Abstract
Background
Papillary Microcarcinoma (PMC) of thyroid is a rare type of differentiated thyroid cancer (DTC), which according to the World Health Organization measures 1.0 cm or less. The gold standard of treatment of PMC is still controversy. Our aim was to contribute in resolving the debate on the therapeutic choices of the surgical and adjuvant I-131 (RAI) treatment in PMC.
Methods
From 2000 to 2012, 326 patients were found to have PMC and were retrospectively reviewed for clinicopathological characteristics, treatment outcomes and prognostic factors.
Results
Mean age of cohort was 42.6 years (range: 18–76) and the mean tumor size was 0.61 cm ± 0.24; lymph node involvement was seen in 12.9 % of cases. Median follow up period was 8.05 years (1.62–11.4). Total 23 all site recurrences (7.13 %) were observed; more observed in patients without I-131 ablation (p <0.0001). Ten year DFS rates were 89.6 %. Cox regression Model analysis revealed size, histopathologic variants, multifocality, extrathyroidal extension, lymphovascular space invasion, nodal status, and adjuvant RAI ablation the important prognostic factors affecting DFS.
Discussion
Despite excellent DFS rates, a small proportion of patients with PMC develop recurrences after treatment. Adjuvant RAI therapy improves DFS in PMC patients with aggressive histopathologic variants, multifocality, ETE, LVSI, tumor size (> 0.5 cm) and lymph node involvement. Failure of RAI ablation to decrease risk in N1a/b supports prophylactic central neck dissection during thyroidectomy, however more trials are warranted.
Conclusion
Adjuvant I-131 ablation following thyroidectomy in PMC patients, particularly with poor prognostic factors improves DFS rates.
Keywords: Papillary microcarcinoma, Optimal treatment, Adjuvant radioiodine ablation, Disease free survival, Saudi Population
Background
In Saudi Arabia, the incidence of differentiated thyroid cancers (DTC) especially papillary thyroid cancers (PTC) is increasing exponentially over the past years accounting for more than 10 % of all cancers among females [1, 2]. Higher rates for identification of PTC in recent years are attributed to the use of high resolution neck ultrasonography (USG) and USG-guided fine needle aspiration biopsy (FNAB) [3]. With the use of these high resolution transducers, papillary microcarcinoma (PMC), i.e. tumor size 1 cm or less can easily be detected [4, 5]. Patients with PMC have generally an excellent outlook with use of surgery, radioactive iodine-131 (RAI) ablation, suppression of thyroid-stimulating hormone (TSH) secretion with levothyroxine, with long term disease-free survival (DFS) of 84–97 % [6]. However, still there is much debate regarding the most appropriate treatment of PMC ranging from observation alone to over-treatment with surgery followed by adjuvant RAI ablation [7–10].
In the present study, we aimed to evaluate the different prognostic factors for DFS in PMC patients in our population, and also to determine the DFS in patients with PMC treated with or without adjuvant RAI ablation following thyroidectomy.
Methods
After formal approval from the institutional ethical committee, medical records of 1192 patients with confirmed papillary thyroid cancers (PTC) who were treated or followed up in two major referral hospitals of Riyadh, Saudi Arabia, during the period of July 2000 and December 2012 were reviewed using computer based departmental database system. Patients with PMC were retrieved in following manner;
Definition
PMC was defined according WHO classification system for thyroid tumors as “PTC is measuring ≤ 1 cm in greatest dimension” [5].
Demographic, clinicopathological and radiological variables
Demographic and clinical data including age at the diagnosis, gender, and symptomatology were reviewed. A detailed second review of all histopathological specimens was performed by experienced histopathologist. Different histopathological parameters, including the location of tumor, tumor size, histopathologic variants, multifocality, extrathyroidal extension (ETE), lymphovascular space invasion (LVSI), surgical margin status, and cervical lymph node status and background thyroid tissue were also recorded. Data from different imaging modalities, including neck ultrasonography, whole body I-131 scintigraphy (WBS), computed tomography (CT) scan of neck and chest, flourodeoxyglucose positron emission tomography (FDG-PET) was collected. Periodic postoperative thyroid function tests (TFTs), thyroid antibodies and stimulated thyroglobulin (TG) levels (off thyroxin or thyrotropin-Alfa injection) were also reviewed. Different treatment modalities, including hemi-thyroidectomy (removal of lobe and isthmus), total thyroidectomy (removal of entire gland), neck dissection, adjuvant RAI ablation, different doses used in millicurie (mCi) and the details of neck irradiation details (if given) were also reviewed.
The primary endpoint was the disease free survival (DFS). Secondary points were; the frequency of PMC and histologic variants, local recurrence free survival (LRFS), distant metastasis free survival (DMFS) and overall survival (OS) according to (a) treatment with or without adjuvant I-131 ablation and (b) according to primary tumor size (≤0.5 cm vs. > 0.5 cm).
