Abstract
The administration of chemotherapy at reduced doses given at regular, frequent time intervals, termed ‘metronomic’ chemotherapy, presents an alternative to standard maximal tolerated dose (MTD) chemotherapy. The primary target of metronomic chemotherapy was originally identified as endothelial cells supporting the tumor vasculature, and not the tumor cells themselves, consistent with the emerging concept of cancer as a systemic disease involving both tumor cells and their microenvironment. While anti-angiogenesis is an important mechanism of action of metronomic chemotherapy, other mechanisms, including activation of anti-tumor immunity and a decrease in acquired therapeutic resistance, have also been identified. Here we present evidence supporting a mechanistic explanation for the improved activity of cancer chemotherapy when administered on a metronomic, rather than an MTD schedule and discuss the implications of these findings for further translation into the clinic.
Graphical abstract
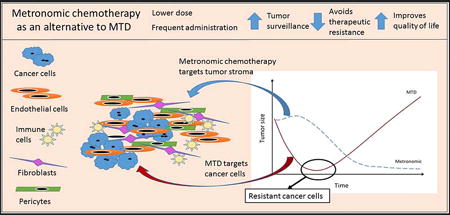
Introduction
Standard clinical protocols for cancer chemotherapy typically employ the maximal drug dose that can be tolerated by the patient. This, in turn, necessitates prolonged time intervals between treatment cycles to allow for normal tissue recovery from the cytotoxic assault, which are ideally designed to maximize tumor cell kill without lethal damage to the patient. This concept of maximum tolerated dose (MTD) chemotherapy derives from the success of treating acute lymphoblastic leukemia (ALL) in children [82]. Childhood ALL is highly responsive to MTD chemotherapy primarily because it represents a rare instance when the leukemic tumor clone can be completely eradicated. This is not always possible in other, more genetically complex leukemias, such as bcrabl and MLL-positive leukemias, where this treatment strategy has not been as successful [74]. Cancers in which MTD chemotherapy has proven to be successful rarely have a complex network of activating mutations, and include gestational choriocarcinomas [14, 62], testicular cancer [94], certain germ-cell tumors [62], Hodgkin disease [40] and B-cell non-Hodgkin lymphomas [43, 80]. In contrast, complex cancers, such as sarcomas, breast, prostate, pancreas and lung cancers, are less effectively treated by up front tumor cell eradication using MTD doses, primarily because these cancers engage the host microenvironment extensively [4, 67–69, 78].
In addition to its high toxicity and detrimental effects on the patients’ quality of life, MTD chemotherapy is often followed by the development of therapeutic resistance. Particularly in the case of solid tumors, MTD chemotherapy kills off chemotherapy-sensitive cancer cell populations, leaving chemoresistant cells behind to re-colonize the tumor bed, ultimately leading to disease relapse. One strategy to prevent disease relapse has been to develop increasingly intense and thus more toxic drug regimens, including combination chemotherapy regimens, in the hope of achieving more complete a priori eradication of all cancer cells [78], subscribing to the philosophy of “more must be better”. However, recent advances in tumor biology point away from focusing on the cytotoxicity of drugs and toward modification of the biology of the tumor using targeted approaches that disengage the tumor microenvironment. This latter approach re-defines the therapeutic goals to aim for prolonged responses rather than the short-term tumor regression responses, which do not necessarily translate into an increase in long-term patient survival.
In contrast to MTD drug regimens, metronomic chemotherapy is characterized by the administration of a cytotoxic agent at a lower, less toxic dose given at regular, more frequent time intervals. A review of clinical trials comparing the effectiveness of metronomic chemotherapy to MTD chemotherapy [6, 13, 36, 69,77] indicates a growing appreciation of the concept. This trend is also evident at www.clinicaltrials.gov, which currently lists over 150 clinical trials of metronomic chemotherapy for various cancers. Of particular interest are results of the CAIRO3 clinical trial [53], which reported highly encouraging results of metronomic maintenance treatment in metastatic colorectal cancer patients. Many more clinical trials using metronomic chemotherapy in combination with molecular agents are ongoing and were recently reviewed [13]. The growing popularity of metronomic chemotherapy reflects the common finding that combining standard chemotherapeutic regimens with non-traditional agents, such as anti-angiogenic drugs, proteasome inhibitors and anti-inflammatory agents, while increasing the response rate, may also increase host toxicity beyond the tolerable level. Metronomic chemotherapy has the potential to preserve efficacy while avoiding the increase in toxicity commonly seen when biologic response modifiers are used.
While metronomic chemotherapy may have been extensively reviewed in the literature and its multiple mechanisms of actions have been well debated [5, 6, 12, 54, 55, 68, 77], for most clinicians, metronomic chemotherapy remains a mostly palliative care tool rather than active, upfront therapy. This categorization of metronomic chemotherapy as palliative tool leads to a disregard of its synergism when used in combination with targeted biological agents and infrequent use in active treatment. By reviewing the mechanisms of action of metronomic chemotherapy in this manuscript in detail, we show the benefits of preferentially using low-dose frequent chemotherapy in facilitating the recent shift in clinical oncology from cytotoxic therapies to molecularly based agents. Furthermore, during the recent Fourth Metronomic and Anti-Angiogenic Therapy meeting (June 2014) one of the main topics of discussion was the “lack of a clear understanding of the exact mechanisms of action, optimal dosages and most efficacious metronomic schedules” [13]. In what follows, we present how changing both the timing and the dosage of chemotherapy, metronomic treatment regimens can effectively address other primary drawbacks of MTD, namely, the development of therapeutic resistance and suppression of anti-tumor immune responses. The argument is supported by the recognition that cancer is a disease not solely of cancer cells, but also of the tumor microenvironment, a point increasingly accepted in the scientific literature [38, 39].
Cancer as a disease of both tumor cells and their microenvironment
Genetically complex tumors grow and develop within a dynamic microenvironment derived from the host’s tissues. From this perspective, it may be useful to think of tumor cells as parasites that are hosted by tumor-associated endothelial cells (TECs) and stromal cells, including fibroblasts, pericytes, inflammatory cells, and immune cells, all coexisting within the larger “ecosystem” of the human body. Just as in other ecosystems, the survival of the parasitic tumor cells primarily depends not on the state of the entire ecosystem, but on the state of their local host, in this case the tumor microenvironment. Experience with anti-parasitic treatments has shown that effective eradication strategies require high doses of toxic chemicals, however, such doses cannot be reached because of unacceptable damage to the host. Furthermore, parasites often develop drug resistance resulting in decreased efficacy in subsequent rounds of therapy [1]. These same obstacles are encountered in cancer treatment [29, 30], reinforcing the concept that increased toxicity to the target tumor cell does not equate with an overall increase in efficacy. Attacking ‘the immediate host’, i.e., TECs and other cells within the tumor microenvironment might in fact prove to be a more successful long-term strategy.
