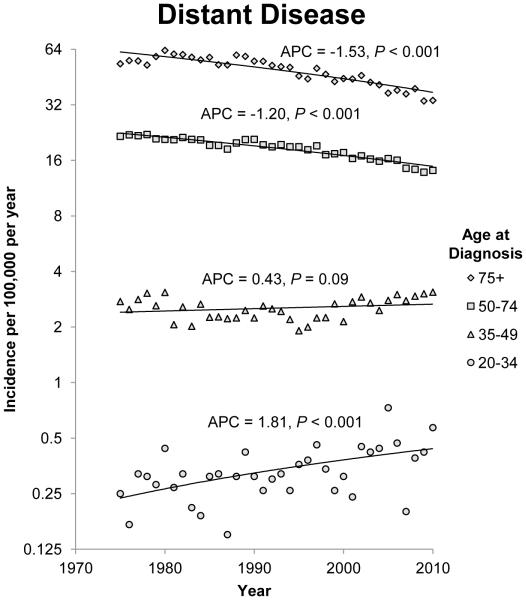
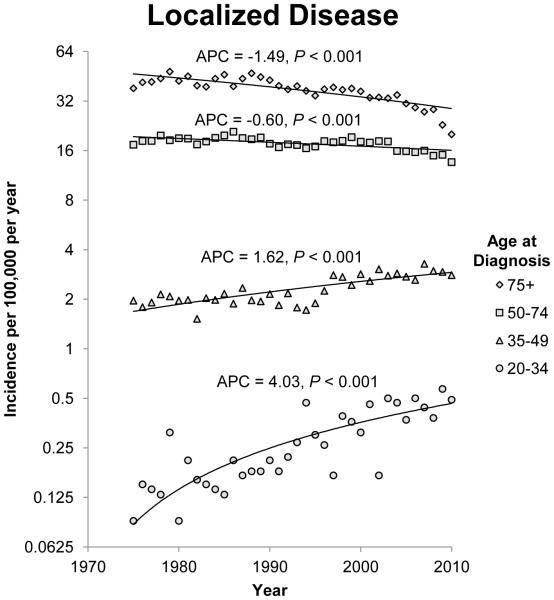
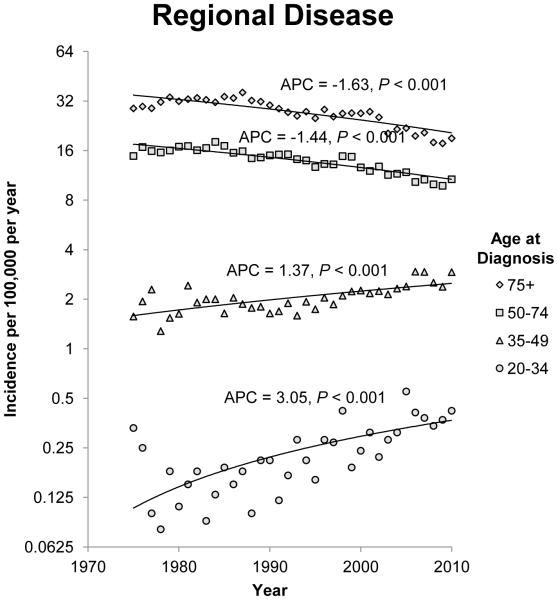
The annual incidence rates of colon cancer from 1975 to 2010 by 15 year intervals and stage of disease at diagnosis are shown in figure 1. Since 1975, there has been a steady decline in the incidence rate of CRC in patients 50 years of age and older for each stage category (localized, regional, and distant). The most notable declines were noted in regional and distant disease. For regional disease, the APC for patients 50 to 74 years of age and patients older than 75 years of age was −1.41 (95% CI −1.75 to −1.07; P<0.001) and −1.46 (95% CI −1.86 to −1.07; P<0.001) respectively. For patients with distant disease, the APCs for patients 50 to 74 years of age and patients older than 75 years of age were −1.20 (95% CI −1.35 to −1.05; P<0.001) and −1.53 (95% CI −1.79 to −1.28; P<0.001) respectively. The incidence rates of localized and regional disease in patients 35 to 49 years of age also decreased, but there was a non-significant increase in the incidence rate of distant disease in this age group. Opposite trends were observed in patients younger than 34 years when compared to patients older than 50 years of age. The annual incidence rate for colon cancer diagnosed in this group of patients is increasing across all three stage groups. The APC for patients with localized, regional and distant disease at diagnosis in the 20 to 34 year age group was 1.10 (95% CI 0.28 to 1.93;P=0.01), 0.91 (95% CI 0.23 to 1.61; P=0.01) and 1.81 (95% CI 0.88 to 2.75; P<0.001) respectively.
