Abstract
This study investigated the neural basis of individual variation in emotion regulation, specifically the ability to reappraise negative stimuli so as to down-regulate negative affect. Brain functions in young adults were measured with functional Magnetic Resonance Imaging during three conditions: (i) attending to neutral pictures; (ii) attending to negative pictures and (iii) reappraising negative pictures. Resting-state functional connectivity was measured with amygdala and dorsolateral prefrontal cortical (DLPFC) seed regions frequently associated with emotion regulation. Participants reported more negative affect after attending to negative than neutral pictures, and less negative affect following reappraisal. Both attending to negative vs neutral pictures and reappraising vs attending to negative pictures yielded widespread activations that were significantly right-lateralized for attending to negative pictures and left-lateralized for reappraising negative pictures. Across participants, more successful reappraisal correlated with less trait anxiety and more positive daily emotion, greater activation in medial and lateral prefrontal regions, and lesser resting-state functional connectivity between (a) right amygdala and both medial prefrontal and posterior cingulate cortices, and (b) bilateral DLPFC and posterior visual cortices. The ability to regulate emotion, a source of resilience or of risk for distress, appears to vary in relation to differences in intrinsic functional brain architecture.
Keywords: fMRI, resting state, reappraisal, amygdala, medial prefrontal cortex
Introduction
Emotion regulation refers to the ability to effectively modulate emotional responses to affective stressors. Effective emotion regulation can be a source of psychological resilience in the face of adversity, and ineffective emotion regulation has been associated with psychological distress, depression and anxiety (Keenan, 2000; Mennin et al., 2005; Biederman et al., 2012a,b). One powerful strategy for adaptive emotion regulation is cognitive reappraisal, by which an individual can purposefully alter the interpretation of an emotionally distressing situation and thereby diminish subjective negative affect. Reappraisal can be examined not only by self reports of affective states, but also by physiological measures (Gross, 1998). The experimental study of reappraisal is well aligned with effective clinical practices that promote reappraisal, such as cognitive behavioral therapy. The present study asked whether there is an intrinsic functional brain architecture (a pattern of functional connectivity) that is associated with variation among individuals in relation to more or less effective emotion regulation through cognitive reappraisal.
A decade of research has examined the brain basis of cognitive reappraisal (Ochsner et al., 2002; Ochsner et al., 2004). A meta-analysis of this research concludes that successful reappraisal that down-regulates negative affect most consistently involves increased activation of brain regions associated with cognitive control, such as prefrontal cortices, and decreased activation of the amygdala (Buhle et al., 2013). Prefrontal cortices are thought to support the implementation of cognitive reappraisal through the modulation of the amygdala, which reflects the affective consequence of the emotion regulation.
Support for this neuroanatomical model of reappraisal regulation comes from evidence for individual differences such that across people greater activation in cognitive control regions is associated with more successful emotion regulation (Ochsner et al., 2002; Ochsner et al., 2004; Phan et al., 2005; Urry et al., 2006; Wager et al., 2008). Furthermore, during emotion regulation individuals who are more effective in reappraisal exhibit greater interactions between the amygdala and both lateral and medial prefrontal cortex (MPFC) (Banks et al., 2007). MPFC is a major component of the default mode network (DMN), which is associated with self-reflection (Raichle et al., 2001; Kelley et al., 2002; Whitfield-Gabrieli et al., 2011), and intentional emotion regulation may involve self-reflection.
Although individual differences in emotion regulation effectiveness have been associated with individual differences in brain functions occurring during an emotion regulation task, it is unknown as to whether such individual differences reflect transitory (state-like) factors, such as fluctuations in mood, fatigue, or motivation, vs stable (trait-like) variation in functional brain architecture. One approach to addressing this question is to relate variation in emotion regulation effectiveness to variation in resting-state functional Magnetic Resonance Imaging (fMRI) measures of intrinsic functional brain architecture that reflects long-term brain network organization. Spontaneous fluctuations of blood oxygenation level-dependent (BOLD) signals across the brain in functionally related brain regions are correlated with each other in the absence of external stimuli, and the patterns of these correlations have been thought to reveal intrinsic relations of brain regions (Biswal et al., 1995; Greicius et al., 2003; Beckmann et al., 2005). Presumably, the frequent co-activation of brain regions sculpts an intrinsic functional brain architecture or functional connectivity of neural networks that are detected in the resting state. Here, we asked if individual differences in emotion regulation are associated with differences in the intrinsic functional architecture of the brain measured in the resting state.
Our resting-state analyses focused on the two brain regions most consistently associated with emotion regulation, the amygdala and the dorsolateral prefrontal cortical (DLPFC) (Buhle et al., 2013). Prior resting-state studies have associated the amygdala with the major midline nodes of DMN, MPFC and the posterior cingulate cortex (PCC) (Banks et al., 2007; Johnstone et al., 2007), suggesting the importance of the interplay between these brain regions in healthy emotional regulation. Consistent with the possibility that functional connectivity between the amygdala and DMN regions may play a role in emotion regulation are findings of amygdala-DMN hyperconnectivity in psychiatric disorders conceptualized as involving impaired emotion regulation, such as depression and anxiety (Gee et al., 2013; Xu et al., 2013; Baeken et al., 2014). Several studies have also reported decreased resting-state connectivity within the cognitive control network (e.g. DLPFC) in patients with depression (Veer et al., 2010; Alexopoulos et al., 2012; Ye et al., 2012) that might contribute to difficulty in cognitive control and emotion regulation. We therefore hypothesized that variation DLPFC and in amygdala-DMN resting state functional connectivity would be associated with variation in emotion regulation ability.
The experimental design followed the typical measurement of brain activations associated with the cognitive reappraisal as a strategy for down-regulating negative affect (Ochsner et al., 2002; Ochsner et al., 2004; Wager et al., 2008). Participants viewed pictures in three conditions: (i) an Attend Neutral condition in which they saw neutral pictures; (ii) an Attend Negative condition in which they saw negative pictures and (iii) a Reappraise Negative condition in which they saw negative pictures and attempted to regulate their negative emotional responses via cognitive reappraisal. After each picture, participants rated their current state of neutral-to-negative affect.
