Abstract
Shyness is a fundamental trait associated with social-emotional maladaptive behaviors, including many forms of psychopathology. Neuroimaging studies have demonstrated that hyper-responsivity to social and emotional stimuli occurs in the frontal cortex and limbic system in shy individuals, but the relationship between shyness and brain-wide functional connectivity remains incompletely understood. Using resting-state functional magnetic resonance imaging, we addressed this issue by exploring the relationship between regional functional connectivity strength (rFCS) and scores of shyness in a cohort of 61 healthy young adults and controlling for the effects of social and trait anxiety scores. We observed that the rFCS of the insula positively correlated with shyness scores regardless of sex. Furthermore, we found that there were significant sex-by-shyness interactions in the dorsal anterior cingulate cortex and insula (two core nodes of the salience network) as well as the subgenual anterior cingulate cortex: the rFCS values of these regions positively correlated with shyness scores in females but negatively correlated in males. Taken together, we provide evidence for intrinsic functional connectivity differences in individuals with different degrees of shyness and that these differences are sex-dependent. These findings might have important implications on the understanding of biological mechanisms underlying emotional and cognitive processing associated with shyness.
Keywords: shyness, sex difference, resting-state fMRI, insula, salience network
Introduction
Shyness has attracted increasing attention in recent years as a common condition which can impact negatively on social interactions and confidence and represent a long-term personality trait. The Stanford Shyness Survey revealed that over 90% of the population is reported to have experienced shyness at some point in their lives (Zimbardo, 1977). However, 10–25% of the population is characterized by temperamental shyness (Cheek and Melchior, 1990; Battaglia et al., 2005), a stable dispositional trait which describes anxious self-preoccupation and behavioral inhibition in social contexts due to the prospect of interpersonal evaluation (Amico et al., 2004).
Individual differences in shyness are known to be related to human psychopathology (Henderson, 2010), and are presumed to arise from abnormality in different neural systems (Beaton et al., 2008). Recent brain imaging studies have demonstrated that the frontal cortex and forebrain limbic areas play key roles in shyness. For example, using a voxel-based morphometry analysis we observed that shyness was associated with focal differences in gray matter density in many brain regions such as the insula, parahippocampal gyri, temporal pole and cerebellum (Yang et al., 2013). Several task-dependent functional magnetic resonance imaging (fMRI) studies have reported greater amygdalar activation in response to novel faces in shy adults (Schwartz et al., 2003), and increased inferior frontal and insular activation in shy relative to non-shy subjects when processing emotional faces (Beaton et al., 2010). However, these structural and task-dependent fMRI studies focus mainly on segregating different brain networks which are specialized for processing distinct forms of information. A new concept that has emerged from analysis of complex networks is that substantively different systems can share key organizational mechanisms in common (Bassett and Bullmore, 2009). For example the amygdala, which is a key part of the limbic system, has rich anatomical connections with other cortical and subcortical regions involved in the control of fear and anxiety. How this key node associated with shyness work is interacting with other connected regions is unclear.
Resting-state functional MRI (R-fMRI) is increasingly used as a functional imaging technique to help establish changes in intrinsic or spontaneous brain networks in an ecologically valid manner and which avoids some of the constraints of task-dependent paradigms. Using seed-based R-fMRI connectivity analysis, we reported that shyness is associated with various brain functional connectivity differences, involving the superior temporal gyrus, parahippocampal gyrus and insula (Yang et al., 2013). However, such hypothesis driven seed-based approaches strongly depend on the seed regions selected and are thus inherently biased and may not necessarily reveal the extent or even the fundamental nature of network changes occurring. It is therefore important to employ unbiased methods to investigate brain-wide intrinsic connectivity patterns related to shyness.
Here, we used a data-driven R-fMRI approach, termed regional functional connectivity strength (rFCS), that provides a voxel-wise measurement of ‘degree centrality’ for the functional connectome (Bassett and Bullmore, 2009; Buckner et al., 2009), to investigate the whole-brain functional connectivity pattern associated with shyness. Importantly, this rFCS method can allow us to identify highly connected brain hub regions by capturing the complexity of the functional connectome as a whole without the constraints of a priori hypotheses (see Materials and methods). Using this approach, several studies have identified brain hubs with higher rFCS located primarily in the medial and lateral prefrontal cortex, insula and parietal cortex (Buckner et al., 2009; Liang et al., 2013). Moreover, these hub regions have been found to have higher rates of cerebral blood flow (Liang et al., 2013) and glucose metabolism (Tomasi et al., 2013), suggesting that the rFCS metric is biologically plausible. Given that previous brain imaging studies have shown different functional and structural features in the insula and frontal cortex in shy individuals (Schwartz et al., 2003; Beaton et al., 2010; Yang et al., 2013), we hypothesized that shyness would be associated with the rFCS patterns in these regions. Considering that previous studies have reported sex-related differences in shyness in humans and other species (Kerr et al., 1996; Dall 2004), sex-by-shyness interaction effects were also evaluated.
Materials and methods
Participants
We recruited 61 (29 males and 32 females, mean age 21.9 ± 1.94 years, age range 19–27 years old) right-handed, Han ethnicity subjects who were the same as the participants in our previous study (Yang et al., 2013). All participants were interviewed using the Structured Clinical Interview by experienced psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorder-IV (DSM-IV). Inclusion criteria for all participants were physical and neurological health. Individuals were excluded if they had a history of neurological or psychiatric disorders, smoking within 2 months prior to inclusion, current or past substance abuse or use of psychoactive drugs. All participants were right-handed which was determined using the Edinburgh Handedness Scale. The study was approved by the local research ethics committee of the West China Hospital and written informed consent was obtained from each participant.