Figure 1.
Annual incidence rates of colon cancer from 1975 to 2010. Rates are per 100,000 and age-adjusted to the 2000 US standard population. The trendlines are logarithmic. A) Localized disease. B) Regional disease. C) Distant disease.
Quantitative Estimates of Colon Cancer Incidence
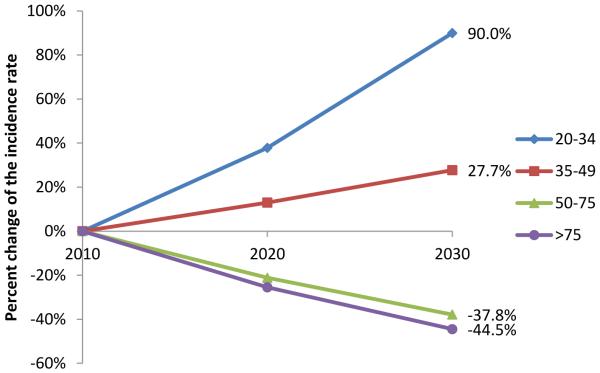
The APC-based predicted incidence rate of colon cancer in 2020 and 2030 compared to 2010 for the 4 different age groups are shown in figure 3A. Based on the predictive model, the incidence rate of colon cancer will continue to decrease for patients older than 50 years of age. The largest decrease is expected for patients older than 75 years of age. The opposite trend is noted in patients younger than 50 years of age and the largest predicted increase in the incidence rate of colon cancer will occur in the 20 to 34 years of age group. By 2020 and 2030, the incidence rate of colon cancer will increase by 37.8% and 90.0% respectively for patients 20 to 34 year of age, while decreasing by 23.2% and 41.1% for patients older than 50 years of age. For the year 2030, this represents an arithmetic difference of 131.1% in the incidence rate change of colon cancer in younger patients compared to patients older than 50 years of age.
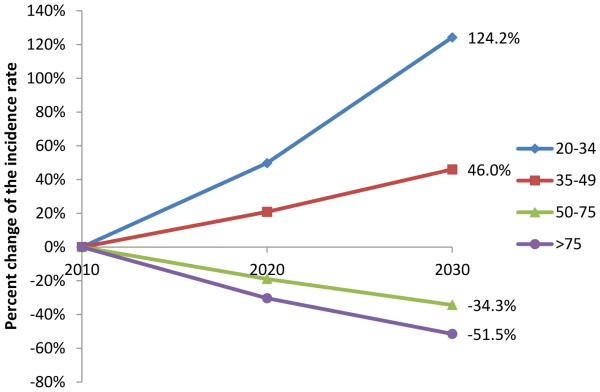
Figure 3.
Percent change of APC-based predicted incidence rate by age compared to incidence rate in 2010. A) Colon cancer. B) Rectosigmoid and rectal cancer.