Behaviorally, we expected greater negative affect following negative than neutral pictures, but a reduction of negative affect for negative pictures following reappraisal. For brain activation, we expected that participants in the Reappraise Negative condition relative to the Attend Negative condition would exhibit greater activations in cognitive control regions, such as prefrontal and parietal cortices, and lesser activation in the amygdala. The critical analyses related to individual differences in reappraisal success (or failure) calculated as the difference in negative affect for the Attend Negative vs Reappraise Negative conditions, and the relation of those differences to variation in amygdala and DLPFC functional connectivity measured in the same individuals in the resting state.
In order to relate emotion regulation ability (i.e. reappraisal success) as measured during imaging to trait measures of affective characteristics, we also administered the State-Trait Anxiety Index, Trait Scale (STAI-T) (Spielberger et al., 1983), which measures anxiety experienced in daily life, the Positive Affect Negative Affect Scale, Form X (PANAS-X) (Watson and Clark, 1994), which measures ongoing positive or negative emotional experiences, and the Difficulties in Emotional Regulation Scale (DERS) (Gratz and Roemer, 2004), which measures self-reported emotion regulation. We hypothesized that superior emotion regulation in the scanner task would be positively correlated with these widely used measures of affective traits.
Methods
Participants
The final sample consisted of 62 right-handed participants (30 males, 32 females; mean age = 22.3 ± 1.6 years); of these 75% were Caucasian, 10% Asian American, 5% multi-racial, 3% African-American, 3% Pacific Islander and 2% declined to report race/ethnicity. From the original 72 participants enrolled from the community through advertising in the local media, eight participants either did not complete the experimental tasks or had excessive movement in the scanner (>10% outlier volumes), which rendered their data invalid, and two participants enrolled in the study were later found to be ineligible (one could not fit in the scanner, and one was currently taking medication for ADHD). One additional participant was removed only from the resting-state analysis due to excessive motion.
In order to have participants reflect a broad range of emotion regulation ability, they were selected on the basis of their responses to DERS (Gratz and Roemer, 2004), a 36-item scale measuring self report of emotions and emotion regulation. Equal numbers of participants were selected to score below 53, 54–72 or above 73 points on the DERS. Consent was obtained for all participants, and the Institutional Review Boards at Massachusetts General Hospital and Massachusetts Institute of Technology approved this study.
Exclusion criteria included major sensorimotor handicaps (paralysis, deafness, blindness), history of psychosis, autism, currently taking psychiatric medication, inadequate command of the English language, IQ below 80, any conditions incompatible with Magnetic Resonance Imaging (MRI) scanning (e.g. certain types of metal in the body, claustrophobia), positive pregnancy test or history of traumatic head injuries. All study procedures were approved by the Institutional Review Board and all participants signed a written consent form. Participants were compensated $125.00 for completing all study procedures ($25 were given for completing the questionnaires, $50 for cognitive assessments and $50 for MRI). If participants could not complete the MRI procedures, they were compensated $25.00 per 15 min of scan time. In addition, participants were reimbursed for parking and child care when needed.
Participants also filled out two additional questionnaires. One questionnaire was STAI-T (Spielberger et al., 1983). The STAI-T consists of 20 statements, each of which the participant rates as to how well it describes him or her on a four-point scale (‘almost never’ to ‘almost always’). The STAI-T has strong internal consistency (α = 0.89) and concurrent validity (r = 0.71–0.85) (Bieling et al., 1998), and good reliability (Spielberger et al., 1983). The other questionnaire was PANAS-X. The PANAS-X is a psychometrically sound measure of positive and negative affect (Watson and Clark, 1994) that consists of a list of 60 feelings (30 positive, 30 negative), and instructs participants to rate on a five-point scale (‘not at all/very slightly’ to ‘extremely’) the degree to which they experienced each feeling that day.
Experimental design
Reappraisal task
The design of the paradigm was based on a study by Wager et al. (2008). Participants viewed pictures drawn from the International Affective Picture System (IAPS) (Lang et al., 2005), normed for elicitation of negative or neutral emotional response. Participants were presented with a negative picture and were instructed to either: (i) attend to the picture, by naturally experiencing the emotional state elicited by the picture, or (ii) to reappraise, whereby participants reinterpreted the picture in an effort to reduce their negative feelings about it. Neutrally valenced pictures were always preceded by instruction to attend to them. At the end of each trial, participants rated the degree to which they were experiencing a negative emotional reaction to the preceding picture on a five-point Likert scale (0 = ‘not at all negative’ to 4 = ‘extremely negative’), similarly designed as the scale used in the reappraisal task by Wager et al. (2008). Before entering the scanner, participants received brief training on how to respond to the pictures (i.e. how to respond to ‘attend’ vs ‘decrease’ instructions, as described next) and were given a chance to practice the task. A ‘reappraisal score’ for each participant was calculated as the difference (i.e. the reduced negative affect) between the mean reported negative affect for the Attend Negative condition minus the Reappraise Negative condition during scanning.
Sixty stimuli from the IAPS were used for the three conditions. One condition was comprised of 20 neutral pictures for the Attend Neutral condition (valence mean = 5.1, arousal mean = 3.3). Two sets of 20 equated negative pictures (set A: valence mean = 2.2, arousal mean = 5.7); set B: valence mean = 2.2, arousal mean = 5.7) were used in either the Attend Negative or Reappraise Negative conditions, with each set counterbalanced across conditions across participants. Optimized trial schedules were created (Dale, 1999), which determined the order based on a block-randomization of stimuli such that every 12 stimuli included four trials of each condition.
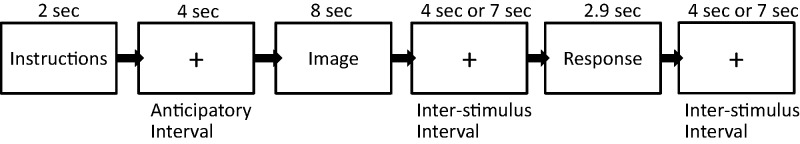
Each trial started with a 2-s display instructing the participant to attend or reappraise either neutral or negative images in the current trial. The instructions were followed by 4 s of anticipatory interval during which an image of a fixation cross was shown. This was followed by 8 s of the showing of the image. This was followed by a 4- or 7-s jittered interstimulus interval (ISI; a period of null event where a fixation cross was presented on the screen); 4 and 7 s ISIs were evenly distributed between blocks and were randomized within blocks. Following the ISI, participants were asked to rate their negative affect on the Likert scale (2.9 s). The trial concluded with a 4- or 7-s jittered ISI (Figure 1).