Tumor microenvironment and endothelial cells as targets
To better understand how the tumor microenvironment becomes engaged and modified by malignant disease, one first needs to understand the functionality of the various components of normal tissues. Most tissues reach a level of dynamic equilibrium under normal conditions, and the local microenvironment can be thought of as a dynamic community composed of a multitude of cells of different lineages and functions, including resident cells and responders [10]. Resident tissue cells include supportive cells, such as fibroblasts, pericytes, astrocytes, and health surveillance cells, such as histiocytes, macrophages and lymphocytes. The resident cells provide support to the tissue, while the responders ensure tissue maintenance and protection. Responders are recruited to the tissue site in times of acute need, and can be further subdivided into primary (early) responders, such as platelets, lymphocytes and neutrophils, and secondary responders, such as hematopoietic progenitor cells and monocytes. Both primary and secondary responders are summoned when local health surveillance cells are unable to contain the damage, resulting in recruitment of specialized cells of the adaptive immune response to aid the innate immune cells, or when there is a need to repair tissue damage (Figure 1).
Figure 1. Role of the tumor microenvironment during tumor progression.
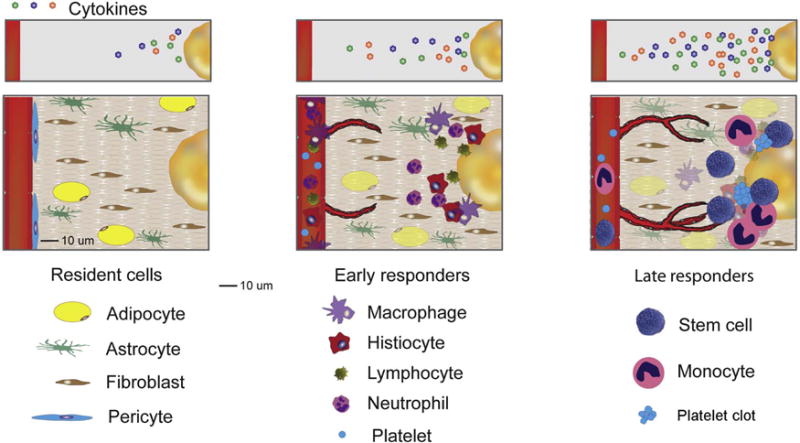
Therapeutically resistant tumors engage their microenvironment as cancer cells recruit normal tissue cells, such as fibroblasts, pericytes, histiocytes, platelets and hematopoetic progenitor cells. Through engagement and modification of their microenvironment, cancer cells ‘simulate’ the conditions of a wound, evoking normal physiological responses such as new blood vessel formation without allowing for angiogenesis termination.
In response to wounding, endothelial cells and fibroblasts send out proangiogenic signals that initiate formation of new blood vessels and stimulate tissue repair. This process is largely mediated by coagulation factors and by platelets, which actively sequester growth factors critical for blood vessel formation, including VEGF, bFGF, PDGF, TSP-1, and PF-4 [51]. Contrary to the widely-held view that platelets release angiogenesis regulators en mass, it is now understood that platelet clots retain angiogenesis regulators and create reciprocally interactive concentration gradients of pro – and anti-angiogenic growth factors [44, 51]. The process of angiogenesis is regulated by the creation of sequential concentration gradients in tissues. More specifically, growth factors such as VEGF initiate formation of sprouts [33], while others, such as bFGF, provide signals for endothelial cell proliferation and tube formation [11, 32, 45], followed by vessel stabilization by PDGF and eventually collagen cleavage and vessel pruning, mediated by such stabilizing and angiogenesis inhibiting factors such as TSP-1 and PF-4 [22, 52]. Thus, under normal physiological conditions, angiogenesis is largely limited to wound healing and placental development. In the tumor microenvironment, however, oncogenic stimulators, such as RAS, c-myc, and EGFR, overcome this inhibition of angiogenesis [65, 75, 76], leading to unrestrained release of angiogenesis-promoting signals. This results in continuous formation of new blood vessels that feed the tumor and further contribute to tumor growth, making angiogenesis an important therapeutic target in cancer.
An important distinction needs to be made between the anti-angiogenic effects of conventional anti-angiogenic drugs, which target individual molecules or signaling pathways, and the anti-angiogenic actions of metronomic chemotherapy, which inhibit the production of growth factors at the source. For instance, bevacizumab, an anti-angiogenic monoclonal antibody, binds to extracellular VEGF, rendering it incapable of activating cell surface VEGF receptors and thus incapable of initiating sprout formation [81]. In contrast, metronomic chemotherapy damages the source of these growth factors, namely, fibroblasts and TECs [28, 48, 79]. Therefore, while metronomic chemotherapy and anti-angiogenic drugs can both induce anti-angiogenesis, the underlying mechanisms are different, with metronomic therapy potentially having more lasting effects due to its targeting the source of vascular growth factors rather than the growth factors themselves.
There are important differences between TECs and normal endothelial cells [42]. TECs, especially those from highly metastatic tumors have more proangiogenic counterpart than TECs from less metastatic tumors or normal endothelial cells. The quiescence of normal endothelial cells is a well-documented finding and represents the basis of higher intrinsic sensitivity of TECs to cytotoxic drugs. In some cases TECs lose functionality when exposed to cancer chemotherapeutic agents at much lower concentrations than those needed to cause tumor cell damage. Picomolar to nanomolar concentrations of therapeutic agents such as vinblastine [90], taxol [91], carboplatinum [49] and adriamycin [49] show intrinsic toxicities to TECs, whereas much higher doses – typically nanomolar to micromolar levels – of the same agents are required for tumor cell toxicity.
