Abstract
Research suggests psychopathy is associated with structural brain alterations that may contribute to the affective and interpersonal deficits frequently observed in individuals with high psychopathic traits. However, the regional alterations related to different components of psychopathy are still unclear. We used voxel-based morphometry to characterize the structural correlates of psychopathy in a sample of 35 healthy adults assessed with the Triarchic Psychopathy Measure. Furthermore, we examined the regional grey matter alterations associated with the components described by the triarchic model. Our results showed that, after accounting for variation in total intracranial volume, age and IQ, overall psychopathy was negatively associated with grey matter volume in the left putamen and amygdala. Additional regression analysis with anatomical regions of interests revealed total triPM score was also associated with increased lateral orbitofrontal cortex (OFC) and caudate volume. Boldness was positively associated with volume in the right insula. Meanness was positively associated with lateral OFC and striatum volume, and negatively associated with amygdala volume. Finally, disinhibition was negatively associated with amygdala volume. Results highlight the contribution of both subcortical and cortical brain alterations for subclinical psychopathy and are discussed in light of prior research and theoretical accounts about the neurobiological bases of psychopathic traits.
Keywords: psychopathy, boldness, meanness, disinhibition, grey matter volume
Introduction
Psychopathy has been described as the conjunction of interpersonal dominance, low anxiety, callous-unemotional traits and externalizing behavioral tendencies (Cooke and Michie, 2001; Frick and White, 2008; Patrick et al., 2009). According to the triarchic model (Patrick et al., 2009), these characteristics may be best captured by three phenotypic components, boldness, meanness and disinhibition, which may result from impairments in core emotional and executive processes, reflecting atypical development of their underlying neural systems. A number of studies have reported associations between overall psychopathy and structural brain abnormalities (Blair, 2010; Koenigs et al., 2011), but little is known regarding the alterations that may underlie different subcomponents of psychopathy; despite clear evidence that these subcomponents are often associated with distinct patterns of neural functioning (Carre et al., 2013; Decety et al., 2013). A fuller understanding of the neural bases of distinct dimensions of psychopathy could help to clarify their developmental origins, and assess their relevance for the manifestation of the psychopathic phenotype. Therefore, in the present study, we have characterized for the first time the regional grey matter alterations associated with distinct components of the psychopathic personality in healthy adults.
Prior research has focused primarily on the structural bases of psychopathy in antisocial adults drawn from forensic (e.g. Bertsch et al., 2013) and clinical populations (e.g. de Oliveira-Souza et al., 2008), or in youths with conduct disorder (e.g. Wallace et al., 2013). Such research has identified alterations as a function of psychopathic traits in subcortical structures, including the amygdala (Yang et al., 2009; Yang et al., 2010; Boccardi et al., 2011; Ermer et al., 2012; Pardini et al., 2014), parahippocampal gyrus (Cope et al., 2012; Ermer et al., 2012; Ermer et al., 2013) and hippocampus (Cope et al., 2012; Ermer et al., 2012). These findings are consistent with the hypothesis that psychopathy is associated with a basic failure in emotional reactivity and associative learning, driven by dysfunctional affective-motivational systems reliant on the amygdala and interconnected structures (Patrick, 1994; Blair, 2013; Marsh, 2013).
Striatal alterations have also been reported and may be related to reward processing and decision-making impairments in individuals with elevated psychopathic traits (Blair, 2013). Previous findings suggested increased volume (Glenn et al., 2010), and atypical morphology (Boccardi et al., 2013) in the striatum of incarcerated psychopathic adults. However, in a community sample of adolescents with conduct disorder, striatal volume was shown to be reduced (Wallace et al., 2013).
