Abstract
Theoretically, normal developmental variation in amygdala volumes may be altered under conditions of severe stress. The purpose of this article was to examine whether posttraumatic stress moderates the association between age and amygdala volumes in youth exposed to traumatic events who are experiencing symptoms of post-traumatic stress disorder (PTSD). Volumetric imaging was conducted on two groups of youth aged 9–17 years: 28 with exposure to trauma and PTSD symptoms (boys = 15, girls = 13) and 26 matched (age, IQ) comparison youth (Controls; boys = 12, girls = 14). There was a significant group by age interaction in predicting right amygdala volumes. A positive association between age and right amygdala volumes was observed, but only in PTSD youth. These associations with age remained when controlling for IQ, total brain volumes and sex. Moreover, older youth with PTSD symptoms had relatively larger right amygdala volumes than controls. Findings provide evidence that severe stress may influence age-related variation in amygdala volumes. Results further highlight the importance of utilizing age as an interactive variable in pediatric neuroimaging research, in so far as age may act as an important moderator of group differences.
Keywords: sMRI, traumatic, severe stress, development, amygdala, FreeSurfer
Introduction
The amygdala is a subcortical brain region that is involved in the evaluation of the emotional significance of incoming stimuli (Tottenham and Sheridanm, 2009; Tottenham et al., 2010). The amygdala projects to several brain structures in the frontal cortex, hippocampus, striatum, hypothalamus and brain stem (LeDoux, 2000; Gordon and Hen, 2004; Tottenham and Sheridanm, 2009; Tottenham, 2012) and is considered to have critical involvement in the behavioral activation and inhibition system that characterizes emotional responses such as fear and anxiety (Gray, 1994; Gray and McNaughton, 2000). Volumetric imaging work in pediatric patient populations, such as youth with autism, bipolar disorder and post-traumatic stress disorder (PTSD) symptoms has tended to focus on amygdala volume comparisons with age and gender-matched comparison youth (De Bellis et al., 1999; Carrión et al., 2001, 2010; Chen et al., 2004; Groen et al., 2010). However, studies examining volumetric differences of the amygdala have often failed to show significant differences (Weems et al., 2013). For example, Morey et al. (2012) reviewed 12 studies on amygdala volumes among individuals (nine adult samples and three youth samples) with post-traumatic stress and examination of effect sizes revealed four studies indicating relatively smaller volumes (in both sides); one relatively smaller in the left, but larger in the right; one study relatively smaller in the right, larger in left; and six relatively larger in both sides (but only one of the 12 reported P-values reaching the typical 0.05 alpha).
Among five pediatric imaging studies reporting amygdala volumes, only Mehta et al. (2009) reported significantly increased right amygdala volumes (but not left amygdala volumes) among severely deprived Romanian adoptees (N = 14, mean age 16.2 years) compared with controls. De Bellis et al. (1999) reported no significant difference in amygdala volumes between maltreated children and adolescents with PTSD (N = 44 aged 6–17 years) and controls. De Bellis et al. (2001) (N = 9, aged 10–13 years) reported no significant difference in amygdala volume between children with maltreatment-related PTSD and healthy controls at baseline, after 2 years’ follow-up, and across time. De Bellis et al. (2002), in a sample including both children and adolescents with maltreatment-related PTSD (N = 28, aged 4.9–16.5 years), reported no significant differences in amygdala volume. Similarly, Carrión et al. (2001) reported that amygdala volume in PTSD youth (N = 24, aged 7–14 years) was 5.1% smaller than that of control subjects, but there were not significant differences after controlling for total brain volume, total gray matter or temporal lobe volume. While these studies matched or otherwise controlled for age, none of these studies tested age as a moderator of the effect of traumatic stress on amygdala volumes. An important possible reason for the failure to show statistically significant effects, especially among youth samples, may be developmental (age-related) variation in amygdala volume differences (Weems et al., 2013).
Structurally, there is evidence to suggest that the amygdala may continue to grow and develop until late childhood (Weems et al., 2013). Most recently, Uematsu et al. (2012) examined the association of age with amygdala volumes among 109 healthy participants aged from infancy to 25 years and found that a curvilinear association best defined the relationship. The nature of the association in Uematsu et al. (2012) has critical developmental implications for research with youth samples. The authors report a strong linear association from infancy to around age 11 years that leveled off or declined at that point (implying no correlation or a weak negative correlation between age and amygdala volumes from around 10 or 11 years and beyond); this association was found for both the right and left amygdala. The peak growth (end of a linear association in the Uematsu et al. scatterplots) was earlier in the left side, generally, and earlier in females (years 9 left, 11 right) than in males (years 11 left, 12 right).
