SUMMARY
In response to infections and tissue damage, ASC-containing inflammasome protein complexes are assembled that promote caspase-1 activation, IL-1β and IL-18 processing and release, pyroptosis, and the release of ASC particles. However, excessive or persistent activation of the inflammasome causes inflammatory diseases. Therefore, a well-balanced inflammasome response is crucial to maintain homeostasis. We show that the PYD-only protein POP1 inhibited ASC-dependent inflammasome assembly by preventing inflammasome nucleation, and consequently interfered with caspase-1 activation, IL-1β and IL-18 release, pyroptosis and the release of ASC particles. There is no mouse ortholog for the POP1 gene, but transgenic expression of human POP1 in monocytes, macrophages and dendritic cells protected mice from systemic inflammation triggered by molecular PAMPs, inflammasome component NLRP3 mutation and ASC danger particles. POP1 expression was regulated by TLR- and IL-1R–signalling, and we propose that POP1 provides a regulatory feedback loop that shuts down excessive inflammatory responses and thereby prevents systemic inflammation.
INTRODUCTION
Inflammation is an essential and tightly controlled process initiated by the innate immune system in response to infection and tissue damage and is responsible for pathogen clearance, wound healing, and restoring homeostasis. It is triggered by the sensing of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs or danger signals) by germline-encoded pattern recognition receptors (PRRs). Particularly, cytosolic PRRs of the AIM2-like receptor (ALR) and Nod-like receptor (NLR) families facilitate the activation of the pro-inflammatory caspase-1 within large macromolecular protein complexes in macrophages (MΦ), referred to as inflammasomes. Inflammasomes promote the proteolytic maturation and release of the leaderless pro-inflammatory cytokines interleukin (IL)-1β and IL-18 and the induction of pyroptotic cell death, which causes the release of IL-1α and HMGB1 (Martinon et al., 2002; Khare et al., 2010; Wen et al., 2013). Inflammasomes are composed of a sensory PRR, which is linked to caspase-1 via the adaptor protein ASC (Srinivasula et al., 2002; Stehlik et al., 2003; Martinon et al., 2002). This complex exhibits specific protein-protein interactions, which are mediated by homotypic PYRIN domain (PYD) interactions between PRRs and ASC and by homotypic caspase recruitment domain (CARD) interactions between ASC and pro-caspase-1. Within this protein complex, pro-caspase-1 is activated by induced proximity-mediated oligomerization (Martinon et al., 2002; Lu et al., 2014). The underlying assembly mechanism of this ternary complex has recently been delineated, and indicates that PRR activation triggers prion-like, self-perpetuating ASC polymerization into filaments, which is initialized by PRR induced ASCPYD nucleation (Cai et al., 2014; Lu et al., 2014; Franklin et al., 2014; Dorfleutner et al., 2015) . PRRs localize to the end of the hollow ASC filaments and allow efficient self-propagation of the ASCPYD, whereby the ASCCARD is flexible linked to the outside of the filaments, allowing recruitment and clustering of pro-caspase-1 (Lu et al., 2014). Eventually, these filaments may assemble into a spherical structure, where caspase-1 is placed to the hollow core (Man et al., 2014). Thus, the PYD is essential for inflammasome assembly following activation of NLRP3 and other PYD-containing PRRs, and disassembly of this ternary signalling complex is required for its termination. NLRP3 is one of the best-studied inflammasome-activating PRRs and the NLRP3 inflammasome is regulated by a two-step mechanism, referred to as priming and activation (Khare et al., 2010). Following priming with LPS or other NF-κB-inducing signals, NLRP3 is activated in a 2nd step by a wide variety of PAMPs that cause potassium (K+) efflux, including exogenous ATP, nigericin or uric acid crystals, as well as environmental and endogenous mediators released in response to stress and tissue damage (Khare et al., 2010; Muñoz-Planillo et al., 2013). Besides inflammasome-linked cytokines, oligomeric ASC particles are also released and phagocytised by neighbouring cells to perpetuate inflammasome responses (Franklin et al., 2014; Baroja-Mazo et al., 2014). Overall, inflammasomes play an essential role in host defense. However, impaired inflammasome activation results in failure to restrict colitogenic microbiota species and subsequently promotes metabolic dysfunction. In contrast, constitutive inflammasome activation through disease-associated mutations in NLRP3 promotes excessive IL-1β release and causes inflammatory diseases, including Cryopyrinopathies (or Cryopyrin-associated periodic syndromes; CAPS) (Henao-Mejia et al., 2012; Hoffman and Brydges, 2011). Therefore, a controlled and well-balanced inflammasome response is essential for maintaining homeostasis. However, the molecular mechanisms regulating assembly and disassembly of inflammasomes are largely unknown. We discovered a family of PYD-only proteins (POPs), which are encoded in humans, but not in mice (Stehlik and Dorfleutner, 2007; Khare et al., 2014; Stehlik et al., 2003; Dorfleutner et al., 2007; Bedoya et al., 2007; Dorfleutner et al., 2007; Johnston et al., 2005). We recently demonstrated that POP3 functions as a specific inhibitor for ALR inflammasomes (Khare et al., 2014). POP3 binds to the PYD of ALRs, but not to ASC or NLRs, and thereby prevents ALR interactions with the inflammasome adaptor ASC. However, the function of endogenous POP1 (PYD-containing 1, PYDC1) has not yet been established. POP1 is highly similar to the PYD of ASC and in vitro over expression experiments showed that POP1 interacts with the PYD of ASC, however, its role in vivo is unknown.
Here we report that POP1 functions in an IL-1β–induced regulatory loop to inhibit the assembly and consequently the activity of ASC-containing inflammasomes by preventing nucleation of ASC. POP1 also prevented the release of oligomeric ASC danger particles, and incorporation of POP1 into ASC particles rendered them inactive, thus preventing self-perpetuation of inflammasome responses in neighbouring cells. To demonstrate the importance of inflammasomes particularly in monocytes and MΦ, we engineered mice that usually lack POP1 to specifically express transgenic POP1 in monocytes, MΦ and DCs. We used the NLRP3 inflammasome as an example to demonstrate that transgenic POP1 expression in the monocyte-MΦ-DC lineage was sufficient to blunt excessive systemic inflammation in response to PAMPs, ASC danger particles and CAPS-associated mutations. Interestingly, CAPS patients exhibited reduced POP1 expression, suggesting that impaired POP1 expression may contribute to excessive inflammasome-driven inflammation. Our data reveal a mechanism by which human inflammasome assembly is regulated and detail how healthy tissue puts the brakes on inflammasome-induced systemic inflammation. In addition our results emphasize the crucial role of the monocyte-MΦ-DC lineage in this response.
RESULTS
POP1 inhibits inflammasome-mediated cytokine release in human macrophages
To investigate the role of POP1 in inflammasome signalling, we established stable shRNA-mediated POP1 silencing in human monocytic THP-1 cells (THP-1shPOP1). POP1 knockdown using two different shRNAs caused elevated IL-1β release in response to LPS and POP1 silencing was confirmed by qPCR (Figure 1A). Similarly, siRNA-mediated silencing of POP1 in primary human MΦ (hMΦ) resulted in elevated IL-1β and IL-18, but not IL-6 release in response to LPS. POP1 silencing was again confirmed by qPCR (Figure 1B). Conversely, THP-1 cells stably expressing POP1 (THP-1GFP-POP1) displayed diminished IL-1β secretion in response to LPS, and also in response to non-canonical inflammasome activation with LPS transfection or cytosolic delivery of LPS with cholera toxin subunit B (CTB) (Figure 1C). Cytokine release after non-canonical inflammasome activation requires NLRP3. Consistently, POP1 blocked IL-1β release after NLRP3 inflammasome activation with nigericin, calcium pyrophosphate dehydrate (CPPD) crystals or K+ depletion. Furthermore, POP1 blocked IL-1β release after AIM2 and NLRC4 activation with poly(dA:dT) or flagellin transfection, respectively (Figure 1D), and POP1 expression in THP-1GFP-POP1 was confirmed by qPCR (Figure 1E). Hence, POP1 inhibits inflammasome-dependent cytokine release. Comparable results were also obtained for myc-tagged POP1, thus establishing that the GFP tag does not affect the of POP1 (Figure S1A).
Figure 1. POP1 inhibits the NLRP3 inflammasome in human macrophages (See also Figure S1A).