At the cortical level, grey matter volume (GMV) reductions (de Oliveira-Souza et al., 2008; Yang et al., 2010; Ermer et al., 2012) and malformations (Yang et al., 2009) have been observed in the orbital and ventromedial prefrontal cortices of adults with psychopathy. These findings are congruent with the impairments shown by this population in processes subserved by these regions, such as reinforcement learning and response reversal (Mitchell et al., 2002; Blair, 2004; Budhani et al., 2006). However, positive associations between orbitofrontal cortex (OFC) volume and psychopathic traits have also been reported, primarily in pediatric samples (De Brito et al., 2009; Fairchild et al., 2013), and in a community substance abuse sample (Cope et al., 2012). Mixed results have also been described in the anterior cingulate cortex (ACC), which has been alternately shown to be augmented (De Brito et al., 2009; Cope et al., 2012), or not altered (Glenn et al., 2010) in individuals with psychopathic traits. Previous studies have documented volume reductions in the insula as well (de Oliveira-Souza et al., 2008; Cope et al., 2012), which could be linked to empathic deficits in adults (Decety et al., 2013), and youths (Lockwood et al., 2013; Marsh et al., 2013) with high psychopathic tendencies. Finally, volume reductions have been demonstrated in cortical regions for which relevance for the behavioral profiles of psychopathy is less clear, including the anterior temporal cortex (de Oliveira-Souza et al., 2008), temporal pole (Yang et al., 2009; Ermer et al., 2012, 2013; Gregory et al., 2012;) and superior temporal sulcus (de Oliveira-Souza et al., 2008; Muller et al., 2008; Yang et al., 2009).
A number of factors may account for remaining discrepancies in the literature, starting with differences between the populations being studied (for instance, differences in brain maturation are likely to explain some incongruent findings between adult and youth samples). In adult populations, a potentially relevant aspect is the way psychopathy has typically been assessed. Studies of adults have relied on forensic samples assessed for overall psychopathy with Psychopathy Checklist (PCL)-based instruments (e.g. Yang et al., 2005; de Oliveira-Souza et al., 2008; Ermer et al., 2012). PCL-based instruments assess psychopathy as a unitary construct, despite emerging evidence indicating this syndrome may be better represented as a constellation of lower order personality dimensions than as a one-dimensional clinical taxon (Lilienfeld, 1998; Marcus et al., 2004; Skeem et al., 2011). Although previous studies have attempted to characterize the structural alterations associated with PCL factors (e.g. Cope et al., 2012), PCL items were selected based on their internal consistency with one another and its higher-order factors are inter-related (Hare, 1980; Hare et al., 1990). Conversely, self-report instruments developed in light of more recent dimensional accounts of psychopathy (Lilienfeld and Widows, 2005; Patrick, 2010) cover the full spectrum of traits that characterize the psychopathic personality, irrespective of their correlation with each other, therefore assessing traits that are not captured by the PCL (e.g. positive adjustment features). It has been suggested that distinct psychopathic personality traits may result from specific neural deficits and developmental pathways (Patrick et al., 2009), consistent with previous demonstrations of dissociable neural functioning patterns as a function of those traits (e.g. Sadeh et al., 2013). Therefore, characterizing the structural alterations related to subcomponents of the psychopathic personality could enable a clearer picture of its neural bases, and perhaps help to clarify some of the previous contradictory findings.
The goal of the present study was 2-fold. First, we aimed to investigate whether structural alterations previously linked to overall psychopathy in forensic and clinical samples also predicted psychopathic traits in healthy adults from a community sample. In line with prior research and leading neurocognitive models of psychopathy (e.g. Blair, 2013), we expected to find structural alterations in five key structures: amygdala, OFC, ACC, insula and striatum. More precisely, we hypothesized overall psychopathy would be associated with GMV reduction in the amygdala, OFC and insula and GMV increase in the striatum and ACC.
Second, in addition to overall psychopathy, we sought to characterize the volumetric alterations associated with its subcomponents, as operationalized by the triarchic model (Patrick et al., 2009). Boldness entails traits of dominance, reduced stress and anxiety, emotional stability and venturesomeness, being often regarded as the ‘adaptive’ component of psychopathy (Drislane et al., 2013). It has been suggested that these features represent the phenotypic manifestation of low dispositional fear, which results from an underactive defensive system (Patrick and Bernat, 2009). We therefore hypothesized that boldness would be associated with volume alterations in structures implicated in defensive processes, namely the amygdala, insula and OFC.