Behavioral assessment
All of the subjects completed the self-report measurements of shyness [Cheek and Buss Shyness Scale (CBSS)], a 13-item self-report questionnaire designed to measure shyness (Cheek and Buss, 1981). Each item was measured on a 5-point Likert scale, with higher scores reflecting greater shyness. CBSS was developed as a unidimensional measure of shyness. The 9 item (Chou, 2005) and 13 item (Yang et al., 2013) versions of this scale have previously been validated in Chinese subjects. The Cronbach’s alpha of the Chinese version of the CBSS scale in the present sample was 0.826.
Since significant correlations between shyness and anxiety scores have been reported in previous research on healthy subjects (Iancu et al., 2011), we also measured social anxiety using the Liebowitz social anxiety scale (LSAS) and trait anxiety using the Chinese state-trait anxiety inventory (CSTAI-T) in all participants. The LSAS is composed of 24-items that assess levels of fear and avoidance in social or performance situations using a 0–3 scale. An overall total score may also be derived by summing the fear and avoidance ratings for all items. The LSAS has been shown to have high internal consistency (alpha coefficient = 0.95; 0.83 and 0.77 for Chinese patients and normal controls), good convergent and discriminant validity and high test–retest reliability (0.83 for 12-week test–retest) (Fresco et al., 2001; Barnes et al., 2002; Pang et al., 2006). The CSTAI-T was designed to measure a stable propensity to experience anxiety, and tendencies to perceive stressful situations as threatening (Shek, 1993). The scale consists of 20 items, with higher scores indicating greater anxiety. This scale has been shown to have high internal consistency (Cronbach’s alpha = 0.81; Split-half reliability = 0.83; Shek, 1988).
MRI acquisition
Brain imaging was carried out on a 3.0 T MR scanner (Siemens Trio, Erlangen, Germany) with a 12-channel head coil as signal receiver. Foam pads were used to restrict subjects’ head motion.fMRI runs detecting Blood oxygenation level dependent (BOLD) signal were acquired using a gradient-echo planar imaging sequence with TR = 2000 ms; TE = 30 ms; FA = 90; acquisition matrix = 64 × 64; FOV = 240 mm × 240 mm; thickness = 5.0 mm; gap = 0 mm; flip angle = 90°; voxel size = 3.75 × 3.75 × 5 mm3. Each brain volume comprised 30 axial slices, and each functional-run contained 205 volumes, with a total scan time of 414 s. During the scan all participants were instructed to lie still with their eyes closed, not to think of anything and not to fall asleep.
Data preprocessing
R-fMRI data preprocessing was performed using the SPM8 package (http://www.fil.ion.ucl.ac.uk/spm/software/SPM8) and DPARSF software (http://resting-fmri.sourceforge.net/). The processing steps mainly included: conversion of DICOM data to NIFTI format; removal of the first ten time points from each subjects’ data; slice timing correction; realignment to the middle image; spatial normalization to the Montreal Neurological Institute (MNI) echo-planar imaging template; resampling of each voxel to 3 × 3 × 3 mm3 and spatially smoothed at 4 mm full width at half maximum (FWHM). The smoothed data were temporally bandpass filtered (0.01–0.1 Hz) to reduce the effect of low-frequency drift and high-frequency noise. Finally, the nuisance signals involving six head motion parameters, global mean signal, cerebrospinal fluid signal and white matter signal were regressed out from the filtered data. The resultant residuals were used for further functional connectivity analysis.
Whole-brain FCS analysis
We performed a voxel-wise whole-brain functional connectivity analysis on the preprocessed R-fMRI data. This method has been used in several previous studies (Buckner et al., 2009; Liang et al., 2013). Briefly, for each participant we first computed the Pearson’s correlations between the residual time series of all pairs of brain voxels, resulting in an individual functional connectivity matrix. This analysis was confined within a gray-matter mask (Nvoxels = 40 716) which was generated by multiplying a thresholded prior gray-matter probability mask (a threshold of 0.2) with a prior automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002). Then, for a given voxel, i, we calculated its rFCS using the equation (Buckner et al., 2009; Liang et al., 2013):
| (1) |
where zij was the Fisher’s Z-transformed version of correlation coefficient, rij, between voxel i and voxel j, and r0 was a correlation threshold that was employed to eliminate weak correlations possibly arising from noise (here, r0 = 0.2). Notably, taking account of the shift in the distribution of correlation coefficients introduced by the removal of global signal (mainly the presence of negative correlations) which can make biological interpretation ambiguous (Murphy et al., 2009; Weissenbacher et al., 2009), we restricted our explorations to positive correlations above a threshold of rth = 0.2, as in previous studies (Buckner et al., 2009; Wang et al., 2014). We also evaluated the effects of other correlation thresholds (rth = 0.1 and 0.3) on our main findings. Prior to statistical analysis all resulting individual rFCS maps were spatially smoothed with a 4 mm FWHM of the Gaussian kernel.
Correlation analysis between FCS map and shyness
To determine the relationship between the FCS and individual shyness measurements, a voxel-wise multiple linear regression analysis was conducted as follows:
In this model, the LSAS and CSTAI-T scores were used as covariates to remove effects of related anxiety. Given that the shyness scales were highly correlated with either social anxiety scales or trait anxiety scales (see Results), we also performed the voxel-wise regression analysis without considering the LSAS and CSTAI-T as covariates to avoid removing the overlapping component of anxiety and shyness. The significance of resulting statistical maps for each βi was set at a corrected value of P < 0.05. Correction for multiple comparisons was performed by Monte Carlo simulations (Ledberg et al., 1998) using the AFNI AlphaSim program (http://afni.nih.gov/afni/docpdf/AlphaSim.pdf). A correction significance level of 0.05 was obtained with a combined P < 0.05 and the cluster size > 1809 mm3.