Fig. 1.
Schematic diagram of trial.
MRI procedures
Acquisition
Data were collected with a Siemens Tim Trio 3.0T. The functional MRI task acquisition parameters were TR = 2000 ms, TE = 30 ms, slice thickness = 4 mm, and a total of 841 acquisitions in addition to four dummy scans. In addition, all participants underwent a resting fMRI scan for which they were instructed to keep their eyes open, and the screen was blanked. The voxel size was 2 × 2 × 2 mm, TR = 6000 ms, TE = 30 ms, slices = 67 with a total of 62 acquisitions. A structural MPRAGE image was also collected (slices = 176, TR = 2530 ms, TE = 3.42 ms, voxel size = 1 × 1 × 1 mm; 5 min 53 s). The total duration of the scanning session was ∼ 1 h. All fMRI stimuli were generated using a MacBook Pro using Matlab software (Mathworks, Natick, MA).
Data analyses
FMRI data were preprocessed using Statistical Parametric Mapping (SPM8; Welcome Department of Cognitive Neurology) and using custom routines in MATLAB (Mathworks, Inc.). Preprocessing included correction for bulk head motion, spatial normalization and spatial smoothing with a Gaussian filter. To address spurious correlations in resting-state networks caused by head motion, we used quality assurance software Artifact Detection Tools (http://www.nitrc.org/projects/artifact_detect) to identify problematic time points during the scan. Specifically, an image was defined as an outlier image if the head displacement in x, y or z direction was >0.5 mm from the previous frame, or if the global mean intensity in the image was greater than three standard deviations from the mean image intensity for the entire resting scan. A single regressor for each outlier image was included in the first level general linear model along with motion parameters and first order derivatives.
Task analysis
Following preprocessing, statistical analysis was performed at the single-participant level using SPM. SPM treated each voxel according to a general linear model, taking into account the intrinsic autocorrelation in fMRI data imposed by the slow hemodynamic response. Low-frequency components of the fMRI signal were modeled as confounding covariates using a set of cosine bias functions in order to increase sensitivity to signals of interest.
We examined two condition contrasts. Greater activation for the Attend Negative than the Attend Neutral conditions validated the response to negative stimuli. Greater or lesser activation for the Reappraise Negative than the Attend Negative conditions identified brain regions engaged in reappraisal. To examine the neural correlates of individual differences in reappraisal effectiveness, we performed a whole-brain regression between individual reappraisal scores and activation differences between the Reappraisal Negative and Attend Negative conditions.
Resting-state analysis
Functional connectivity analysis was performed using the Connectivity Toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). We performed seed-voxel correlations by estimating maps showing temporal correlations between the BOLD signal from seed regions and every other voxel in the brain.
Seed definitions
We defined a priori left and right anatomical amygdalae seed regions based on the WFU_pickatlas (Maldjian et al., 2003). We also defined left and right DLPFC seed regions based on a 10 mm sphere around the peak voxel of the fMRI group Reappraisal > Look Negative contrast located within BA areas 9 and 46.
Noise removal
Physiological and other spurious sources of noise were estimated and regressed using the anatomical CompCor method (aCompCor) (Behzadi et al., 2007) rather than global signal regression, a widely used preprocessing method known to mathematically generate negative correlations and systematically alter network structure (Murphy et al., 2009; Saad et al., 2012). The anatomical image for each participant was segmented into white matter (WM), gray matter and cerebrospinal fluid (CSF) masks using SPM8. To minimize partial voluming with gray matter, the WM and CSF masks were eroded by one voxel, which resulted in substantially smaller masks than the original segmentation. The eroded WM and CSF masks were then used as noise regions of interest (ROIs). Signals from the WM and CSF noise ROIs were extracted from the unsmoothed functional volumes to avoid additional risk of contaminating WM and CSF signals with gray matter signals.
Resting state functional connectivity and reappraisal
To examine the neural correlates of individual differences in reappraisal effectiveness, we performed a whole-brain regression between reappraisal scores and resting state functional connectivity with the anatomically defined amygdalae and functionally defined DLPFC seed regions.
Overlap of DMN and amygdala seeded clusters
We calculated the mean DMN resting state functional connectivity for all participants. First, we defined DMN seeds as 10 mm spheres around the peak coordinates of the MPFC, PCC and right/left parietal (RLP/LLP) from the literature (Fox et al., 2005). Then, we took the mean of these four seeds to define one overall group DMN seed and performed a one sample test on the Fischer transformed r-maps. Finally, we overlaid the amygdala seed based clusters, which were significantly correlated with the reappraisal success, onto the group DMN.
Statistical thresholds
All imaging analyses were corrected for multiple comparisons with an initial height threshold of P = 0.001 and a family wise error (FWE) cluster level corrected of P > 0.05.
Results
Behavioral responses to reappraisal task
Participants reported significantly greater negative affect when attending to negative pictures (mean 3.43 ± 0.72) than neutral pictures (1.19 ± 0.20) [t (61) = 25.10, P < 0.001], and reported that reappraisal (2.48 ± 0.68) significantly reduced negative affect for negative pictures [t (61) = 11.68, P < 0.001].
Correlation between reappraisal score and self-report trait measures
Reappraisal scores had a significant negative correlation with STAI-T scores (r = −0.29, P < 0.05) and a significant positive correlation with the PANAS positive emotion scores (r = 0.34, P < 0.01) (but no significant correlation with PANAS negative emotion scores (r = −0.13, P = 0.32). DERS scores did not correlate with reappraisal scores (r = −0.019, P = 0.88) or PANAS negative scores (r = 0.02, P = 0.90), but had a significant positive correlation with STAI-T scores (r = 0.61, P < 0.0001) and a significant negative correlation with PANAS positive scores (r = −0.31, P = 0.02).