(A) THP-1 cells stably expressing shRNAs targeting POP1 (POP1#1, #2) or a scrambled Ctrl were analysed by Real-time PCR for POP1 transcripts and for IL-1β release in culture supernatants (SN) in untreated cells (Ctrl) or in response to LPS.
(B) Primary hMΦ transfected with a scrambled Ctrl or a POP1-specific siRNA were analysed for POP1 transcripts by Real-time PCR and culture SN for IL-1β, IL-18 and IL-6 release in response to LPS.
(C, D)Culture SN from THP-1 cells stably expressing GFP or GFP-POP1 were analysed for IL-1β release by ELISA in untreated cells (Ctrl) or in response to LPS treatment, LPS transfection or incubation with LPS complexed with CTB (C) and nigericin or CPPD treatment or K+ depletion in LPS-primed cells or transfection of poly(dA:dT) or flagellin (D).
(E) Real-time PCR of POP1 transcripts in above cells.
(A) *P=0.0009, **P=0.0128, ***P<0.0001, ****P<0.0001; (B) *P=0.0284, **P=0.0315, ***P=0.0001; (D, left panel) *P<0.0001, **P<0.0001, ***P<0.0001; (D, right panel) *P<0.0001, **P<0.0001; (E) *P=0.0024, **P=0.0022, ***P=0.0139; all two-tailed unpaired t-test; data are representative of two (A) or three (B–E) replicates; error bars represent s.e.m.
POP1 impairs ASCPYD nucleation and caspase-1 activation in human macrophages
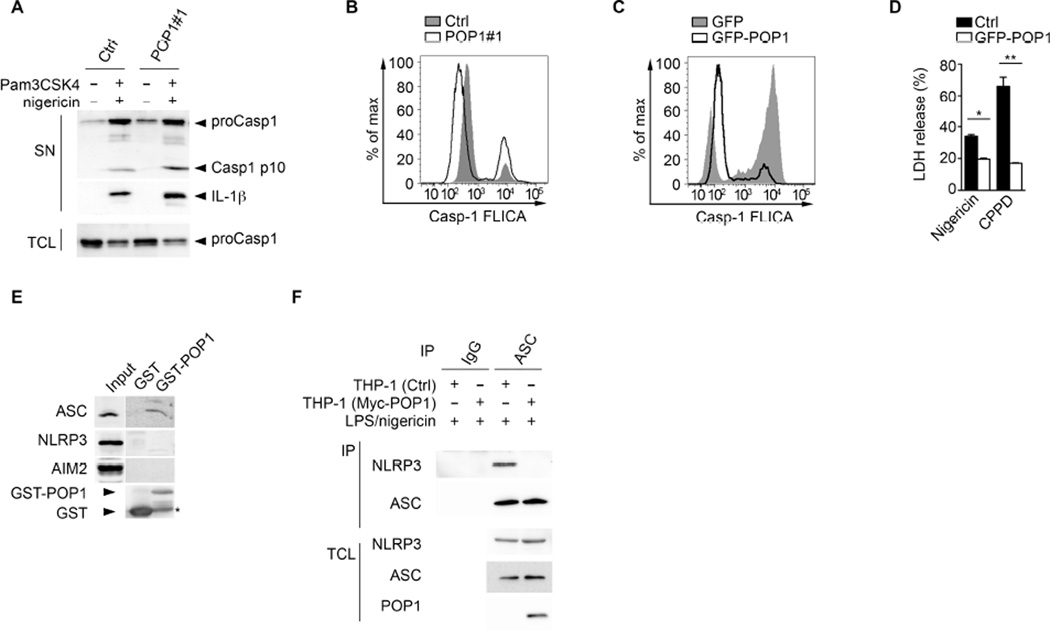
Caspase-1 activation in canonical inflammasomes of MΦ is required for IL-1β and IL-18 release and for pyroptosis. Hence, we determined caspase-1 activity in the presence or absence of POP1. Treatment of Pam3CSK4-primed POP1 knockdown cells (THP-1shPOP1) with nigericin resulted in elevated active caspase-1 p10 and mature IL-1β in culture supernatants when compared to THP-1Ctrl cells (Figure 2A). Further, intracellular caspase-1 activation was also augmented in THP-1shPOP1 compared to THP-1Ctrl, as shown by caspase-1 FLICA assay (Figure 2B). Conversely, expression of POP1 in primed THP-1GFP-POP1 cells showed drastically reduced caspase-1 activity in response to nigericin compared to THP-1GFP cells (Figure 2C). Consequently POP1 expression also caused reduced pyroptosis, as determined by LDH release (Figure 2D). TLR-mediated priming is necessary for NLRP3 inflammasome activation (Bauernfeind et al., 2009; Juliana et al., 2012; Schroder et al., 2012; Lin et al., 2014). However, stable POP1 expression in THP-1 cells did not affect transcription of IL1B (Figure S1B) and resulted in mild increased transcription of NLRP3 or PYCARD (ASC) in response to LPS (Figure S1C), and contrary to transient over-expression in epithelial cell lines (Stehlik et al., 2003), stable POP1 expression also did not affect phosphorylation of IκBα (Figure S1D). Furthermore, silencing of POP1 in THP-1 cells also did not affect transcription of PYCARD and NLRP3 (Figure S1E). Thus POP1 seems to directly regulate inflammasome assembly or activation in MΦ. Recruitment of ASC to upstream sensors is essential for inflammasome activation and we hypothesized that POP1 interferes with this interaction. POP1 specifically bound to endogenous ASC, but not to the PYD-containing PRRs NLRP3 and AIM2 in LPS-primed THP-1 cells (Figure 2E). Binding of ASC to NLRP3 induces ASCPYD nucleation, which provides the oligomeric platform essential for caspase-1 activation (Cai et al., 2014; Lu et al., 2014). Therefore we hypothesized that POP1 prevents ASCPYD-NLRP3PYD interactions, and thus ASCPYD nucleation (Chu et al., 2015). Indeed, the nigericin-induced interaction of NLRP3 and ASC was abolished in LPS-primed THP-1GFP-POP1 cells, indicating that POP1 may prevent NLRP3-mediated ASC nucleation (Figure 2F). Upon ASC nucleation the ASCPYD polymerizes in a prion-like self-perpetuating manner that further promotes caspase-1 activation (Lu et al., 2014). Since ASC-ASC, ASC-POP1 and ASC-NLRP3 interactions utilize the same key residues (Vajjhala et al., 2012), POP1 binding to the ASCPYD could also directly prevent ASCPYD self-polymerization. Surprisingly, POP1 expression in HEK293 cells did not impair the ASCPYD self-interaction, as determined by in coIP experiments between Myc-ASC and HA-ASCPYD (Figure S1F). However, over expression in HEK293 cells may lead to inaccurate results. Our data indicate that, in the context of PRR-mediated inflammasome activation, POP1 prevents the essential ASC nucleation step in human MΦ, which is required for inflammasome assembly.
Figure 2. POP1 impairs inflammasome nucleation in human macrophages (See also Figure S1B–F).
(A, B) Pam3CSK4 primed THP-1 cells stably expressing shRNA#1 targeting POP1 or a scrambled Ctrl were treated with nigericin and total cell lysates (TCL) and culture supernatants (SN) were analysed for caspase-1 and IL-1β by immunoblot (A) or active caspase-1 by flow cytometry (B).
(C) LPS primed THP-1 cells expressing GFP or GFP-POP1 were treated with nigericin, and active caspase-1 was determined by flow cytometry.
(D) LPS primed THP-1 cells expressing GFP or GFP-POP1 were treated with nigericin or CPPD crystals, and LDH release was quantified in culture supernatants.
(E) Interaction of GST-POP1 with endogenous ASC in THP-1 TCL using GST as negative control and showing 10% TCL as input. * marks a degradation product of GST-POP1.
(F) Immunoprecipitation (IP) of proteins with antibodies to ASC or control immunoglobulin G (IgG) from LPS primed and nigericin-treated THP-1 cells stably expressing GFP or GFP-POP1, followed by immunoblot analysis alongside TCL. (D) *P=0.001, **P=0.0004; all two-tailed unpaired t-test; data are representative of two (A–F) replicates; error bars represent s.e.m.