Validation analysis
To further evaluate if our results are influenced by threshold selection and head motion during scan we conducted the following procedures.
Effects of different correlation thresholds
While computing FCS, we utilized a single correlation coefficient threshold (rth = 0.2) to reduce the contributions of spurious correlations possibly arising from signal noise. To determine whether our main results depended on the choices of different correlations thresholds, we recomputed FCS maps using two other different correlation thresholds (i.e. rth = 0.1, 0.3) and performed the statistical analysis.
Effects of head motion
Head motion has been considered as a confounding factor on resting-state functional connectivity in the recent literature (Power et al., 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012). In this study, we therefore performed another statistical analysis with head motion (the root mean squares of both overall head motion displacement and rotation) as an extra covariate in the multiple regression model.
Results
A total of 61 healthy young adults were included in this study. Shyness scores of the entire sample were normally distributed (Shapiro–Wilk normality test, P = 0.205). There were no significant sex differences in age and scale measures. For all subjects, the mean CBSS scores were 37.31 (range = 15–65, s.d. = 2.42), the mean LSAS scores were 40.68 (range = 1–88, s.d. = 22.58) and the CSTAI-T were 40.72 (range = 20–64, s.d. = 10.71). The detailed demographic and behavior characteristics of the participants are provided in Table 1. We found that the shyness scores were significantly correlated with both social anxiety scores (LSAS: r = 0.376, P = 0.003) and trait anxiety scores (CSTAIT-T: r = 0.257, P = 0.046). In the males, shyness scores were not significantly correlated with LSAS (r = 0.340, P = 0.071) and CSTAIT-T (r = 0.119, P = 0.540); in contrast, in the females shyness scores were significantly correlated with LSAS (r = 0.506, P = 0.003) and CSTAIT-T (r = 0.444, P = 0.011). Because a focus of the study was to establish potential sex differences in the impact of shyness on neural networks, and in view of the fact that there was a trend for women to have lower scores than men on the CBSS (P = 0.079), we also carried out an item-based analysis. This revealed a significant difference for the question ‘I am socially somewhat awkward’ with males scoring much higher than females (3.10 vs 2.34, P = 0.009) and for the question ‘I am more shy with members of the opposite sex’ (3.34 vs 2.56, P = 0.037).
Table 1.
Demographic characteristics of participants
| Subjects | Male | Female | Total | Pa | Age or score range |
|---|---|---|---|---|---|
| Gender (M/F) | 29 | 32 | 61 | — | — |
| Age (m ± s.d.) | 22.13 ± 1.90 | 21.68 ± 1.99 | 21.90 ± 1.94 | 0.371 | 19–27 |
| CBSS | 40.24 ± 12.64 | 34.65 ± 11.79 | 37.31 ± 12.42 | 0.079 | 15–65 |
| LSAS | 37.31 ± 18.89 | 43.75 ± 25.38 | 40.68 ± 22.58 | 0.263 | 1–88 |
| CSTAI-T | 40.51 ± 11.73 | 40.90 ± 9.88 | 40.72 ± 10.71 | 0.889 | 20–64 |
CBSS, Cheek and Buss Shyness Scale; LSAS, Liebowitz Social Anxiety Scale; CSTAI-T, the Chinese State-Trait Anxiety Inventory (Trait version).
aAge and the behavioral scores were compared between males and females using two-sample t-tests.
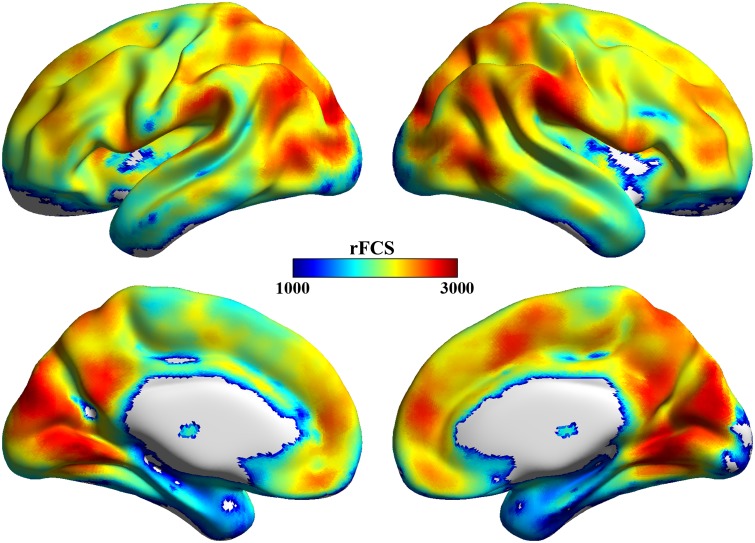
Figure 1 presents the spatial distribution of the average rFCS values across the 61 participants included in this study. Regions with high rFCS were primarily located in the posterior cingulate cortex, anterior cingulate cortex/medial prefrontal cortex, inferior and superior parietal cortex, visual cortex and insula, which was largely consistent with previous R-fMRI studies (Buckner et al., 2009; Zuo et al., 2012; Liang et al., 2013; Wang et al., 2014).
Fig. 1.
Average regional FCS maps. Regions with high FCS were primarily located in the posterior cingulate cortex, anterior cingulated cortex/medial prefrontal cortex, inferior and superior parietal cortex, visual cortex and insula. The maps were obtained by averaging FCS maps across all 61 subjects and were visualized by using in-house BrainNet Viewer software (Xia et al., 2013). The color bar represents rFCS values.