Lower dosages can decrease the rate of acquired therapeutic resistance
The use of lower dosages of cytotoxic drugs for attacking TECs and other supporting cells in the tumor microenvironment can have the added benefit of minimizing the induction of acquired therapeutic resistance [47, 49], particularly in the setting of combination therapy. Tumors are characterized by high levels of both genotypic and phenotypic intratumoral heterogeneity, and as a consequence, most tumors are likely to contain one or even multiple cancer cell clones that are resistant to even the highest doses of cytotoxic drugs that can be given to a patient. High doses of cytotoxic chemotherapy (i.e., MTD chemotherapy) impose severe selective pressure on a heterogeneous tumor population, thereby killing drug-sensitive tumor cell clones and leading to the selection of the most drug-resistant clones [35, 64].
Consider the schematic dose-response graphs for killing tumor cells and for TECs presented in Figure 2. With some anticancer agents, the minimal dose needed to inflict significant damage to TECs is so low that tumor cells are spared. All tumor cells depend on TECs and on the stromal compartment for pro-angiogenic signals that recruit the blood vessels needed to access oxygen and nutrients [59, 87], and as consequence, chemotherapeutic drug doses and schedules that selectively target these and other critical cells within the tumor microenvironment can inflict severe damage on both resistant and sensitive tumor cell clones. This weakens the entire tumor cell population without specifically selecting for resistant clones. In some cases the combination of metronomic chemotherapy with anti-angiogenic therapy yields a superior outcome [9, 15, 48]. Low-dose chemotherapy damages TECs, while the direct acting angiogenesis inhibitors interfere with TEC survival signals, preventing regrowth of new blood vessels. TECs may acquire therapeutic resistance [41], but the mechanisms of TEC resistance are different than the mechanisms of tumor cell resistance, indicating a need for multi-targeted approaches.
Figure 2. Dose-response curves for cancer cells and tumor endothelial cells (TECs).
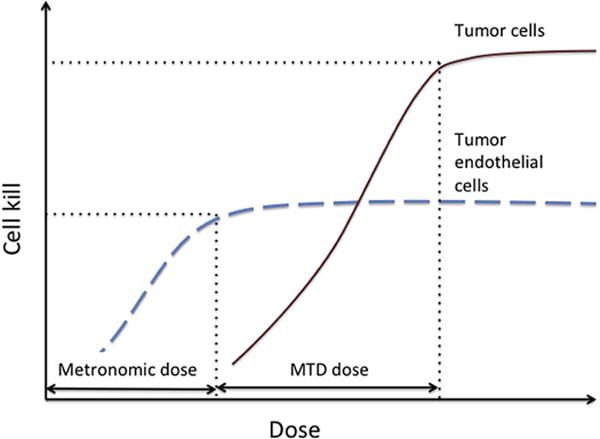
The dosage of therapeutic agents that achieve maximal cell kill of TECs can be orders of magnitude lower than the dosage necessary to inflict significant damage on cancer cells. Therefore, damaging tumor-supporting endothelial cells may inflict equal damage on all tumor cells, effectively preventing selection for resistant cell clones.
One of the primary arguments against administering low dose chemotherapy is based on the experience with infectious diseases and antibiotics, where low (inadequate) drug doses leads to selection for antibiotic-resistant superbugs [21]. However, this concept is not applicable to low-dose metronomic chemotherapy. Whereas antibiotics still act on the bacteria themselves when given at low dosages, low dose chemotherapy primarily affects the stromal cells on which the tumor cells rely for support and sustenance. Therefore, the mechanisms that account for the increased resistance of bacteria treated with low dose antibiotics are largely not applicable to cancer chemotherapy.
Metronomic chemotherapy and cancer stem cells (CSCs)
Another important question concerns the effect of metronomic chemotherapy on cancer stem-like cells (CSCs), i.e. tumor-initiating cells, which are often intrinsically resistant to classic anticancer drugs. In a study of subcutaneous rat C6 gliomas, metronomic chemotherapy in combination with direct anti-angiogenic drug treatment, but not several other treatment regimens, was effective at reducing the CSC population [26]. Further, neither targeted anti-angiogenic therapy nor cytotoxic chemotherapy alone reduced the fraction of CSCs. Furthermore, others found that an MTD regimen followed by metronomic chemotherapy (a chemo-switch schedule), was more effective in blocking metastatic dissemination in an orthotopic pancreatic adenocarcinoma model compared to MTD treatment [92]. An increase in TSP-1 expression and a decrease in the number of CSCs bearing CD133+ and CD133+/CD44+/CD24+ markers was also seen, indicating that a cytoreductive MTD regimen followed by metronomic chemotherapy may be a promising strategy for eradicating chemotherapy-resistant CSCs.
More frequent timing can activate the immune system
Both innate and adaptive immune responses play an important role in keeping cancer progression in check. These responses can be compromised by high dose chemotherapy, which triggers host inflammatory immune response [27] and ablates immune surveillance [97]. However, these deleterious effects on the patient’s immune system can be managed through changes in both dosage and timing of chemotherapy, which can lead to stimulation of anti-tumor immunity and suppression of pro-tumor immune responses.
Low-dose chemotherapy can reduce immune suppressive populations of CD4+CD25+ regulatory T cells (Tregs) [7, 31, 34]. However, metronomic administration of chemotherapeutic drugs can also have effects on other subsets of immune cells. For instance, changing the dosage of commonly used chemotherapeutic drugs can affect antigen presenting cells, such as dendritic cells (DCs), which are crucial for activation of adaptive immune responses [85]. In one study, Tanaka and colleagues [84] evaluated and classified chemotherapeutic agents with respect to their effect on DCs and identified a class of drugs that induced DC maturation. Specifically, vinblastine, which is highly suppressive of anti-tumor immunity at high concentrations, promoted maturation of DCs at low concentrations, as indicated by the increased expression of markers such as MHC-II, CD40, CD80 and CD86. In another study [85], vinblastine increased the activity of cytotoxic lymphocytes against mouse B16 melanoma targets, interfering with the otherwise progressive growth of B16 melanoma. Low doses of chemotherapeutic agents also affect myeloid-derived suppressor cells, alleviating suppression of adaptive immune responses and allowing for improved anti-tumor [83]. An extensive review of the effects of specific chemotherapeutic agents on a variety of immune cell subsets has been published [63].