POP1 prevents caspase-1 activation and cytokine release in mouse macrophages
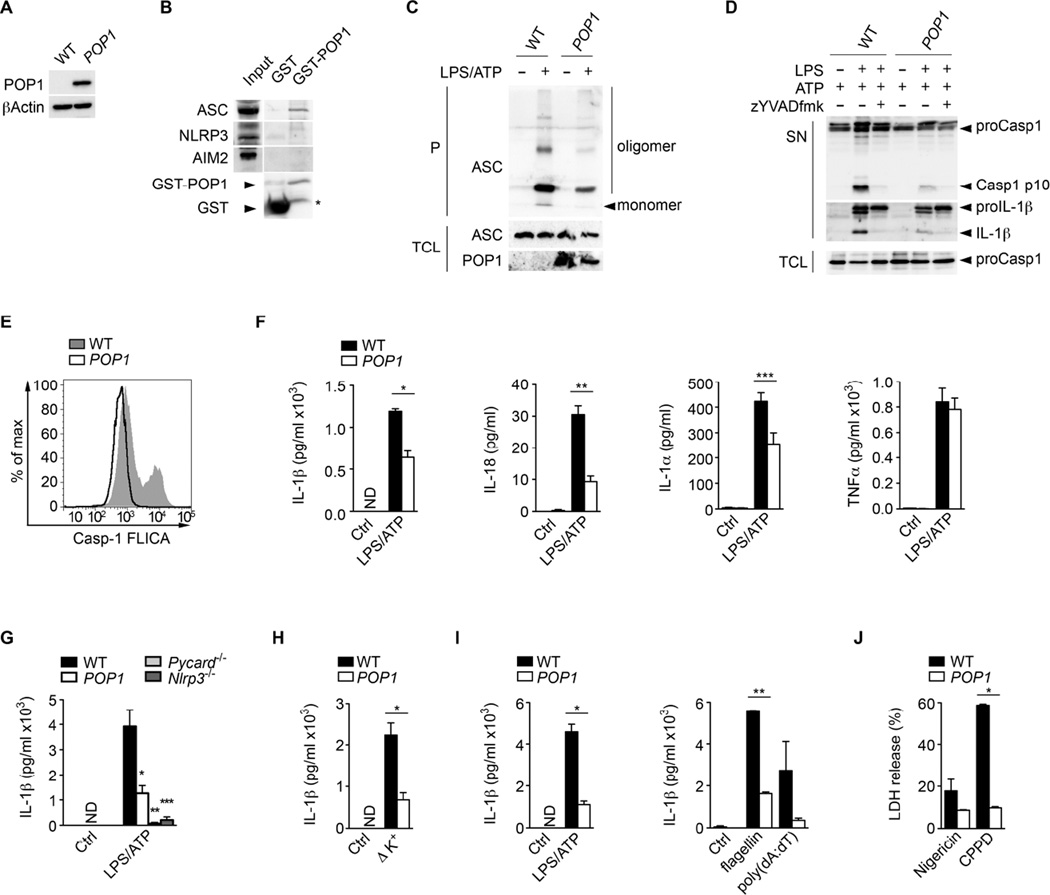
Since the genes for all POPs, including POP1, are absent in mice (Stehlik and Dorfleutner, 2007; Khare et al., 2014), we generated GFP-POP1 transgenic (TG) mice. Caspase-1 is essential for IL-1β and IL-18 release in monocytes and MΦ and we detected POP1 expression in CD68+ macrophages in inflamed lung tissue (Figure S2A). Therefore, to limit POP1 expression to MΦ, we used the hCD68/IVS-1 promoter/enhancer (Iqbal et al., 2014; Khare et al., 2014; Gough et al., 2001) for GFP-POP1 expression in TG mice. We confirmed POP1 expression specifically in CD68-POP1 TG but not in wild-type (WT) mice by qPCR analysis of whole blood cell RNA (Figure S2B). Accordingly, we also detected POP1 expression in transgenic BMDM (BMDMPOP1) by immunoblot (Figure 3A). Human and mouse ASCPYD have a high degree of homology (Figure S2C) and therefore it was not surprising that similar to human ASC in THP-1 cells, POP1 also interacted with mouse ASC, but not NLRP3 or AIM2 in BMDM (Figure 3B). ASC polymerization can be captured by non-reversible cross-linking and functions as a read-out for inflammasome activation (Fernandes-Alnemri et al., 2007), which was markedly reduced in LPS primed and ATP-treated BMDMPOP1 compared to BMDMWT (Figure 3C). Consequently, BMDMPOP1 lacked active caspase-1 p10 and mature IL-1 β in culture supernatants of LPS/ATP treated cells to a similar extent as the caspase-1 inhibitor zYVAD-fmk (Figure 3D). Reduced caspase-1 activity was also detected by flow cytometry in intact cells (Figure 3E), indicating that POP1 also inhibits caspase-1 activation in mouse MΦ.
Figure 3. POP1 inhibits the NLRP3 inflammasome in mouse macrophages (See also Figure S2).
(A) Western Blot of POP1 expression in BMDM using a GFP antibody.
(B) Interaction of GST-POP1 with endogenous ASC from LPS-primed BMDM total cell lysates (TCL) using GST as negative control and showing 10% TCL as input. * marks a degradation product of GST-POP1.
(C) Immunoblot analysis of ASC polymerization (oligomerization) in untreated or LPS/ATP treated WT and POP1 BMDM after cross linking of pellets (P) and in TCL.
(D) Immunoblot analysis of caspase-1 and IL-1β in culture SN of LPS/ATP treated WT and POP1 BMDM. Pro-caspase-1 expression in TCL confirms equal loading.
(E) Flow Cytometric quantification of active caspase-1 in WT and POP1 BMDM in response to LPS/ATP.
(F) Analysis of culture supernatants (SN) for IL-1β, IL-18, IL-1α and TNF-α by ELISA in LPS/ATP treated WT and POP1 BMDM.
(G) Analysis of culture SN for IL-1β by ELISA in LPS primed and ATP treated WT, POP1, Pycard−/− and Nlrp3−/− BMDM.
(H) LPS primed WT and POP1 BMDM cultured in K+ depleted medium
(I) WT and POP1 PM treated with LPS/ATP or transfected with flagellin or poly(dA:dT).
(J) LPS primed WT and POP1 BMDM were treated with nigericin or CPPD crystals and released LDH in culture SN was quantified.
(F) *P=0.0027, **P=0.003, ***P=0.0403; (G) *P=0.0214, **P=0.0043, ***P=0.0052; (H) *P=0.01; (I) *P=0.0009, **P<0.0001; (J) *P<0.0001; all two-tailed unpaired t-test; data are representative of four (A,H,I) or two (B-G,J) replicates; error bars represent s.e.m.
As expected from impaired caspase-1 activation, LPS/ATP treated BMDMPOP1 also displayed significantly reduced levels of IL-1β, IL-1α and IL-18 in culture supernatants by ELISA. However, secretion of TNFα, which occurs independently of caspase-1, was not affected (Figure 3F). Significantly, reduced IL-1β release in BMDMPOP1 was comparable to BMDMPycard−/− and BMDMNlrp3−/− (Figure 3G). K+ efflux is the unifying mechanism of NLRP3 activation in BMDM (Muñoz-Planillo et al., 2013), and culturing BMDMPOP1 in K+-free medium showed impaired IL-1β release compared to BMDMWT (Figure 3H). Similarly, peritoneal MΦ (PMPOP1) showed impaired IL-1β release in response to activation of ASC-dependent inflammasomes containing NLRP3 with ATP, AIM2 with poly(dA:dT) and NLRC4 with flagellin (Figure 3I). BMDMPOP1 also showed reduced LDH release, and thus pyroptosis, when compared to BMDMWT in response to NLRP3 activation (Figure 3J), but did not reveal any altered LPS-induced activation of NF-κB, p38, JNK or ERK (Figure S2D), or altered transcription of Il1b, Il18, Pycard and Nlrp3 (Figure S2E), ruling out POP1 effects on inflammasome priming in mouse MΦ. Collectively, these data indicate that POP1 impairs assembly of the NLRP3 inflammasome in human and mouse MΦ, by impairing the PRR-mediated nucleation of ASC and consequently, the release of inflammasome-dependent cytokines.
