Abstract
Background
Tivozanib is a potent and selective tyrosine kinase inhibitor of vascular endothelial growth factor receptors (VEGFR)-1, −2 and −3, with a long half-life. Tivozanib has demonstrated clinical activity and acceptable tolerability in renal cell carcinoma (RCC). This phase Ib study determined the recommended phase II dose (RP2D) and evaluated the safety and clinical activity of tivozanib plus temsirolimus, a mammalian target of rapamycin inhibitor.
Patients and methods
Patients with advanced RCC were administered open-label tivozanib 0.5, 1.0 or 1.5 mg/d orally (3 weeks on/1 week off) and temsirolimus 15 or 25 mg/week intravenously in a 3 + 3 dose–escalation design and subsequent expansion cohort.
Results
Of 27 patients treated, 20 patients had received ≥1 prior VEGF-targeted therapy. No dose-limiting toxicities occurred; the RP2D was determined to be tivozanib 1.5 mg/d plus temsirolimus 25 mg/week. Combination of tivozanib plus temsirolimus demonstrated acceptable tolerability and suggested no synergistic toxicity. The most common grade ≥3 adverse events were fatigue and thrombocytopenia (15% each). One patient each required dose reduction of tivozanib or temsirolimus due to an adverse event. Confirmed partial responses and stable disease were achieved at 23% and 68%, respectively. Pharmacokinetic analyses may suggest lack of an interaction between tivozanib and temsirolimus.
Conclusions
In this small phase Ib study, tivozanib and temsirolimus were safely combined at the fully recommended dose and schedule of both agents. The observed clinical activity and manageable toxicity profile of this combination warrant further exploration in patients with RCC.
Keywords: Dose escalation, Maximum tolerated dose, Pharmacokinetics, Renal cell carcinoma, Tivozanib, Temsirolimus
1. Introduction
Among expanding choices for targeted therapy of renal cell carcinoma (RCC), available drugs act via inhibition of the vascular endothelial growth factor (VEGF)1 and mammalian target of rapamycin (mTOR) pathways.2
Combination therapy with VEGF and mTOR pathway inhibitors is appealing because of cooperative mTOR inhibition downstream of VEGFR signalling in epithelial cells and the effect of mTOR inhibition on tumour cell growth, as well as differing side-effect profiles.3,4 Side-effects observed with mTOR inhibitors (e.g. mouth sores, non-infectious pneumonitis)2 appear generally distinct from those observed with VEGFR inhibitors (e.g. fatigue, diarrhoea, hand–foot skin reaction, rash)1. However, combinations of mTOR inhibitors with sunitinib or sorafenib were either not tolerated5 or tolerated only as lower-dose combinations.6,7 While the combination of bevacizumab with full-dose temsirolimus or everolimus appears feasible,8,9 recent reports of bevacizumab plus temsirolimus have been less promising.10,11
Tivozanib is a tyrosine kinase inhibitor (TKI) with picomolar potency against VEGFR-1, −2 and −3, and minimal c-kit inhibition.12 Tivozanib has demonstrated a VEGFR-2 potency 2 orders of magnitude greater than sunitinib, sorafenib or pazopanib (cell-based IC50 of 160 pmol/L13 versus 15–34 nmol/L14) and a lower relative extent of off-target inhibition,13 similar to reports for axitinib (cell-based IC50 of 200 pmol/L15). Additionally, tivozanib’s half-life is longer than all four other TKIs (~4.5 d16 versus <0.1–2 d17–20). At a dose of 1.5mg/d for 3 weeks followed by a 1-week break, single-agent tivozanib demonstrated clinical activity in a phase II trial in RCC, with an overall response rate (ORR) of 24% and median progression-free survival of 11.7 months.21 An ongoing, randomised phase III trial recently reported superior progression-free survival with tivozanib (11.9 months) versus sorafenib (9.1 months).22 Tivozanib has also demonstrated a well-tolerated toxicity profile primarily characterised by manageable hypertension.21,22
The current phase Ib study evaluated the feasibility of combining tivozanib with temsirolimus in patients with clear cell RCC.
2. Patients and methods
2.1. Patients
Adults with a histologically confirmed diagnosis of RCC with a clear cell component were eligible. Additional eligibility criteria included a history of progressive disease, ≤1 prior VEGF-targeted therapy; measureable disease by Response Evaluation Criteria In Solid Tumours (RECIST); Karnofsky performance status >70%; adequate bone marrow, hepatic, and renal function; and no prior mTOR-targeted therapy. Patients with prior chemotherapy or cytokine therapy were eligible. Key exclusion criteria included active/clinically symptomatic central nervous system metastases, haematologic malignancies and significant cardiovascular disease (including uncontrolled hypertension). Full inclusion/exclusion criteria are provided in Supplementary Table S1 (online only).
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided a written informed consent. The protocol was approved by institutional review boards at each site.