Neuroimaging
Attend negative > attend neutral
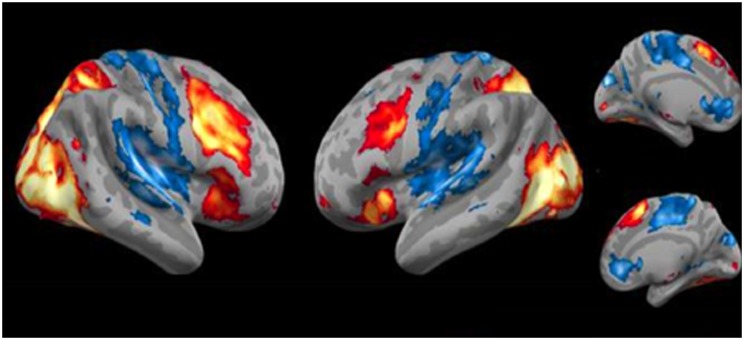
There was widespread bilateral activation for attending to negative relative to neutral pictures (Figure 2; Table 1a). There was significant positive activation in clusters that included bilateral occipital regions, frontal middle gyrus (BA9/46), supplementary motor areas (BA6/8), insula and cerebellum. The activation extended bilaterally into the amygdala. There were also negative activations (i.e. Attend Neutral > Attend Negative) primarily in regions of the DMN (e.g. BA 10).
Fig. 2.
Task activation analysis (negative attend > neutral attend). Lateral and medial views of brain regions more activated for negative attend vs neutral attend conditions. Red/Yellow clusters depict significant activations; blue clusters depict significant deactivations.
Table 1.
Activations in emotion regulation task conditions
| BA | x, y, z | T | k | p-corrected | |
|---|---|---|---|---|---|
| (a) Look negative > look neutral | |||||
| Positive activation | |||||
| Mid temporal R/mid occipital | 19/37 | 54, −64, 2 | 12.10 | 26209 | <0.001 |
| Frontal inferior triangularis R/frontal inferior operon | 9/46 | 44, 8, 32 | 8.02 | 9775 | <0.001 |
| Insula L/temporal pole L | 38/47 | −30, 24, −2 | 7.45 | 4630 | <0.001 |
| Supplementary motor area R | 8/6 | 6, 18, 52 | 7.89 | 2601 | <0.001 |
| Cerebellum | −2, −52, −38 | 5.29 | 334 | 0.021 | |
| Negative activation | |||||
| Insula R | 13/40 | 44, −12, 14 | 8.24 | 27277 | <0.001 |
| Medial frontal gyrus/sub-gyral | 10 | 12, 36, −6 | 7.39 | 3875 | <0.001 |
| (b) Reappraisal > look negative | |||||
| Positive activation | |||||
| Middle frontal L | 6/8 | −38, 22, 44 | 13.1 | 31267 | <0.001 |
| Angular gyrus L | 39/40 | −44, −58, 30 | 10.75 | 9137 | <0.001 |
| Angular gyrus R | 40 | 58, −40, −4 | 8.25 | 5128 | <0.001 |
| Cerebellum L | 34, −60, −30 | 7.96 | 7991 | <0.001 | |
| Precuneus R | 7/31 | 12, −48, 36 | 5.41 | 1566 | <0.001 |
| Caudate L | wm | −14, 8, 12 | 5.65 | 519 | <0.001 |
| Negative activation | |||||
| Rolandic operon L | 6/13 | −48, −22, 20 | 4.55 | 607 | <0.001 |
Regions with increased or decreased activation in the (a) Look Negative greater than Look Neutral contrast and (b) Reappraisal greater than Look Negative contrast. Coordinates (x y z) are based on MNI brain (Montreal Neurologic Institute). k, cluster size. BA, Brodmann area. P-corrected, cluster-level FWE-corrected value.
Reappraise negative > attend negative
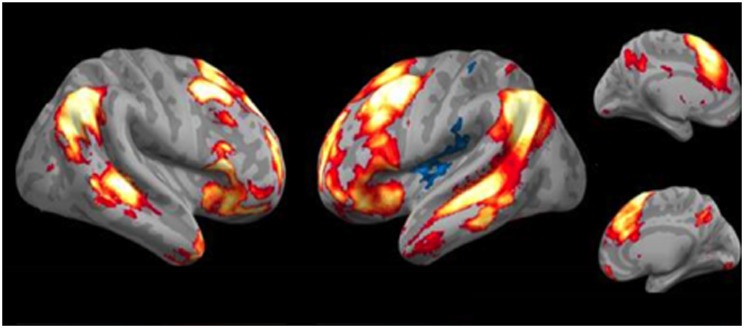
There was also widespread bilateral activation for the cognitive reappraisal of negative pictures (Figure 3; Table 1b). There were significant positive activations in multiple prefrontal regions including laterally in bilateral middle and inferior frontal gyri and medially in anterior cingulate cortex and dorsomedial prefrontal cortex. There were also activations posteriorly in inferior parietal lobule (IPL), angular gyrus and precuneus. There was one small region of negative activation.
Fig. 3.
Task activation analysis (reappraisal > negative attend). Lateral and medial views of brain regions more activated for reappraisal vs negative attend conditions. Red/yellow clusters depict significant activations; blue clusters depict significant deactivations.
Laterality of activations
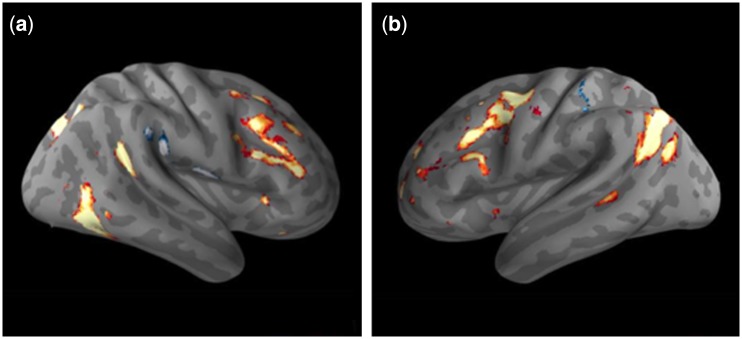
It appeared that activations for attending to negative vs neutral pictures were more extensive in the right hemisphere, whereas activations related to cognitive reappraisal of negative pictures were more extensive in the left hemisphere. In order to quantitatively evaluate this apparent cerebral asymmetry, we compared activations between homologous regions of the two hemispheres in each contrast. There were significantly greater activations for attending to negative relative to neutral pictures in the right hemisphere (Figure 4a), whereas there were significantly greater activations for reappraising relative to attending to negative pictures in the left hemisphere (Figure 4b).