Meanness is associated with callousness, manipulativeness and cruelty (Sellbom and Phillips, 2013). Like boldness, it is believed to arise from low dispositional fear (Patrick et al., 2009). However, boldness-related traits may reflect more directly a low fear genotype, while meanness results from the combination of low fear with negative development experiences and inadequate socialization, leading to impaired capacity for affiliation and empathy and to deviant patterns of interpersonal functioning (Patrick et al., 2009, 2012). Given its supposedly shared etiological basis with boldness, we predicted meanness would also be associated with structural alterations in the amygdala, insula and OFC. In addition, we expected meanness to correlate with GMV in the ACC, which, together with the anterior insula, has been previously implicated in empathy (Singer et al., 2004; Gu et al., 2010) and shown atypical activity as a function of callousness (e.g. Lockwood et al., 2013; Marsh et al., 2013).
Finally, disinhibition encompasses a general propensity to externalizing and impulse control problems and is associated with traits such as impulsivity, irresponsibility and hostility. It has been suggested that these traits reflect dysfunctional anterior brain systems implicated in affect regulation and behavioral control, which include the ACC and the prefrontal cortex (Patrick et al., 2012). Hence, we expected disinhibition to correspond to GMV alterations in the ACC and OFC. Following suggestions that striatal dysfunction may also contribute to the computational impairments associated with externalizing features of psychopathy (Blair, 2013), we hypothesized that disinhibition would also be associated with structural alterations in the striatum.
To test these predictions, two complementary approaches were used: first, we performed voxel-wise multiple regression analyses to identify regions in which GMV was predicted by overall psychopathy, and by each triarchic component; second, we examined the differential contributions of GMV within predefined anatomical regions to overall psychopathy and to each subcomponent. To our knowledge, this is the first study to both investigate structural brain alterations as a function of psychopathy in a sample of healthy adults recruited from the community, and to characterize the neuroanatomical bases of the psychopathy components described by the triarchic model.
Materials and methods
Participants
Thirty-five participants (all right-handed; 18–24 years old; 20 females) were recruited from the Georgetown University community through advertisements developed for psychopathy research (Widom, 1977), which have been shown to result in oversampling of high psychopathy scorers in previous studies (e.g. Marsh and Cardinale, 2014; Sellbom et al., 2014). All participants reported not having any prior neurological or psychiatric diagnoses, history of brain injuries or substance abuse, which constituted exclusion criteria. In addition, all participants reported not taking any psychotropic medication at the time of screening. Average IQ was assessed using the Kaufman Brief Intelligence Test (K-BIT; Kaufman, 1990) (Table 1) and socio-demographic information was collected.
Table 1.
Sample characterization: age, IQ and psychopathy scores
| Range | M (SD) | |
|---|---|---|
| Age | 18–24 | 21.06 (1.80) |
| IQ | 94–132 | 112.43 (12.02) |
| TriPM total | 23–120 | 71.31 (25.42) |
| Boldness | 20–55 | 39.83 (9.49) |
| Meanness | 1–51 | 15.71 (11.02) |
| Disinhibition | 0–37 | 15.77 (10.58) |
The study was approved by the Institutional Review Board at Georgetown University. Participants provided informed written consent in accordance with the Declaration of Helsinki and were compensated for their time.
Psychopathy measures
Psychopathy was assessed with the Triarchic Psychopathy Measure (TriPM; Patrick, 2010), a self-report instrument based on the triarchic model (Patrick et al., 2009). The TriPM assesses psychopathic traits in a dimensional manner, consistent with the idea that psychopathy, like other personality disorders, can be more reliably investigated continuously than categorically (Marcus et al., 2004). It includes 58 items, scored in a 4-point scale (0 = true; 1 = somewhat true; 2 = somewhat false; 3 = false), and provides subscale scores for boldness (e.g. ‘I’m a born leader’), meanness (e.g. ‘It doesn’t bother me to see someone else in pain’) and disinhibition (e.g. ‘I often act on immediate needs’). Kolmogorov–Smirnov tests revealed no significant deviations from normality for boldness, meanness and total score distributions (P values = 0.17, 0.20 and 0.20, respectively). Disinhibition scores did not follow a normal distribution (P = 0.02), but were symmetrically distributed (skewness = 0.64; descriptive statistics provided in Table 1). The TriPM has been reported to have good construct validity and to be able to successfully tap the core traits of psychopathy (Sellbom and Phillips, 2013; Stanley et al., 2013; Hall et al., 2014). Although this instrument has been previously used in studies investigating the neural correlates of psychopathic traits (Vieira et al., 2014), it is still a relatively new measure of psychopathy. Therefore, we also screened participants using another self-report instrument, the Psychopathic Personality Inventory—Revised (PPI-R; Lilienfeld and Widows, 2005), to provide confirmatory validation for the TriPM.