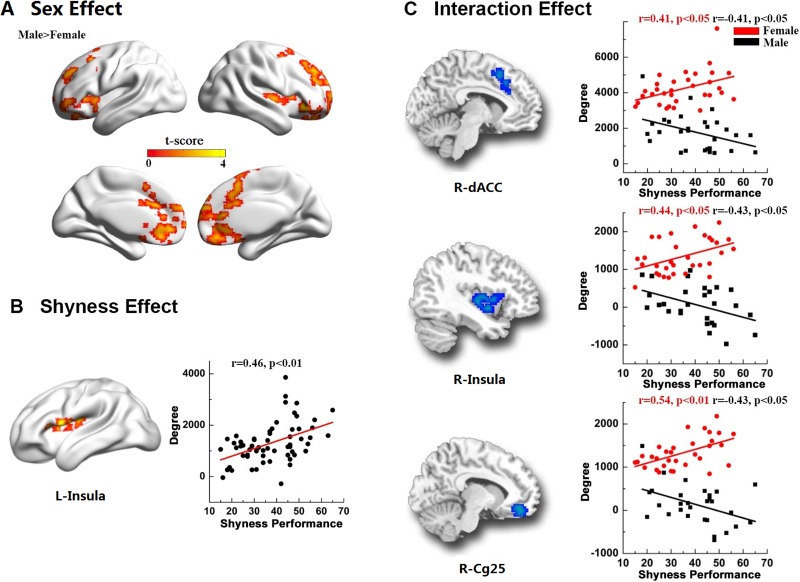
To explore the correlation between rFCS and shyness scores, a multiple regression analysis was performed with age, sex, sex-by-shyness interaction, LSAS and CSTAI-T scores as covariates. Significant sex differences (regression coefficient, β2; male > female) in rFCS were found in many brain regions, including bilateral anterior cingulate cortex/medial frontal cortex, bilateral orbitofrontal gyri, bilateral inferior frontal gyrus and bilateral insula (Figure 2A and Table 2).
Fig. 2.
A voxel-wise rFCS regression analysis on sex, shyness and interaction effects while controlling the LSAS and CSTAI-T as covariates. (A) Significant sex differences were observed in rFCS in the bilateral anterior cingulate cortex/medial prefrontal cortex, bilateral orbitofrontal gyri, bilateral inferior frontal gyri and bilateral insula. The color bar represents t values of group differences in rFCS. (B) The most significant correlation between shyness and rFCS was found in the left insula regardless of gender. (C) Sex-by-shyness interaction was mainly located in the dACC, insula and subgenual cingulate (Cg25). Scatter plots show significant positive correlations in females and negative correlations in males. The maps were visualized by using in-house BrainNet Viewer software (Xia et al., 2013).
Table 2.
Brain regions using a voxel-wise rFCS regression analysis with controlling the LSAS and CSTAI-T scores as covariates
| Correlation | Brain regions | Voxel | Peak coordinates |
Peak intensity | P | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Gender effect | R Anterior Cingulate/Medial frontal gyrus | 550 | 15 | 43 | −21 | 4.32 | <0.001 |
| R Inferior orbital frontal cortex | 152 | 36 | 36 | −3 | 3.29 | <0.001 | |
| L Insula | 171 | −42 | 57 | −3 | 3.13 | <0.005 | |
| R Superior frontal gyrus | 961 | 15 | 54 | 21 | 3.77 | <0.001 | |
| R Insula | 98 | 36 | −9 | 3 | 3.19 | <0.005 | |
| L Superior frontal gyrus | 114 | −27 | 51 | 36 | 3.66 | <0.001 | |
| Shy effect | L Insula | 105 | −45 | −3 | 9 | 4.32 | <0.01 |
| Interaction effect | R Anterior Cingulate | 215 | 3 | 15 | 51 | −3.21 | <0.005 |
| R Insula | 112 | 39 | −3 | −9 | −3.46 | <0.005 | |
| R Medial frontal gyrus(Cg25) | 190 | 15 | 45 | −21 | −3.86 | <0.005 | |
A statistically significant positive association between shyness and rFCS (regression coefficient β3) was found in the left insula (P < 0.01, cluster size = 105 voxels, peak MNI coordinates (x = −45, y = −3, z = 9, t = 4.32; Figure 2B and Table 2). Interestingly, we found a significant shyness-by-sex interaction (regression coefficient β4) in the dorsal anterior cingulate cortex (dACC), the right insula and the right ventral anterior cingulate cortex and ventral medial prefrontal cortex, namely subgenual anterior cingulate (Cg25) or Brodmann area 25 (Figure 2C and Table 2). Notably, these regions showed significant positive correlations in rFCS with shyness scores in females but negative correlations in males.
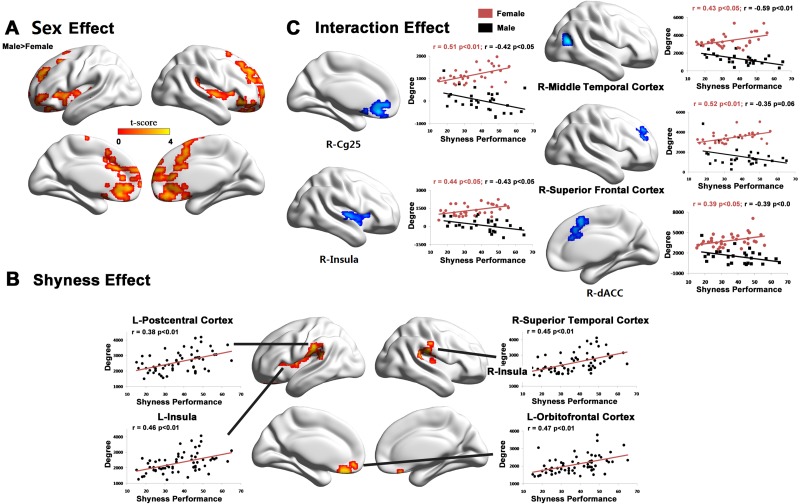
When social and trait anxiety were not included as covariates the same regions were still found (Figure 3 and Table 3). However, several additional regions were also observed to show significant gender (left superior temporal gyrus) and shyness (right superior orbitofrontal cortex, right superior temporal gyrus, left postcentral cortex) effects and a shyness-sex interaction (right middle temporal gyrus and right middle frontal gyrus; Figure 3 and Table 3).