Monocyte-MΦ-DC-specific expression of POP1 ameliorates LPS-induced peritonitis
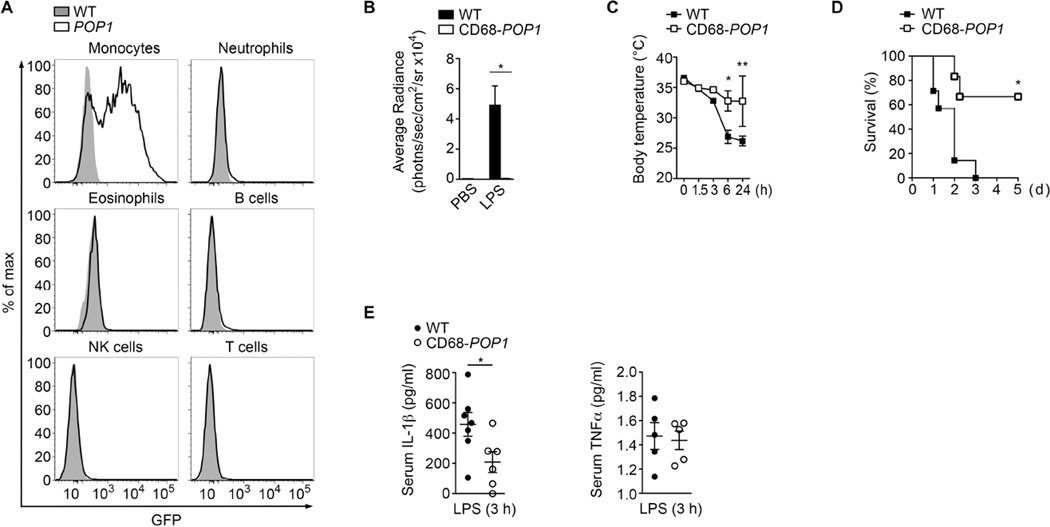
NLRP3 senses endogenous danger signals and PAMPs and promotes inflammatory responses that can be detrimental to the host. We therefore used the NLRP3 inflammasome to investigate the role of POP1 in vivo. To initially characterize the CD68-POP1 TG mice, we analysed peripheral blood, which revealed POP1 expression selectively in monocytes (Figure 4A and Figure S3), with equal expression in classical Ly6ChiCD43−, intermediate Ly6CintCD43+ and non-classical Ly6CloCD43+ monocytes (Figure S4A). POP1 was also expressed in myeloid precursor (MP), MΦ and DC precursor (MDP) and common DC precursor (CDP) in the bone marrow (Figure S4B and S4C), large peritoneal MΦ (LPM), small peritoneal MΦ (SPM) and peritoneal DC cells (Figure S4D and S4E), as well as in splenic red pulp MΦs (RPMs), monocytes and CD11b+ DCs, but not plasmacytoid DCs (pDCs) (Figure S5A and S5B). Monocyte-MΦ-DC -specific POP1 expression was also observed in other tissues, with no detectable expression in CD45− cells (Figure S5C and data not shown). Collectively, these results demonstrate POP1 expression in the monocyte-MΦ-DC lineage.
Figure 4. Monocyte-MΦ-DC-specific expression of POP1 ameliorates LPS-induced peritonitis (See also Figures S3, S4, S5).
(A) Analysis of POP1 expression by flow cytometry in peripheral blood cell populations from wild-type (WT) and CD68-POP1 (POP1) mice. Data are representative of three replicates.
(B) Quantification of MPO activity by in vivo imaging in mice 3h after i.p. injection of PBS or E. coli LPS (2.5 mg/kg body weight) in WT (n=6) and CD68-POP1 (n=5) mice.
(C, D) Endotoxic shock was induced by i.p. injection of E. coli LPS (20 mg/kg body weight) and body temperature (C) and survival (D) was determined in WT (n=5) and CD68-POP1 (n=5) mice.
(E) ELISA of IL-1 β and TNF-α in the serum of WT (n=5) and CD68-POP1 (n=5) mice 3h after i.p. E. coli LPS challenge (20 mg/kg body weight).
(B) two-tailed unpaired t-test *P=0.0036; (C) two way ANOVA + Bonferoni posttest, *P<0.005, **P<0.005; (D) asymmetrical Log-rank Mantel-Cox survival test, *P<0.005; (E) two-tailed unpaired t-test *P=0.0392; error bars represent s.e.m.
Caspase-11 is responsible for LPS- and Gram negative bacteria-induced lethal shock, but ASC and NLRP3 are both necessary for amplifying this response to LPS in vivo (Kayagaki et al., 2011). Accordingly, Pycard−/− and Nlrp3−/− mice are protected from LPS-induced lethality in response to moderate LPS doses (Mariathasan et al., 2004; Mariathasan et al., 2006; Kayagaki et al., 2011). NLRP3 inflammasome-released IL-1β is essential for neutrophil recruitment during sterile inflammation (McDonald et al., 2010). Therefore we injected WT and CD68-POP1 TG mice i.p. with a low dose of LPS and determined neutrophil infiltration 3 hours after LPS challenge by quantifying myeloperoxidase (MPO) activity in vivo. Contrary to PBS, injection of LPS recruited a substantial number of neutrophils into the peritoneal cavity, which was completely abolished in CD68-POP1 TG mice (Figure 4B). Consequently, CD68-POP1 TG mice experienced significantly less hypothermia (Figure 4C) and were significantly more protected from a lethal LPS dose (Figure 4D). Compared to 100% lethality in WT mice, only 30% of CD68-POP1 TG mice died within 96 hours, which is similar to Pycard−/− mice (Mariathasan et al., 2004). Consistent with reduced neutrophil infiltration and increased survival, serum IL-1β was reduced, but TNFα levels remained unchanged (Figure 4E), indicating that expression of POP1 in the monocyte-MΦ-DC lineage is sufficient to impair inflammasome activation in response to PAMPs in vivo, thereby blocking the secretion of IL-1β and ameliorating an excessive host response.
Monocyte-MΦ-DC-specific expression of POP1 ameliorates CAPS
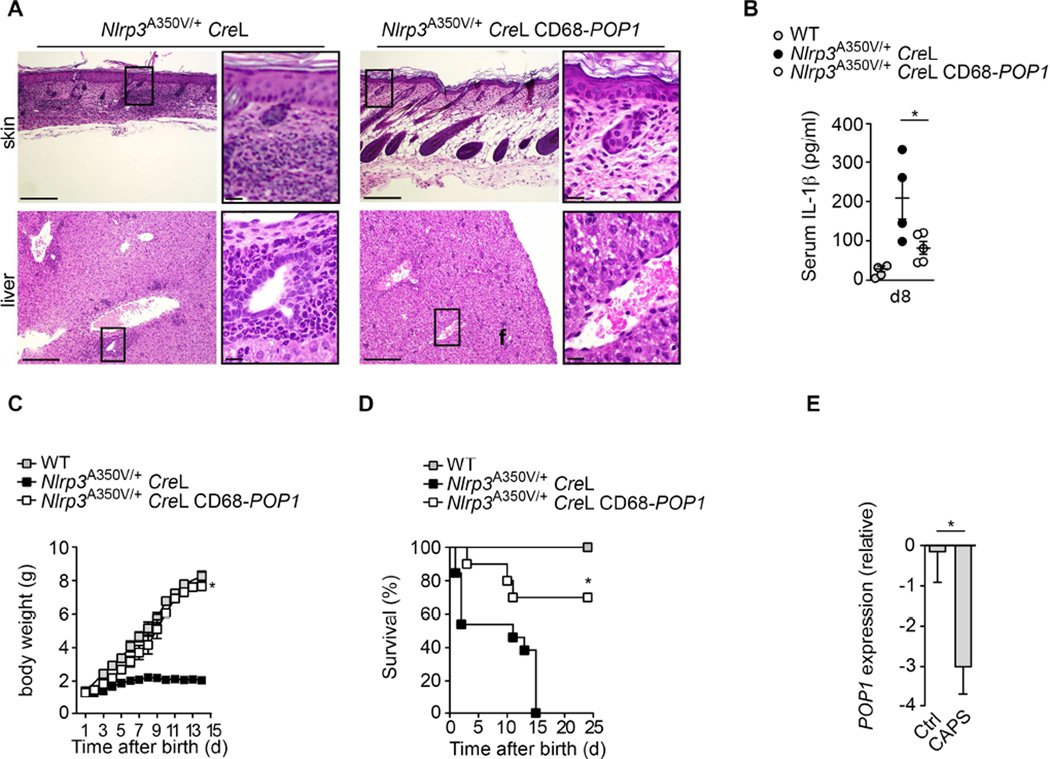
Since sepsis is a rather complex disease, we investigated a disease model, for which the pathology is absolutely dependent on the NLRP3 inflammasome. Cryopyrinopathies (or Cryopyrin-associated periodic syndromes; CAPS) are caused by mutations in NLRP3 (Hoffman et al., 2001), are therefore directly linked to the NLRP3 inflammasome, and can be recapitulated in mice by knocking-in CAPS-associated NLRP3 mutations (Brydges et al., 2009; Meng et al., 2009; Brydges et al., 2013). We employed a mouse model for Muckle Wells Syndrome (MWS), where floxed Nlrp3A350V, corresponding to human NLRP3A352V, is expressed exclusively in myeloid cells in the presence of lysozyme M-Cre (CreL) (Brydges et al., 2009; Brydges et al., 2013). Nlrp3A350V/+ CreL mice develop systemic inflammation that affects multiple organs, display characteristic skin inflammation and die within two weeks of birth. This phenotype is caused by excessive IL-1β and IL-18 release as well as pyroptosis (Brydges et al., 2009; Brydges et al., 2013). Nlrp3A350V/+ CreL mice had inflammatory skin abscesses and lesions shortly after birth, which developed into scaling erythema (Brydges et al., 2009), but Nlrp3A350V/+ CreL CD68-POP1 mice did not display this phenotype (Figure S6A). Histological analysis revealed that POP1 expression prevented leukocytic infiltrates in multiple organs, including the liver and the skin and also restored skin architecture (Figure 5A). Also, the systemic IL-1β levels were reduced (Figure 5B). Significantly, POP1 expression rescued the severe growth delay (Figure 5C) and prevented mortality of Nlrp3A350V/+ CreL mice from multi-system organ failure (Figure 5D). Since expression of POP1 efficiently ameliorated CAPS, we analysed POP1 expression in a previously published patient cohort (Boisson et al., 2012) and found that CAPS patients displayed significantly lower POP1 expression compared to healthy controls (Figure 5E). We also observed the same trend in in leukocytes from two large, independent septic patient cohorts, when compared to healthy controls (Figure S6B) (Tang et al., 2008; Wong et al., 2009), albeit the reduced POP1 expression in patients was less pronounced. These findings demonstrate that POP1 inhibits excessive NLRP3 inflammasome activity in vivo and thereby ameliorates auto-inflammatory disease. We also identified reduced POP1 expression in CAPS patients, which may circumvent appropriate inflammasome control.