Magnetic Resonance Imaging (MRI) acquisition and preprocessing
Participants were scanned at Georgetown University’s Center for Functional and Molecular Imaging (CFMI), on a 3.0 T MRI system (Siemens Magnetom Trio, Erlangen, Germany), fitted with a circularly polarized 12-channel head coil. Head movements were minimized through padding. A high-resolution T1-weighted structural scan (MPRAGE) was acquired (repetition time = 1900 ms, echo time = 2.52 ms, inversion time = 900 ms, flip angle = 9°, slice thickness = 1.0 mm, matrix size = 176 × 256 mm), yielding 176 sagittal slices with an in-plane resolution of 1 × 1 mm. Total scan time was ∼ 4 min.
Preprocessing and analyses of anatomical data were performed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). T1 images were visually inspected and manually realigned as needed to ensure proper spatial normalization. Following realignment, segmentation, bias correction and spatial normalization were performed in one integrated step (Ashburner and Friston, 2005). Visual inspection of grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) distributions confirmed there were no deviations from normality, which could compromise the segmentation process. After being spatially normalized and segmented into GM, WM and CSF, images were registered using DARTEL to increase the accuracy of inter-subject alignment (Ashburner, 2007). Images were then normalized to Montreal Neurological Institute (MNI) space, modulated to compensate for the effect of spatial normalization, resliced to 1.5 × 1.5 × 1.5 mm, and smoothed using a 10-mm full-width at half-maximum (FWHM) Gaussian kernel following previous similar analyses (Cope et al., 2012; Ermer et al., 2012). Absolute masking with a 0.2 threshold (Ashburner, 2010) was performed prior to statistical analysis to exclude unstable voxels.
Statistical analysis
Variance in brain structure explained by psychopathy
We performed voxel-wise multiple regression analyses to determine how variation in overall psychopathy and unique variance associated with each component explained GMV, quantified through voxel-based morphometry (VBM). Because age and IQ showed significant correlations with some TriPM components (Table 2), these variables were included as covariates in all analyses. Likewise, we accounted for individual variation in brain size by including total intracranial volume (TIV) as a covariate in every regression (Ashburner, 2010; Wallace et al., 2013; Marsh et al., 2014).
Table 2.
Correlation coefficients (df = 33) between TriPM scores, PPI-R, age and IQ
| TriPM total | Boldness | Meanness | Disinhibition | |
|---|---|---|---|---|
| Age | −0.31 | −0.16 | −0.36* | −0.23 |
| IQ | −0.16 | −0.09 | 0.14 | −0.44** |
| PPI-R total | 0.93*** | 0.75*** | 0.77*** | 0.76*** |
*, P < 0.05; **, P < 0.01; ***, P < 0.001.
To identify regions in which GMV was correlated with overall psychopathy, we conducted a regression with TriPM total score as predictor. The pattern of structural alterations obtained with the TriPM was confirmed by performing an additional regression using the total PPI-R score as predictor of whole-brain GMV. To investigate the neural correlates of each psychopathy component, we performed a regression with boldness, meanness and disinhibition as predictors of whole-brain GMV.
A cluster-size threshold was used to control for multiple comparisons. This procedure balances Type I and Type II errors and increases the power of the analysis (Ward, 2000; Huettel et al., 2008; Ermer et al., 2012). A Monte Carlo simulation implemented in AlphaSim (Ward, 2000) determined that an extent threshold of 341 contiguous voxels at P < 0.001 uncorrected yielded a Familywise error (FWE) corrected threshold of P < 0.05. For additional exploratory analysis, we set the threshold at P < 0.005 uncorrected, with the same extent threshold.
Variance in psychopathy explained by brain structure
We conducted additional multiple regression analyses to address the inverse question: how unique variance of GMV in specific cortical and subcortical structures accounted for variation in overall psychopathy and its subcomponents. Identification of regions of interest (ROI) was based on the literature (e.g. De Brito et al., 2009), and targeted five bilateral structures: left and right amygdala, left and right striatum, left and right insula, left and right ACC, and left and right OFC (lateral and medial). These structures were previously associated with psychopathic traits in both adult and youth samples and feature in dominant theoretical models of psychopathy (Patrick, 1994; Kiehl, 2006; Blair, 2013).