Local recurrence was defined as, clinically or radiologically detectable recurrences in the thyroid bed or in cervical lymph nodes on imaging (ultrasonography, WBS and CT-PET) after evaluating for elevated thyroglobulin (TG) levels. Distant metastasis was defined as, clinically or radiologically detectable disease outside the neck on imaging (WBS, CT imaging and CT-PET) after evaluating for elevated thyroglobulin (TG) levels. The DFS was defined as, the duration between the surgery date and the date of documented disease reappearance/relapse, death from cancer and/or last follow-up (censored). The OS was defined as, the duration between the surgery date and the date of patient death or last follow-up (censored).
Statistical analysis
Chi-square test, Student’s t test, or Fisher exact tests were used to determine the differences in various clinical variables. Multivariate logistic regression was done using Cox proportional hazards modeling. Probabilities of LRFS, DMFS, DFS and OS were shown with the Kaplan-Meier method and the comparisons for various survival curves were performed using log rank. All statistical analyses were performed using the computer program SPSS version 16.0.
Results
Demographic and clinicopathological features of cohort
Among the 1192 PTC patients in our departmental database, 377 (31.6 %) patients were found to have PMC. Fifty one (13.3 %) patients with insufficient data regarding size, treatment and follow-up period were excluded. The remaining study cohort (n = 326) consisted of 271 (83.1 %) women and 55 (16.9 %) men; the median age at diagnosis was 42.6 years ±11.6. The majority of patients had total thyroidectomy (n = 299, 91.7 %); only 27 (8.3 %) patients underwent lobectomy. The mean tumor size was 0.61 cm ± 0.24, with 12.9 % (n = 42) involvement of cervical lymph nodes (level VI in 34 patients). The predominant histopathologic variants were, classic (265 patients), follicular (41 patients), and tall cell (11 patients). Other clinicopathological features are described in Table 1.
Table 1.
Patients characteristics
| Variable | Whole cohort N (%) | RAI ablation N (%) | Without RAI ablation N (%) | P value* |
|---|---|---|---|---|
| Total patients | 326/1192 (27.4 %) | 182/326 (55.8) | 144/326 (44.2) | 0.06 |
| Age (years) | 42.6 (18–76) SD ±11.6 | 43.2 (18–76) SD ± 12.4 | 41.8 (19–71) SD ± 10.2 | |
| ≤45 years | 201 (61.7) | 110 (60.4) | 94 (65.3) | 0.81 |
| ≥45 years | 125 (38.3) | 72 (39.6) | 50 (34.7) | |
| Gender | ||||
| Female | 271 (83.1) | 146 (80.2) | 125 (86.8) | 0.06 |
| Male | 55 (16.9) | 36 (19.8) | 19 (13.2) | |
| Female to male ratio | 4.9 | 4.0 | 6.5 | |
| Type of surgery | ||||
| Total thyroidectomy | 299 (91.7) | 182 (100) | 117 (81.3) | 0.04 |
| Hemi-thyroidectomy | 27 (8.3) | - | 27 (18.7) | |
| Lymph node surgery | ||||
| Central neck dissection | 88 (27.0) | 54 (29.7) | 34 (23.6) | |
| Lateral neck dissection | 18 (5.5) | 9 (4.9) | 9 (6.3) | 0.9 |
| Sampling | 55 (16.9) | 25 (13.7) | 30 (20.8) | |
| None | 165 (50.6) | 94 (51.7) | 71 (49.3) | |
| Mean size (cm) | 0.61 (0.1–1.0) ± 0.24 | 0.72 (0.2–1.0) ± 0.21 | 0.44 (0.1–0.9) ± 0.2 | |
| ≤0.5 cm | 161 (49.4) | 50 (27.5) | 111 (77.1) | <0.0001 |
| ≥0.5 cm | 165 (50.6) | 132 (72.5) | 33 (22.9) | |
| Histopathologic variants | ||||
| Classic | 265 (81.3) | 143 (78.6) | 122 (84.7) | |
| Follicular | 41 (12.6) | 21 (11.5) | 20 (13.9) | |
| Hurthle cell | 8 (2.5) | 6 (3.3) | 2 (1.4) | |
| Tall cell | 11 (3.4) | 11 (6.0) | - | 0.001 |
| Sclerosing | 1 (0.3) | 1 (0.5) | - | |