Figure 5. Monocyte-MΦ-DC-specific expression of POP1 ameliorates CAPS (See also Figures S6A, B).
(A) H&E staining of skin sections at day 8 in Nlrp3A350V/+ CreL CD68-POP1 and Nlrp3A350V/+CreL mice . Scale bar 100 µm (original) and 10 µm (magnification).
(B) Serum IL-1β levels in 8d old Nlrp3A350V/+ CreL (n=4) and Nlrp3A350V/+ CreL CD68-POP1 (n=5) mice.
(C) Body weight of WT (n=15), Nlrp3A350V/+CreL CD68-POP1 (n=11) and Nlrp3A350V/+CreL (n=11) mice.
(D) Survival of WT (n=11), Nlrp3A350V/+CreL CD68-POP1 (n=10) and Nlrp3A350V/+CreL (n=8) mice.
(E) POP1 mRNA expression (relative to control) in whole blood drawn from healthy controls (n=23), CINCA (n=2) and MWS (n=5) patients (GEO accession number GSE40561).
(B) *P=0.0394; (C) *P<0.005; non-linear regression fit and Kruskal-Wallis one-way ANOVA (P=0.0021) with Dunn’s Multiple Comparison post test; (D) *P<0.0001; asymmetrical Log-rank Mantel-Cox survival test; (E) *P=0.0359; all two-tailed unpaired t-test; data are representative of n=two (A) replicates; error bars represent s.e.m.
POP1 prevents ASC particle release and ameliorates ASC particle-induced inflammation
Recently, polymerized ASC particles were detected in the serum of active CAPS patients (Baroja-Mazo et al., 2014), which are released from MΦ through inflammasome-dependent pyroptosis and act as danger signals on neighbouring cells (Baroja-Mazo et al., 2014; Franklin et al., 2014). POP1 prevented ASC nucleation and the subsequent ASC polymerization, caspase-1 activation and pyroptosis, which are all required for the ASC particle response. Hence, culture supernatants from LPS primed and nigericin or ATP-treated control THP-1GFP cells and BMDMWT contained ASC, but supernatants from THP-1GFP-POP1 cells (Figure 6A) or BMDMPOP1 (Figure 6B) did not contain any ASC. Particularly, the release of polymeric ASC was inhibited by POP1 (Figure 6C). Extracellular ASC particles are phagocytized by MΦ and activate caspase-1 in an NLRP3- and ASC-dependent process (Baroja-Mazo et al., 2014; Franklin et al., 2014). FACS-purified ASC-GFP particles (Figure S6C) induced IL-1β release in LPS primed THP-1GFP cells, but not in THP-1GFP-POP1 cells (Figure 6D), suggesting that POP1 prevents ASC nucleation downstream of NLRP3 activation. POP1 is an ASC binding protein, which can be incorporated into ASCPYD filaments (Figure S1F). This may result in reduced ASCCARD density and subsequently prevent caspase-1 nucleation and activation (Lu et al., 2014). To directly prove that POP1 incorporation into ASC particles renders them inactive, we generated mixed ASC-GFP/RFP-POP1 particles (Figure S6C), which, contrary to ASC-GFP particles, failed to cause IL-1β release in THP-1 cells (Figure 6E). Importantly, i.p. injection of ASC-GFP particles into WT mice resulted in neutrophil recruitment (Figure 6F) and IL-1β release (Figure 6G), which was substantially reduced in CD68-POP1 TG mice.
Figure 6. Expression of POP1 prevents ASC particle release and ameliorates ASC particle-induced inflammation (See also Figures S6C–E).
(A) LPS primed THP-1 cells expressing GFP or GFP-POP1 were treated with nigericin and released ASC determined by immunoblot in total cell lysates (TCL) and culture supernatants (SN).
(B) WT and POP1 BMDM were treated with ATP and released ASC determined by immunoblot in TCL and SN.
(C) Immunoblot analysis of ASC polymerization in untreated or LPS/ATP treated WT and POP1 BMDM after cross linking of pellets (P) and in TCL.
(D) ASC-GFP particles were FACS purified and imaged by immunofluorescence microscopy, showing the characteristic filamentous structure. Scale bar is 2 µm. Culture SN from THP-1 cells stably expressing GFP or GFP-POP1 were analyzed for IL-1β release by ELISA in LPS primed cells before (Ctrl) and after treatment with 1×103 FACS-purified ASC-GFP particles.
(E) Mixed ASC-GFP/RFP-POP1 particles were FACS purified as above, showing comparable size and overall structure. Scale bar is 2 µm. Culture SN from THP-1 cells were analyzed for IL-1β release by ELISA in LPS primed cells before (Ctrl) and after treatment with 1×103 FACS-purified ASC-GFP and ASC-GFP/RFP-POP1 particles.
(F) Quantification of MPO activity by in vivo imaging in WT and CD68-POP1 mice 4h after i.p. injection of PBS or 1×105 FACS-purified ASC-GFP particles (n=2/genotype).
(G) ELISA of total IL-1β in the peritoneal cavity from above mice 4h after ASC-GFP particle challenge.
(H) 30 min. after i.p. injection of TAT-GFP or TAT-POP1 (40 µg/kg), mice were i.p. injected with LPS (2.5 mg/kg). After 1h MPO activity was determined by in vivo imaging and the MPO luminescent signal quantified in LPS injected WT mice pre-treated with TAT-GFP (n=2) and TAT-POP 1 (n=3) mice.
(D) *P=0.0488; (E) *P=0.0022; (F) *P=0.0488; (G) *P=0.0038; all two-tailed unpaired t-test; data are representative of two (A–E) replicates; error bars represent s.e.m.
Based on the reduced POP1 expression in CAPS patients and its inflammasome inhibitory function in MΦ, we designed a proof of concept treatment approach targeting ASC inflammasomes. Cell penetrating peptides are frequently employed for the delivery of molecules targeting intracellular signalling pathways (Schwarze et al., 1999), and hence we produced recombinant POP1 and GFP as a control fused to the cell penetrating HIV TAT sequence (TAT-POP1 and TAT-GFP) (Figure S6D). TAT-GFP was efficiently taken-up by PM after i.p. injection in vivo (Figure S6E), and injection of TAT-POP1, but not TAT-GFP, ameliorated LPS-induced peritonitis (Figure 6H), reminiscent to transgenic POP1 expression. Collectively, these data show that POP1 also blocks the release of ASC danger particles and consequently, propagation of secondary inflammasome responses in neighbouring cells and that delivery of POP1 may potentially be used as the basis for future therapeutic approaches in CAPS patients and other inflammasomopathies.