We used the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) to extract volume estimations (mm3) of each region and of total grey matter (TGM). Proportional ROI volumes [(ROI/TGM) × 100] were then entered in separate multiple regression models, predicting total TriPM score, boldness, meanness and disinhibition, respectively. Multicollinearity was avoided by estimating separate models for each hemisphere, given that the volume of most ROIs was strongly correlated with that of its bilateral counterpart. In each regression, ROIs were included as predictors using a forward selection method, following Cope et al. (2012). This procedure selects and retains the predictor with the highest correlation with the dependent variable, provided that it increases the predictive power of the model above and beyond the intercept alone. Predictors that improve the model are sequentially added, until no predictors significantly alter its predictive power (Field, 2009). Age and IQ were not significantly correlated with GMV of any ROI and thus were not included in the regressions. Regression analyses were conducted in IBM SPSS 20 (IBM Corp., Armonk, NY).
Results
Variance in brain structure explained by total psychopathy score
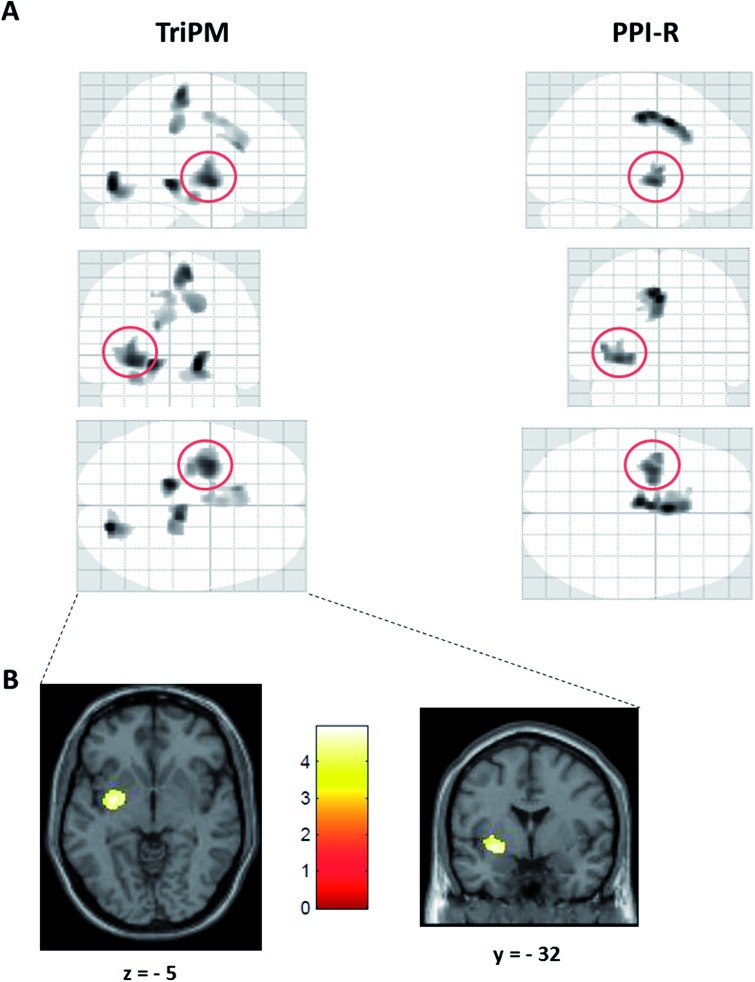
Controlling for TIV, age and IQ, total TriPM score was negatively associated with GMV volume in a cluster including the left striatum, specifically in the putamen (xyz = −30, −2, −8), which extended to the left amygdala when the threshold was set at P < 0.005 (Table 3; Figure 1). Results with total PPI-R score overlapped with those obtained with the TriPM in this cluster (Table 3; Figure 1A), consistent with the strong correlation observed between the two measures (r = 0.93, P < 0.001). However, this overlap was only apparent at an exploratory threshold, as PPI-R scores were not significantly associated with regional GMV at P < 0.001.
Table 3.