Theoretically, maturational variation in the amygdala may be altered by exposure to early life stress (Tottenham and Sheridan, 2009; Tottenham et al., 2010, Tottenham, 2012). The work of Tottenham and colleagues (2009, 2010) showing an effect of early life stress on amygdala volumes, coupled with the idea of strong positive associations between age and amygdala volumes in school-aged youth (Giedd et al., 1996; Uematsu et al., 2012), implies that testing the role of severe stress as a moderator of developmental variation in amygdala volumes is an important approach to understanding group differences in the amygdala. Specifically, the potential for maturational differences in amygdala volumes(Tottenham and Sheridan, 2009; Tottenham, 2012) among different patient populations with high or traumatic stress exposure (Chen et al., 2004) suggests that exposure to traumatic stress may moderate the association between age and amygdala volumes. In this view, age is a main driver of variation in amygdala volumes and stress may alter normal developmental trends. Such a perspective follows from an evolutionary account wherein severe stress serves to trigger an adaptive delay in the completion of developmental changes (Del Giudice et al., 2011).
Recently, Weems et al. (2013) tested this theory in a sample of 24 youth (aged 7–14) with PTSD1 symptoms and a matched control group. Using a 1.5-T scanner and manual delineation of volumes, results indicated a positive association between age and right amygdala volumes among the trauma exposed sample, but a non-significant negative correlation within a sample of controls. This association in the PTSD group was observed for Tanner Stage, as well as after controlling for total cerebral volumes and IQ. While the Weems et al. study is encouraging in that it shows the potential importance of age in understanding the effects of traumatic stress on the amygdala, it is critical to replicate these findings in independent samples of youth exposed to trauma to ensure sampling did not bias initial results and using higher field magnets with improved image resolution (i.e. 3T scans). The purpose of this article was thus to provide a test of the theory that stress is associated with atypical developmental variation of the amygdala and provide a replication test of Weems et al. (2013) by using higher-resolution images within a new participant sample. We also sought to move the age range to an older period where data from Uematsu et al. (2012) would more clearly predict that age should no longer be associated with amygdala volumes (i.e. findings from Weems et al. may have been a result of the age range of the sample; moreover, the comparison sample was not matched on IQ scores). We predicted that traumatic stress exposure would impact developmental variation in amygdala volumes such that it would moderate the association between age and amygdala volumes.
Methods and materials
Participants and assessments
Imaging data from 59 youth (30 PTSD and 29 controls) were first visually inspected. Five participants had amygdala structures (two PTSD and three controls) that were excluded because of movement artifact, hyper-intensities within the region or poor quality. The final sample for analysis was 54 participants—ages 9–17. None of these participants were included in Weems et al. Twenty-eight participants (PTSD group; 13 female participants and 15 male participants) had experienced interpersonal violence and exhibited PTSD symptoms (described below) and 26 were typically developing control participants (Control group; 14 female participants and 12 male participants); the two groups were matched on age, IQ and sex. The majority (62%) of the PTSD group experienced multiple traumatic events including physical abuse (50%), witnessing interpersonal violence (58%) and sexual abuse (27%). Differences between the PTSD and control groups on measures of IQ, age and sex were tested. Participants with PTSD had a mean age of 13.8 years and control participants had a mean age of 14.0 years (t(52) = 0.34, P = 0.74). PTSD participants had a mean IQ of 108 and controls had a mean IQ of 112 (t(52) = 1.24, P = 0.22) and the distribution of sex was not significantly different (χ2 (1) = 0.30, P = 0.59). Depression (major depressive disorder, depressive disorder NOS or depressive episode) was the only significant comorbid disorder and was present in 36% of the PTSD group.