POP1 functions in an inducible inflammasome regulatory loop
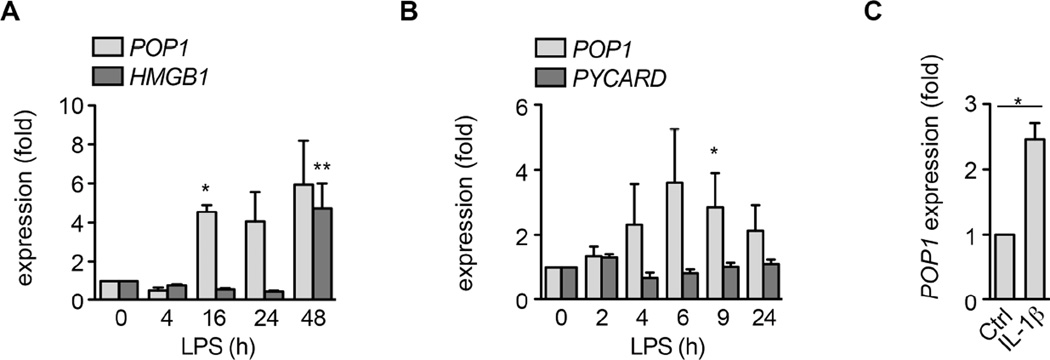
Overwhelming evidence supports the necessity for a balanced inflammasome response to maintain tissue homeostasis (Henao-Mejia et al., 2012). Thus, we hypothesized that POP1 expression needs to be tightly regulated. We observed LPS-induced late response gene expression of POP1 in hMΦ (Figure 7A) and THP-1 cells (Figure S7A), but contrary to POP3, POP1 was not up-regulated in response to IFN-b (Khare et al., 2014), which emphasizes the distinct function of individual POPs. Notably, POP1 expression peaked right before the inducible expression of HMGB1, which is released through pyroptosis (Lamkanfi et al., 2010; Willingham et al., 2009) and contributes to inflammatory disease (Harris et al., 2012). Thus, the late response expression of POP1 potentially enables inflammasome functions in early host defense and may provide a mechanism to counter excessive release of late mediators that perpetuate systemic inflammation, such as HMGB1. Importantly, this LPS-inducible expression of POP1 was also observed in leukocytes isolated from human subjects following in vivo LPS infusion (Calvano et al., 2005) (Figure 7B). TLR4 is required for inducible POP1 expression by LPS, as a TLR4 inhibitor reduced POP1 expression (Figure S7B). TLR signalling leads to NF-κB activation and blocking NF-κB also reduced POP1 transcription (Figure S7B). Inducible POP1 transcription was not only caused by TLR4, but also TLR2 activation by Pam3CSK4 (Figure S7C). IL-1R and IL-18R share signalling components with TLRs and accordingly, POP1 expression was also elevated in hMΦ after IL-1β (Figure 7C) and IL-18 treatment (Figure S7D). Thus, TLR, IL-1R and IL-18R engagement contributes to NF-κB dependent inducible transcription of POP1. At the transcriptional level, we observed ~4 fold increased POP1 expression in hMΦ after TLR or IL-1R stimulation and our stable THP-1GFP-POP1 and THP-1Myc-POP1 cells showed ~3 fold and ~7 fold more POP1 compared to baseline expression levels, respectively (Figure 1E, S1A, E). Hence our stable cell lines closely mimicked the induced POP1 expression levels, which were therefore also sufficient to impair inflammasome activity. At the protein level, PMGFP-POP1 expressed less POP1 than THP-1GFP-POP1, as determined by flow cytometry and western blot (Figure S7E), indicating that POP1 expression in our TG mice was decreased relative to hMΦ. Still, we found that POP1 expression levels in CD68-POP1 TG mice varied slightly and correlated inversely with inflammasome activity, as determined by measuring IL-18 levels 4 hours after i.p. LPS injection (R2=0.9316) (Figure S7F). Overall, our results demonstrate that POP1 regulates the inflammasome-mediated IL-1β and IL-18 release, which in turn regulates POP1 expression.
Figure 7. POP1 expression is regulated by TLRs, IL-1β (See also Figure S7).
(A) POP1 and HMGB1 transcripts were measured by Real-time PCR in LPS-treated primary hMΦ.
(B) POP1 and PYCARD mRNA expression in leukocytes from LPS infused human subjects.
(C) POP1 mRNA expression in primary h MΦ after 4h IL-1β treatment.
(A) *P=0.0097, **P=0.0443; (B) *P=0.0284; (C) *P=0.0266; all two-tailed unpaired t-test; data are representative of two (A,C,D) and (n=8) (B) replicates; error bars represent s.e.m.
DISCUSSION
ASC-containing inflammasomes are responsible for cytokine release through canonical and non-canonical inflammasomes (Mariathasan et al., 2004; Kayagaki et al., 2011; Kayagaki et al., 2013) and consequently play a central role in facilitating the beneficial inflammatory responses to clear pathogen infections and initiate wound healing after tissue damage. However, uncontrolled inflammasome responses cause inflammatory disease through excessive cytokine release (Strowig et al., 2012; Hoffman and Brydges, 2011). Thus, a well-balanced inflammasome response is crucial for maintaining homeostasis. Therefore, inflammasome regulatory proteins likely exist to maintain an appropriate level of activity and in particular to limit inflammasome activity during the resolution phase of these responses. We proposed that POP1 is one of these proteins. Assembly of the inflammasome platform is initiated by nucleation of ASC through PYD interactions with PYD-containing PRRs followed by prion-like ASC self-polymerization (Cai et al., 2014; Franklin et al., 2014; Lu et al., 2014; Chu et al., 2015) . Hence, the PYD plays a crucial role in inflammasome assembly. We recently demonstrated that POP3 selectively regulates ALR inflammasomes by binding to the PYD of ALRs but not to ASC, which is sufficient to prevent ALR-ASC interactions (Khare et al., 2014). In contrast to POP3, our data support a model where POP1 acted more broadly by binding to the ASCPYD, which prevented ASC interaction with PYD-containing PRRs and consequently the crucial ASC nucleation event. Similarly, POP1 prevented exogenous ASC particle responses, which are only partially dependent on NLRP3, but depend on ASC-nucleated ASC polymerization (Cai et al., 2014; Franklin et al., 2014; Lu et al., 2014). Moreover, mixed particles composed of ASC and POP1 were inactive, likely by reducing the necessary ASC-CARD density required for caspase-1 activation. Thus, POP1 expression not only prevented inflammasome assembly and cytokine release, but also release of ASC danger particles and propagation of secondary inflammasome responses to bystander cells. Previous studies suggested that POP1 enhances IL-1β release in vitro (Stehlik et al., 2003). However, based on our results, this is likely a consequence from over expression in HEK293 cells, as we also observed a weak nucleation of ASC polymerization by POP1 in these cells. Other PYDs, including PYD from NLRP3 can also induce weak nucleation of ASC polymerization in this cell type (Lu et al., 2014), and the NLRP3PYD also binds to the same ASC region as POP1 (Vajjhala et al., 2012).
Overexpression studies of several PYD proteins in HEK293 cells have implicated PYDs in NF-κB regulation, but these results where not observed in MΦ and in vivo. Similarly, we previously observed an inhibitory effect of POP1 on NF-κB activity in HEK293 cells (Stehlik et al., 2003) but did not observe such activity in THP-1 cells, hMΦ and BMDM in response to all tested stimuli. However, POP1 expressing THP-1 cells showed a slightly reduced IκBα phosphorylation in response to LPS, but this cannot be responsible for the potent inhibition of caspase-1 and we did not observe significant altered transcription of inflammasome components or release of IL-6 and TNFα. Filament formation of prion activity containing signalling components is one of the mechanisms of innate immune pathway activation. While ASCPYD, AIM2PYD and NLRP3PYD contain such intrinsic prion activity (Lu et al., 2014; Cai et al., 2014), POP1 does not appear to have this activity, as it does not form these characteristic filaments (Stehlik et al., 2003), but POP1 expression levels likely are crucial for its inflammasome inhibitory function. Inflammasome activity increased upon POP1 silencing in hMΦ and THP-1 cells but decreased upon stable expression of POP1 at levels that are comparable to its induced endogenous expression levels. POP1 transgene expression in mice was lower than in our stable cells and by extension also likely lower than in hMΦ, and therefore closely mimicked physiologically relevant POP1 levels. Despite this lower expression, POP1 was still able to inhibit the inflammasome, and variable POP1 expression levels in individual TG mice even correlated with its inflammasome inhibitory activity. An earlier study mapping the interaction of ASC with NLRP3, did not observe reduced NLRP3-ASC interaction in the presence of POP1 when using in vitro binding assays with recombinant proteins (Vajjhala et al., 2012). However, protein folding and posttranslational modifications may not be recapitulated in vitro and may yield different results compared to our studies of endogenous inflammasome assembly in human and mouse MΦ. Posttranslational modifications, such as ubiquitination (Py et al., 2013) and phosphorylation (Lin et al., 2015; Hara et al., 2013), have been implicated in NLRP3 inflammasome activity, and POP1 phosphorylation (Stehlik et al., 2003) may also be involved in the binding mechanism.