Clusters in which GMV was associated with TriPM and PPI-R total scores controlling for age, IQ and TIV (MNI coordinates are reported; P < 0.001, uncorrected, 341 voxel threshold - results presented in bold)
| Cluster | BA | x | y | z | t | Voxels | BA | x | y | z | t | Voxels | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TriPM total | PPI-R total | ||||||||||||
| Positive associations | Positive associations | ||||||||||||
| L precentral gyrus* | 6 | −45 | −3 | 62 | 4.27 | 459 | R brainstem* | 6 | −39 | −48 | 3.91 | 658 | |
| R superior frontal gyrus* | 8, 31, 6 | 18 | 14 | 50 | 4.20 | 355 | |||||||
| Negative associations | Negative associations | ||||||||||||
| R lingual gyrus* | 18 | 20 | −77 | −9 | 4.93 | 742 | |||||||
| L parahippocampal gyrus/hippocampus* | 30 | −15 | −33 | −9 | 4.64 | 565 | |||||||
| R paracentral lobule/medial frontal gyrus* | 6, 31 | 11 | −26 | 62 | 4.40 | 1014 | |||||||
| L striatum/insula | 13 | −30 | −2 | −8 | 4.44 | 564 | L striatum/insula/amygdala* | 13 | −32 | −5 | −8 | 3.42 | 766 |
| L striatum/insula/amygdala* | 13 | −30 | −2 | −8 | 4.44 | 1411 | L cingulate gyrus* | 24, 32 | −2 | 8 | 41 | 3.72 | 1130 |
| L cingulate gyrus* | 24 | −11 | 20 | 21 | 3.71 | 664 |
*Clusters found at an exploratory threshold of P < 0.005, uncorrected, 341 voxels.
Fig. 1.
(A) Glass brains depicting clusters in which GMV was negatively associated with total TriPM (left) and total PPI-R (right) after controlling for age, IQ and TIV. TriPM and PPI-R results overlap in a cluster including the left striatum, mid-posterior insula and amygdala (red circle), in which lower GMV was associated with higher total psychopathy scores (results displayed at an exploratory threshold of P < 0.005 uncorrected, 341 voxel threshold). (B) Negative association between TriPM total score and GMV in the left striatum (P < 0.001, uncorrected, 341 voxel threshold).
Variance in total psychopathy score explained by brain structure
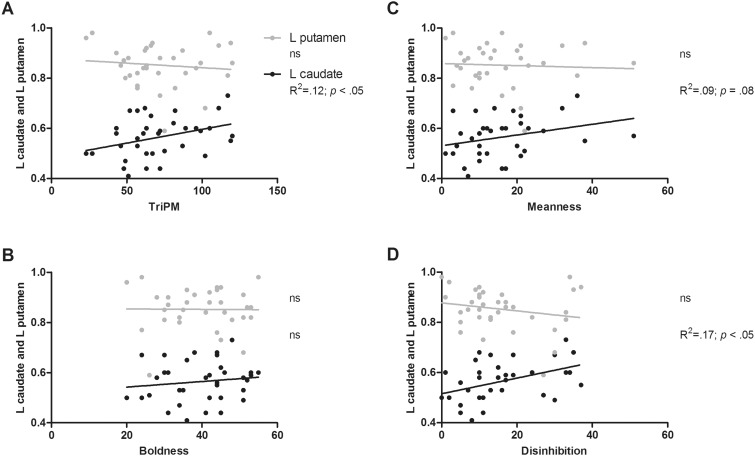
Regression analyses showed that volume within the left lateral OFC and left striatum predicted 24% of the variance in total TriPM score, with increased volume in these structures being associated with higher TriPM scores (lateral OFC: β = 0.51, P = 0.005; striatum: 0.35, P = 0.05). Results concerning the striatum seemed inconsistent with the whole-brain analysis, in which GMV in the left striatum (particularly putamen) was negatively associated with total TriPM. However, follow-up correlation analyses using TriPM scores and GMV within striatal structures (i.e. caudate, putamen and globus pallidus) showed that TriPM was only significantly correlated with GMV increase in the left caudate (r = 0.35, P = 0.04; Figure 2 A). Also, although not statistically significant, the correlation coefficient between TriPM and left putamen volume was in the direction predicted by the whole-brain analysis (r = –0.11; Figure 2A).