All participants filled out demographic questionnaires and were examined by a board certified psychiatrist prior to study entrance. The PTSD group was recruited from community agencies and mental health clinics. Control participants were recruited through advertisement. Informed consent and assent, as approved by Stanford University’s Human Subjects Institutional Review Board (IRB), were obtained from all participants and their legal guardians. In cases where children were wards of the court, consent was also obtained from the counties’ superior courts. Participants with a history of interpersonal trauma completed the Kiddie Schedule for Affective Disorders (KSADS) and the Clinician-Administered PTSD Scale for Children and Adolescents (CAPS-CA). The CAPS-CA interview assesses the 17 symptoms for PTSD outlined in DSM-IV and was designed to be a developmentally adjusted counterpart to the CAPS for adults (Blake et al., 1990, 1995; Nader et al., 1996). The inclusion criteria for children with a history of interpersonal trauma were the following: (1) at least one exposure to an interpersonal traumatic event that occurred at least 6 months prior to participation in the study (traumatic events included sexual abuse, physical abuse and/or witnessing violence); (2) meeting threshold or sub-threshold criteria for PTSD as determined by the CAPS-CA; (3) availability of a non-abusing caretaker who was willing to participate with the study protocol; (4) a completed child protective services report and an evaluation substantiating the report; (5) a stable home environment with no exposure to perpetrators during the screening phase of the study.
Control participants were screened with in person interviews that included either the KSADS (Kaufman et al., 1997) or the Child Behavior Check List (CBCL) (Achenbach, 1991) to assess psychopathology and the KSADS or the PTSD Reaction Index for Children (PTSD-RI) (Steinberg et al., 2004) trauma exposure items assess trauma exposure. Exclusion criteria for both control and participants with a history of interpersonal trauma included: (1) usage of medications with central nervous system activation, autonomic nervous system activation and hypothalamic–pituitary–adrenal axis effects for 2 weeks prior to the evaluations; (2) clinically significant medical illness; (3) gross obesity (weight > 150% of ideal body weight) or growth failure (height under 3rd percentile); (4) diagnosis of autism or schizophrenia according to DSM-IV criteria; (5) IQ <80; (6) metal or electrical conductive implants or foreign bodies (in addition to other MRI contraindications); (7) history of alcohol or drug dependence or abuse; (8) history of neurological disorder, such as a seizure disorder; (9) history of drug or alcohol exposure in utero; (10) left-handedness.
Procedures
Prior to being scanned at the Lucas Center for Magnetic Resonance Spectroscopy and Imaging (Stanford University, Palo Alto, CA, USA), participants entered a mock scanner in order to help familiarize them to the scanner environment, and completed a protocol that trained participants to minimize movement. Participants were scanned with a 3.0-T General Electric Signa whole body MR system (GE Healthcare Systems, Milwaukee, WI, USA) using a standardized head coil. We acquired 124 sagittal high-resolution anatomical brain slices (1.5-mm-thick) using a spoiled gradient echo pulse sequence for each subject (TR = 35 ms, TE = 6 ms, flip angle = 45 degrees, matrix = 256 × 256, FOV = 240 × 240 mm). A custom-built head stabilizer was used to help reduce head movement during the scan. Participants appeared to tolerate the scan well and were able to successfully complete the imaging procedure.
MRI data were visually inspected to eliminate scans with significant head movement and flow artifact. FreeSurfer processing is described in detail within a series of publications. In brief, processing is performed on weighted images, beginning with the removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Ségonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (in this study, the amygdala) (Fischl et al., 2002, 2004); intensity normalization (Sled et al., 1998), tessellation of the gray matter–white matter boundary, automated topology correction (Fischl et al., 2001; Ségonne et al., 2007), and surface deformation (Dale and Sereno, 1993; Dale et al., 1999; Fischl and Dale, 2000). Once the cortical models were complete, a number of deformable procedures were performed which included surface inflation (Fischl et al., 1999), registration to a spherical atlas (which utilized individual cortical folding patterns to match cortical geometry across subjects) (Fischl et al., 1999), parcellation of the cerebral cortex into units based on gyral and sulcal structure (Fischl et al., 2004; Desikan et al., 2006), and creation of a variety of surface based data including maps of curvature and sulcal depth. Gray–white and pial surfaces were visually inspected and, where needed, were manually corrected by someone trained in examining and editing cortical surfaces. The rater was trained to achieve inter-rater reliability of v ≥0.95 (intraclass correlation coefficient) with gold-standard datasets developed in our laboratory for volumetric regions of interest. As noted all amygdala images were visually inspected and amygdala volumes were also checked for outliers after generating and visually checking outlier and residual plots. No additional amygdala editing was indicated from visual inspection or these checks after initial FreeSurfer parcellations.