| Multifocal | ||||
| Yes | 125 (38.3) | 122 (67.1) | 3 (2.1) | <0.0001 |
| No | 201 (61.7) | 60 (32.9) | 141 (97.9) | |
| ETE | ||||
| Yes | 62 (19.0) | 57 (31.3) | 5 (3.5) | <0.0001 |
| No | 264 (81.0) | 125 (68.7) | 139 (96.5) | |
| LVSI | ||||
| Yes | 55 (16.9) | 49 (26.9) | 6 (4.2) | <0.0001 |
| No | 271 (83.1) | 133 (73.1) | 138 (95.8) | |
| Surgical margins | ||||
| Positive | 35 (10.7) | 30 (16.5) | 5 (3.5) | <0.0001 |
| Negative | 291 (89.3) | 152 (83.5) | 139 (96.5) | |
| Lymph node metastasis | ||||
| Yes | 42 (12.9) | 42 (23.1) | - | <0.0001 |
| N1a | 34 (73.8) | 34 (73.8) | ||
| N1b | 8 (19.2) | 8 (19.2) | ||
| No | 284 (87.1) | 140 (76.9) | 144 (100) | |
| Background thyroid tissue | ||||
| Normal | 98 (30.1) | 47 (25.8) | 51 (35.4) | |
| Multi-nodular goiter | 106 (32.5) | 60 (32.9) | 46 (31.9) | |
| Lymphocytic thyroiditis/Hashimotos’ thyroiditis | 122 (37.5) | 75 (41.3) | 47 (32.6) | 0.052 |
| Distant Metastasis at presentation | 3 (0.9) | 3 (1.65) | - | <0.0001 |
| AJCC staging | ||||
| I | 217 (66.5) | 73 (40.1) | 139 (96.5) | |
| II | - | - | - | |
| III | 96 (29.5) | 96 (52.6) | 5 (3.5) | <0.0001 |
| IVA | 10 (3.1) | 10 (5.6) | - | |
| IVB | - | - | - | |
| IVC | 3 (0.9) | 3 (1.7) | - | |
| Mean postoperative TG (ng/ml) | 1.39 (0.1–42890) | 2.44 (0.1–42890) | 0.39 (0.1–8.9) | 0.62 |
| RAI dose | ||||
| 30 mCi | 50 (27.5) | - | <0.0001 | |
| 100 mCi | 85 (46.7) | - | ||
| 150-200 mCi | 47 (25.8) | - | ||
| RT to Neck | 2 (0.61) | 2 (1.1) | - | <0.0001 |
| Recurrences | ||||
| Locoregional | 13 (3.9) | 4 (2.2) | 9 (6.2) | <0.001 |
| Distant | 10 (3.1) | 4 (2.2) | 6 (4.2) | |
*P value pertaining to the variation in clinicopathological characteristics between two groups
RAI radioactive iodine 131, N number, SD standard deviation, ETE extra-thyroidal extension, LVSI lymphovascular space invasion, AJCC American joint committee on cancer, TG thyroglobulin, mCi millicurie, RT radiation therapy
Clinicopathological features and DFS Comparison in PMC patients treated with and without I-131 ablation
Among 326 patients, 182 (55.8 %) patients were given adjuvant RAI ablation as shown in Table 1. Major indications for adjuvant RAI ablation were multifocality (67.1 %), extra-thyroidal extension (ETE) in 31.3 % of cases, aggressive histopathologic variants (tall cell, sclerosing), lymph node metastasis (23.1 %) and distant metastasis at time of presentation (1.65 %). Primary tumor size was not a primary indication in our series; however the observed mean tumor size was bigger in patients treated with adjuvant RAI ablation (0.72 cm vs. 0.44 cm). RAI ablation doses were as; 30 m-curie (mCi) for tumors with multifocality and focal ETE (27.5 %); 100 mCi for tumors with multifocality, ETE, LVSI, positive surgical margins, poor histopathologic variants, and elevated postoperative stimulated TG levels (>2 ng/ml) (46.7 %); 150 mCi for positive lymph nodes (24.7 %), and 200 mCi for distant metastasis at the time of diagnosis (1.65 %). RAI ablation was tolerated well without any grade 3 or 4 side effects. Additional neck irradiation was given in two patients with adherent tumors; trachea (one patient; 0.5 %) and skeletal muscle (one patient; 0.5 %).
A median follow-up period was 8.05 years (range: 1.62–11.4). For whole cohort, the 5 and 10 years LRFS were 98.4 % and 96.8 % respectively; DMFS rates were 92.4 % at 5 years and 90 % at 10 years. Five and 10 years OS rates were 99.3 % and 98.6 % (two deaths) and the 5 and 10 years DFS rates were 94.7 % and 89.6 %.