Fig. 3.
A voxel-wise rFCS regression analysis without controlling the LSAS and CSTAI-T as covariates. (A) Significant sex differences were observed in rFCS in the right anterior cingulate cortex/medial frontal cortex, right orbitofrontal gyrus, left superior temporal gyrus and bilateral insula. The color bar represents t values of group differences in rFCS. (B) The most significant correlation between shyness and rFCS was found in the right superior orbital frontal cortex, left insula, right superior temporal gyrus and left postcentral cortex, regardless of sex. (C) Sex-by-shyness interaction was mainly located in the right Cg25, right insula, right middle temporal gyrus, right middle frontal gyrus and right dACC. Scatter plots in part B showed significant correlations between FCS and shyness scores, whereas scatter plots in part C showed significant positive correlations in females and negative correlations in males. The maps were visualized by using in-house BrainNet Viewer software (Xia et al., 2013).
Table 3.
Brain regions using a voxel-wise rFCS regression analysis without controlling the LSAS and CSTAI-T scores as covariates
| Correlation | Brain regions | Voxel | Peak coordinates |
Peak intensity (t value) | P | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Gender effect | R Anterior Cingulate/ Medial frontal gyrus | 611 | 15 | 45 | −21 | 4.28 | <0.001 |
| R Superior frontal gyrus | 1186 | 15 | 54 | 21 | 3.88 | <0.001 | |
| R Inferior orbital frontal cortex | 151 | 42 | 30 | −9 | 3.46 | <0.001 | |
| L Insula | 155 | −33 | 48 | −15 | 3.18 | <0.005 | |
| L Superior temporal gyrus | 79 | −39 | −3 | −12 | 2.95 | <0.005 | |
| R Insula | 161 | 36 | −9 | 3 | 3.26 | <0.001 | |
| Shy effect | R Superior orbital frontal cortex | 113 | −12 | 27 | −21 | 3.59 | <0.001 |
| L Insula | 114 | −42 | 0 | 6 | 3.50 | <0.001 | |
| R Superior temporal gyrus | 133 | 54 | −33 | 18 | 3.39 | <0.001 | |
| L Postcentral cortex | 115 | −54 | −21 | 27 | 2.82 | <0.005 | |
| Interaction effect | R Medial frontal gyrus(Cg25) | 264 | 15 | 45 | −21 | −3.81 | <0.001 |
| R Insula | 157 | 39 | −3 | −9 | −3.54 | <0.001 | |
| R Middle temporal gyrus | 69 | 42 | −66 | 18 | −4.21 | <0.001 | |
| R Middle frontal gyrus | 73 | 21 | 45 | 36 | −3.28 | <0.001 | |
| R Anterior Cingulate (extend to Supplementary motor area) | 229 | 3 | 15 | 51 | −3.17 | <0.005 | |
Finally, our main findings were largely preserved after considering the effects of different correlation thresholds and head motion corrections (data not shown).
Discussion
Using R-fMRI and a data-driven rFCS analysis, we found that shyness is positively associated with the rFCS in the insula regardless of sex. Intriguingly, we demonstrated that the rFCS properties associated with shyness are sex related: positive correlations between rFCS and shyness were observed in the dACC and the insula [two core nodes in the salience network (SN)] as well as in the subgenual cingulate cortex (Cg25) in females, whereas negative correlations were found in males. These results provide the first evidence that intrinsic connectivity networks might reflect the sex-divergent neural architectures that support fundamental aspects of human shyness.
Shyness often emerges as an independent factor in factor analysis and can be treated as a distinct trait (Paulhus and Trapnell, 1998), albeit significantly correlated with both social and trait anxiety. Our results reveal specific intrinsic brain networks associated with shyness, lending further support to the view that shyness should be considered as distinct characteristic despite a degree of behavioral correlation with anxiety measures. However, given the similarity between some of the items in the shyness and social anxiety scales (Yang et al., 2013), we further ran a voxel-wise regression analysis without including social and trait anxiety as covariates to avoid removing the common components in shyness and anxiety traits. Our results confirmed a similar pattern of regions involved as with shyness alone although with the addition of the orbitofrontal cortex, superior temporal gyrus and postcentral cortex. The insula often show highly correlated brain activity with the orbitofrontal cortex, as well as superior temporal gyrus, and together they are purported to show mirroring properties, producing the constellation of anxiety related symptoms that characterize impairments in reciprocal social interactions (Cattaneo and Rizzolatti, 2009). Our results show that an expanded shyness construct involving the anxiety component engages the insula network even more extensively than shyness alone. It provides strong evidence supporting the specificity of intrinsic brain alterations underlying shyness as distinct from social anxiety although also for some overlap between the two traits.