FreeSurfer image analysis suite version 5.0 was used to process the data and perform cortical reconstruction and volumetric segmentation. FreeSurfer image analysis suite is a software package frequently used in neuroimaging publications that analyze cortical and subcortical measurement; it is freely available for download online (http://surfer.nmr.mgh.harvard.edu/). FreeSurfer processing was performed on T1 weighted images (Dale and Sereno, 1993; Sled et al., 1998; Dale et al., 1999; Fischl et al., 1999a, b, 2001, 2002, 2004a, b; Fischl and Dale, 2000; Ségonne et al., 2004, 2007; Desikan et al., 2006).
Statistical analysis
Formal tests of the interaction hypothesis were conducted with hierarchical linear models (Bryk et al., 2013). Specifically, the analyses nested amygdala volumes as a function of age (on level 1) and diagnostic group (PTSD vs Control) and sex (on level 2) controlling for total brain volume (on level 1). These analyses allow flexibility in testing the theorized interactive roles of age and traumatic stress exposure on amygdala volumes, while testing other variables and covariates as well (e.g. sex, total volumes). Given significant interactions, we decomposed the interactions by group status (PTSD vs Control), and at upper and lower quartiles of age (Holmbeck, 2002) using PROCESS (Hayes, 2014). Follow-up analyses also used Pearson’s r correlations to test differential correlations between age and amygdala volumes by group. We used an alpha level of 0.05. Given we have one main analysis to test the hypothesis of an age by group interaction, no corrections were necessary.
Results
No significant main effect differences for controls vs PTSD youth were found on left or right amygdala volumes. We also correlated level of PTSD symptoms with left and right amygdala volumes and found no significant association [right r (52) = 0.02, P = 0.91; left r (52) = 0.10, P = 0.46] there was also not a significant association between age and PTSD symptoms [r (52) = 0.11, P = 0.45].
HLM analyses tested amygdala volumes as a function of age (level 1) diagnostic group (PTSD vs Control) and sex (level 2) controlling for total brain volume (level 1). The results of the HLM analysis on right amygdala volumes indicated that there was a significant effect of group as well as the predicted age by group interaction. Total volumes were a significant covariate. Results are summarized in Table 1. IQ was also tested as a covariate, but was non-significant and its inclusion did not affect results. This same analysis on left amygdala volumes did not produce a significant age by group interaction (P > 0.2) controlling for total brain volumes.2 Similar analyses using the PTSD symptoms as continuous variable did not produce a significant age by PTSD symptom interaction (P > 0.2).3
Table 1.
Summary of hierarchical linear model predicting right amygdala volumes
| Fixed effect | Coefficient | Standard error | t-ratio | d.f. | P-value |
|---|---|---|---|---|---|
| Intercept, β0 | |||||
| Intercept, γ00 | 1939.54 | 207.66 | 9.34 | 51 | <0.001 |
| PTSD vs control, γ01 | −545.55 | 208.83 | −2.61 | 51 | 0.012 |
| Sex, γ02 | −361.28 | 242.55 | −1.49 | 51 | 0.143 |
| For age slope, β1 | |||||
| Intercept, γ10 | −20.11 | 14.02 | −1.43 | 48 | 0.158 |
| PTSD vs control, γ11 | 40.88 | 14.91 | 2.74 | 48 | 0.009 |
| Sex, γ12 | 29.83 | 17.03 | 1.75 | 48 | 0.086 |
| For total brain volumes slope, β2 | |||||
| Intercept, γ20 | 0.00098 | 0.00035 | 2.84 | 48 | 0.007 |
| PTSD vs control, γ21 | −0.00009 | 0.00040 | −0.23 | 48 | 0.818 |