Excessive and uncontrolled release of inflammasome mediators contributes to auto-inflammatory and auto-immune diseases (Strowig et al., 2012). Hence, blocking IL-1β has proved beneficial in various inflammatory diseases in human and mice (Dinarello, 2011). Furthermore, oligomeric ASC particles have been identified in CAPS and pulmonary disease (Franklin et al., 2014; Baroja-Mazo et al., 2014). Despite the tight regulation of inflammasome responses, even a single point mutation in NLRP3 can drive excessive systemic inflammation (Hoffman and Brydges, 2011). Thus, inflammasome regulatory mechanisms may be also impaired. In line with this possibility, we observed reduced POP1 expression in CAPS patients. This observation suggested that in addition to uncontrolled activation of NLRP3, recruitment and oligomerization of ASC and extracellular release of ASC danger particles proceed uncontrolled, due to reduced POP1 expression. The presence of POP1 may also increase the required threshold for inflammasome assembly. At low expression levels, as observed in resting MΦ, POP1 would likely not interfere with acute host defense and maintenance of metabolic health. However, upon inducible expression as a late response gene, POP1 may become involved in the resolution phase of inflammasome responses (Figure S7G), which is still poorly understood. In particular, POP1 expression before the onset of HMGB1 expression may be important, since inflammasome-dependent release of HMGB1 is directly linked to inflammatory disease (Yang et al., 2013). Accordingly, in mice that normally lack POP1 and other POP members (Stehlik and Dorfleutner, 2007; Khare et al., 2014), POP1 expression potently ameliorated systemic inflammasome-driven inflammation. Interestingly, CAPS is not only caused by excessive IL-1β secretion, but IL-18 secretion and pyroptosis also contribute to its pathology (Brydges et al., 2009; Brydges et al., 2013). Furthermore, ASC inflammasomes are also responsible for cytokine release by the non-canonical inflammasome (Kayagaki et al., 2011). By blocking all inflammasome-dependent mediators, POP1 exhibited a potent anti-inflammatory function in peritonitis, sepsis and CAPS, and this function can likely be extended to other ASC-dependent inflammatory diseases. Employing a cell permeable recombinant POP1 provided proof-of-concept that a POP1-based therapy could be effective in CAPS and other inflammasomopathy patients with impaired POP1 expression, where it may function as a novel broad-spectrum anti-inflammatory treatment strategy targeting all inflammasome effectors.
Inflammasomes do not only assemble in macrophages, but the cell types that are responsible for systemic inflammation have not been elucidated yet. We demonstrated that monocyte-MΦ-DC-lineage-specific expression of POP1 was sufficient to prevent systemic inflammation in three different inflammatory disease models, which strongly implicates that inflammasome activation and defects in inflammasome control in this lineage are crucial for promoting systemic inflammation. Many studies highlight the importance of inflammasomes for homeostasis and disease pathology and suggest that understanding the mechanism by which healthy tissues put the brakes on inflammasome-induced systemic inflammation will be the next important step for designing future therapies. With the results from our study, we started to provide some answers to this important question. POP1 provides a unique mechanism that evolved in humans to possibly allow tighter control of an essential host defense system by guarding against excessive and out of control responses that cause inflammatory disease.
EXPERIMENTAL PROCEDURES
Animals
B6.TgN(CD68-POP1) TG mice were generated as described using GFP-POP1 (Khare et al., 2014; Iqbal et al., 2014). C57BL/6 wild type and Lysozyme M-Cre knock-in mice (CreL) were obtained from the Jackson Laboratories and Nlrp3−/−, Pycard−/− and floxed Nlrp3A350V knock-in mice were described earlier (Mariathasan et al., 2004; Mariathasan et al., 2006; Brydges et al., 2009). Mice were housed in a specific pathogen-free animal facility and all experiments were performed on age and gender-matched, randomly assigned 8–14 week old mice conducted according to procedures approved by the Northwestern University Committee on Use and Care of Animals. Floxed Nlrp3A350V mice (Brydges et al., 2009; Brydges et al., 2013) were crossed with CreL and CD68-POP1 TG mice and male and female offsprings were analysed for body weight and survival. Histological analysis was performed at day 8 after birth.
Macrophage isolation, culture and transfection
Peripheral blood-derived hMΦ, BMDM and Peritoneal MΦ (PM) were isolated as described (Khare et al., 2014; Khare et al., 2012).
Quantitative real-time PCR
Total RNA was isolated and analyzed as described (Khare et al., 2012; Khare et al., 2014).
LPS-induced peritonitis
8–12 week old female WT and CD68-POP1 TG mice had their abdomen shaved under anaesthesia, and were randomly selected for i.p. injection with PBS or LPS (2.5 mg/kg, E. coli 0111:B4, Sigma). After 3h, mice were i.p. injected with XenoLight Rediject Inflammation probe (200 mg/kg, PerkinElmer) (Gross et al., 2009) and in vivo bioluminescence was captured by imaging (IVIS Spectrum, PerkinElmer) 10 min post injection with a 5 min exposure on anesthetized mice (Khare et al., 2014). Images were quantified with Living Image software (PerkinElmer). Endotoxic shock was induced by i.p. injection of a lethal dose of 20 mg/kg LPS (E. coli 0111:B4) and mice were monitored 4 times daily for survival. Body temperature was measured with an animal rectal probe. Blood was collected 3h post LPS injection by mandibular bleed, and serum cytokine levels were quantified by ELISA.
ASC-particle-induced peritonitis
ASC-GFP-containing particles were FACS purified and verified by microscopy. 14 weeks old male WT and CD68-POP1 TG mice had their abdomen shaved under anaesthesia, and were randomly selected for i.p. injection with PBS or FACS-purified ASC-GFP particles (1×105 particles/mouse). After 4h, MPO activity was determined as above. Peritoneal lavage fluids were collected and assayed for IL-1β by ELISA.
Plasmids
pcDNA3 and pGEX-based expression constructs for ASC, POP1, NLRP3, ASCPYD and NLRP3PYD were described earlier (Khare et al., 2014; Stehlik et al., 2003; Stehlik et al., 2003)
Antibody-based detection
Co-immunoprecipitations (IP), GST pull down, ASC cross-linking, immunohistochemistry, ELISA, Flow cytometry and caspase-1 activity were performed as previously described (Khare et al., 2014).
Cell penetrating recombinant proteins
6xHIS-POP1 and a 6xHIS-GFP cDNAs were fused with the HIV TAT sequence and purified from E. coli. 12 week old male WT mice had their abdomen shaved under anaesthesia, were randomly selected for i.p. injection with TAT-GFP or TAT-POP1 (40 µg/kg) for 30 min prior LPS i.p. injection (2.5 mg/kg, E. coli 0111:B4, Sigma), and were quantified for MPO activity in vivo 1h later, as described above.
Statistics
Graphs represent the mean +/− s.e.m. A standard two-tailed unpaired t-test was used for statistical analysis of two groups with all data points showing a normal distribution and Kaplan-Meier survival curves were used to investigate differences in survival. Values of P<0.05 were considered significant and listed in the figure legends (Prism 5, GraphPad). The investigators were not blinded to the genotype of the mice/cells. Sample sizes were selected on the basis of preliminary results to ensure a power of 80% with 95% confidence between populations.
Supplementary Material
HIGHLIGHTS.