Fig. 4.
Laterality analysis. (a) Regions significantly more activated for negative attend vs neutral conditions in the right than left hemisphere (red/yellow). (b) Regions significantly more activated for reappraisal vs negative attend conditions in the left than right hemisphere (red/yellow). Blue clusters depict greater activations in the contralateral hemisphere.
Correlation between reappraisal success and activations
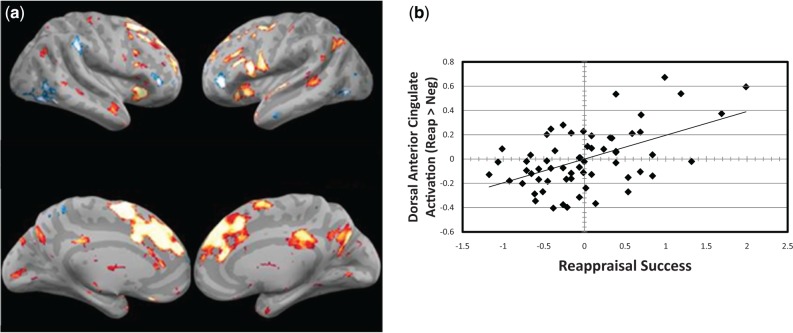
Across participants, greater reappraisal success correlated significantly and positively with greater activation in dorsomedial and lateral prefrontal cortices bilaterally (Figure 5; Table 2).
Fig. 5.
Brain activations correlated with reappraisal success. (a) Greater lateral (top) and medial (bottom) prefrontal cortex activations for reappraisal vs negative attend conditions significantly correlated with greater reappraisal success. (b) Scatter plot depicting that greater activation in dorsal anterior cingulate for reappraisal vs negative attends condition significantly correlated with greater reappraisal success (r = 0.52).
Table 2.
Correlations of activation for reappraisal vs negative conditions with reappraisal success
| BA | x, y, z | T | k | P-corrected | |
|---|---|---|---|---|---|
| Correlation with reappraisal success | |||||
| Positive correlation | |||||
| Frontal middle R/medial frontal superior | 6 | 30, 18, 54 | 4.53 | 16469 | <0.001 |
| Negative correlation | |||||
| No suprathreshold clusters | |||||
Regions in the reappraisal vs negative attend conditions that correlate with reappraisal success activation. Coordinates (x y z) are based on MNI brain (Montreal Neurologic Institute). k, cluster size. BA, Brodmann area. P-corrected, cluster-level FWE-corrected P value.
Correlation between reappraisal success and resting-state functional connectivity
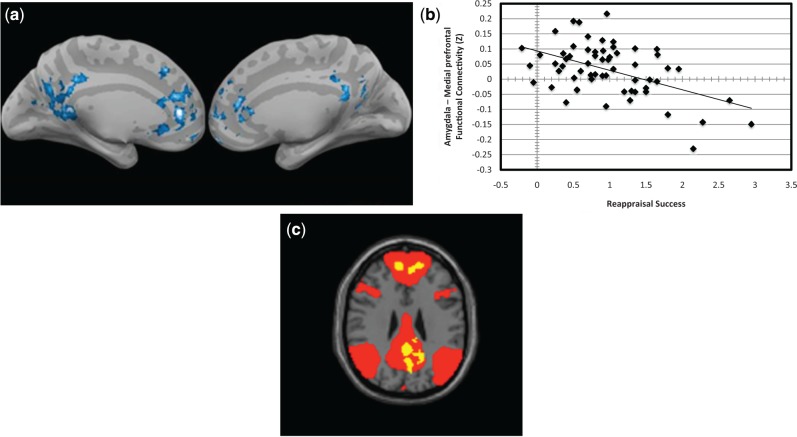
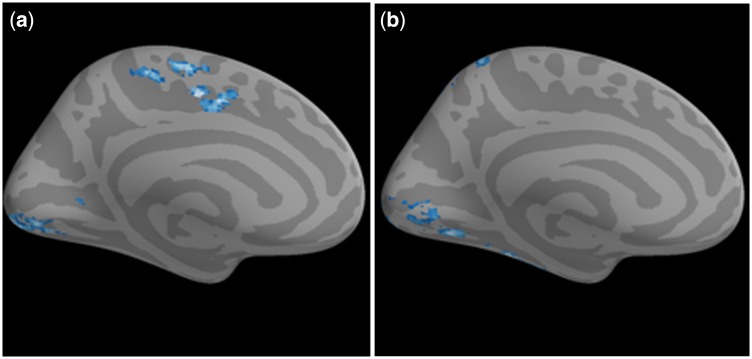
Across participants, greater reappraisal success correlated significantly and negatively with functional connectivity between the right amygdala seed and clusters in the MPFC and the PCC (Figure 6a and b; Table 3) (there was no correlation with the left amygdala). The locations of these MPFC and PCC regions overlapped with the groups’ mean DMN (Figure 6c). Across participants, greater reappraisal success correlated significantly and negatively with functional connectivity between the left DLPFC and also the right DLPFC and ipsilateral posterior regions of occipital cortex and fusiform gyrus (Figure 7a and b; Table 4). Connectivity between right DLPFC and anterior cingulate cortex also correlated significantly and negatively with greater reappraisal success.
Fig. 6.
Amygdala resting state functional connectivity correlated with reappraisal success. (a) Lesser resting-state functional connectivity between right amygdala seed and clusters in MPFC and PCC regions correlated with greater reappraisal success. (b) Scatter plot depicting that lesser resting-state functional connectivity between right amygdala seed and a cluster in MPFC correlated with greater reappraisal success. (c) Overlap of the group mean DMN resting state network (red) and the clusters (a) that significantly correlated with reappraisal success (yellow).
Table 3.