| Sex, γ22 | 0.00067 | 0.00039 | 1.70 | 48 | 0.096 |
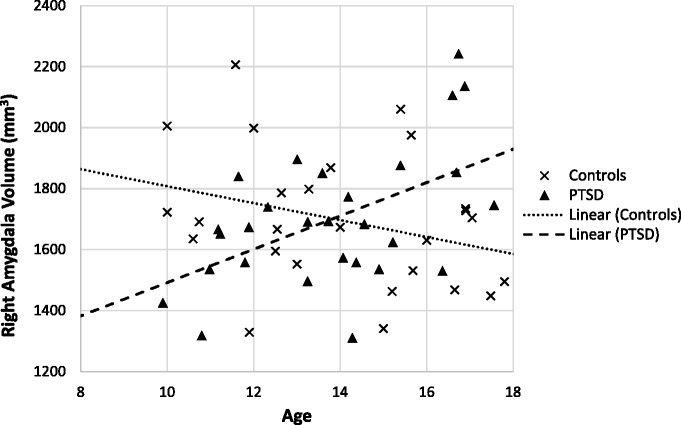
Two sets of follow-up analyses were conducted to explore the nature of the observed interaction in more detail. In the first, left and right amygdala volumes were correlated with age separately for the control and PTSD youth. In the second, the effect of PTSD group at older and younger ages was the focus. First, correlation analyses by group indicated a significant positive correlation between age and right amygdala volumes in the PTSD youth (r = 0.52, P < 0.01) beyond the 0.05 level, but not in the control group where correlations were negative (r = −0.30), but did not reach statistical significance. A scatterplot of the correlation between age and right amygdala volumes by group is depicted in Figure 1. Fisher’s R-toZ tests indicated that the differences in the correlation between age and right amygdala volumes were statistically significant (z for r = 3.09, P < 0.01) between the two groups. The pattern of association with age observed in the right amygdala was similar in left amygdala volumes [left amygdala correlation with age in the PTSD youth (r = 0.36, ns) and control group (r = − 0.15, ns); the group difference in these correlations were z for r = 1.82, P = 0.069].
Fig. 1.
Scatterplot of age and right amygdala volumes showing regression lines by group status.
To examine if findings were influenced by IQ or total brain volumes, separate partial correlations between age and right amygdala volumes were calculated for the PTSD group controlling for total brain volumes (PTSD partial r = 0.50, P < 0.01; Control partial r = −0.15, ns). The partial correlation between age and right amygdala volumes controlling for IQ was also significant (PTSD partial r = 0.53, P < 0.01; Control partial r = −0.32, ns). Separate partial correlations between age and left amygdala volumes were calculated for the PTSD group controlling for total brain volumes and unlike the findings on the right amygdala, and were not significant (PTSD partial r = 0.28, ns; Control partial r = 0.10, ns). The partial correlation between age and left amygdala volumes controlling for IQ was also not significant (partial r = 0.37, P = 0.057; Control partial r = −0.13, ns). Amygdala volumes (both sides) were not significantly correlated with IQ, with values in the 0.2 range or less.
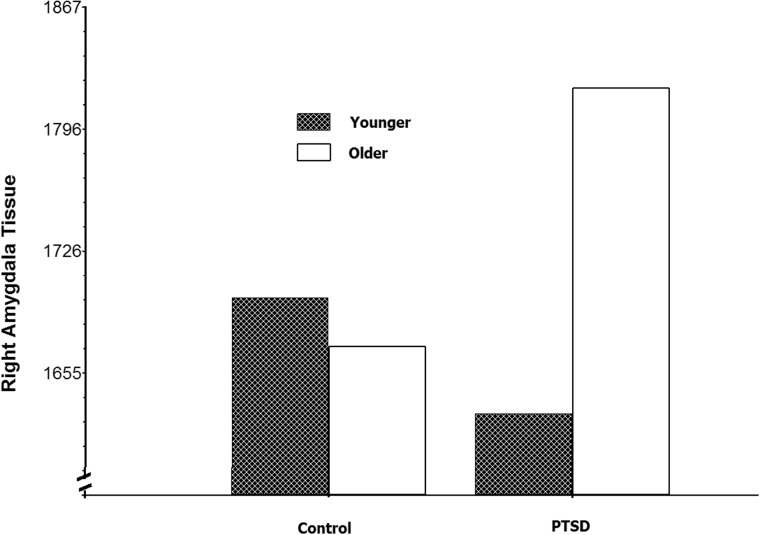
In this second set of follow-up analyses, the effect of PTSD group at older and younger ages was the focus. Holmbeck (2002) and others have suggested decomposing significant interactions using the full sample degrees of freedom and plotting the effect at high (e.g. upper quartile) and low levels (e.g. lower quartile) as opposed to dichotomizing a continuous variable such as age. Figure 2 shows the effect of PTSD group at the upper and lower quartiles of age. Follow-up analyses (Holmbeck, 2002) on results depicted in Figure 2 showed that older youth (upper quartile of age) with PTSD have significantly larger right amygdala volumes (t(52) = 2.09, P < 0.05 controlling for total brain volumes and gender), whereas, younger youth [lower quartile) with PTSD showed smaller volumes than controls (this was not significant controlling for total brain volumes t(52) = − 1.50, P = 0.14]. The Johnson–Neyman technique (Johnson and Fay, 1950) was used in PROCESS (Hayes, 2014) to determine the age at which the PTSD group had significantly larger volumes (P < 0.05) and this difference began at age 15.5 years.