Fig. 2.
Bivariate associations between left caudate and putamen volumes, and total TriPM (A), Boldness (B), Meanness (C) and Disinhibition (D). Proportional ROI volumes calculated as a percentage of TGM are depicted in the y axis. Estimated left caudate and putamen volume varied between 2826 and 5864 mm3, and 4418 and 7560 mm3, respectively.
Variance in brain structure explained by boldness, meanness and disinhibition
To investigate volumetric alterations associated with each triarchic component, we performed a whole-brain multiple regression analysis with boldness, meanness and disinhibition as predictors, while controlling for age, IQ and TIV. Contrary to our predictions, results showed Boldness was only positively associated with GMV in the right pons (xyz = 9, −29, −41, t = 4.77, 481 voxels). Meanness and Disinhibition were not significantly associated with GMV in any brain regions.
Variance in boldness, meanness and disinhibition explained by brain structure
Regression analysis results showed that right insula volume explained 11% of the variance of boldness scores, with increased GMV in this region being associated with higher boldness scores (β = 0.34, P = 0.049).
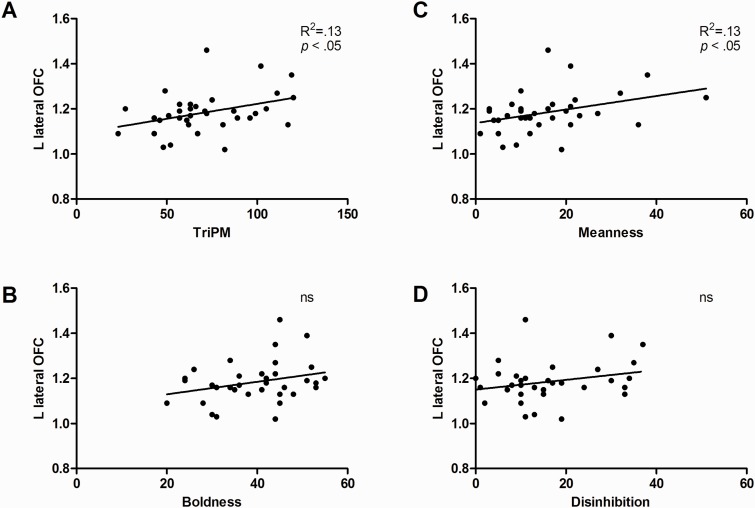
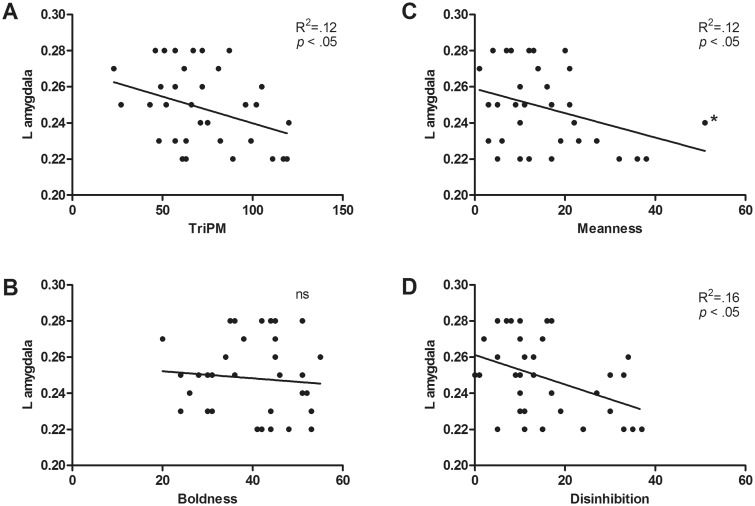
Volume in the left lateral OFC, striatum and amygdala volume explained 35% of the variance of meanness, such that higher scores in this subscale were associated with increased lateral OFC and striatum volumes (lateral OFC: β = 0.49, P < 0.01; striatum; β = 0.36, P = 0.03) and reduced amygdala volume (β =−0.30, P = 0.04: Figures 2, 3 , and 4C).
Fig. 3.
Bivariate associations between left lateral OFC volume and total TriPM (A), Boldness (B), Meanness (C) and Disinhibition (D). Proportional ROI volumes calculated as a percentage of TGM are depicted in the y axis. Estimated left lateral OFC volume varied between 6126 and 10798 mm3.