2.2. Study design
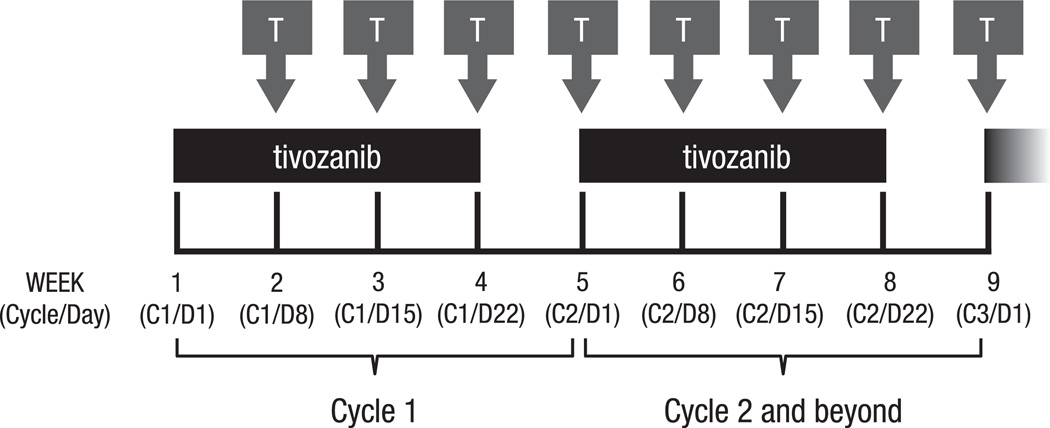
This was a phase Ib, open-label, multicenter, dose– escalation trial. Eligible patients received tivozanib orally once daily for 3 weeks, followed by a 1-week break (4 weeks = 1 cycle),21 and temsirolimus intravenously once weekly, starting on day 8 of cycle 1 for a minimum of 8 weeks (two cycles), if tolerated (Fig. 1). A standard 3 + 3 dose–escalation design was employed to determine the recommended phase II dose (RP2D), the primary study objective. Patients were sequentially enrolled at escalating dose levels beginning at tivozanib 0.5 mg/d and temsirolimus 15 mg/week. If 1/3 patients experienced a dose-limiting toxicity (DLT) in cycle 1, that dose level was expanded to six patients; enrolment to the next dose level occurred if 0/3 or 1/6 patients experienced a DLT. If ≥2/6 patients experienced a DLT in cycle 1, dose escalation stopped and the prior dose level was considered the RP2D. Additional patients could be enrolled if a patient discontinued prior to completing cycle 1. Detailed DLT criteria are provided in Supplementary Table S2 (online only). An expansion cohort of 12 additional patients was enrolled at the RP2D for further evaluation of safety as well as clinical activity. After completion of cycle 2, patients with stable disease (SD) or response could continue treatment for up to 1 year from their first tivozanib dose. A rollover study was available for further tivozanib therapy.
Fig. 1.
Dosing schedule. Tivozanib was administered once daily for 3 weeks, followed by a 1-week break (4 weeks = 1 cycle), and temsirolimus was administered weekly starting on day 8 of cycle 1. Treatment continued for a minimum of 8 weeks (two cycles), but could be continued for up to 1 year for patients achieving response or stable disease. T, temsirolimus.
2.3. Safety assessments
Safety parameters included adverse event (AE) reporting and laboratory abnormalities, recorded through ≥1 month after the last study dose. Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Dose reductions were allowed for patients with grade 3/4 AEs (except initial control of hypertension), based on clinical judgment. Guidelines for hypertension management were provided to investigators; patients with uncontrolled hypertension despite optimal management (including standard anti-hypertensives or tivozanib dose reduction/interruption) were discontinued from treatment. Temsirolimus was withheld if the absolute neutrophil count was <1000 /mm3, platelets were <75,000 /mm3, or any grade 3/4 toxicity except hypertension was present.
2.4. Response assessments
Response was evaluated as a secondary objective. Radiological tumour assessments were performed during screening, at the end of cycle 2 and every other cycle thereafter during treatment. Response was determined by the investigator using standard RECIST criteria, with partial response (PR) and complete response (CR) confirmed by a repeat evaluation ≥4 weeks after the initial assessment.
2.5. Pharmacokinetic assessments
Pharmacokinetic parameters were estimated to evaluate potential interaction between tivozanib and temsirolimus. Fifteen blood samples were drawn to measure tivozanib serum concentrations at the following time points: cycle 1 day 1, prior to the first tivozanib dose and at 1, 2, 4 and 8 (±2) h postdose; day 2, at 24 h post-dose; days 8 and 15, predose; day 22, predose and at 1, 2, 4 and 8 (±2) h postdose; day 23, at 24 h after the day 22 dosing; and cycle 2 day 1, predose. On days when both tivozanib and temsirolimus were administered, tivozanib administration immediately followed temsirolimus administration.
2.6. Statistical analyses
Safety evaluations included all patients who received ≥1 dose of tivozanib. Safety events were tabulated as maximum severity, and relationship to study drug was determined.
Response analyses included all eligible patients who completed ≥2 cycles of tivozanib. Patients who withdrew prior to completion of cycle 2 due to disease progression were also included in the analysis as non-responders. The ORR (CR + PR) was calculated along with a 95% confidence interval (CI). Duration of response and time to progression were estimated using Kaplan–Meier methodology.
Pharmacokinetic parameters were calculated using standard non-compartmental methods using WinNonlin version 6.2 (Pharsight Corp., Mountain View, CA, United States of America (USA)). Pharmacokinetic parameters determined for tivozanib, temsirolimus and sirolimus (metabolite of temsirolimus) included maximum serum concentration (Cmax), time to Cmax (Tmax) and area under the curve (AUC).
3. Results
3.1. Patient characteristics
Twenty-eight patients with clear cell RCC were screened. One patient withdrew before tivozanib administration due to atrial fibrillation and was excluded from safety and response analyses. The remaining 27 patients were enrolled in the following cohorts: (1) tivozanib 0.5 mg/d plus temsirolimus 15 mg/week; (2) tivozanib 1.0 mg/d plus temsirolimus 15 mg/week; (3) tivozanib 1.5 mg/d plus temsirolimus 15 mg/week and (4) tivozanib 1.5 mg/d plus temsirolimus 25 mg/week (Table 1). The median duration of treatment was 5.3 months; of the 15 patients treated at the RP2D, nine patients received >6 treatment cycles. Baseline patient characteristics are summarised in Table 2.
Table 1.
Dose escalation.
| Dose level | Tivozanib, mg/d |
Temsirolimus, mg/week |
Patients enrolled, n |
|---|---|---|---|
| 1 | 0.5 | 15 | 5 |
| 2 | 1.0 | 15 | 4 |
| 3 | 1.5 | 15 | 3 |
| 4 | 1.5 | 25 | 3 |
| RP2D expansion | 1.5 | 25 | 12 |
RP2D, recommended phase II dose.
Table 2.