Correlations of resting state amygdalae functional connectivity with reappraisal success
| BA | x, y, z | k | P-corrected | ||
|---|---|---|---|---|---|
| Right amygdalae seed | |||||
| Increased correlation with reappraisal scores | |||||
| No suprathreshold clusters | |||||
| Decreased correlation with reappraisal scores | |||||
| Anterior prefrontal cortex L, dorsal lateral prefrontal cortex L | 9/32 | −12, 38, 4 | 3023 | <0.001 | |
| Dorsal PCC L | 31 | −10, −48, 8 | 1441 | 0.015 | |
Regions with decreased connectivity to the amygdala correlated with reappraisal scores. Coordinates (x y z) are based on MNI brain (Montreal Neurologic Institute). k, cluster size. BA, Brodmann area. P-corrected, cluster-level FWE-corrected P value.
Fig. 7.
Prefrontal resting state functional connectivity correlated with reappraisal success. (a) Lesser resting-state functional connectivity between the right DLPFC seed and visual regions and anterior cingulate correlated with greater reappraisal success. (b) Lesser resting-state functional connectivity between the left DLPFC seed and visual regions correlated with greater reappraisal success.
Table 4.
Correlations of resting state dorsal lateral prefrontal cortex functional connectivity with reappraisal success
| BA | x, y, z | k | P-corrected | |
|---|---|---|---|---|
| Right dorsal lateral prefrontal cortex seed | ||||
| Increased correlation with reappraisal scores | ||||
| No suprathreshold clusters | ||||
| Decreased correlation with reappraisal scores | ||||
| Primary/secondary visual cortex, associative cortex | 17/18,19 | 0, −84, −10 | 2724 | <0.001 |
| Premotor cortex L, anterior cingulate cortex | 6, 24 | −6, −20, 60 | 1071 | 0.039 |
| Left dorsal lateral prefrontal cortex seed | ||||
| Increased correlation with reappraisal scores | ||||
| No suprathreshold clusters | ||||
| Decreased correlation with reappraisal scores | ||||
| Primary/secondary visual cortex R, fusiform gyrus | 7, 18/19 | 26,−38,−24 | 4517 | <0.001 |
| Associative visual cortex L, somatosensory cortex | 17/18,37 | −6, −76, 56 | 1372 | <0.001 |
Regions with decreased connectivity to the dorsal lateral prefrontal cortex correlated with reappraisal scores. Coordinates (x y z) are based on MNI brain (Montreal Neurologic Institute). k, cluster size. BA, Brodmann area. P-corrected, cluster-level FWE-corrected p value. The seeds were 10 mm spheres around local maxima from the Reappraisal > Negative Attend. Left Dorsal Lateral Prefrontal Seed was centered around (−28, 48, 6), and Right Dorsal Lateral Prefrontal Seed was centered around (26, 50, 14).
Discussion
Individual differences in emotion regulation ability, specifically the ability to down-regulate negative emotions in response to negative experiences, were associated with variation in the intrinsic functional brain architecture of the human brain. Successful regulation resulted in a positive emotional benefit with participants reporting significantly less negative emotion after successful reappraisal than after attending to negative pictures. There was a corresponding increase of activation for reappraising relative to attending to negative pictures that was especially pronounced in medial and lateral prefrontal and parietal regions that are known to be associated with cognitive control. The effectiveness of reappraisal varied behaviorally across participants, with more successful regulation associated with greater activation in lateral and medial prefrontal regions. effectiveness was also related to variation in the intrinsic functional architecture of the brain. At rest, individuals with greater regulation ability exhibited (i) lesser functional connectivity between the right amygdala and the two major components of the DMN, MPFC and PCC; and (ii) lesser functional connectivity between both left and right DLPFC and ipsilateral posterior visual regions.
The task-related activations in the present study paralleled those reported in prior neuroimaging studies comparing reappraisal and attending conditions for the down-regulation of negative emotion in response to negative experiences. Simply attending to negative vs neutral pictures resulted in participants reporting significantly more negative affect, and invoked greater activation in many regions, including lateral and medial prefrontal cortices, parietal cortex, and amygdala. Reappraising vs attending to negative pictures resulted in participants reporting significantly less negative affect, and invoked greater activation in similar brain regions, including prefrontal, parietal and temporal regions. Activation of these brain regions that are thought to support cognitive control may reflect the cognitive restructuring needed to override an initial negative emotional response to an overtly negative stimulus (Ochsner et al., 2002). The overall pattern of brain activations observed resembles those reported in the initial studies of reappraisal (Ochsner et al., 2002; Ochsner et al., 2004) as well as a meta-analysis of 48 neuroimaging studies of reappraisal (Buhle et al., 2013). The main exception was that in the present study there was not the expected measureable decrease of activation in the amygdala during reappraisal (although there was amygdala activation for viewing negative vs neutral pictures).
We also found evidence for a functional cerebral asymmetry in viewing vs reappraising negative pictures. There was significantly greater activation in the right than the left hemisphere for viewing negative vs neutral pictures. In contrast, there was significantly greater activation in the left than right hemisphere for reappraising than viewing negative pictures [as in Johnstone et al. (2007)]. Most prior neuroimaging studies of emotion regulation have noted bilateral activations for both viewing and reappraising negative pictures, as was found in the present study, and not examined statistically whether there was cerebral asymmetry of activation for either contrast. The cerebral asymmetry revealed in the present study for viewing vs regulating negative stimuli can be related to evidence that there is hemispheric specialization for negative and positive emotions. The valence hypothesis of the representation of emotions in the brain posits that positive (or approach-related) emotion processes are cortically left-lateralized whereas negative (or avoidance-related) emotion processes are cortically right-lateralized (Sackheim et al., 1982; Silberman and Weingartner, 1986; Davidson, 1992). In this study, there were bilateral activations in both contrasts, so the cerebral asymmetry is one of relative specialization rather than absolute dominance. Nevertheless, there was a relatively right-lateralized pattern of neocortical activation for the negative emotional experience associated with viewing negative pictures, and a relatively left-lateralized pattern of neocortical activation for the reappraisal of negative pictures that enhanced positive emotional experience.
Participants varied in their ability to regulate negative emotions, and this ability as measured in scanner task performance correlated with scores on two questionnaires widely used in personality and clinical research. Successful behavioral emotion regulation correlated with lesser trait anxiety as measured by STAI-T (Spielberger et al., 1983) and with greater positive affect as measured by PANAS-X (Watson and Clark, 1994). STAI-T asks people about the typical anxiety they experience in daily life, and the PANAS positive affect scale asks people about their positive emotional experiences that day as an index of ongoing positive emotional experiences. Thus, better emotion regulation during neuroimaging was associated with less anxiety and more positive affect in everyday life (and conversely, worse emotion regulation was associated with greater anxiety and less positive affect). This is congruent with the extant literature documenting that poor emotion regulation through reappraisal of negative stimuli is associated with a number of clinical states, including measures of depression and anxiety (e.g. Gross and John, 2003). Thus, the variation in emotion regulation during scanning appears to reflect ecological variation in everyday positive and negative mental health.