Fig. 2.
Older youth (upper quartile) with PTSD have significantly larger right amygdala volumes than controls, while younger youth (lower quartile) with PTSD have relatively smaller volumes (but the difference was not significant for younger youth).
Discussion
Results supported the hypotheses and provided a replication and extension of Weems et al. (2013) in an independent sample of older youth using higher-resolution scans and advanced segmentation techniques. The non-significant correlation in our control sample might represent the tail end of the curvilinear trend observed in Uematsu et al. (2012) with the positive correlation in the PTSD group representing a delay in pruning, atypical or prolonged growth. The findings help contextualize research on youth with a history of traumatic stress and offer suggestions for the use of age in comparing other patient populations. In particular, the findings augment the work of Tottenham and colleagues (Tottenham and Sheridan, 2009; Tottenham, 2012) linking exposure to stress with atypical developmental variation in amygdala volumes. One implication of our findings is the need to not simply match patient populations on age (or co-vary age) when examining amygdala differences, but to also examine age by stress interactions. Specifically, findings indicated that, depending on age, youth with exposure to stress could have relatively smaller (younger) and relatively larger (older) amygdala volumes than controls, thought the difference in younger youth was not significant controlling for total brain volumes. This finding of relative increased volumes in the right amygdala is consistent with Mehta et al. (2009) who reported significantly increased right amygdala volumes (but not left amygdala volumes) in older youth (mean age 16 years, standard deviation 0.72 with range not reported). The finding is also consistent with the relatively smaller but not significant finding in Carrión et al. (2001) among a younger sample (age range 7–14).
Our follow-up analyses treated both PTSD group and age as the potential moderator variable (i.e. we show that age is differentially associated with right amygdala volumes depending on PTSD vs control group and also a differential effect of PTSD group on volumes depending on age). Our interpretation is that these findings are, at minimum, suggestive of the need to consider age as a potential moderator of group differences. Future studies testing maturation as a moderator of the main effects (or lack of main effects) of clinical groupings may help address some of the inconsistencies in the literature noted by previous researchers (Morey et al., 2012). In addition, pediatric studies attempting to understand amygdala development might also benefit from testing stress as a moderator of developmental (age) trends in amygdala volumes. Indeed, given the finding of an age by group interaction as reported here the use of age as a covariate in an ANCOVA design would be a violation of the homogeneity of regression assumption.
The data are consistent with the theory that age is a critical driver of variation in amygdala volumes, but that stress may alter the normal developmental trends. While this study cannot disentangle which variable is the moderator and which is the main driver of amygdala volumes, findings can be seen as consistent with an evolutionary account wherein severe stress may serve to prolong the process of age-related amygdala growth (Del Giudice et al., 2011). It is important to recognize an alternative that pre-disposition in amygdala size and development may be the reason for these findings. The findings add to the existing knowledge base on amygdala development in youth with exposure to traumatic stress, but also raise additional questions. In particular, our results do not allow us to conclude if the findings are simply prolonged age-related growth or atypical amygdala development due to stress. Alternatively, children with PTSD may have a qualitatively different developmental trajectory, not a delayed (or accelerated) trajectory. In addition, the cross-sectional design of this study prohibits causal or directional inferences.