Demographic and baseline characteristics (safety population).
| Characteristic | N = 27 |
|---|---|
| Median age (range), years | 61 (43–71) |
| Male sex, n (%) | 25 (93) |
| Caucasian,a n (%) | 24 (89) |
| Median time since diagnosis (range), mo | 24 (0–146) |
| Karnofsky performance status,b n (%) | |
| 100% | 18 (67) |
| 90% | 5 (19) |
| 80% | 4 (15) |
| ECOG Performance Status, n (%) | |
| 0 | 23 (85) |
| 1 | 4 (15) |
| No. of organs involved,c n (%) | |
| 1 | 4 (15) |
| 2 | 11 (41) |
| 3 | 6 (22) |
| 4 | 3 (11) |
| ≥5 | 3 (11) |
| No. of prior VEGF treatments, n (%) | |
| 0 | 7 (26) |
| 1 | 19 (70) |
| 2 | 1 (4) |
| Prior VEGF treatments, n (%) | |
| Bevacizumab | 3 (11) |
| Sorafenib | 10 (37) |
| Sunitinib | 8 (30) |
| Prior cytokine therapy,d n (%) | 7 (26) |
| Prior surgery, n (%) | 26 (96) |
| Prior radiotherapy, n (%) | 4 (15) |
ECOG, Eastern Cooperative Oncology Group; VEGF, vascular endothelial growth factor.
Includes patients self-reported as Caucasian.
Percentages may not add to 100% due to rounding.
The most common sites for metastases were lung (n = 21 [78%]), lymph nodes (n = 17 [63%]) and liver (n = 9 [33%]).
Two patients in cohort 1 (n = 1 each in adjuvant and metastatic settings), two patients in cohort 2 (both in metastatic setting) and three patients in the R2PD cohort (all in metastatic setting) had received prior cytokine therapy.
3.2. Maximum tolerated dose
Among 27 patients evaluable for safety, no DLTs were observed in cycle 1. Therefore, the RP2D was determined to be tivozanib 1.5 mg/d plus temsirolimus 25 mg/week, corresponding to the full recommended doses of each drug. An additional 12 patients were subsequently enrolled at the RP2D in an expansion cohort. Of note, treatment-emergent AEs (TEAEs; described below) were tabulated separately and differently from DLTs.
3.3. Safety and tolerability
The most common TEAEs, regardless of causality, were fatigue (74%), stomatitis (59%), diarrhoea (56%), decreased appetite (52%) and nausea (48%; Table 3). Hypertension was observed in seven (26%) patients, with no grade 3/4 events. Fatigue and thrombocytopenia were the most common grade 3/4 TEAEs, reported by four (15%) patients each. Twenty-nine serious TEAEs were observed in 14 (52%) patients; of these, seven patients reported ≥1 serious TEAE related to tivozanib and/or temsirolimus (flank and back pain; pneumonia; gastric ulcer; nausea, diarrhoea, vomiting, fatigue and dehydration; pancreatitis; left ventricular dysfunction and hypothyroidism; and colitis and rectal abscess).
Table 3.
Treatment-emergent AEs in >15% of patients, any causality
| AE, all grades/ grade ≥3, n (%) |
Tivozanib 0.5 mg/d, temsirolimus 15 mg/week (n = 5) |
Tivozanib 1.0 mg/d, temsirolimus 15 mg/week (n = 4) |
Tivozanib 1.5 mg/d, temsirolimus 15 mg/week (n = 3) |
Tivozanib 1.5 mg/d, temsirolimus 25 mg/week (n = 15)a |
Total (N = 27) |
|---|---|---|---|---|---|
| Fatigue | 1/1 | 3/0 | 3/0 | 13/3 | 20 (74)/4 (15) |
| Stomatitis | 2/0 | 4/1 | 1/0 | 9/1 | 16 (59)/2 (7) |
| Diarrhoea | 1/0 | 2/0 | 3/0 | 9/2 | 15 (56)/2 (7) |
| Decreased appetite | 3/0 | 2/0 | 2/0 | 7/0 | 14 (52)/0 |
| Nausea | 2/0 | 2/0 | 1/0 | 8/1 | 13 (48)/1 (4) |
| Constipation | 0 | 2/0 | 2/0 | 7/1 | 11 (41)/1 (4) |
| Thrombocytopenia | 3/1 | 0 | 1/0 | 6/3 | 10 (37)/4 (15) |
| Dysponea | 0 | 1/0 | 2/0 | 7/1 | 10 (37)/1 (4) |
| Increased triglycerides | 2/0 | 0 | 0 | 7/3 | 9 (33)/3 (11) |
| Decreased weight | 0 | 1/0 | 2/0 | 5/0 | 8 (30)/0 |
| Dehydration | 0 | 1/0 | 1/0 | 5/2 | 7 (26)/2 (7) |
| Vomiting | 2/0 | 1/0 | 1/0 | 3/1 | 7 (26)/1 (4) |
| Cough | 1/0 | 1/0 | 1/0 | 4/0 | 7 (26)/0 |
| Hypertension | 1/0 | 0 | 1/0 | 5/0 | 7 (26)/0 |
| Hyperglycaemia | 2/1 | 1/1 | 0 | 3/1 | 6 (22)/3 (11) |
| Abdominal pain | 0 | 0 | 1/0 | 5/2 | 6 (22)/2 (7) |
| Back pain | 1/1 | 0 | 1/0 | 4/0 | 6 (22)/1 (4) |
| Rash erythematous | 1/0 | 1/1 | 1/0 | 3/0 | 6 (22)/1 (4) |
| Anaemia | 0 | 1/0 | 2/0 | 3/0 | 6 (22)/0 |
| Dysphonia | 0 | 1/0 | 1/0 | 4/0 | 6 (22)/0 |
| Epistaxis | 0 | 0 | 1/0 | 5/0 | 6 (22)/0 |
| Pyrexia | 0 | 0 | 2/0 | 4/0 | 6 (22)/0 |
| Rash | 0 | 1/0 | 0 | 5/0 | 6 (22)/0 |
| Arthralgia | 0 | 0 | 0 | 5/0 | 5 (19)/0 |
| Chills | 0 | 0 | 2/0 | 3/0 | 5 (19)/0 |
| Dizziness | 0 | 2/0 | 1/0 | 2/0 | 5 (19)/0 |
| Dry skin | 1/0 | 2/0 | 0 | 2/0 | 5 (19)/0 |
| Dysgeusia | 1/0 | 1/0 | 0 | 3/0 | 5 (19)/0 |
| Headache | 2/0 | 0 | 0 | 3/0 | 5 (19)/0 |
| Insomnia | 1/0 | 2/0 | 0 | 2/0 | 5 (19)/0 |
| Musculoskeletal chest pain | 0 | 1/0 | 1/0 | 3/0 | 5 (19)/0 |
AE, adverse event.