Total 23 recurrences (7.13 %) were observed; 8/182 in patients with RAI ablation and 15/144 in patients without RAI ablation. The pattern of recurrences was as: three patients had disease in thyroid bed only, 10 had cervical nodes, and 10 failed at distant sites (9 patients in lungs and one patient in bones). Combined locoregional and distant recurrences were seen in 3 patients. The elevated TG levels were always seen with local recurrences and distant metastasis. The isolated locoregional recurrences were salvaged by surgery (lateral neck dissection; 7 patients, completion thyroidectomy; 2 patients and excision in one patient), followed by RAI ablation (12 patients) and distant failures were salvaged by RAI ablation (9 patients) and palliative irradiation for bony lesion (one patient). Time to initial local recurrence was 0.8 years and time to initial distant metastasis was 1.5 year. The 5 and 10 year DFS rates were 95.7 % vs. 90.9 % and 92.2 % vs. 84 % in patients with and without RAI ablation respectively (p = 0.04) Fig. 1a. The 5 and 10 year DFS rates according to different prognostic factors are summarized in Table 2. The overall 5 and 10 year DFS rates were significantly dropped in the presence of poor histopathologic variants (p < 0.001) and ETE. In addition to these factors, multifocality (p < 0.001) LVSI (p = 0.001) and elevated postoperative thyroglobulin levels > 2 ng/ml (p = 0.04) resulted in inferior 5 and 10 year DFS in patients treated without RAI ablation.
Fig. 1.
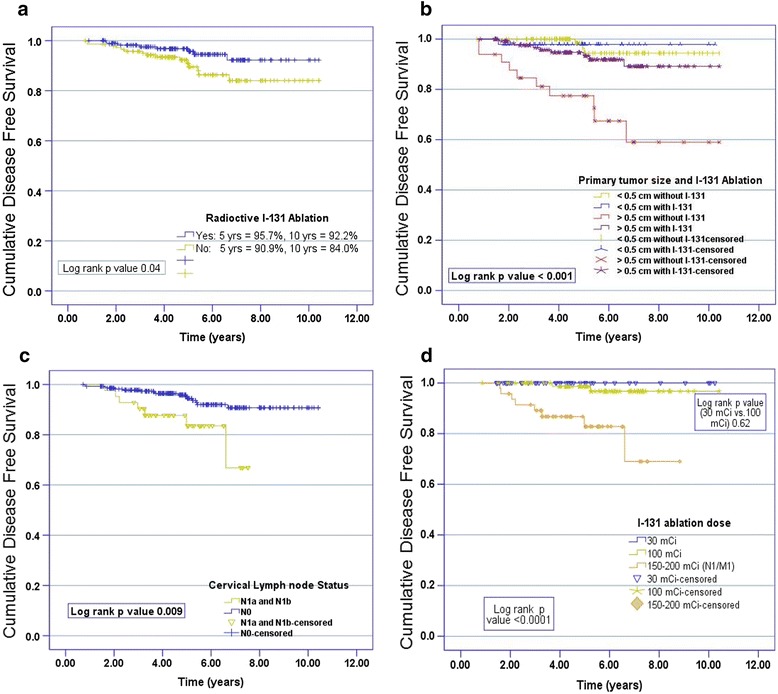
Kaplan-Meier curves of disease free survival (a) according to treated with or without radioactive iodine 131 ablation, (b) primary size (≤0.5 cm vs. > 0.5 cm) treated with or without radioactive iodine 131 ablation, (c) lymph node status and (d) dose regimens (30 mCi vs. 100 mCi)
Table 2.
Disease free survival according to different prognostic factors
| Variable | RAI ablation | Without RAI ablation | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 years-DFS | p | 10 years-DFS | p | 5 years-DFS | p | 10 years-DFS | p | |
| Age | ||||||||
| ≤45 years | 97.8 % | 93.5 % | 87.6 % | 83.6 % | ||||
| ≥45 years | 92.5 % | NS | 85.3 % | NS | 88.5 % | NS | 84.5 % | NS |
| Gender | ||||||||
| Female | 95.4 % | 91.2 % | 93.8 % | 82.2 % | ||||
| Male | 96.8 % | NS | 89.4 % | NS | 90.4 % | NS | 82.0 % | NS |
| Histopathologic variants | ||||||||
| Classic | 96.1 % | 93.5 % | 95.5 % | 92.3 % | ||||
| Follicular | 94.7 % | 90.9 % | 78.9 % | 59.6 % | ||||
| Hurthle cell | 96.1 % | 92.7 % | 90.0 % | 0.001 | 78.9 % | <0.001 | ||
| Tall cell | 68.2 % | - | - | |||||
| Sclerosing | 55.0 % | 0.002 | - | <0.001 | - | |||
| Multifocal | ||||||||
| Yes | 95.2 % | 90.9 % | 66.7 % | 33.3 % | ||||
| No | 96.6 % | NS | 93.4 % | NS | 90.0 % | <0.001 | 88.3 % | <0.0001 |
| Surgical margins | ||||||||
| Positive | 96.6 % | 91.5 % | 86.3 % | 84.0 % | ||||
| Negative | 96.8 % | NS | 95.3 % | NS | 93.2 % | NS | 87.9 % | NS |
| ETE | ||||||||
| Yes | 89.5 % | 85.5 % | 40.0 % | 0.0 % | ||||
| No | 98.2 % | 0.03 | 95.1 % | 0.02 | 91.7 % | <0.0001 | 80.1 % | <0.0001 |
| LVSI | ||||||||
| Yes | 89.5 % | 85.5 % | 80.0 % | 60.0 % | ||||
| No | 92.5 % | NS | 89.4 % | NS | 93.2 % | 0.02 | 84.4 % | 0.001 |
| Mean postoperative TG | ||||||||
| ≤2 ng/ml | 96.6 % | 93.4 % | 93.2 % | 91.7 % | ||||
| >2 ng/ml | 89.5 % | NS | 85.5 % | NS | 87.9 % | NS | 80.1 % | 0.04 |
| Surgery | ||||||||
| Total thyroidectomy | 96.0 % | 94.4 % | 93.2 % | 87.5 % | ||||
| Hemi-thyroidectomy | 91.5 % | 0.03 | 86.0 % | 0.03 | 85.1 % | 0.04 | 80.2 % | 0.02 |
RAI radioactive iodine 131, yr year, DFS disease free survival, SD standard deviation, ETE extra-thyroidal extension, LVSI lymphovascular space invasion, TG thyroglobulin