With respect to innate immune responses, Doloff and Waxman [25] demonstrated that dramatic regression of implanted brain tumor xenografts treated with cyclophosphamide on an intermittent, every 6 day metronomic schedule (Q6day cycle) was accompanied by significant recruitment and activation of innate immune cells, specifically, natural killer (NK) cells, dendritic cells and macrophages. Notably, these responses were achieved with little or no anti-angiogenesis. Selective depletion of NK cells using anti-asialo-GM1 antibody resulted in delayed and incomplete tumor regression, which were both reversed following termination of asialo-GM1 antibody treatment. The efficacy of the Q6day cycle was hypothesized to reflect the life span of NK cells and perhaps other first-line immune responder cells. More frequent administration of cytotoxic therapy was ineffective in these brain tumors models, where it may interfere with the immune-stimulating effects of the every 6-day metronomic regimen by inflicting severe damage to the NK cells themselves [17, 95]. Thus, the timing of metronomic chemotherapy appears to be critical: it needs to be sufficiently frequent to activate a strong innate anti-tumor immune response, but it also needs to be sufficiently well-spaced in time to minimize damage to the immune cells recruited to the tumor microenvironment. Further, longer intervals between metronomic drug treatments (cyclophosphamide given every 9 or every 12 days, instead of every 6 days), and drug doses that are too low, can both lead to tumor escape [95], highlighting the importance of regular, repeated drug treatment for an effective innate anti-tumor response. Other studies show that VEGFR2 signaling is essential for metronomic cyclophosphamide to stimulate robust innate immune cell recruitment [24]. See Figure 3B. Moreover, anti-angiogenic drugs that primarily act by a VEGFR2-independent mechanism do not interfere with innate immune cell recruitment, indicating that the interference with immune cell recruitment is not due to the loss of the tumor vasculature [24]. Avoiding damage to the immune surveillance system might be of crucial importance if the growth of a particular tumor type is dependent on its ability to evade anti-tumor immunity. This contention is supported by Young et al. [96], who suggest that in a clinical setting, optimization of exact dosage and timing may need to be adjusted with respect to the patient’s immune response and the type of tumor.
Figure 3. Impact of metronomic chemotherapy frequency and dose on anti-tumor innate immune responses.
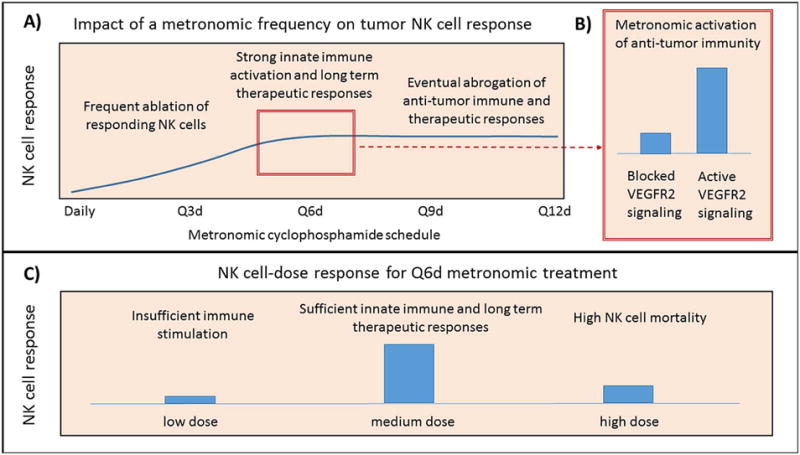
A) Impact of metronomic frequency on tumor NK cell response. Chemotherapy administration that is too frequent (daily and Q3d in the glioma models studied; [17]) causes ablation of responding NK cells, while chemotherapy administration that is insufficiently frequent (Q9d and Q12d schedules), while initially effective at activating an innate immune response, leads to abrogation of the anti-tumor immune response and tumor escape [95]. In these models, maximal immunostimulatory and therapeutic responses have been observed on a 6-day schedule. B) Metronomic activation of anti-tumor immunity on a 6-day schedule is impaired when VEGFR2 signaling is inhibited [24, 25]. C) NK cell dose response on a 6-day schedule is dose-dependent: reduction in dose causes insufficient immune stimulation [17], while a dose that is too high increases NK cell mortality [95].
Not all tumors respond to the above intermittent, every 6-day metronomic schedule of cyclophosphamide with NK cell recruitment leading to tumor regression, as seen in a KM12 colon carcinoma model [46]. This may reflect the fact that NK cells are rare in human colorectal carcinoma tissues, even in the presence of high levels of chemokines that activate and recruit these cells, with the capacity for NK cell migration into colorectal carcinoma being impaired early during colorectal carcinoma development [37]. The impact of changes in dose and schedule of metronomic chemotherapy on the innate immune response is presented in Figure 3.
Clinical implications and future directions
Metronomic administration of cancer chemotherapeutic drugs holds much promise to address several of the major drawbacks of MTD regimens. These include the emergence of drug resistance, suppression of anti-tumor immunity, toxicity and poor quality of life during therapy. Further, metronomic chemotherapy lowers the financial burden for the patient when compared to targeted therapies [50], while maintaining efficacy. Unfortunately, the time lag between anti-tumor effect and a visible reduction in tumor bulk may in some cases decrease the utility of metronomic chemotherapy for advanced disease. For example, in the care of brain stem glioma, even minimal progression can be lethal to the patient, calling for more drastic intervention with immediate tumor bulk reduction such as surgery or radiation. Similarly, treatment protocols for ALL include a period of high-intensity induction, followed by a milder dose consolidation, followed by 2–3 years of lower-dose, higher-frequency maintenance therapy [88]. This strategy gives 90–95% survival rates, and any attempts to omit the maintenance therapy yield inferior results [23, 73, 93]. Choi et al. [18] reported encouraging results for a small group of children with tumors of central nervous system treated with upfront high-dose chemotherapy, followed by metronomic maintenance therapy. In that study, 8 of the 10 patients, including six with metastatic disease, continued to have stable clinical and radiographic disease 20 months from the time of diagnosis. Encouraging results were also reported when using metronomic therapy for children with medulloblastoma, with over 65% survival rates after 24 months [73], warranting further investigation.
In summary, although the long-standing goal of MTD chemotherapy has been immediate tumor shrinkage, an immediate anti-tumor response that leads to recurrence of disease does not improve patient outcomes. Further, as illustrated in Figure 4, while the anti-tumor responses to metronomic chemotherapy may be delayed, e.g., due to the time required to ablate tumor blood vessels or activate an anti-tumor immune response, the anti-tumor response is more likely to be sustained [48, 68], owing to the decreased selection of resistant tumor cell clones and the suppression of anti-tumor immunity with a decreased likelihood of disease relapse.
Figure 4. Predicted response to changes in tumor size over time after MTD compared to metronomic chemotherapy.