The increased strength of functional connectivity in the insula which was specifically associated with shyness was important for distinguishing individual differences in shyness. Recent studies have suggested that regions with high FCS may play a critical role for the efficient integration of specific different systems via minimizing the number of steps to connect with other regions (Tomasi and Volkow, 2012). As a key component of the ‘limbic integration cortex’, the insula has efferent projections to the amygdala, ACC, lateral orbitofrontal cortex, olfactory cortex and superior temporal sulcus (STS), and receives input from orbito-frontal cortex, olfactory cortex, ACC and STS (Menon and Uddin, 2010). This pattern of extensive interconnectivity exhibited by the insula node allows it to play an important integrative role in monitoring, processing and interpreting external salient stimuli and interoceptive changes (Augustine, 1996; Menon and Uddin, 2010). Shy individuals may experience fluctuations in bodily state (e.g. blush, increased heart rate and heart rate variability) when meeting strangers, and experience negative emotions leading them to engage in self-referential somatic-cognitive interpretations (Critchley et al., 2005). The insula also serves as an integral hub in mediating dynamic interactions between other large-scale networks involved in externally oriented attention, internally oriented or self-related cognition and also motor responses to salient stimuli. In line with this, our previous resting fMRI study found increased functional connectivity between the insula and precentral gyrus and inferior parietal lobule in shyness (Yang et al., 2013), perhaps reflecting enhanced unconscious attention and motor responses such as involuntary mouth or face movements. The core integrative function of the proposed insula node is also supported by anatomical evidence. For example, white matter pathways between insula and the parietal cortex (Uddin and Menon, 2009) and frontal cortex (Wiech et al., 2014) have recently been demonstrated. Thus, our finding of a positive correlation between rFCS in the insular cortex and shyness may provide the physiological substrate that links autonomic arousal and experience and regulation of social emotion and cognition in shyness, and also works as an integral hub for switching to other brain networks to facilitate dedication of further attentional and other resources to processing salient stimuli.
Importantly, our data show that the rFCS patterns related to shyness are dependent on sex in the dACC, and the insula, which are two interconnected hubs in the SN. The SN network plays a crucial role in switching between central-executive and default-mode networks. Critically, both the dACC and the insula contain a unique class of neurons called von Economo neurons (VENs), which have large diameter axons to facilitate rapid transmission of signals from these two nodes signals (Menon and Uddin, 2010). Thus VENs may constitute the neuronal basis for the core function of theses two key nodes in the SN. Within the SN, the dACC is involved in conflict monitoring in the engagement of cognitive control, whereas the insula is involved in generating the signals to trigger hierarchical control (Kerns et al., 2004; Sridharan et al., 2008). Considering the sex difference in these two nodes, the dACC node is more frequently reported in women than in men and this difference is related to withdrawal-related behavior, such as be worrying about potential problems and shyness with strangers (Pujol et al., 2002). For the insula node, a previous functional imaging study demonstrated a female predominant response during negative memory retrieval (Piefke et al., 2005). Thus, these regions with increased FCS value may be the functional hubs in the brain SN where sex differences in processing external salience stimuli occur (Bonnelle et al., 2012). These sex differences could be associated with the maturation of the human brain, because network expansion occurs earlier in females than in males up until late adolescence and can influence neural connectivity and integration by functional specialization and anatomical underpinning of this network (Zielinski et al., 2010).
Another key region related to the sex-by-shyness interaction effect is located in the subgenual cingulate cortex (Cg25). This area shares predominantly ipsilateral anatomical connections with the amygdala, orbitomedial prefrontal cortex, nucleus accumbens and subiculum (Ongur et al., 2003). Possibly abnormal synaptic interactions between Cg25 and these connected areas may contribute to dysfunction in the ventral ‘emotion’ circuit network associated with mood disorders (Drevets et al., 2008). Previous studies have reported increased Cg25 activity in women relative to men in response to pain (Derbyshire et al., 2002) and negative affective pictures (Wrase et al., 2003). Notably, a meta-analysis study has demonstrated Cg25 as one of key regions more commonly activated in women than in men during emotional activation paradigms (Wager et al., 2003). Moreover, Cg25 has been recognized to be involved in modulating anxiety, fear and other negative mood states (Etkin et al., 2011), and is implicated in the pathogenesis of psychiatric disorders (Butler et al., 2005). Indeed, we found previously that unipolar depression patients showed decreased activation in Cg25 during a pretreatment depressed state (Lui et al., 2009; Zhang et al., 2009; Du et al., 2012). Moreover, dysfunction in this target region disappeared after treatment with fluoxetine, deep brain stimulation and cognitive behavior therapy (Mayberg et al., 2000, 2005; Siegle et al., 2006). In relation to the emotional hyper-reactivity theory of shyness (Yang et al., 2013), the observation that increased FCS of this area is associated with the degree of shyness may reflect an enhanced sensitivity and response bias to negative social stimuli similar to the inability to suppress negative affect seen in mood disorders. Thus, the increased FCS of the Cg25 node maybe the basis for pathologically enhanced salience information processing. These previous observations, together with the current results, suggest that the Cg25 acts as a sex related core network node involved in shyness.
Although we showed sex differences in functional connectivity associated with overall shyness scores, there were no overall significant behavioral differences between males and females. Nonetheless, there were some indications of sex differences in specific CBSS items: males being significantly more socially awkward and also more shy when meeting members of the opposite sex. Thus shy males may be more strongly characterized by feelings of awkwardness in social situations, particularly involving members of the opposite sex, whereas perhaps for females feelings of anxiety rather than awkwardness may predominate. A sex-specific difference in shy-bold animal personality has been demonstrated in birds (Dall, 2004), suggesting that a sex difference in this trait may have ecological and evolutionary significance. In humans, earlier behavior studies suggested that shyness in girls was associated with positive outcome at both home and school whereas the opposite was true for boys (Chen et al., 1998, 2011). This difference may reflect cultural expectations and socialization patterns according to sex, such that some degree of inhibition/shyness and increased responsivity may be considered ‘gender appropriate’ in girls, but not encouraged in boys. In line with this, we did find that shyness was only positively coupled with brain connectivity in females. Our findings are also in accordance with a previous report of increased Electroencephalography (EEG) activation in shy females when processing emotional stimulus (Theall-Honey and Schmidt, 2006). However, behavioral studies on sex-specific shyness effects have produced inconsistent results. Although both sexes share a similar symptomatology, the cues evoking the various symptoms of shyness may be processed differently in terms of salience and conflict resolution outcomes. The subtle behavioral differences observed here, together with inconsistent findings across other studies, suggest the need for caution in interpreting the potential functions of sex differences and indicate the need for further task-dependent fMRI studies to help resolve this question.