Clinicopathological features and DFS comparison among PMC of size ≤ 0.5 cm and > 0.5 cm
With regard to the difference in DFS (locoregional and distant failure), a comparative analysis was performed according to primary tumor size (≤0.5 cm vs. > 0.5 cm) as described in the Table 3. About 161 (49.4 %) patients had tumors of size ≤ 0.5 cm and 165 (50.6 %) patients had tumors of size above 0.5 cm in greatest dimension. Significant demographic and clinicopathological differences were observed between two groups. Patients with tumor size ≤ 0.5 cm were younger (mean age 36.7 years), with higher female to male ratio (6.3), and with more aggressive histopathologic variants (tall cell, sclerosing). The cervical lymph node metastases were seen in 9.3 % of patients with tumor size ≤ 0.5 cm as compared to patients with tumor size > 0.5 cm (16.4 %) with p < 0.001. Patients with tumor size ≤ 0.5 cm had high rates of hemi-thyroidectomy (18.7 %), less adjuvant RAI ablation (31.1 %) with low recurrence rates. There was also no significant difference in 5 and 10 year DFS rates in in patients with tumor size ≤ 0.5 cm treated with or without adjuvant RAI ablation (p = 0.71) Fig. 1b. Further it was seen that adjuvant RAI ablation did better in N0 as compared to N1 neck status Fig. 1c. Also in patients treated with adjuvant RAI ablation, no significant difference was observed between two dose regimens (30 mCi vs. 100 mCi) with p = 0.62 (Fig. 1d).
Table 3.
Comparative analysis of clinicopathological characteristics based on the size of primary tumors
| Variable | Tumor size ≤ 0.5 cm N (%) | Tumor size > 0.5 cm N (%) | P value |
|---|---|---|---|
| Total patients | 161/326 (49.4) | 165/326 (50.6) | - |
| Age (years) | 36.7 (8–71) | 47.8 (8–76) | |
| ≤45 years | 107 (66.5) | 94 (56.9) | 0.034 |
| ≥45 years | 54 (33.5) | 71 (43.1) | |
| Gender | |||
| Female | 139 (86.4) | 132 (80.0) | 0.08 |
| Male | 22 (13.6) | 33 (20.0) | |
| Mean size (cm) | 0.38 (0.1–0.5) | 0.68 (0.6–1.0) | <0.001 |
| Histopathologic variants | |||
| Classic | 126 (78.2) | 139 (84.3) | |
| Follicular | 26 (16.2) | 14 (8.5) | |
| Hurthle cell | 4 (2.5) | 4 (2.4) | 0.023 |
| Tall cell | 4 (2.5) | 7 (4.3) | |
| Sclerosing | 1 (0.6) | - | |
| Multifocal | |||
| Yes | 36 (22.4) | 89 (53.9) | |
| No | 125 (77.6) | 76 (46.1) | <0.001 |
| ETE | |||
| Yes | 16 (9.9) | 46 (27.9) | <0.001 |
| No | 145 (90.1) | 119 (72.1) | |
| LVSI | |||
| Yes | 14 (8.7) | 41 (24.9) | <0.001 |
| No | 147 (91.3) | 124 (75.1) | |
| Surgical margins | |||
| Positive | 5 (3.1) | 30 (18.2) | |
| Negative | 156 (96.9) | 135 (81.8) | <0.001 |
| Background thyroid tissue | |||
| Normal | 60 (37.3) | 38 (23.0) | |
| Multi-nodular goiter | 48 (29.8) | 58 (35.2) | |
| Lymphocytic thyroiditis/Hashimotos’ thyroiditis | 53 (32.9) | 69 (41.8) | 0.05 |
| Lymph node metastasis | |||
| Yes | 15 (9.3) | 27 (16.4) | |
| No | 146 (90.7) | 138 (83.6) | <0.001 |
| RAI ablation | |||
| Yes | 50 (31.1) | 132 (67.3) | <0.001 |
| No | 111 (68.9) | 22 (13.3) | |
| Recurrences | |||
| Locoregional | 4 (2.5) | 9 (5.5) | |
| Thyroid bed | 1/4 | 2/9 | |
| Lymph nodes | 3/4 | 7/9 | <0.001 |
| Distant | 2 (1.3) | 8 (4.9) | |
| Lungs | 2 | 7/8 | |
| Bone | - | 1/8 | |
I-131 radioactive iodine 131, N number, ETE extra-thyroidal extension, LVSI lymphovascular space invasion, RAI radioactive iodine
Prognostic factors
Cox regression Model using univariate and multivariate analysis for DFS to predict important prognostic factors Table 4. Important prognostic factors were, histopathologic variants (p < 0.0001), multifocality (p < 0.0001), ETE (p < 0.0001), LVSI (p =0.03), nodal status (p < 0.0001), and adjuvant RAI ablation (p < 0.0001).