Includes the expansion cohort at the recommended phase II dose.
Grade 3/4 laboratory abnormalities (any causality) are shown in Table 4. Hyperglycaemia and hypophos-phatemia were most common, observed in four (15%) patients each; three (11%) patients each demonstrated grade 3/4 elevated gamma-glutamyl transpeptidase, lymphopaenia, thrombocytopenia and hypertriglyceridemia.
Table 4.
Grade 3/4 laboratory abnormalities, any causalitya
| Laboratory abnormality, grade 3/4, n (%) | Tivozanib 0.5 mg/d, temsirolimus 15 mg/week (n = 5) |
Tivozanib 1.0 mg/d, temsirolimus 15 mg/week (n = 4) |
Tivozanib 1.5 mg/d, temsirolimus 15 mg/week (n = 3) |
Tivozanib 1.5 mg/d, temsirolimus 25 mg/week (n = 15)b |
Total (N = 27) |
|---|---|---|---|---|---|
| Hyperglycaemia | 1 | 1 | 0 | 2 | 4 (15) |
| Hypophosphatemia | 0 | 0 | 1 | 3 | 4 (15) |
| Elevated GGT | 1 | 0 | 0 | 2 | 3 (11) |
| Lymphopenia | 0 | 1 | 0 | 2 | 3 (11) |
| Thrombocytopenia | 1 | 0 | 0 | 2 | 3 (11) |
| Hypertriglyceridemia | 0 | 0 | 0 | 3 | 3 (11) |
| Hypokalemia | 0 | 0 | 0 | 2 | 2 (7) |
| Hyponatremia | 0 | 1 | 0 | 1 | 2 (7) |
| Elevated alkaline phosphatase | 1 | 0 | 0 | 0 | 1 (4) |
| Hypermagnesemia | 0 | 0 | 0 | 1 | 1 (4) |
| Neutropenia | 0 | 0 | 1 | 0 | 1 (4) |
| Elevated uric acid | 0 | 0 | 0 | 1 | 1 (4) |
GGT, gamma-glutamyl transpeptidase; RP2D, recommended phase II dose.
Patients with laboratory parameters that shifted from grade ≤2 severity to grade ≥3 severity.
Includes the expansion cohort at the RP2D.
One patient died after completion of five cycles of tivozanib and temsirolimus, due to cardiopulmonary arrest. The patient had metastases to the lung and pleura at study entry and experienced grade 4 pleural effusion after five cycles of treatment. Study drug was discontinued and, 17 d later, a therapeutic thoracentesis was performed, immediately after which the patient experienced severe respiratory distress and supraventricular tachycardia leading to hypotension, cardiac arrest and ultimately death. Because of the patient’s medical history and the time since the last dose of study drug, the death was considered unrelated to the study drug by the investigator and more likely related to the thoracentesis procedure.
Seventeen (63%) patients had ≥1 reduction/interruption of temsirolimus, including 16 (59%) patients who had ≥1 dose reduction/interruption of tivozanib. The majority of dose modifications were treatment interruptions; one patient each required a dose reduction of tivozanib (grade 2 fatigue) and temsirolimus (grade 3 hyponatremia). Eight (30%) patients experienced AEs resulting in discontinuation of both study drugs, including three patients who withdrew due to possibly drug-related AEs: ventricular dysfunction (possibly related to tivozanib), fatigue (possibly related to temsirolimus) and colitis and rectal abscess (possibly related to either study drug).