The brain basis for better or worse emotion regulation was identified by correlations between emotion regulation success and activation differences in reappraisal vs attending to negative pictures. Participants who had more successful emotion regulation tended to have greater activation in dorsomedial prefrontal, lateral prefrontal and PCC bilaterally. These brain-behavior/emotion correlations are similar to those reported in a prior study of individual differences in emotion regulation success (Wager et al., 2008). Other studies have reported that successful reappraisal involves increased activation of lateral and medial prefrontal regions that, in turn, down-regulate amygdala activation (Ochsner et al., 2002; Ochsner et al., 2004; Phan et al., 2005), thus confirming the importance of prefrontal activations and the functional interplay between frontal and limbic regions in the process of the healthy regulation of emotions.
Analysis of resting-state correlations with the amygdala revealed that variation in the intrinsic functional organization of the brain (functional connectivity) was also related to variation in reappraisal success even in the absence of emotional stimuli. Specifically, individuals with greater reappraisal ability exhibited less functional connectivity between (i) right amygdala and both MPFC and PCC, and (ii) left and right DLPFC and ipsilateral visual cortices. No prior study has related resting-state intrinsic functional organization to variation in successful or impaired emotion regulation. One study examined task-rest interactions that occurred in a similar emotion regulation paradigm during inter-trial intervals (Lamke et al., 2014). Regulation of negative emotion had differential effects during stimulation (when there was an image and an active task that participants needed to engage in) and fixation (when participants were instructed to simply look at the screen) periods in the amygdala vs DLPFC and DMN regions.
It is noteworthy that greater emotion regulation ability was associated with lesser functional connectivity with the two brain regions most associated with emotion regulation, prefrontal cortex and amygdala. Speculatively, greater intrinsic independence of these brain regions may promote the ability to reconstruct through reappraisal the emotional interpretation of negative experiences. Thus, weaker functional connectivity between the prefrontal cortices and ispilateral visual cortices may diminish the bottom-up, stimulus-driven capture of thought from visual inputs (negative scenes), and permit greater flexibility in top-down, goal-driven prefrontal mediation of reappraisal. Weaker functional connectivity between the right amygdala and the two major hubs of the DMN (MPFC and PCC) may also facilitate emotion regulation because the DMN is also implicated in self-referential processing (Raichle et al., 2001; Kelley et al., 2002; Whitfield-Gabrieli et al., 2011). Weaker functional connectivity between the right amygdala and the DMN may facilitate goal-driven modulation of the amygdala (although it is unclear why this was observed only in the right amygdala).
Aligned with the present finding that superior emotion regulation was associated with lesser amygdala-DMN intrinsic functional connectivity, prior studies indicated that greater functional relations between the amygdala and the DMN increase the risk for maladaptive affective and anxiety states. Increased amygdala-DMN functional connectivity has been reported in adults with traits associated with high levels of anxiety, pessimism and inhibition, as well as in previously institutionalized children presenting with anxiety (Gee et al., 2013; Xu et al., 2013; Baeken et al., 2014). Hyperconnectivity between the DMN and the limbic system has been reported in people with subsyndromal and clinical major depressive disorders (Greicius et al., 2007; Felder et al., 2012). These deficits in functional connectivity also may be malleable with treatment such as antidepressants (Anand et al., 2007).
Resting-state measures of temporal correlations among brain regions are thought to reveal the intrinsic functional architecture of the brain into networks that reflect ongoing experiences. Although activation studies, and individual differences in such activations, allow for well-controlled experimental conditions, they reveal only transitory responses to stimulus provocation. In contrast, resting-state functional networks cannot be characterized by controlled experimental conditions, but they may reflect stable functional brain organization sculpted by long-term experiences that induce the networks. The nature of these networks may, in turn, encourage or discourage particular patterns of associated emotions and behaviors. The present study indicates that greater ongoing functional connectivity between the right amygdala and major hubs of the DMN and between DLPFC and ipsilateral visual cortices may jeopardize the ability to cognitively regulate negative emotions and thus promote negative emotional experiences.
Several limitations of this study can be noted. First, it is unclear why scores on the DERS measure of self-reported emotion regulation ability was unrelated to either emotion regulation performance or the neural correlates of that performance. Second, the final sample of 62 participants were mostly Caucasian (75%), so the findings should be examined in a more diverse sample. Third, there were no specific instructions given to participants during the 4–7 s ITI. Due to the lack of tasks, stimuli, or instructions, the participants may have ruminated, self-referenced, or continued to reappraise during this time, and this may have affected their emotional ratings in the reappraisal task. Additionally, participants were instructed to keep their eyes open and were monitored by study staff to confirm that their eyes were open during the resting state scanning, but we did not perform electroencephalography (EEG) to confirm that they were not asleep during any of the 6 min of the resting state procedure.
Results from this study suggest that successful reappraisal is associated with (i) less trait anxiety and more positive emotion in everyday life; (ii) greater activation in medial and lateral prefrontal regions bilaterally; (iii) lesser intrinsic functional connectivity between right amygdala and both medial prefrontal and PCC that are the major hubs of the DMN and (iv) lesser intrinsic functional connectivity between left and right DLPFC and ipsilateral visual cortices. These findings are congruent with the literature that documents impaired activitation and functional connectivity in similar neural circuits in diagnoses such as major depressive disorder and anxiety disorders that are known to be associated with reappraisal failure (Aldao et al., 2010). Further research is needed to evaluate whether these abnormalities in the level of activation and intrinsic brain functional architecture associated with reappraisal failure could represent neurobiological biomarkers of risk for mood and anxiety disorders, an area of high clinical, scientific and public health relevance. Effective treatment for or prevention of such mental health difficulties may require a beneficial restructuring of the identified intrinsic functional networks so as to support more effective reappraisal, less anxiety and greater positive emotion.