Table 4.
Cox regression model of various prognostic factors for disease specific survival
| Variable | All patients | |||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| RR (95 % CI) | p | RR (95 % CI) | p | |
| Age | ||||
| ≤45 years | 1.05 (0.7–1.3) | 1.07 (0.8–1.3) | ||
| ≥45 years | 1.10 (0.8–1.4) | 0.6 | 1.10 (0.9–1.3) | 0.06 |
| Gender | ||||
| Female | 1.07 (0.9–1.4) | 1.05 (0.7–1.3) | ||
| Male | 1.05 (0.7–1.3) | 0.6 | 1.40 (1.2–1.6) | 0.05 |
| Histopathologic variants | ||||
| Classic | 1.05 (0.7–1.2) | 1.20 (0.8–1.6) | ||
| Follicular | 1.00 (0.6–1.8) | 1.18 (0.7–1.5) | ||
| Hurthle cell | 1.30 (1.1–1.7) | 2.00 (1.6–2.4) | ||
| Tall cell | 2.70 (1.6–4.5) | 2.82 (2.4–4.6) | ||
| Sclerosing | 1.80 (1.6–2.9) | <0.0001 | 2.00 (1.6–3.0) | <0.0001 |
| Multifocal | ||||
| Yes | 3.1 (2.8–4.2) | 2.94 (2.2–3.4) | ||
| No | 1.0 (0.8–1.2) | <0.0001 | 1.07 (0.9–1.3) | <0.0001 |
| Surgical margins | ||||
| Positive | 1.10 (0.9–1.4) | 1.20 (0.8–1.6) | ||
| Negative | 1.07 (0.9–1.4) | 0.7 | 1.17 (0.6–1.2) | 0.68 |
| ETE | ||||
| Yes | 4.2 (3.5–5.1) | 3.31 (1.7–4.2) | ||
| No | 1.05 (0.7–1.1) | <0.0001 | 1.17 (0.9–1.4) | <0.0001 |
| LVSI | ||||
| Yes | 2.0 (1.7–2.9) | 1.81 (1.6–2.8) | ||
| No | 1.0 (0.8–1.2) | 0.02 | 1.04 (0.9–1.5) | 0.03 |
| Lymph nodes | ||||
| Positive | 4.45 (3.7–6.8) | 3.74 (3.4–5.9) | ||
| Negative | 1.17 (0.9–1.4) | <0.0001 | 1.01 (0.8–1.3) | <0.0001 |
| Mean postoperative TG | ||||
| ≤2 ng/ml | 1.01 (0.7–1.2) | 1.05 (0.7–1.2) | ||
| >2 ng/ml | 1.04 (0.9–1.5) | 0.6 | 1.00 (0.6–1.8) | 0.6 |
| RAI ablation | ||||
| Yes | 0.35 (0.2–0.7) | 0.30 (0.2–0.8) | ||
| No | 1.09 (1.0–1.9) | <0.0001 | 1.00 (0.6–1.8) | <0.0001 |
I-131 radioactive iodine 131, RR relative risk, CI confidence interval, ETE extra-thyroidal extension, LVSI lymphovascular space invasion, TG thyroglobulin, RAI radioactive iodine
Discussion
Despite excellent DFS rates in patients with PMC, about 3–16 % of patients develop local and distant failures [11]. In present study, we were able to determine overall five and ten year DFS rates of 94.7 % and 89.6 % respectively after aggressive treatment by total thyroidectomy followed by RAI ablation in the majority of cases. These results were found in consistent with similar reported data [12–15]. Several clinicopathological and treatments related prognostic factors were observed. An important prognostic factor, the age > 45 years was not found a prognosticator to predict DFS in our study, suggesting that other risk factors, such as aggressive histopathologic variants, multifocality, ETE, and LVSI are more important clinicopathological predictors than age in PMC [16]. Similarly, in contrast to other reported data, gender was also not found an important predictor of DFS [17]. Improved DFS was observed in patients who underwent total thyroidectomy. Possible explanation for this could be (a) high percentage of multifocality, and (b) more aggressive histopathological variants (tall cell and diffuse sclerosing variants) in our series, which is in agreement with few previously published studies of PMC [18–20].