Our current study suggested that that the rFCS in the whole-brain functional networks is related to the degree of shyness; however, the underlying structural basis of shyness remains unclear. In future studies it will be important to combine R-fMRI with other modalities (e.g. diffusion tensor imaging and structural MRI) to clarify structure–function relationships in the brain associated with shyness. Several recent studies have demonstrated that brain regions with higher rFCS (i.e. brain hubs) show higher rates of cerebral blood flow and cerebral glucose metabolism (Liang et al., 2013, Tomasi et al., 2013). It might therefore be important to explore the metabolic basis underlying rFCS in shyness. Finally, further research comparing brain connectivity patterns in healthy shy subjects with those of individuals with psychiatric disorders such as social anxiety disorder and depression, could be important to clarify the boundary between them and trait shyness.
Acknowledgements
Drs. Yong He and Qiyong Gong contributed equally to playing the role of corresponding author, and in particular, Dr. Qiyong Gong is the Changjiang Professor of Education Ministry of China, and also acknowledges the support from his CMB Distinguished Professorship Award (Award No. F510000/ G16916411) administered by the Institute of International Education, USA, and he is an appointed visiting professor at the Department of Psychiatry, Yale School of Medicine, Yale University, USA.
Funding
This study was supported by the National Natural Science Foundation (Grant 81030027, 81030028, 81225012, 81227002, 81220108013 and 91132720), National Key Technologies R&D Program (Program No. 2012BAI01B03) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT1272) of China
Conflict of interest. None declared.
References
- Amico K.R., Bruch M.A., Haase R.F., Sturmer P.J. (2004). Trait shyness, actual-ought self-discrepancy and discomfort in social interaction. Personality and Individual Differences, 36, 1597–610. [Google Scholar]
- Augustine J.R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Brain Research Reviews, 22, 229–44. [DOI] [PubMed] [Google Scholar]
- Barnes L.L.B., Harp D., Jung W.S. (2002). Reliability generalization of scores on the Spielberger state-trait anxiety inventory. Educational and Psychological Measurement, 62, 603–18. [Google Scholar]
- Bassett D.S., Bullmore E.T. (2009). Human brain networks in health and disease. Current Opinion in Neurology, 22, 340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M., Ogliari A., Zanoni A., et al. (2005). Influence of the serotonin transporter promoter gene and shyness on children's cerebral responses to facial expressions. Archives of General Psychiatry, 62, 85–94. [DOI] [PubMed] [Google Scholar]
- Beaton E.A., Schmidt L.A., Schulkin J., et al. (2008). Different neural responses to stranger and personally familiar faces in shy and bold adults. Behavioral Neuroscience, 122, 704–9. [DOI] [PubMed] [Google Scholar]
- Beaton E.A., Schmidt L.A., Schulkin J., Hall G.B. (2010). Neural correlates of implicit processing of facial emotions in shy adults. Personality and Individual Differences, 49, 755–61. [Google Scholar]
- Bonnelle V., Ham T.E., Leech R., et al. (2012). Salience network integrity predicts default mode network function after traumatic brain injury. Proceedings of the National Academy of Sciences of the United States of America, 109, 4690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience, 29, 1860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T., Pan H., Epstein J., et al. (2005). Fear-related activity in subgenual anterior cingulate differs between men and women. Neuroreport, 16, 1233–6. [DOI] [PubMed] [Google Scholar]
- Cattaneo L., Rizzolatti G. (2009). The mirror neuron system. Arch Neurol, 66(5), 557–60. [DOI] [PubMed] [Google Scholar]
- Cheek J.M., Buss A.H. (1981). Shyness and sociability. Journal of Personality and Social Psychology, 41, 330. [Google Scholar]
- Cheek J.M., Melchior L.A. (1990). Shyness, self-esteem, and self-consciousness. In: Leitenberg H., editor. Handbook of Social and Evaluation Anxiety. New York: Plenum, 47–82. [Google Scholar]
- Chen X., Hastings P.D., Rubin K.H., Chen H., Cen G., Stewart S.L. (1998). Child-rearing attitudes and behavioral inhibition in Chinese and Canadian toddlers: a cross-cultural study. Developmental Psychology, 34(4), 677. [DOI] [PubMed] [Google Scholar]
- Chen X., Wang L., Cao R. (2011). Shyness-sensitivity and unsociability in rural Chinese children: relations with social, school, and psychological adjustment. Child Development, 82(5), 1531–43. [DOI] [PubMed] [Google Scholar]
- Chou K.L. (2005). Assessing shyness in Chinese older adults. Aging and Mental Health, 9, 456–60. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Rotshtein P., Nagai Y., et al. (2005). Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage, 24, 751–62. [DOI] [PubMed] [Google Scholar]
- Dall S.R. (2004). Behavioural biology: fortune favours bold and shy personalities. Current Biology, 14, R470–2. [DOI] [PubMed] [Google Scholar]
- Derbyshire S.W., Nichols T.E., Firestone L., et al. (2002). Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. The Journal of Pain, 3, 401–11. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Savitz J., Trimble M. (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums, 13(8), 663–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M.Y., Wu Q.Z, Yue Q., et al. (2012). Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Progress in Neuro-psychopharmacology and Biological Psychiatry, 36, 11–6. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco D.M., Coles M.E., Heimberg R.G., et al. (2001). The Liebowitz social anxiety scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine, 31, 1025–35. [DOI] [PubMed] [Google Scholar]
- Henderson H.A. (2010). Electrophysiological correlates of cognitive control and the regulation of shyness in children. Developmental Neuropsychology, 35, 177–93. [DOI] [PubMed] [Google Scholar]
- Iancu I., Sarel A., Avital A., et al. (2011). Shyness and social phobia in Israeli Jewish vs Arab students. Comprehensive Psychiatry, 52, 708–14. [DOI] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., III, et al. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303, 1023–6. [DOI] [PubMed] [Google Scholar]