3.4. Clinical response
Twenty-two patients completed ≥2 treatment cycles and were included in the response evaluations; of the remaining five patients, two patients received <2 cycles of tivozanib before withdrawing for reasons other than progressive disease, and it was later determined that three others did not satisfy the entry criteria.
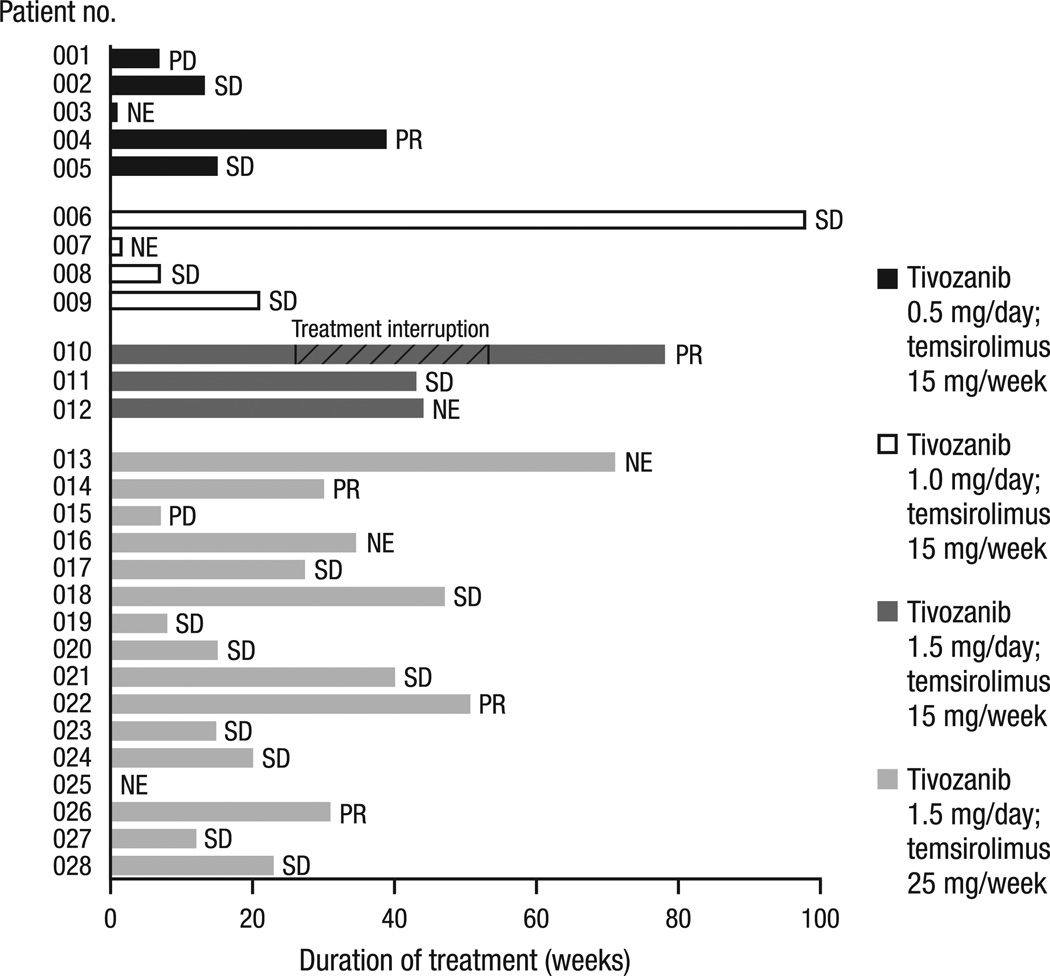
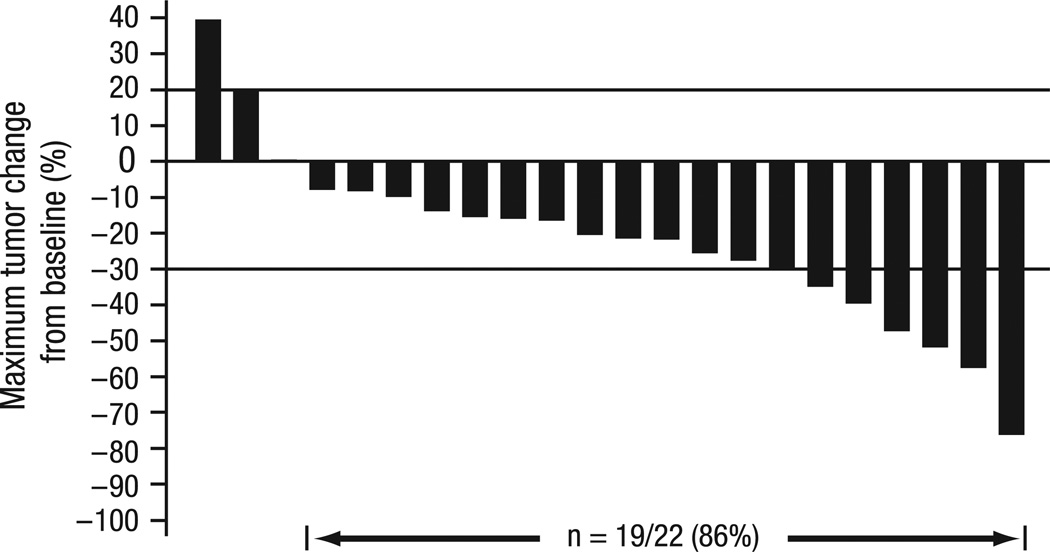
The ORR was 23% (95% CI, 8–45%). Although no CRs were observed, PR was confirmed in five (23%) patients and SD was achieved in 15 (68%) patients (Fig. 2). Of these, all five patients with PR and 10 of 15 patients with SD had received prior VEGFR-targeted therapy. Among the 11 patients in the RP2D expansion cohort who were evaluable for response, two patients achieved PR and the remaining nine patients achieved SD. In this cohort, the median duration of SD was 9.2 months (95% CI, 9.2 months to not reached). The maximum changes in tumour size from baseline for all evaluable patients are shown in Fig. 3.
Fig. 2.
Duration of treatment and best response. Response was determined for the 22 patients evaluable for response: five (23%) patients achieved a best response of PR, and 15 (68%) patients achieved a best response of SD. Patient 010 received six cycles of study treatment before the patient’s treatment was interrupted (September 2009) when a suspected abscess appeared in the peripheral lung. He had antimicrobial treatment and resection, showing no malignancy in that area. The mediastinal lymph nodes that were the site of the metastatic RCC were about the same (SD) after the treatment hiatus, and he restarted on treatment (March 2010) following IRB approval. The patient continued study treatment for another 6 months before rolling over to a long-term treatment study. Of the six patients who were NE for response, one patient withdrew prior to tivozanib administration, two patients received <2 cycles of tivozanib before withdrawing for reasons other than progressive disease, and it was later determined that three others did not satisfy the entry criteria (no histologically confirmed RCC with clear cell component; received herbal preparations/supplements within 2 weeks prior to or during the study; and ongoing hemoptysis/clinically significant bleeding and experimental therapy within 4 weeks prior to and during the study). PD, progressive disease; SD, stable disease; NE, not evaluable; PR, partial response; RCC, renal cell cancer; IRB, Institutional Review Board.
Fig. 3.
Tumour response. Maximum changes in tumour size from baseline were determined for the 22 patients evaluable for response.