Recent studies regarding PMC have reported that patients with multinodular goiter (MNG) and with lymphocytic or Hashimotos’ thyroiditis are associated with better prognosis; however, we could not reproduce the same results. Reason could be (a) few cases of histopathological proven MNG (32.5 %); (b) lack of preoperative TFTs in MNG patients; and (c) few number of patients with lymphocytic/Hashimoto’s thyroiditis (37.5 %) [21].
Further, present study showed the lymph node involvement and tumor size as the most significant independent risk factors for recurrence. Although we found tumor size > 0.5 cm seem to be associated with high recurrence rates, we were not able to identify a size threshold below which there was no lymph node involvement and no risk of recurrence; as in tumor of size ≤ 0.5 cm, 9.3 % lymph node metastasis were seen along with 2.5 % local and 1.3 % distant failures. This supports the hypothesis, that lymph node involvement status is higher in PMC of size > 0.8 cm, but is independent of tumor size [22]. Patients tolerated adjuvant RAI ablation very well with minimal toxicity. Failure of RAI ablation to decrease local or distant failure risk in N1a/b as compared to N0 disease is an indicator of underlying tumor burden in neck and this supports the idea of prophylactic central neck dissection during thyroidectomy [23]. However, still there is much debate over the prophylactic central neck dissection because of potential increased risk of hypoparathyroidism associated with central neck dissection [24].
Strengths of our study were; (a) reasonable sample size of Saudi patients with PMC, and (b) long term follow up period. Limitations of our study were; (a) retrospective data; (b) no intention to treat based analysis, and (c) lack of availability of preoperative clinical data, diagnostic methods (FNAC and radiology), tumor characteristics and baseline TFTs.
Conclusions
In conclusion, among all PTC, 31.6 % of patients are diagnosed as PMC. Despite excellent DFS rates, a small proportion of patients with PMC develop recurrences after treatment. These recurrences not only badly affect physical health, but also mental and social health and overall quality of life. Based on our results we conclude that;
High percentage of multifocality in our population of PMC favors near total or total thyroidectomy against lobectomy, which can be an option for unifocal PMC.
Age > 45 years and gender were not found strong prognostic factors of DFS.
Adjuvant RAI therapy improves DFS in PMC patients with aggressive histopathologic variants, multifocality, ETE, LVSI, tumor size (>0.5 cm in absence of other features) and lymph node involvement (≥150 mCi). In absence of N0 neck, there is significant difference of DFS in two doses (30 mCi vs. 100 mCi).
Failure of RAI ablation to decrease risk in N1a/b supports prophylactic central neck dissection during thyroidectomy, however more trials are warranted.
Abbreviations
- DTC
Differentiated thyroid cancer
- DFS
Disease free survival
- DMC
Distant metastasis control
- ETE
Extrathyroid extension
- PMC
Papillary microcarcinoma
- PTC
Papillary thyroid cancer
- FTC
Follicular thyroid cancer
- LVSI
Lymphovascular invasion
- LR
Locoregional recurrence
- LRC
Locoregional control
- mCi
Millicurie
- OS
Overall survival
- RAI
Radioactive iodine-131
- TG
Thyroglobulin
- WBS
Whole body scintigraphy
Footnotes
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Authors’ contribution
KAQ conceived the study. MAA, KAQ, AAH collected the data. MAT and YB performed the statistical analysis. NAJ and HF performed histopathological data collection and review. All authors read and approved the final manuscript.
Contributor Information
Khalid Hussain AL-Qahtani, Phone: +96612889999, Email: kalqahtani@KSU.EDU.SA.
Mushabbab Al Asiri, Phone: +96612889999, Email: masiri@kfmc.med.sa.
Mutahir A. Tunio, Phone: +96612889999, Email: drmutahirtonio@hotmail.com
Naji J. Aljohani, Phone: +96612889999, Email: njaljohani@kfmc.med.sa
Yasser Bayoumi, Email: y_bayoumi@yahoo.com.