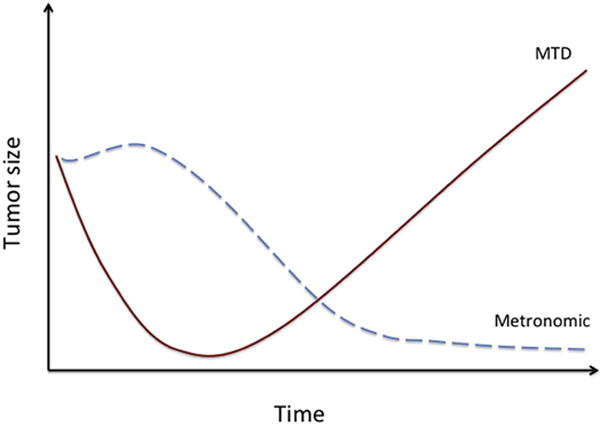
MTD therapy induces early onset, short-term tumor regression, which is frequently followed by a relapse. Metronomic administration of chemotherapy may have a delayed anti-tumor effect; it may initially lead to tumor growth stasis or even an increase in tumor size, followed by a slow but persistent decrease in overall tumor mass, yielding a potentially alarming short-term effect but superior long-term outcome.
More widespread adoption of metronomic chemotherapy as a main up front therapeutic modality will require improved ways to measure therapeutic efficacy, including the identification of biomarkers that can be used to evaluate therapeutic effectiveness. Such biomarkers may already exist, and include cancer antigens CA 15-3 and CA 19-9, prostate-specific antigen, platelet biomarkers [16, 51, 57, 71, 72], serum VEGF and other angiogenic cytokines [86], plasma levels of PDGF-BB [89], thrombospondin-1 expression [3], VEGF SNPs [66] and CD133 gene expression [2], and various immune response genes[98]. There is also an effort to monitor tumor response using ratios of angiogenesis regulators[70]. Further work is needed however to establish the utility of these biomarkers compared to the old paradigm of measuring the effectiveness of MTD chemotherapy by the degree of myelosuppression or tumor shrinkage.
It remains to be determined whether metronomic chemotherapy will ultimately be more effective than MTD-based therapies in the treatment of metastatic disease, although early indicators suggest this may be the case [19, 20, 56, 58, 60, 61]. The introduction and increased use of computational models to assist with identification of patient-specific optimal dosage and timing protocols will also facilitate the implementation of metronomic chemotherapy in the clinic [8]. The body of experimental and clinical evidence, coupled with theoretical considerations, outlined above, point to metronomic chemotherapy as a preferred course of action.
Highlights.
Metronomic chemotherapy involves administering lower doses of chemotherapeutic drugs at more frequent intervals
Lower dosage allows targeting supporting tumor stroma without selecting for resistant cells, unlike in case of antibiotic resistance
Lower dosage and more frequent administration allow preservation and maintenance of anti-tumor immunity
Metronomic chemotherapy yields long-term improved clinical outcome despite slower initial decreases in tumor size
Cancer is a disease of both tumor cells and their microenvironment
Acknowledgments
The authors thank the organizers of the Second Annual Workshop on Cancer Systems Biology of Metronomic Chemotherapy 2012 in Boston, Massachusetts, which was devoted to identifying factors that impede the introduction of metronomic chemotherapy to the clinic. This workshop was funded by funded by an ICBP NIH/NCI – U54 CA149233-01 grant to Dr.Lynn Hlatky. The first author of this manuscript (I.K.) was partially supported by the Office of Science (BER), U.S. Department of Energy, under Award Number DE-SC0001434 (to Philip Hahnfeldt, her postdoctoral fellowship mentor). Supported in part by NIH grant CA049248 (to DJW) and NIH/NIGMS R01 GM093050-01A1 (to GLK). The authors thank Clare Lamont for her artistic help with Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None
References
- 1.Antibiotic Resistance Questions & Answers” [Are antibacterial-containing products (soaps, household cleaners, etc.) better for preventing the spread of infection? Does their use add to the problem of resistance?] Atlanta, Georgia: 2011. [Google Scholar]
- 2.Allegrini G, Di Desidero T, Barletta MT, Fioravanti A, Orlandi P, Canu B, Chericoni S, Loupakis F, Di Paolo A, Masi G, Fontana A, Lucchesi S, Arrighi G, Giusiani M, Ciarlo A, Brandi G, Danesi R, Kerbel RS, Falcone A, Bocci G. Clinical, pharmacokinetic and pharmacodynamic evaluations of metronomic UFT and cyclophosphamide plus celecoxib in patients with advanced refractory gastrointestinal cancers. Angiogenesis. 2012;15:275–286. doi: 10.1007/s10456-012-9260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allegrini G, Falcone A, Fioravanti A, Barletta MT, Orlandi P, Loupakis F, Cerri E, Masi G, Di Paolo A, Kerbel RS, Danesi R, Del Tacca M, Bocci G. A pharmacokinetic and pharmacodynamic study on metronomic irinotecan in metastatic colorectal cancer patients. Br J Cancer. 2008;98:1312–1319. doi: 10.1038/sj.bjc.6604311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andre N, Abed S, Orbach D, Alla CA, Padovani L, Pasquier E, Gentet JC, Verschuur A. Pilot study of a pediatric metronomic 4-drug regimen. Oncotarget. 2011;2:960–965. doi: 10.18632/oncotarget.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andre N, Banavali S, Snihur Y, Pasquier E. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol. 2013;14:e239–248. doi: 10.1016/S1470-2045(13)70056-1. [DOI] [PubMed] [Google Scholar]
- 6.Andre N, Carre M, Pasquier E. Metronomics: towards personalized chemotherapy?, Nature reviews. Clinical oncology. 2014;11:413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 7.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58:1627–1634. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbolosi D, Ciccolini J, Meille C, Elharrar X, Faivre C, Lacarelle B, Andre N, Barlesi F. Metronomics chemotherapy: time for computational decision support. Cancer Chemother Pharmacol. 2014;74:647–652. doi: 10.1007/s00280-014-2546-1. [DOI] [PubMed] [Google Scholar]
- 9.Bello L, Carrabba G, Giussani C, Lucini V, Cerutti F, Scaglione F, Landre J, Pluderi M, Tomei G, Villani R, Carroll RS, Black PM, Bikfalvi A. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001;61:7501–7506. [PubMed] [Google Scholar]
- 10.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harbor perspectives in medicine. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocci G, Francia G. Metronomic Chemotherapy. Springer; 2014. [Google Scholar]