Rectosigmoid and Rectal Cancer Trends by Stage and Age
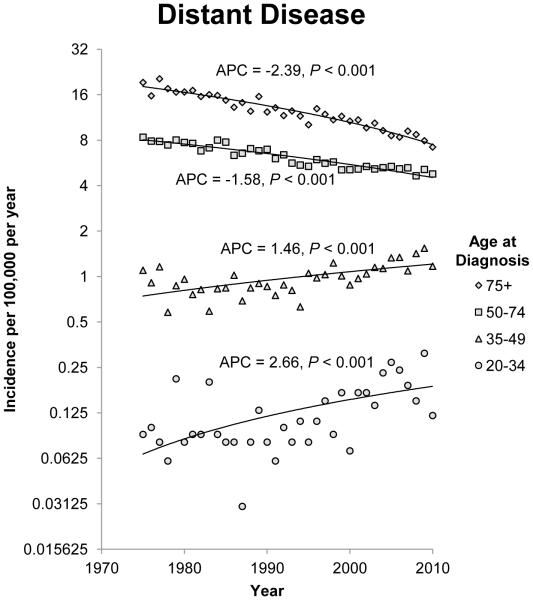
The annual incidence rates of rectosigmoid and rectal cancer from 1975 to 2010 by 15 year intervals and stage of disease at diagnosis are shown in figure 2. Since 1975, there has been a steady decline in localized, regional and distant disease in patients 50 years of age and older. The most notable decline was observed in patients older than 75 years, for which the APC for localized, regional and distant disease is −1.49 (95% CI −1.85 to −1.12; P<0.001), −1.63 (95% CI −1.97 to −1.28; P<0.001) and −2.39 (95% CI −2.63 to −2.15; P<0.001), respectively. The trend observed in patients between the ages of 50 and 74 was less dramatic, but APC is decreasing for all three stages of disease. The opposite trend was observed in younger patients. The APC is increasing for patients younger than 50 years of age for all three stages of cancer, but the increase is most significant in the 20 to 34 year age group. In the youngest age group, the APC for localized, regional and distant disease is 4.03 (95% CI 3.02 to 5.05; P<0.001), 3.05 (95% CI 1.95 to 4.17;P<0.001) and 2.66 (95% CI 1.33 to 3.99; P<0.001) respectively.
Figure 2.
Annual incidence rates of rectosigmoid and rectal cancer from 1975 to 2010. Rates are per 100,000 and age-adjusted to the 2000 US standard population. The trendlines are logarithmic. A) Localized disease. B) Regional disease. C) Distant disease.
Quantitative Estimates of Rectosigmoid and Rectal Cancer Incidence
The APC-based predicted incidence rate of rectosigmoid and rectal cancer in 2020 and 2030 compared to 2010 for the 4 different age groups are shown in figure 3B. Similar to colon cancer, the incidence rate of rectosigmoid and rectal cancer will continue to decrease for patients older than 50 years of age with the largest decrease occurring in patients older than 75 years of age. Conversely, the incidence rate of rectosigmoid and rectal cancer will continue to increase in patients younger than 50 years of age. As in colon cancer, the largest predicted increase in incidence rate is observed in the 20 to 34 years of age group. By 2020 and 2030, the incidence rate for rectosigmoid and rectal cancer will increase by 49.7% and 124.2% respectively for patients 20 to 34 year of age, while decreasing by 23.2% and 41% for patients older than 50 years of age. For the year 2030, this represents a difference of 165% in the incidence rate change of rectosigmoid and rectal cancer in younger patients compared to patients older than 50 years of age.
DISCUSSION
Our population-based analysis demonstrates dramatically increasing disparities in age-related incidence rates of colon and rectal cancer in the United States over the past 25 years. The incidence rate of colon and rectal cancer in young patients is increasing without a corresponding increase in patients older than 50 years of age. At the present rate, the incidence rate for young patients with newly diagnosed colon or rectal cancer will nearly double by 2030 while it will similarly decline by more than 1/3 among patients above the screening age of 50 years. By 2030, 10.9% of all colon and 22.9% of all rectal cancers will be diagnosed in patients under the screening age compared to 4.8% and 9.5% in 2010. Stated another way, more than 1 in 10 colon cancers and nearly 1 in 4 rectal cancers will be diagnosed in people below the traditional screening age. These finding highlight an important growing public health problem of increasing risks of CRC among young patients.
Our finding of increasing incidence of CRC in young patients is consistent with prior studies of CRC trends utilizing large population based databases (2, 8-10). A 1.5% per year increase in the incidence of CRC in men and a 1.6% per year increase in women from 1992 to 2005 aged 20 to 49 years of age has been previously observed (8). In this study we further provide quantitative estimates of the exponentially rising risk of CRC among young patients compared to the dramatic declines among patients over 50 years of age. Our study thus provides further insight into the growing concern of age-related disparities in the incidence rates of CRC. Moreover, these relationships are not unique to colorectal cancer as similar findings have been observed for breast cancer with increasing incidence among young women (11).