Hanadi Fatani, Phone: +96612889999, Email: h.fatani@kfmc.med.sa.
Abdulrehman AlHadab, Phone: +96612889999, Email: arehaman@kfmc.med.sa.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hussain F, Iqbal S, Mehmood A, Bazarbashi S, ElHassan T, Chaudhri N. Incidence of thyroid cancer in the Kingdom of Saudi Arabia, 2000–2010. Hematol Oncol Stem Cell Ther. 2013;6:58–64. doi: 10.1016/j.hemonc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DS, Doherty GM, Haugen BR, Haugen BR, Kloos RT, Lee SL, et al. The american thyroid association guidelines taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–41. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 4.Senchenkov A, Staren ED. Ultrasound in head and neck surgery: thyroid, parathyroid, and cervical lymph nodes. Surg Clin North Am. 2004;84:973–1000. doi: 10.1016/j.suc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd R, De Lellis R, Heitz R, Eng C. World health organization classification of tumours: pathology and genetics of tumours of the endocrine organs Lyon. France: IARC Press International Agency for Research on Cancer; 2004. [Google Scholar]
- 6.Pellegriti G, Scollo C, Lumera G, Regalbuto C, Vigneri R, Belfiore A. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endo Metabol. 2004;89:3713–20. doi: 10.1210/jc.2003-031982. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27–34. doi: 10.1089/thy.2013.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254:653–60. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Kim SW. Radioactive iodine ablation does not prevent recurrences in patients with papillary thyroid microcarcinoma. Clin Endocrinol (Oxf) 2013;79:445. doi: 10.1111/cen.12131. [DOI] [PubMed] [Google Scholar]
- 11.Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid. Thyroid. 2009;19:707–16. doi: 10.1089/thy.2008.0270. [DOI] [PubMed] [Google Scholar]
- 12.Besic N, Pilko G, Petric R, Hocevar M, Zgajnar J. Papillary thyroid microcarcinoma: prognostic factors and treatment. J Surg Oncol. 2008;97:221–5. doi: 10.1002/jso.20935. [DOI] [PubMed] [Google Scholar]
- 13.Ross DS, Litofsky D, Ain KB, Bigos T, Brierley JD, Cooper DS, et al. Recurrence after treatment of micropapillary thyroid cancer. Thyroid. 2009;19:1043–8. doi: 10.1089/thy.2008.0407. [DOI] [PubMed] [Google Scholar]
- 14.McDougall IR, Camargo CA. Treatment of micropapillary carcinoma of the thyroid: where do we draw the line? Thyroid. 2007;17:1093–6. doi: 10.1089/thy.2007.0203. [DOI] [PubMed] [Google Scholar]
- 15.Pelizzo MR, Merante Boschin I, Toniato A, Piotto A, Bernante P, Pagetta C, et al. Papillary thyroid microcarcinoma. Long-term outcome in 587 cases compared with published data. Minerva Chir. 2007;62:315–25. [PubMed] [Google Scholar]
- 16.Karatzas T, Vasileiadis I, Kapetanakis S, Karakostas E, Chrousos G, Kouraklis G. Risk factors contributing to the difference in prognosis for papillary versus micropapillary thyroid carcinoma. Am J Surg. 2013;206:586–93. doi: 10.1016/j.amjsurg.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Creach KM, Siegel BA, Nussenbaum B, Grigsby PW. Radioactive iodine therapy decreases recurrence in thyroid papillary microcarcinoma. ISRN Endocrinol. 2012;2012:816386. doi: 10.5402/2012/816386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–81. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstein J, Virk RK, Hui P, Prasad A, Westra WH, Tallini G, et al. Tall cell variant of papillary thyroid microcarcinoma: clinicopathologic features with BRAF (V600E) mutational analysis. Thyroid. 2013;23:1525–31. doi: 10.1089/thy.2013.0154. [DOI] [PubMed] [Google Scholar]
- 20.Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol. 2012;19:1874–80. doi: 10.1245/s10434-011-2129-x. [DOI] [PubMed] [Google Scholar]
- 21.Koo JS, Hong S, Park CS. Diffuse sclerosing variant is a major subtype of papillary thyroid carcinoma in the young. Thyroid. 2009;19:1225–31. doi: 10.1089/thy.2009.0073. [DOI] [PubMed] [Google Scholar]
- 22.Elisei R, Molinaro E, Agate L, Bottici V, Masserini L, Ceccarelli C, et al. Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J Clin Endocrinol Metab. 2010;95:1516–27. doi: 10.1210/jc.2009-1536. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg. 2007;31:2085–91. doi: 10.1007/s00268-007-9224-y. [DOI] [PubMed] [Google Scholar]
- 24.Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20:3477–83. doi: 10.1245/s10434-013-3125-0. [DOI] [PubMed] [Google Scholar]