Findings are also limited by the relatively small sample size. Another limitation is that there are likely a number of intermediary factors, not assessed in this study that could influence the link between age and amygdala volumes. While no single study can hope to capture all the potential influences, the present findings help identify developmental differences as a potential source of inconsistencies in the literature. Findings also raise questions about the mechanisms (mediators) whereby stress and maturation exerts its influences on brain development. A final limitation from the perspective of PTSD as a discrete disorder is the absence of a trauma-exposed non-PTSD comparison group. This limits our ability to interpret the structural differences in terms of (1) traumatic stress exposure, versus (2) PTSD diagnosis (regardless of whether pre-existing vulnerability and/or sequelae) versus (3) some combination of 1 and 2. From the perspective of a PTSD symptoms being continuous or an indicator of underlying dimensional pathology (our point of view since multiple studies call into question the utility of dichotomous perspectives on PTSD in youth) (Scheeringa et al., 2001, 2012; Carrion et al., 2012), one can view the sample as trauma exposed with varying levels of PTSD symptoms. Moreover, we did test PTSD levels as a continuous variable and did not find a link to Amygdala volumes, suggesting trauma exposure vs PTSD, per se. Similarly, we found no effects by depressive disorders. Both of these analyses are limited by the sample size precluding firm conclusion about there being no effect in the absence of a statistical significant difference. Future research with larger samples is needed to clarify the nature of unique and interactive effects of comorbidity and PTSD diagnostic status. Despite the limitations, a clear implication is that future research may benefit from not just matching or controlling for age/maturation, but examining age as a moderator of group differences.
In summary, this study used FreeSurfer data analysis within 3T structural MRI scans to provide evidence that severe stress may influence the rate of amygdala development in youth. The results highlight the importance of examining stress by age interactions in pediatric neuroimaging research.
Acknowledgements
We acknowledge the children and families who participated in the project.
Footnotes
1 Because research calls into question the utility of simple dichotomous perspectives on the disorder of PTSD in youth (Scheeringa et al., 2001, 2012; Carrion et al., 2002), we consider these samples as trauma exposed with varying levels of PTSD symptoms. Thus, we use the phrases ‘PTSD symptoms’ and ‘PTSD group’ simply as concise terms for the longer phrase “trauma exposed with varying levels of PTSD symptoms”.
2 We tested non-linear curvilinear effects and they did not provide better fit than the linear models tested. One reason for the difference here and Weems et al. may be due to this being an older sample.
3 Given that 36% of the PTSD group were comorbid for depressive disorder, we examined linkage to left and right volumes and found no significant association (right r = −0.03, left r = −0.08). We also tested comorbid depression in the HLM analyses and did not find any age by depression or depression by PTSD interactions in the prediction of amygdala volumes.
Funding
The work was supported by awards from the National Institute of Mental Health (MH63893), the National Alliance for Research on Schizophrenia and Depression, the American Foundation for Suicide Prevention and the Aloha Foundation.
Conflict of interest. None declared.
References
- Achenbach T.M. (1991). Integrative Guide for the 1991 CBCL/4-18, YSR, and TRF Profiles. Burlington, VT: University of Vermont, Department of Psychiatry. [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., et al. (1990). A clinician rating scale for assessing current and lifetime PTSD: the CAPS-1. Behavior Therapist , 13(8), 187–8. [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., et al. (1995). The development of a clinician - administered PTSD scale. Journal of Trauma Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Bryk A.S., Raudenbush S.W., Congdon R.T. (2013). HLM 7.01 for Windows. Skokie, IL: Scientific Software International, Inc.. [Google Scholar]
- Carrión V.G., Weems C.F., Eliez S., et al. (2001). Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry, 50(12), 943–51. [DOI] [PubMed] [Google Scholar]
- Carrion V.G., Weems C.F., Ray R., Reiss A.L. (2002). Toward an empirical definition of pediatric PTSD: The phenomenology of PTSD symptoms in youth. Journal of the American Academy of Child and Adolescent Psychiatry, 41(2), 166–73. [DOI] [PubMed] [Google Scholar]
- Carrión V.G., Weems C.F., Richert K., Hoffman B.C., Reiss A.L. (2010). Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biological Psychiatry, 68(5), 491–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.K., Sassi R., Axelson D., et al. (2004). Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biological Psychiatry, 56(6), 399–405. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–94. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Sereno M.I. (1993). Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. Journal of Cognitive Neuroscience, 5(2), 162–76. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Hall J., Boring A.M., Frustaci K., Moritz G. (2001). A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry, 50(4), 305–9. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Clark D.B., et al. (1999). Developmental traumatology part II: brain development. Biological Psychiatry, 45(10), 1271–84. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Shifflett H., et al. (2002). Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological Psychiatry, 52(11), 1066–78. [DOI] [PubMed] [Google Scholar]