3.5. Pharmacokinetics
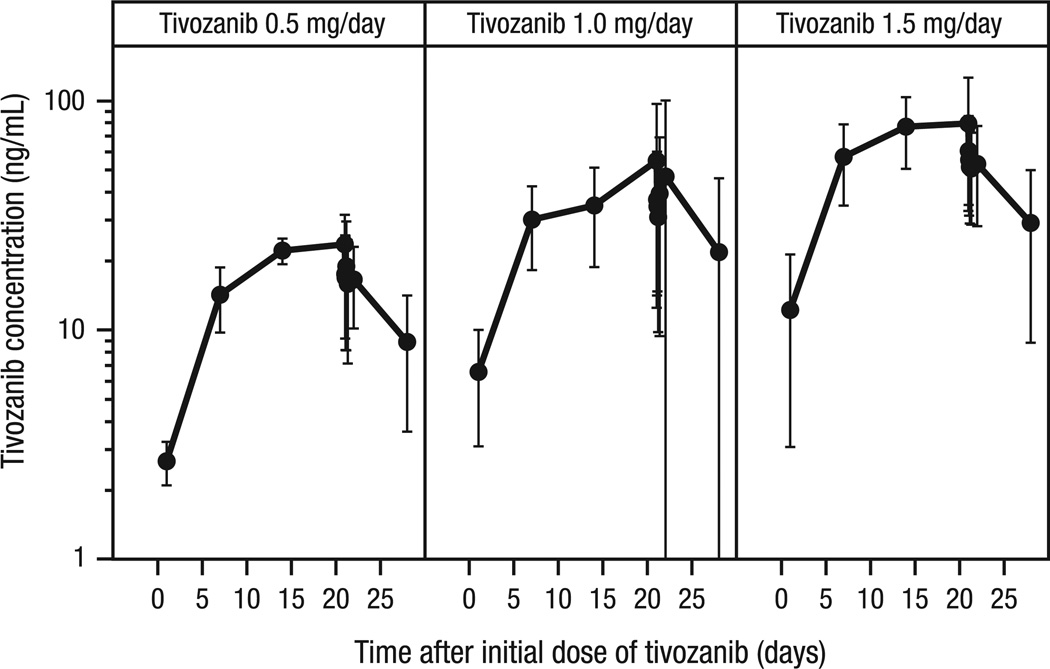
The tivozanib serum concentration–time profile showed expected increases in concentration with increasing dose as well as accumulation to steady-state levels over the initial 21-d treatment period (Fig. 4). At the recommended therapeutic dose of tivozanib 1.5 mg/d, mean (standard deviation) pharmacokinetic parameters on day 1 were 13.16 (9.21) ng/mL for Cmax, 17.33 (10.35) h for Tmax, and 238.9 (140) h·ng/mL for AUC0–τ (from time 0 to immediately prior to the next dose). Day 22 pharmacokinetic parameters for temsirolimus 15 and 25 mg/week and its metabolite sirolimus also demonstrated a dose-related increase in exposure (Table 5).
Fig. 4.
Individual and mean (shown in bold) tivozanib (1.5 mg) serum concentration versus time by dose level throughout cycle 1. Although substantial individual variation was observed, there was a clear relationship between tivozanib concentration and dose, and an accumulation of tivozanib to nearly steady-state levels over the 21-d treatment period.
Table 5.
Pharmacokinetic Parameters of Temsirolimus and Sirolimus at day 22
| Parameter, mean (SD) | Temsirolimus 15 mg/week | Temsirolimus 25 mg/week |
|---|---|---|
| Temsirolimus | ||
| Evaluable, n | 7 | 9 |
| Cmax, ng/mL | 164.3 (88.1) | 199.6 (33.4) |
| Tmax, h | 2.32 (1.20) | 1.81 (0.54) |
| AUC0–last, h•ng/mL | 3191 (1925) | 4245 (2033) |
| Sirolimus | ||
| Evaluable, n | 7 | 9 |
| Cmax, ng/mL | 51.33 (25.44) | 93.79 (25.04) |
| Tmax, h | 3.18 (1.34) | 1.81 (0.54) |
| AUC0–last, h•ng/mL | 3559 (1629) | 6566 (4110) |
| Ratio of sirolimus AUC0–last /temsirolimus AUC0–last | 1.26 (0.67) | 1.48 (0.66) |
SD, standard deviation; Cmax, maximum serum concentration; Tmax, time to maximum concentration; AUC0–last, area under the concentration– time curve from time 0 to the last non-zero concentration value.
4. Discussion
The combination of mTOR and VEGFR TKIs holds promise for the treatment of advanced RCC, as each class inhibits different mechanisms of tumourigenesis and tumour angiogenesis. Although preclinical data appeared promising,23 initial clinical trials combining mTOR and multi-targeted TKIs reported considerable toxicities, and often with administration at doses lower than those recommended for each agent as monotherapy.5,6,9,10,24
In the current study, tivozanib and temsirolimus were safely combined at the fully recommended doses of each agent for the treatment of advanced RCC, with no DLTs encountered during the dose–escalation phase of the study. Therefore, the RP2D was determined to be tivozanib 1.5 mg/d and temsirolimus 25 mg/week, and higher doses were not evaluated.
The combination of tivozanib and temsirolimus had an acceptable safety profile, with most TEAEs of mild to moderate severity. The most commonly reported TEAE in the current study was fatigue (74% all grades; 15% grade 3/4); fatigue was also the most common temsirolimus-related TEAE (51%) in a phase III study of advanced RCC25 and was observed with tivozanib monotherapy (8% with a treatment-related event) in a phase II study of advanced RCC.21 Other common toxicities observed in this study have also been associated with temsirolimus monotherapy, including stomatitis, nausea, rash, dysponea, hyperglycaemia, hypertriglyceridemia and thrombocytopenia.25 Hypertension, a common on-target effect of VEGFR inhibition, was reported for only 26% of patients in the current study, including 33% of patients in the tivozanib 1.5-mg/d cohorts, and was generally well managed. The incidence of hypertension was lower compared with that in the phase II study of tivozanib in advanced RCC (45% with a treatment-related event),21 although roughly in line with reports for other TKIs (sunitinib, 24%26; sorafenib, 17–29%27,28; pazopanib, 40%29 and axitinib, 40%28). However, no grade 3/4 hypertension was reported in the current study, which was unexpected and likely due to the small patient enrolment. In comparison, grades 3 and 4 hypertension was reported in 11% and 1% of patients, respectively, in the tivozanib phase II trial as well as in pivotal phase III clinical trials of other VEGF TKIs in RCC, including sunitinib (8% and 0%),26 sorafenib (4–11% grade 3/4),27,28 pazopanib (4% and 0%)29 and axitinib (16% grade 3/4).28 Further evaluation of hypertension associated with this combination regimen in a larger clinical trial is needed.
Within the current study, the combination of tivozanib and temsirolimus treatment did not suggest synergistic toxicity. This may be due in part to the specificity of tivozanib for the VEGF pathway, suggested by the low incidence of off-target toxicities observed with tivozanib.4 By contrast, multi-targeted TKIs such as sorafenib, sunitinib and pazopanib target multiple pathways in addition to the VEGF pathway, and have been associated with higher incidences of off-target toxicities, including fatigue, stomatitis and diarrhoea.26,27,29 These off-target toxicities overlap with toxicities produced on temsirolimus treatment, preventing successful combination therapy.5 Thus, the data suggest that independent and non-overlapping toxicity profiles of tivozanib and temsirolimus may have contributed to the lack of DLTs in this study. However, eight patients withdrew from ongoing treatment, underscoring concerns of relative sustainability of combination regimens compared with single-agent treatments and the need for larger trials to more fully characterise the safety profile of this regimen.