- 13.Bouche G, Andre N, Banavali S, Berthold F, Berruti A, Bocci G, Brandi G, Cavallaro U, Cinieri S, Colleoni M, Curigliano G, Di Desidero T, Eniu A, Fazio N, Kerbel R, Hutchinson L, Ledzewicz U, Munzone E, Pasquier E, Graciela Scharovsky O, Shaked Y, Sterba J, Villalba M, Bertolini F. Lessons from the Fourth Metronomic and Anti-angiogenic Therapy Meeting, 24–25 June 2014, Milan. Ecancermedicalscience. 2014;8:463. doi: 10.3332/ecancer.2014.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower M, Paradinas FJ, Fisher RA, Nicholson SK, Rustin GJ, Begent RH, Bagshawe KD, Newlands ES. Placental site trophoblastic tumor: molecular analysis and clinical experience. Clin Cancer Res. 1996;2:897–902. [PubMed] [Google Scholar]
- 15.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 16.Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi A, Naumov GN, Bender E, Almog N, Italiano JE, Jr, Folkman J, Klement GL. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood. 2008;111:1201–1207. doi: 10.1182/blood-2007-04-084798. [DOI] [PubMed] [Google Scholar]
- 17.Chen CS, Doloff JC, Waxman DJ. Intermittent metronomic drug schedule is essential for activating antitumor innate immunity and tumor xenograft regression. Neoplasia. 2014;16:84–96. doi: 10.1593/neo.131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi LM, Rood B, Kamani N, La Fond D, Packer RJ, Santi MR, Macdonald TJ. Feasibility of metronomic maintenance chemotherapy following high-dose chemotherapy for malignant central nervous system tumors. Pediatr Blood Cancer. 2008;50:970–975. doi: 10.1002/pbc.21381. [DOI] [PubMed] [Google Scholar]
- 19.Colleoni M, Orlando L, Sanna G, Rocca A, Maisonneuve P, Peruzzotti G, Ghisini R, Sandri MT, Zorzino L, Nole F, Viale G, Goldhirsch A. Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: antitumor activity and biological effects. Ann Oncol. 2006;17:232–238. doi: 10.1093/annonc/mdj066. [DOI] [PubMed] [Google Scholar]
- 20.Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, Peruzzotti G, Robertson C, Orlando L, Cinieri S, de BF, Viale G, Goldhirsch A. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 21.Davies J, Davies D. Origins and evolution of antibiotic resistance, Microbiology and molecular biology reviews. MMBR. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson DW, Bouck N. Thrombospondin as an inhibitor of angiogenesis. In: Teicher BA, editor. Thrombospodin. Humana Press; Totowa, NJ: 1999. pp. 185–203. [Google Scholar]
- 23.Dekker AW, van’t Veer MB, Sizoo W, Haak HL, van der Lelie J, Ossenkoppele G, Huijgens PC, Schouten HC, Sonneveld P, Willemze R, Verdonck LF, van Putten WL, Lowenberg B. Intensive postremission chemotherapy without maintenance therapy in adults with acute lymphoblastic leukemia. Dutch Hemato-Oncology Research Group. J Clin Oncol. 1997;15:476–482. doi: 10.1200/JCO.1997.15.2.476. [DOI] [PubMed] [Google Scholar]
- 24.Doloff JC, Chen CS, Waxman DJ. Anti-tumor innate immunity activated by intermittent metronomic cyclophosphamide treatment of 9L brain tumor xenografts is preserved by anti-angiogenic drugs that spare VEGF receptor 2. Molecular cancer. 2014;13:158. doi: 10.1186/1476-4598-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doloff JC, Waxman DJ. VEGF receptor inhibitors block the ability of metronomically dosed cyclophosphamide to activate innate immunity-induced tumor regression. Cancer Res. 2012;72:1103–1115. doi: 10.1158/0008-5472.CAN-11-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 27.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 28.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–740. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 29.Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459:508–509. doi: 10.1038/459508a. [DOI] [PubMed] [Google Scholar]
- 30.Gatenby RA, Brown J, Vincent T. Lessons from applied ecology: cancer control using an evolutionary double bind. Cancer Res. 2009;69:7499–7502. doi: 10.1158/0008-5472.CAN-09-1354. [DOI] [PubMed] [Google Scholar]
- 31.Generali D, Bates G, Berruti A, Brizzi MP, Campo L, Bonardi S, Bersiga A, Allevi G, Milani M, Aguggini S, Dogliotti L, Banham AH, Harris AL, Bottini A, Fox SB. Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin Cancer Res. 2009;15:1046–1051. doi: 10.1158/1078-0432.CCR-08-1507. [DOI] [PubMed] [Google Scholar]
- 32.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerhardt H, Betsholtz C. How do endothelial cells orientate? EXS. 2005:3–15. doi: 10.1007/3-7643-7311-3_1. [DOI] [PubMed] [Google Scholar]
- 34.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafner CR, Albrecht, Vogt Thomas. New Indications for Established Drugs: Combined Tumor-Stroma-Targeted Cancer Therapy with PPAR Agonists, COX-2 Inhibitors, mTOR Antagonists and Metronomic Chemotherapy. Current Cancer Drug Targets. 2005;5:393–419. doi: 10.2174/1568009054863591. [DOI] [PubMed] [Google Scholar]
- 37.Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, Schirmacher P, Brand K, Grabe N, Falk CS. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17:678–689. doi: 10.1158/1078-0432.CCR-10-2173. [DOI] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 41.Hida K, Akiyama K, Ohga N, Maishi N, Hida Y. Tumour endothelial cells acquire drug resistance in a tumour microenvironment. J Biochem. 2013;153:243–249. doi: 10.1093/jb/mvs152. [DOI] [PubMed] [Google Scholar]
- 42.Hida K, Ohga N, Akiyama K, Maishi N, Hida Y. Heterogeneity of tumor endothelial cells. Cancer science. 2013;104:1391–1395. doi: 10.1111/cas.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang HH, Xiao F, Chen FY, Wang T, Li JM, Wang JM, Cao JN, Wang C, Zou SH. Reassessment of the prognostic value of the International Prognostic Index and the revised International Prognostic Index in patients with diffuse large B-cell lymphoma: A multicentre study. Experimental and therapeutic medicine. 2012;4:475–480. doi: 10.3892/etm.2012.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochemical Society transactions. 2009;37:1233–1236. doi: 10.1042/BST0371233. [DOI] [PubMed] [Google Scholar]