- Del Giudice M., Ellis B.J., Shirtcliff E.A. (2011). The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35(7), 1562–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–80. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging, 20(1), 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–55. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J., et al. (2004a). Sequence-independent segmentation of magnetic resonance images. Neuroimage, 23, S69–84. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., et al. (2004b). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. (1999a). Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. (1999b). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8(4), 272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Vaituzis A.C., Hamburger S.D., et al. (1996). Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology, 366(2), 223–30. [DOI] [PubMed] [Google Scholar]
- Gordon J.A., Hen R. (2004). The serotonergic system and anxiety. Neuromolecular Medicine, 5(1), 27–40. [DOI] [PubMed] [Google Scholar]
- Gray J.A. (1994). Framework for a taxonomy of psychiatric disorder. In: Van Goozen T.H.M., Van de Poll N.E., Sergeant J.A., editors. Emotions: Essays on Emotion Theory. New York: Lawrence Erlbaum Associates; 29–59. [Google Scholar]
- Gray J.A., McNaughton N. (2000). The Neuropsychology of Anxiety: An Enquiry into the Function of the Septo-Hippocampal System. New York: Oxford University Press. [Google Scholar]
- Groen W., Teluij M., Buitelaar J., Tendolkar I. (2010). Amygdala and hippocampus enlargement during adolescence in autism. Journal of the American Academy of Child and Adolescent Psychiatry, 49(6), 552–60. [DOI] [PubMed] [Google Scholar]
- Hayes A. (2014). PROCESS for SPSS and SAS. Available: http://www.afhayes.com/introduction-to-mediation-moderation-and-conditional-process-analysis.html (December 15, 2014). [Google Scholar]
- Holmbeck G.N. (2002). Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology, 27(1), 87–96. [DOI] [PubMed] [Google Scholar]
- Johnson P.O., Fay L.C. (1950). The Johnson–Neyman technique, its theory and application. Psychometrika, 15(4), 349–67. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., et al. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–8. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. (2000). Emotion circuits in the brain. Annual Review Neuroscience, 23(1), 155–84. [DOI] [PubMed] [Google Scholar]
- Mehta M.A., Golembo N.I., Nosarti C., et al. (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry, 50(8), 943–51. [DOI] [PubMed] [Google Scholar]
- Morey R.A., Gold A.L., LaBar K.S., et al. (2012). Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives in General Psychiatry, 69(11), 1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K.O., Kriegler J.A., Blake D.D., Pynoos R.S., Newman E., Weathers F.W. (1996). Clinician-Administered PTSD Scale, Child and Adolescent Version. White River Junction, VT: National Center for PTSD. [Google Scholar]
- Scheeringa M.S., Myers L., Putnam F.W., Zeanah C.H. (2012). Diagnosing PTSD in early childhood: an empirical assessment of four approaches. Journal of Trauma Stress, 25(4), 359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa M.S., Peebles C.D., Cook C.A., Zeanah C.H. (2001). Toward establishing procedural, criterion, and discriminant validity for PTSD in early childhood. Journal of Americal Academy of Child and Adolescent Psychiatry , 40(1), 52–60. [DOI] [PubMed] [Google Scholar]
- Ségonne F., Dale A.M., Busa E., et al. (2004). A hybrid approach to the skull stripping problem in MRI. Neuroimage, 22(3), 1060–75. [DOI] [PubMed] [Google Scholar]
- Ségonne F., Pacheco J., Fischl B. (2007). Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging, 26(4), 518–29. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging, 17(1), 87–97. [DOI] [PubMed] [Google Scholar]
- Steinberg A.M., Brymer M.J., Decker K.B., Pynoos R.S. (2004). The University of California at Los Angeles post-traumatic stress disorder reaction index. Current Psychiatry Reports , 6(2), 96–100. [DOI] [PubMed] [Google Scholar]
- Tottenham N. (2012). Human amygdala development in the absence of species-expected caregiving. Developmental Psychobiology, 54(6), 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. (2009). A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., et al. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A., Matsui M., Tanaka C., et al. (2012). Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One, 7(10), e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems C.F., Scott B.G., Russell J.D., Reiss A.L., Carrión V.G. (2013). Developmental variation in amygdala volumes among children with posttraumatic stress. Developmental Neuropsychology, 38(7), 481–95. [DOI] [PubMed] [Google Scholar]