Acknowledgements
This work was supported by the Tommy Fuss Fund, the MGH Pediatric Psychopharmacology Council Fund, and the Poitras Center for Affective Disorders Research at the McGovern Institute for Brain Research.
Conflicts of interest
Dr A.B. is currently an employee of Alkermes, Inc. Dr J.B. is currently receiving research support from the following sources: The Department of Defense, Food & Drug Administration, Ironshore, Lundbeck, Magceutics Inc., Merck, PamLab, Pfizer, Shire Pharmaceuticals Inc., SPRITES, Sunovion, Vaya Pharma/Enzymotec, and NIH. In 2015, Dr J.B. has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. In 2014, Dr J.B. received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He received research support from AACAP, Alcobra, Forest Research Institute, and Shire Pharmaceuticals Inc. Dr Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. In 2013, Dr J.B. received an honorarium from the MGH Psychiatry Academy for a tuition-funded CME course. He received research support from APSARD, ElMindA, McNeil, and Shire. Dr Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Shire and Sunovion; these royalties were paid to the Department of Psychiatry at MGH. In 2012, Dr J.B. received an honorarium from the MGH Psychiatry Academy and The Children’s Hospital of Southwest Florida/Lee Memorial Health System for tuition-funded CME courses. In previous years, Dr J.B. received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Boston University, Bristol Myers Squibb, Cambridge University Press, Celltech, Cephalon, Cipher Pharmaceuticals Inc., Eli Lilly and Co., Esai, Fundacion Areces (Spain), Forest, Fundación Dr Manuel Camelo A.C., Glaxo, Gliatech, Hastings Center, Janssen, Juste Pharmaceutical Spain, McNeil, Medice Pharmaceuticals (Germany), Merck, MGH Psychiatry Academy, MMC Pediatric, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shionogi Pharma Inc, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma Inc., Veritas, and Wyeth. Dr Uchida, Dr Micco, Dr Gabrieli, Dr Whitfield-Gabrieli, Mr de Los Angeles, Ms Kenworthy and Ms Kagan have no conflicts to report.
References
- Aldao A., Nolen-Hoeksema S., Schweizer S. (2010). Emotion-regulation strategies across psychopathology: a meta-analytic review. Clinical Psychology Review, 30, 217–37. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., Hoptman M.J., Kanellopoulos D., Murphy C.F., Lim K.O., Gunning F.M. (2012). Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders, 139, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Gardner K., Lowe M.J. (2007). Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an fMRI study. Journal of Neuropsychiatry and Clinical Neuroscience, 19, 274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeken C., Marinazzo D., Van Schuerbeek P., et al. (2014). Left and right amygdala—mediofrontal cortical functional connectivity is differentially modulated by harm avoidance. PLoS One, 9, e95740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 360, 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J., Petty C.R., Day H., et al. (2012a). Severity of the aggression/anxiety-depression/attention child behavior checklist profile discriminates between different levels of deficits in emotional regulation in youth with attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics, 33, 236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J., Spencer T.J., Petty C., et al. (2012b). Longitudinal course of deficient emotional self-regulation CBCL profile in youth with ADHD: prospective controlled study. Neuropsychiatric Disease and Treatment, 8, 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieling P.J., Antony M.M., Swinson R.P. (1998). The state-trait anxiety inventory, trait version: structure and content re-examined. Behaviour Research and Therapy, 36, 777–88. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34, 537–41. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2013). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M. (1999). Optimal experimental design for event-related fMRI. Human Brain Mapping, 8, 109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J. (1992). Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition, 20, 125–51. [DOI] [PubMed] [Google Scholar]
- Felder J.N., Smoski M.J., Kozink R.V., et al. (2012). Neural mechanisms of subclinical depressive symptoms in women: a pilot functional brain imaging study. BMC Psychiatry, 12, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., et al. (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110, 15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz K.L., Roemer L. (2004). Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 26, 41–54. [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62, 429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100, 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (1998). Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74, 224–37. [DOI] [PubMed] [Google Scholar]
- Gross J.J., John O.P. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–62. [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience, 27, 8877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K. (2000). Emotion dysregulation as a risk factor for child psychopathology. Clinical Psychology: Science and Practice, 7, 418–34. [Google Scholar]
- Kelley W.M., Macrae C.N., Wyland C.L., Caglar S., Inati S., Heatherton T.F. (2002). Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience, 14, 785–94. [DOI] [PubMed] [Google Scholar]
- Lamke J.P., Daniels J.K., Dorfel D., et al. (2014). The impact of stimulus valence and emotion regulation on sustained brain activation: task-rest switching in emotion. PLoS One, 9, e93098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P., Bradley M.M., Cuthbert B.N. (2005). International Affective Pictures System (IAPS) . Technical report A-6. Gainsville, Fl: University of Florida. [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Mennin D.S., Heimberg R.G., Turk C.L., Fresco D.M. (2005). Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy, 43, 1281–310. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage, 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. (2002). Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14, 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage, 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry, 57, 210–9. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., et al. (2012). Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackheim H.A., Greenberg M.S., Weiman A.L., Gur R.C., Hungerbuhler J.P., Geschwind N. (1982). Hemispheric asymmetry in the expression of positive and negative emotions. Archives of Neurology, 39, 210–8. [DOI] [PubMed] [Google Scholar]
- Silberman E.K., Weingartner H. (1986). Hemispheric lateralization of functions related to emotion. Brain and Cognition, 5, 322–53. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists’ Press. [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience, 26, 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I.M., Beckmann C.F., van Tol M.J., et al. (2010). Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience, 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A. (1994). The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form: Ames: The University of Iowa. [Google Scholar]
- Whitfield-Gabrieli S., Moran J.M., Nieto-Castanon A., Triantafyllou C., Saxe R., Gabrieli J.D. (2011). Associations and dissociations between default and self-reference networks in the human brain. Neuroimage, 55, 225–32. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2, 125–41. [DOI] [PubMed] [Google Scholar]
- Xu P., Gu R., Broster L.S., et al. (2013). Neural basis of emotional decision making in trait anxiety. The Journal of Neuroscience, 33, 18641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T., Peng J., Nie B., et al. (2012). Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. European Journal of Radiology, 81, 4035–40. [DOI] [PubMed] [Google Scholar]