- 46.Jia L, Waxman DJ. Thrombospondin-1 and pigment epithelium-derived factor enhance responsiveness of KM12 colon tumor to metronomic cyclophosphamide but have disparate effects on tumor metastasis. Cancer Lett. 2013;330:241–249. doi: 10.1016/j.canlet.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerbel RS. Inhibition of Tumor Angiogenesis as a Strategy to Circumvent Acquired Resistance to Anti-Cancer Therapeutic Agents. BioEssays. 1991;13:31–36. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 48.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, Hicklin D, Kerbel RS. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res. 2002;8:221–232. [PubMed] [Google Scholar]
- 50.Klement GL, Kamen BA. Nontoxic, fiscally responsible, future of oncology: could it be beginning in the Third World? Journal of pediatric hematology/oncology. 2011;33:1–3. doi: 10.1097/MPH.0b013e3182024918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, Almog N, Kieran MW, Folkman J. Platelets actively sequester angiogenesis regulators. Blood. 2009;113:2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klement GSEVD. The role of platelets in angiogenesis. In: Michelson A, editor. Platelets. Elsevier/Academic Press; San Diego, CA: 2012. [Google Scholar]
- 53.Koopman M, Simkens L, May A, Mol L, Tinteren HV, Punt CJA. Final results and subgroup analyses of the phase 3 CAIRO3 study: Maintenance treatment with capecitabine and bevacizumab versus observation after induction treatment with chemotherapy and bevacizumab in metastatic colorectal cancer (mCRC) 2014 Gastrointestinal cancers Symposium. 2014 [Google Scholar]
- 54.Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U. Low-dose metronomic chemotherapy: a systematic literature analysis. Eur J Cancer. 2013;49:3387–3395. doi: 10.1016/j.ejca.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 55.Maiti R. Metronomic chemotherapy. Journal of pharmacology & pharmacotherapeutics. 2014;5:186–192. doi: 10.4103/0976-500X.136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manso L, Valdiviezo N, Sepulveda J, Ciruelos E, Mendiola C, Ghanem I, Vega E, Manneh R, Dorta M, Cortes-Funes H. Safety and efficacy of metronomic non-pegylated liposomal encapsulated doxorubicin in heavily pretreated advanced breast cancer patients. Clin Transl Oncol. 2013;15:467–471. doi: 10.1007/s12094-012-0954-4. [DOI] [PubMed] [Google Scholar]
- 57.Mayer EL, Isakoff SJ, Klement G, Downing SR, Chen WY, Hannagan K, Gelman R, Winer EP, Burstein HJ. Combination antiangiogenic therapy in advanced breast cancer: a phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Res Treat. 2012;136:169–178. doi: 10.1007/s10549-012-2256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montagna E, Cancello G, Bagnardi V, Pastrello D, Dellapasqua S, Perri G, Viale G, Veronesi P, Luini A, Intra M, Calleri A, Rampinelli C, Goldhirsch A, Bertolini F, Colleoni M. Metronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2-negative breast cancer: clinical and biological activity. Clin Breast Cancer. 2012;12:207–214. doi: 10.1016/j.clbc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Mueller MM, Fusenig NE. Friends or foes – bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 60.Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, Kerbel RS. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 61.Munoz R, Shaked Y, Bertolini F, Emmenegger U, Man S, Kerbel RS. Anti-angiogenic treatment of breast cancer using metronomic low-dose chemotherapy. Breast. 2005;14:466–479. doi: 10.1016/j.breast.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 62.Murugaesu N, Schmid P, Dancey G, Agarwal R, Holden L, McNeish I, Savage PM, Newlands ES, Rustin GJ, Seckl MJ. Malignant ovarian germ cell tumors: identification of novel prognostic markers and long-term outcome after multimodality treatment. J Clin Oncol. 2006;24:4862–4866. doi: 10.1200/JCO.2006.06.2489. [DOI] [PubMed] [Google Scholar]
- 63.Nars MS, Kaneno R. Immunomodulatory effects of low dose chemotherapy and perspectives of its combination with immunotherapy. Int J Cancer. 2013;132:2471–2478. doi: 10.1002/ijc.27801. [DOI] [PubMed] [Google Scholar]
- 64.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 65.Okada F, Rak JW, Croix BS, Lieubeau B, Kaya M, Roncari L, Shirasawa S, Sasazuki T, Kerbel RS. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci U S A. 1998;95:3609–3614. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orlandi P, Fontana A, Fioravanti A, Di Desidero T, Galli L, Derosa L, Canu B, Marconcini R, Biasco E, Solini A, Francia G, Danesi R, Falcone A, Bocci G. VEGF-A polymorphisms predict progression-free survival among advanced castration-resistant prostate cancer patients treated with metronomic cyclophosphamide. Br J Cancer. 2013;109:957–964. doi: 10.1038/bjc.2013.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasquier E, Andre N. New therapeutic advances and perspectives in tumour angiogenesis. Curr Cancer Drug Targets. 2010;10:877–878. doi: 10.2174/156800910793358032. [DOI] [PubMed] [Google Scholar]
- 68.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions, Nature reviews. Clinical oncology. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 69.Pasquier E, Kieran MW, Sterba J, Shaked Y, Baruchel S, Oberlin O, Kivivuori MS, Peyrl A, Diawarra M, Casanova M, Zacharoulis S, Vassal G, Berthold F, Verschuur A, Andre N. Moving forward with metronomic chemotherapy: meeting report of the 2nd International Workshop on Metronomic and Anti-Angiogenic Chemotherapy in Paediatric Oncology. Transl Oncol. 2011;4:203–211. doi: 10.1593/tlo.11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perroud HA, Rico MJ, Alasino CM, Pezzotto SM, Rozados VR, Scharovsky OG. Association between baseline VEGF/sVEGFR-2 and VEGF/TSP-1 ratios and response to metronomic chemotherapy using cyclophosphamide and celecoxib in patients with advanced breast cancer. Indian journal of cancer. 2013;50:115–121. doi: 10.4103/0019-509X.117031. [DOI] [PubMed] [Google Scholar]
- 71.Peterson JE, Zurakowski D, Italiano JE, Jr, Michel LV, Connors S, Oenick M, D’Amato RJ, Klement GL, Folkman J. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis. 2012;15:265–273. doi: 10.1007/s10456-012-9259-z. [DOI] [PubMed] [Google Scholar]