- Kerr M., Lambert W.W., Bem D.J. (1996). Life course sequelae of childhood shyness in Sweden: comparison with the United States. Developmental Neuropsychology, 32, 1100. [Google Scholar]
- Ledberg A., Åkerman S., Roland P.E. (1998). Estimating the significance of 3D clusters in functional brain images. NeuroImage, 8, 113–28. [DOI] [PubMed] [Google Scholar]
- Liang X., Zou Q., He Y., Yang Y. (2013). Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceedings of the National Academy of Sciences of the United States of America, 110(5), 1929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S., Parkes L.M., Huang X., et al. (2009). Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology, 251, 476–84. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Brannan S.K., Tekell J.L., et al. (2000). Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry, 48, 830–43. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Lozano A.M., Voon V., et al. (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45, 651–60. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214, 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., et al. (2009). The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage, 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D., Ferry A.T., Price J.L. (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology, 460(3), 425–49. [DOI] [PubMed] [Google Scholar]
- Pang J., Zhang J., Ma P., et al. (2006). The utility of Liebowitz social anxiety scale in the patients with social anxiety disorder in Chinese. Chinese Journal Nervous and Mental Disease, 32, 206–10. [Google Scholar]
- Paulhus D.L., Trapnell P.D. (1998). Typological measures of shyness: additive, interactive, and categorical. Journal of Research in Personality, 32(2), 183–201. [Google Scholar]
- Piefke M., Weiss P.H., Markowitsch H.J., Fink G.R. (2005). Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Human Brain Mapping, 24, 313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., et al. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59, 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Lopez A., Deus J., et al. (2002). Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage, 15, 847–55. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., et al. (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage, 60, 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C.E., Wright C.I., Shin L.M., et al. , (2003). Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science, 300, 1952–3. [DOI] [PubMed] [Google Scholar]
- Shek D.T.L. (1988). Reliability and factorial structure of the Chinese version of the State-Trait Anxiety Inventory. Journal of Psychopathology and Behavioral Assessment, 10, 303–17. [Google Scholar]
- Shek D.T.L. (1993). The Chinese version of the State-Trait Anxiety Inventory: its relationship to different measures of psychological well-being. Journal of Clinical Psychology, 49, 349–58. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Carter C.S., Thase M.E. (2006). Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. The American Journal of Psychiatry, 163, 735–8. [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105, 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theall-Honey L.A., Schmidt L.A. (2006). Do temperamentally shy children process emotion differently than nonshy children? Behavioral, psychophysiological, and gender differences in reticent preschoolers. Developmental Psychobiology, 48, 187–96. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Wang G.J., Volkow N.D. (2013). Energetic cost of brain functional connectivity. Proceedings of the National Academy of Sciences of the United States of America, 110, 13642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. (2012). Laterality patterns of brain functional connectivity: gender effects. Cerebral Cortex, 22, 1455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Menon V. (2009). The anterior insula in autism: under-connected and under-examined. Neuroscience and Biobehavioral Reviews, 33(8), 1198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 59, 431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Phan K.L., Liberzon I., Taylor S.F. (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage, 19, 513–31. [DOI] [PubMed] [Google Scholar]
- Wang L., Dai Z., Peng H., et al. (2014). Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Human Brain Mapping, 35, 1154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., et al. (2009). Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage, 47, 1408–16. [DOI] [PubMed] [Google Scholar]
- Wiech K., Jbabdi S., Lin C.S., Andersson J., Tracey I. (2014). Differential structural and resting state connectivity between insular subdivisions and other pain-related brain regions. Pain, 155(10), 2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J., Klein S., Gruesser S.M., et al. (2003). Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neuroscience Letters, 348, 41–5. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. (2013). BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One, 8(7), e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Kendrick K.M., Wu Q., et al. (2013). Structural and functional connectivity changes in the brain associated with shyness but not with social anxiety. PLoS One, 8, e63151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.J., Wu Q.Z., Huang X.Q., et al. (2009). Magnetization transfer imaging reveals the brain deficit in patients with treatment-refractory depression. Journal of Affective Disorders, 117, 157–61. [DOI] [PubMed] [Google Scholar]
- Zielinski B.A., Gennatas E.D., Zhou J., Seeley W.W. (2010). Network-level structural covariance in the developing brain. Proceedings of the National Academy of Sciences of the United States of America, 107, 18191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimbardo P.G. (1977). Shyness: What Is it and What To Do About It. New York: Symphony. [Google Scholar]
- Zuo X.N., Ehmke R., Mennes M., et al. (2012). Network centrality in the human functional connectome. Cerebral Cortex, 22(8), 1862–75. [DOI] [PubMed] [Google Scholar]