Although pharmacokinetic sampling was performed in the current study, the small sample groups and the sampling regimen did not support comprehensive pharmacokinetic analysis beyond the summarisation of Cmax, Tmax, and AUC0–τ. For tivozanib, substantial drug accumulation was observed from day 1 to day 15 in every patient, approximating steady-state levels. These data are generally consistent with previously reported pharmacokinetic data for tivozanib, which also suggested slow absorption and a comparatively long terminal half-life.21,30, For temsirolimus, mean Cmax appeared lower than in previously reported data sets,31–33 likely due to the sampling regimen, which specified the first blood sample to be drawn 1 h after the end of temsirolimus infusion, well after the expected Tmax. However, the general pattern of temsirolimus and sirolimus pharmacokinetics was consistent with previous reports.31–33 While there was no evidence of pharmacokinetic interaction between tivozanib and temsirolimus, these results should be interpreted with caution and considered inconclusive. Direct comparison with single-agent pharmacokinetics within the same study would be needed to fully assess a potential drug interaction.
Evaluation of patients who received ≥ 2 cycles of tivozanib and temsirolimus revealed a preliminary indication of clinical activity. Despite 74% of patients having had prior VEGF therapy, the ORR was 23% in the current study, with 20/22 patients achieving a best outcome of PR or SD. Although the current study is small and patient populations between studies are distinct and cannot be directly compared, the ORR in the current study is similar to that reported in the phase II study of tivozanib (24%)21 and better than that reported in the phase III study of temsirolimus (9%; high-risk patients with generally worse disease risk features)25 or the phase III study of everolimus (1%; patients with prior VEGF therapy)34 in advanced RCC. Direct clinical comparison will be needed to address the superiority of the combination compared with tivozanib or temsirolimus monotherapy, and a larger randomised study is needed to evaluate the relationship between duration of treatment and progression-free survival. However, some responses were observed for this combination regimen, without dose limitations imposed by early toxic synergy.
Reports of mTOR inhibitors plus sorafenib or sunitinib generally indicate poor tolerability at full doses.5–7 While reports of mTOR inhibitors plus bevacizumab (which has greater VEGFR specificity) appear more tolerable and were initially thought promising, recent data from the phase III INTORACT35 and phase II RECORD-236 trials have not indicated superiority over the previously studied combination regimen of interferon alfa plus bevacizumab. Similarly, the phase II BeST trial11 found no added efficacy (but increased toxicity) for bevacizumab plus temsirolimus or sorafenib plus temsirolimus over the single-agent arm; while sorafenib plus bevacizumab had better activity, the tolerability was inferior. However, tivozanib is distinct from bevacizumab (TKI versus antibody) as well as sorafenib and sunitinib (selectivity and potency). These findings underscore the need for more mature, larger-scale data for more thorough evaluation of tivozanib plus temsirolimus.
Although this combination appears promising, there are some inherent limitations of the current study. First, no biomarker analysis was included to identify patients who might best respond to the regimen. However, a thorough biomarker analysis of tivozanib therapy in RCC has recently been completed (ClinicalTrials.gov #NCT01297244) to address this need. Additionally, the current study is an early phase trial with limited enrolment, in which patients received different doses and had different extents of pretreatment. Investigation of this combination regimen in a larger population, particularly treatment-naive patients, could provide more thorough evaluation of clinical activity and tolerability. Any subsequent trials with this combination should provide comparison with other regimens.
Tivozanib is the first selective VEGFR TKI to be successfully combined with an mTOR inhibitor at the fully recommended dose and schedule of both agents. The inherently selective VEGFR blockade by tivozanib may contribute to the observed low occurrence of off-target effects while maintaining activity. The clinical activity and manageable toxicity profile associated with combination tivozanib and temsirolimus therapy warrants further exploration in RCC. Ongoing clinical studies are evaluating tivozanib as monotherapy and in combination regimens for treatment of RCC and other solid tumours.
Supplementary Material
Acknowledgements
We thank all the patients, investigators, and staff who participated in the study. This study was supported by AVEO Pharmaceuticals, Inc. AVEO and Astellas Pharma Inc. who are parties to a collaboration agreement for the co-development of tivozanib. Editorial assistance was provided by Kimberly Brooks, PhD, of SciFluent, and was funded by AVEO and Astellas Pharma Inc.
Role of the funding source
This work (ClinicalTrials.gov Identifier: NCT00563147) was supported by AVEO Pharmaceuticals, Inc. AVEO and Astellas Pharma Inc are parties to a collaboration agreement for the co-development of tivozanib. Through the roles of B. Esteves, M.M. Cotreau, A.L. Strahs, W.J. Slichenmyer and P. Bhargava (current/former employees of AVEO) as authors, the study sponsor has participated in all aspects of study conduct and manuscript development.
Footnotes
Conflict of interest statement
M.N. Fishman has served as a consultant/advisor and/ or received research funding from Altor, Amgen, AVEO, Bayer, Bristol-Myers Squibb, Eisai, GlaxoSmithKline, Novartis, Onyx, Pfizer, Prometheus and Roche/Genentech. R.J. Hauke has received research funding from AVEOand Pfizer.B. Esteves, M.M. Cotreau, A.L. Strahs and W.J. Slichenmyer are employees of, and own stock in, AVEO; P. Bhargava is a former employee of AVEO. All remaining authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ejca.2013.04.019.