The increasing incidence of CRC among young adults is concerning and highlights the need to investigate potential causes and external influences such as lack of screening and behavioral factors. Routine screening options for CRC include fecal occult blood test, flexible sigmoidoscopy or colonoscopy. Adults younger than 50 years of age without risk factors are not recommended to undergo routine screening for CRC, whereas high risk patients (i.e. patients with a history of familial polyposis, hereditary nonpolyposis CRC or ulcerative colitis) are recommended to begin screening prior to the age of 50 (5). In the absence of routine screening, patients and their providers may need to have a heightened awareness regarding colorectal cancer risk and its rising incidence in order to minimize delays in both diagnosis and treatment and missed opportunities for treatment of precursor lesions resulting from delays in seeking medical attention. In the pre-Affordable Care Act era, younger patients often lack health insurance, resulting in further delays.
The observed increasing incidence of CRC may also be attributed to behavioral factors. Obesity, physical inactivity and the “Western diet” are all risk factors associated with an increased risk for CRC. The prevalence of obesity has increased markedly in the United States and is a major risk factor for CRC (14-16). Physical activity has been demonstrated to reduce the risk of both colon and rectal cancer (17, 18). Moderate physical activity has been associated with a decreased risk of rectal cancer in both men and women with an odds ratio of 0.70 and 0.51(17). Also of note, the “Western diet” which consists primarily of processed meats, red meat, fast food, and low levels of vegetables and fruits is associated with an increased risk of colon cancer(19). While the net contribution of these factors to the rising incidence among young patients cannot be known, many of these risk factors are potentially modifiable with behavioral changes at little cost to the healthcare system and may impact overall health in addition to reducing the risk of CRC.
Our study demonstrates the potential impact of CRC incidence in young patients if no changes are made in public awareness and policy. Based on our data, the predicted incidence rate of colon and rectal cancer in 2030 will increase by 90% and 124% in patients aged 20 to 34 years. Studies have demonstrated that both screening and risk factor contribute to the overall incidence of CRC. Using the MISCAN-Colon model, Edwards demonstrated that changes in risk factors and screening individually accounted for 50% of the overall decline in CRC incidence rates between 1975 and 2000(2). These findings demonstrate the importance of emphasizing education regarding risk factors, improving healthy lifestyles, and prevention in public policy.
The incidence of colon and rectal cancer is increasing in young adults and declining in adults older than 50 years of age. Evaluation of the predicted incidence rates reveal dramatic rises in the number of young adults who will be diagnosed with colon or rectal cancer over the next two decades. Further studies are needed to determine the cause for these trends and identify potential preventive and early detection strategies.
Acknowledgments
Funding/Support: This work was supported in part by National Institutes of Health/National Cancer Institute grants T32CA009599 (C.E.B.), K07-CA133187 (G.J.C.) and CA16672 (The University of Texas MD Anderson Cancer Center Support Grant).
Footnotes
No authors have any potential conflicts of interest to disclose.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cress RD, Morris C, Ellison GL, Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992-2001. Cancer. 2006;107(5 Suppl):1142–52. doi: 10.1002/cncr.22011. [DOI] [PubMed] [Google Scholar]
- 4.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):132–41. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 5.Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137(2):129–31. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 6.Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93(5):1009–13. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 7.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–8. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 9.O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69(10):866–72. [PubMed] [Google Scholar]
- 10.You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? Archives of internal medicine. 2012;172(3):287–9. doi: 10.1001/archinternmed.2011.602. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309(8):800–5. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 12.Surveillance E, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2011 Sub (1973-2010) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission.
- 13.Bleyer A. CAUTION! Consider cancer: common symptoms and signs for early detection of cancer in young adults. Semin Oncol. 2009;36(3):207–12. doi: 10.1053/j.seminoncol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Aleksandrova K, Pischon T, Buijsse B, et al. Adult weight change and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Bassett JK, Severi G, English DR, et al. Body size, weight change, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2978–86. doi: 10.1158/1055-9965.EPI-10-0543. [DOI] [PubMed] [Google Scholar]
- 16.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 17.Slattery ML, Edwards S, Curtin K, et al. Physical activity and colorectal cancer. Am J Epidemiol. 2003;158(3):214–24. doi: 10.1093/aje/kwg134. [DOI] [PubMed] [Google Scholar]
- 18.Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer. 1996;73(9):1134–40. doi: 10.1038/bjc.1996.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma KN. Eating patterns and risk of colon cancer. Am J Epidemiol. 1998;148(1):4–16. doi: 10.1093/aje/148.1.4-a. [DOI] [PubMed] [Google Scholar]