- 72.Peterson JE, Zurakowski D, Italiano JE, Jr, Michel LV, Fox L, Klement GL, Folkman J. Normal ranges of angiogenesis regulatory proteins in human platelets. American journal of hematology. 2010;85:487–493. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
- 73.Peyrl A, Chocholous M, Kieran MW, Azizi AA, Prucker C, Czech T, Dieckmann K, Schmook MT, Haberler C, Leiss U, Slavc I. Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer. 2012;59:511–517. doi: 10.1002/pbc.24006. [DOI] [PubMed] [Google Scholar]
- 74.Pizzo PA, Poplack DG. Principles and practice of pediatric oncology. 6. Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- 75.Rak J, Filmus J, Finkenzeller G, Grugel S, Marme D, Kerbel RS. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev. 1995;14:263–277. doi: 10.1007/BF00690598. [DOI] [PubMed] [Google Scholar]
- 76.Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria-Petit A, Filmus J, Mansour SJ, Ahn NG, Kerbel RS. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 2000;60:490–498. [PubMed] [Google Scholar]
- 77.Romiti A, Cox MC, Sarcina I, Di Rocco R, D’Antonio C, Barucca V, Marchetti P. Metronomic chemotherapy for cancer treatment: a decade of clinical studies. Cancer Chemother Pharmacol. 2013;72:13–33. doi: 10.1007/s00280-013-2125-x. [DOI] [PubMed] [Google Scholar]
- 78.Savage P, Stebbing J, Bower M, Crook T. Why does cytotoxic chemotherapy cure only some cancers? Nat Clin Pract Oncol. 2009;6:43–52. doi: 10.1038/ncponc1260. [DOI] [PubMed] [Google Scholar]
- 79.Scharovsky OG, Mainetti LE, Rozados VR. Metronomic chemotherapy: changing the paradigm that more is better. Current oncology. 2009;16:7–15. doi: 10.3747/co.v16i2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, Gascoyne RD, Connors JM. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 81.Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 82.Skipper HE, Schabel FM, Jr, Mellett LB, Montgomery JA, Wilkoff LJ, Lloyd HH, Brockman RW. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep. 1970;54:431–450. [PubMed] [Google Scholar]
- 83.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka H, Matsushima H, Mizumoto N, Takashima A. Classification of chemotherapeutic agents based on their differential in vitro effects on dendritic cells. Cancer Res. 2009;69:6978–6986. doi: 10.1158/0008-5472.CAN-09-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka H, Matsushima H, Nishibu A, Clausen BE, Takashima A. Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res. 2009;69:6987–6994. doi: 10.1158/0008-5472.CAN-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang JH, Zhao JH, Lu JW, Yan F, Qin JW, Xu B. Circulating levels of angiogenic cytokines in advanced breast cancer patients with system chemotherapy and their potential value in monitoring disease course. J Cancer Res Clin Oncol. 2011;137:55–63. doi: 10.1007/s00432-010-0859-y. [DOI] [PubMed] [Google Scholar]
- 87.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annual review of pathology. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 88.Toyoda Y, Manabe A, Tsuchida M, Hanada R, Ikuta K, Okimoto Y, Ohara A, Ohkawa Y, Mori T, Ishimoto K, Sato T, Kaneko T, Maeda M, Koike K, Shitara T, Hoshi Y, Hosoya R, Tsunematsu Y, Bessho F, Nakazawa S, Saito T. Six months of maintenance chemotherapy after intensified treatment for acute lymphoblastic leukemia of childhood. J Clin Oncol. 2000;18:1508–1516. doi: 10.1200/JCO.2000.18.7.1508. [DOI] [PubMed] [Google Scholar]
- 89.Treiber G, Wex T, Malfertheiner P. Impact of different anticancer regimens on biomarkers of angiogenesis in patients with advanced hepatocellular cancer. J Cancer Res Clin Oncol. 2009;135:271–281. doi: 10.1007/s00432-008-0443-x. [DOI] [PubMed] [Google Scholar]
- 90.Vacca A, Iurlaro M, Ribatti D, Minischetti M, Nico B, Ria R, Pellegrino A, Dammacco F. Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood. 1999;94:4143–4155. [PubMed] [Google Scholar]
- 91.Vacca A, Ribatti D, Iurlaro M, Merchionne F, Nico B, Ria R, Dammacco F. Docetaxel versus paclitaxel for antiangiogenesis. J Hematother Stem Cell Res. 2002;11:103–118. doi: 10.1089/152581602753448577. [DOI] [PubMed] [Google Scholar]
- 92.Vives M, Ginesta MM, Gracova K, Graupera M, Casanovas O, Capella G, Serrano T, Laquente B, Vinals F. Metronomic chemotherapy following the maximum tolerated dose is an effective anti-tumour therapy affecting angiogenesis, tumour dissemination and cancer stem cells. Int J Cancer. 2013;133:2464–2472. doi: 10.1002/ijc.28259. [DOI] [PubMed] [Google Scholar]
- 93.Wernli M, Tichelli A, von Fliedner V, Brun del Re G, Chapuis B, Fey MF, Fopp M, Gmur J, Grob JP, Jacky E, et al. Intensive induction/consolidation therapy without maintenance in adult acute lymphoblastic leukaemia: a pilot assessment. Working Party on Leukaemia of the Swiss Group for Epidemiologic and Clinical Cancer Research (SAKK) Br J Haematol. 1994;87:39–43. doi: 10.1111/j.1365-2141.1994.tb04867.x. [DOI] [PubMed] [Google Scholar]
- 94.Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435–1440. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 95.Wu J, Waxman DJ. Metronomic cyclophosphamide schedule-dependence of innate immune cell recruitment and tumor regression in an implanted glioma model. Cancer Lett. 2014;353:272–280. doi: 10.1016/j.canlet.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Young SD, Whissell M, Noble JC, Cano PO, Lopez PG, Germond CJ. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin Cancer Res. 2006;12:3092–3098. doi: 10.1158/1078-0432.CCR-05-2255. [DOI] [PubMed] [Google Scholar]
- 97.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 98.Zloza A, Kim DW, Kim-Schulze S, Jagoda MC, Monsurro V, Marincola FM, Kaufman HL. Immunoglobulin-like transcript 2 (ILT2) is a biomarker of therapeutic response to oncolytic immunotherapy with vaccinia viruses. Journal for immunotherapy of cancer. 2014;2:1. doi: 10.1186/2051-1426-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