References
- 1.Rini BI. Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma. Cancer. 2009;115:2306–2312. doi: 10.1002/cncr.24227. [DOI] [PubMed] [Google Scholar]
- 2.Hudes GR. Targeting mTOR in renal cell carcinoma. Cancer. 2009;115:2313–2320. doi: 10.1002/cncr.24239. [DOI] [PubMed] [Google Scholar]
- 3.Mulders P. Vascular endothelial growth factor and mTOR pathways in renal cell carcinoma: differences and synergies of two targeted mechanisms. BJU Int. 2009;104:1585–1589. doi: 10.1111/j.1464-410X.2009.08987.x. [DOI] [PubMed] [Google Scholar]
- 4.Sosman J, Puzanov I. Combination targeted therapy in advanced renal cell carcinoma. Cancer. 2009;115:2368–2375. doi: 10.1002/cncr.24234. [DOI] [PubMed] [Google Scholar]
- 5.Patel PH, Senico PL, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2009;7:24–27. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina AM, Feldman DR, Voss MH, et al. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012;118:1868–1876. doi: 10.1002/cncr.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterhouse DM, Penley WC, Webb CD, et al. Sorafenib and everolimus (RAD001) in the treatment of patients with advanced clear cell renal carcinoma (RCC): a Sarah Cannon Research Institute phase I/II trial. J Clin Oncol. 2011;29:4629. [Google Scholar]
- 8.Hainsworth JD, Spigel DR, III, Burris HA, Waterhouse D, Clark BL, Whorf R, et al. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010;28:2131–2136. doi: 10.1200/JCO.2009.26.3152. [DOI] [PubMed] [Google Scholar]
- 9.Merchan JR, Pitot HC, Qin R, et al. Final phase II safety and efficacy results of study MC0452: phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma. J Clin Oncol. 2011;29 Abstract 4548. [Google Scholar]
- 10.Negrier S, Gravis G, Perol D, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol. 2011;12:673–680. doi: 10.1016/S1470-2045(11)70124-3. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty K. E2804 The BeST trial: a randomized phase II study of VEGF, RAF kinase and mTOR combination targeted therapy with bevacizumab, sorafenib, and temsirolimus in advanced renal cell cancer. The 11th International Kidney Cancer Symposium; October 5–6, 2012; Chicago, IL. [Google Scholar]
- 12.Nakamura K, Taguchi E, Miura T, et al. KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res. 2006;66:9134–9142. doi: 10.1158/0008-5472.CAN-05-4290. [DOI] [PubMed] [Google Scholar]
- 13.Bhargava P, Robinson MO. Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep. 2011;13:103–111. doi: 10.1007/s11912-011-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R, Crouthamel MC, Rominger DH, et al. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br J Cancer. 2009;101:1717–1723. doi: 10.1038/sj.bjc.6605366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 16.Eskens FALM, De Jonge MJA, Esteves B, et al. Updated results from a phase I study of AV-951 (KRN951), a potent and selective VEGFR-1, -2 and -3 tyrosine kinase inhibitor, in patients with advanced solid tumors. Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; April 12–16, 2008; San Diego, CA. Abstract LB-201. [Google Scholar]
- 17.Hurwitz H, Dowlati A, Savage S, et al. Safety, tolerability and pharmacokinetics of oral administration of GW786034 in pts with solid tumors. J Clin Oncol. 2005;23:195s. Abstract 3012. [Google Scholar]
- 18.NEXAVAR (sorafenib) tablets, oral [package insert] Wayne (NJ): Bayer Healthcare Pharmaceuticals Inc.; 2010. [Google Scholar]
- 19.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol. 2005;23:5474–5483. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 20.SUTENT® (sunitinib malate) capsules, oral [package insert] New York (NY): Pfizer Inc; 2011. [Google Scholar]
- 21.Nosov DA, Esteves B, Lipatov ON, et al. Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J Clin Oncol. 2012;30:1678–1685. doi: 10.1200/JCO.2011.35.3524. [DOI] [PubMed] [Google Scholar]
- 22. [accessed February 10, 2012];AVEO Pharmaceuticals Inc. AVEO and Astellas announce tivozanib successfully demonstrated progression-free survival superiority over sorafenib in patients with advanced renal cell cancer in phase 3 TIVO-1 trial. 2012 Available at: http://investor.aveopharma.com/phoenix.zhtml?c=219651&p=irol-newsArticle&ID=1643913&highlight=
- 23.Fuereder T, Jaeger-Lansky A, Hoeflmayer D, et al. mTOR inhibition by everolimus counteracts VEGF induction by sunitinib and improves anti-tumor activity against gastric cancer in vivo. Cancer Lett. 2010;296:249–256. doi: 10.1016/j.canlet.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Patnaik A, Eckhardt SG, Izbicka E, et al. A phase I, pharmacokinetic, and biological study of the farnesyltransferase inhibitor tipifarnib in combination with gemcitabine in patients with advanced malignancies. Clin Cancer Res. 2003;9:4761–4771. [PubMed] [Google Scholar]
- 25.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 27.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 28.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;278:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 29.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 30.Eskens FALM, Oldenhuis CNAM, Bhargava P, et al. A phase 1b study of escalating doses of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor tivozanib and FOLFOX6 in patients with advanced gastrointestinal (GI) tumors. Poster presented at: the EORTC-NCI-AACR International Symposium on Molecular Targets and Cancer Therapeutics; November 16–19, 2010; Berlin, Germany. Poster PP19. [Google Scholar]
- 31.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Hudes GR, Curti BD, et al. Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol. 2007;25:3958–3964. doi: 10.1200/JCO.2006.10.5916. [DOI] [PubMed] [Google Scholar]
- 33.TORISEL® (temsirolimus) injection [prescribing information] Philadelphia (PA): Wyeth Pharmaceuticals Inc.; 2008. [Google Scholar]
- 34.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 35.Rini BI, Bellmunt J, Clancy J, et al. Randomized phase IIIb trial of temsirolimus and bevacizumab versus interferon and bevacizumab in metastatic renal cell carcinoma: results from INTORACT. The Annual Meeting of the European Society for Medical Oncology (ESMO); September 28–October 2, 2012; Vienna, Austria. Abstract LBA21-PR. [Google Scholar]
- 36.Ravaud A, Barrios C, Anak O, et al. Randomized phase II study of first-line everolimus (EVE) + bevacizumab (BEV) versus interferon alfa-2A (IFN) + BEV in patients (pts) with metastatic renal cell carcinoma (MRCC): RECORD-2. The Annual Meeting of the European Society for Medical Oncology (ESMO); September 28–October 2, 2012; Vienna, Austria. Abstract 7830. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.