-
►
POP1 inhibits inflammasome-mediated responses to PAMPs and DAMPs
-
►
POP1 prevents IL-1 β and IL-18 release and pyroptosis
-
►
POP1 prevents ASC danger particle-mediated response propagation to bystander cells
-
►
Transgenic POP1 expression protects mice from systemic inflammation
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (GM071723, HL097183, AI092490, AI082406, AI099009, AI120625 and AR064349 to C.S., AR057532 and AR066739 to A.D., AR050250, AR054796, AI092490 and HL108795 to H.P., AR061593 to A.V.M and AI52430 to H.M.H.), a Cancer Center Support Grant (CA060553), the Skin Disease Research Center (AR057216) and the American Heart Association (13GRNT17110117) to C.S., an ATS/Scleroderma Foundation Grant to A.V.M., The British Heart Foundation (RG/10/15/28578) to D.R.G.. S.K. was an Arthritis Foundation fellow (AF161715), L.d.A. was supported by the American Heart Association (11POST585000) and the NIH (T32AR007611) and H.P. was supported by funds provided by the Solovy/Arthritis Research Society Professor. Plasmids pMD2.G and psPAX2 were kindly provided by Didier Trono (École Polytechnique Fédérale de Lausanne), Nlrp3A350V KI mice by Hal M. Hoffman (University of California at San Diego), and Pycard−/− and Nlrp3−/− mice by Vishva M. Dixit (Genentech). This work was supported by the Northwestern University Transgenic and Targeted Mutagenesis Laboratory, Mouse Histology and Phenotyping Laboratory, and flow cytometry facility. We thank Dr. C.M. Cuda for support with flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
L.d.A., C.S. and A.D. designed the research; L.d.A., S.K., A.V.M., R.P., R.A.R., A.D., C.S. and M.C.W. performed experiments; D.R.G., H.P. and H.M.H. provided essential reagents, expertise and advice; L.d.A., S.K., A.V.M., R.A.R., H.P., A.D. and C.S. analyzed results; L.d.A., A.D. and C.S. wrote the paper; A.D. and C.S. conceived the study and provided overall direction.
REFERENCES
- Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe J, Ruiz-Ortiz E, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya F, Sandler LL, Harton JA. Pyrin-only protein 2 modulates NF-kappaB and disrupts ASC:CLR interactions. J. Immunol. 2007;178:3837–3845. doi: 10.4049/jimmunol.178.6.3837. [DOI] [PubMed] [Google Scholar]
- Boisson B, Laplantine E, Prando C, Giliani S, Israelsson E, Xu Z, Abhyankar A, Israël L, Trevejo-Nunez G, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J. Clin. Invest. 2013;123:4695–4705. doi: 10.1172/JCI71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Chen J, Xu H, Liu S, Jiang Q-X, Halfmann R, Chen ZJ. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- Chu LH, Gangopadhyay A, Dorfleutner A, Stehlik C. An updated view on the structure and function of PYRIN domains. Apoptosis. 2015;20:157–173. doi: 10.1007/s10495-014-1065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfleutner A, Bryan NB, Talbott SJ, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect. Immun. 2007;75:1484–1492. doi: 10.1128/IAI.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfleutner A, Chu L, Stehlik C. Inhibiting the inflammasome: one domain at a time. Immunol. Rev. 2015;265:205–216. doi: 10.1111/imr.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfleutner A, Talbott SJ, Bryan NB, Funya KN, Reed JC, Shi X, Flynn DC, Rojanasakul Y, Stehlik C. A Shope Fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes. 2007;35:685–694. doi: 10.1007/s11262-007-0141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Alnemri ES. Chapter Thirteen Assembly, Purification, and Assay of the Activity of the ASC Pyroptosome. Methods Enzymol. 2008;442:251–270. doi: 10.1016/S0076-6879(08)01413-4. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough PJ, Gordon S, Greaves DR. The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class A scavenger receptor in macrophages in vitro and in vivo. Immunology. 2001;103:351–361. doi: 10.1046/j.1365-2567.2001.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S, Gammon ST, Moss BL, Rauch D, Harding J, Heinecke JW, Ratner L, Piwnica-Worms D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009;15:455–461. doi: 10.1038/nm.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V, Mitsuyama M. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 2013;14:1247–1255. doi: 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Strowig T, Flavell RA. Inflammasomes: far beyond inflammation. Nat. Immunol. 2012;13:321–324. doi: 10.1038/ni.2257. [DOI] [PubMed] [Google Scholar]
- Hoffman HM, Brydges SD. Genetic and molecular basis of inflammasome-mediated disease. J. Biol. Chem. 2011;286:10889–10896. doi: 10.1074/jbc.R110.135491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S, Smart N, Machemer DEW, Stylianou E, et al. Human CD68 promoter directs GFP transgene expression in mouse myeloid cells, allowing analysis of monocyte to macrophage differentiation in vivo. Blood. 2014;124:e33–e44. doi: 10.1182/blood-2014-04-568691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Barrett JW, Nazarian SH, Goodwin M, Ricuttio D, Wang G, McFadden G. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23:587–598. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, Newton K, Qu Y, Liu J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, et al. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. An NLRP7-Containing Inflammasome Mediates Recognition of Microbial Lipopeptides in Human Macrophages. Immunity. 2012;36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S, Luc N, Dorfleutner A, Stehlik C. Inflammasomes and their activation. Crit. Rev. Immunol. 2010;30:463–487. doi: 10.1615/critrevimmunol.v30.i5.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S, Ratsimandresy RA, de Almeida L, Cuda CM, Rellick SL, Misharin AV, Wallin MC, Gangopadhyay A, Forte E, et al. The PYRIN domain-only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat. Immunol. 2014;15:343–353. doi: 10.1038/ni.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-Dependent Release of the Alarmin HMGB1 in Endotoxemia. J. Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K-M, Hu W, Troutman TD, Jennings M, Brewer T, Li X, Nanda S, Cohen P, Thomas JA, Pasare C. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. U. S. A. 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-C, Huang D-Y, Wang J-S, Lin Y-L, Hsieh S-L, Huang K-C, Lin W-W. Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J. Leukoc. Biol. 2015 doi: 10.1189/jlb.3HI0814-371RR. in press. [DOI] [PubMed] [Google Scholar]
- Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, Egelman EH. Unified Polymerization Mechanism for the Assembly of ASC-Dependent Inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Hopkins LJ, Nugent E, Cox S, Glück IM, Tourlomousis P, Wright JA, Cicuta P, Monie TP, Bryant CE. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc. Natl. Acad. Sci. U. S. A. 2014;111:7403–7408. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-1b. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, Beck PL, Muruve DA, Kubes P. Intravascular Danger Signals Guide Neutrophils to Sites of Sterile Inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith B, Rajendiran T, Núñez G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py BF, Kim M-S, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Schroder K, Sagulenko V, Zamoshnikova A, Richards AA, Cridland JA, Irvine KM, Stacey KJ, Sweet MJ. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiol. 2012;217:1325–1329. doi: 10.1016/j.imbio.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Poyet J-L, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for Caspase-1. J. Biol. Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- Stehlik C, Dorfleutner A. COPs and POPs: Modulators of Inflammasome Activity. J. Immunol. 2007;179:7993–7998. doi: 10.4049/jimmunol.179.12.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated NF-kB and pro-Caspase-1 regulation. Biochem. J. 2003;373:101–113. doi: 10.1042/BJ20030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J. Immunol. 2003;171:6154–6163. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Tang BM, McLean AS, Dawes IW, Huang SJ, Cowley MJ, Lin RC. Gene-expression profiling of gram-positive and gram-negative sepsis in critically ill patients. Crit. Care Med. 2008;36:1125–1128. doi: 10.1097/CCM.0b013e3181692c0b. [DOI] [PubMed] [Google Scholar]
- Vajjhala PR, Mirams RE, Hill JM. Multiple binding sites on the ASC pyrin domain allow self-association and interaction with NLRP3. J. Biol. Chem. 2012;287:41732–41743. doi: 10.1074/jbc.M112.381228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Miao EA, Ting JP-Y. Mechanisms of NOD-like Receptor-Associated Inflammasome Activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MTH, Taxman DJ, Duncan JA, Ting JPY. NLRP3 (NALP3, Cryopyrin) Facilitates In Vivo Caspase-1 Activation, Necrosis, and HMGB1 Release via Inflammasome-Dependent and -Independent Pathways. J. Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HR, Cvijanovich N, Allen GL, Lin R, Anas N, Meyer K, Freishtat RJ, Monaco M, Odoms K, et al. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit. Care Med. 2009;37:1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 2013;93:865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.