Fig. 4.
Bivariate associations between left amygdala volume and total TriPM (A), Boldness (B), Meanness (C) and Disinhibition (D). Proportional ROI volumes calculated as a percentage of TGM are depicted in the y axis. Estimated left amygdala volume varied between 1346 and 2247 mm3. *The leverage value of this observation was examined to ensure it was not significantly influencing the slope of the regression (0.03) and follow-up analysis confirmed the meanness × amygdala association remained significant after excluding this subject.
Finally, left amygdala volume predicted 14% of the variance of disinhibition, with higher scores being associated with decreased volume in that structure (β =−0.37, P = 0.027; Figures 4 D).
Discussion
In recent years, research has identified a set of brain regions that may be altered in individuals with heightened psychopathic tendencies, but limited evidence yet exists concerning the structural abnormalities that underlie specific components of psychopathy. Using a dimensional approach, we demonstrated that self-reported psychopathic traits in healthy individuals predict GMV variation in structures previously associated with psychopathy in institutionalized and clinical samples. Furthermore, we characterized the structural correlates of the boldness, meanness and disinhibition components of psychopathy. Two analytic strategies were used: one aimed to determine how psychopathy and the unique variance associated with each component explained variation of GMV across the whole brain; the other investigated how the unique variance of GMV in predefined anatomical regions explained total psychopathy and its subcomponents.
Structural alterations associated with total psychopathy
We expected overall psychopathy to predict GMV variation in five key structures—amygdala, OFC, ACC, insula and striatum. Our predictions were partially confirmed, with results showing volumetric alterations as a function of total psychopathy in the striatum and lateral OFC. Specifically, whole-brain and ROI analyses showed TriPM score was associated with grey volume reduction in the left putamen and volume increase in the left caudate, respectively. These findings are in agreement with previous reports of greater caudate volumes in individuals scoring high in the PCL-R (Glenn et al., 2010; Cope et al., 2012). They are also consistent with the findings reported by Wallace et al. (2013) and Fairchild et al. (2013) of reduced putamen volumes in youths with conduct disorder. Existing literature about structural striatal alterations in psychopathy is still scarce and mixed. For example, contrary to our results, Glenn et al. also found increased volume in the lenticular nuclei (putamen/globus pallidus) as a function of psychopathy, whereas Boccardi et al. (2013) reported atypical morphology in the caudate and putamen of PCL-R-assessed psychopaths, but no volumetric alterations (notably, this study did not control for age or IQ in their analysis, which has been shown to affect caudate measurements before—see Wallace et al. 2013). The present study thus adds a new finding to the picture, suggesting subclinical psychopathy may be associated with GMV alterations in some striatal structures, specifically reduced putamen and augmented caudate. These abnormalities may be linked to reward processing and decision-making impairments in individuals with elevated psychopathic traits (Blair, 2013) and are congruent with reports of alterations in striatal function in community samples varying in psychopathy (e.g. Bjork et al., 2012; Carre et al., 2013).
ROI regression analysis also revealed a positive association between total TriPM score and GMV in the left lateral OFC. This finding is consistent with prior research in antisocial adults assessed with the PCL-R (Cope et al., 2012) and in female adolescents with conduct disorder and high callous-unemotional traits (Fairchild et al., 2013), both recruited from the community. However, it is at odds with other studies pointing to reduced OFC volume in psychopathy. For instance, Yang et al. (2010) reported reduced OFC volume in unsuccessful psychopaths. Yet, not only was this finding not observed in successful psychopaths, but the study did not differentiate medial and lateral OFC. On the other hand, Ermer et al. (2012) reported decreased volume in the left lateral OFC specifically, using a sample of inmates with high PCL-R scores. The OFC has been considered one of the main neural candidates implicated in the affective and decision-making impairments observed in psychopathy (Blair, 2010, 2013), which raises questions about the reason for these inconsistencies. One possibility is that they are due to sampling differences across studies. It is noteworthy that our study, Cope et al., and Fairchild et al. all used participants recruited from the community and reported OFC findings in the same direction. It is known that institutionalization may be related to cortical structure (Sheridan et al., 2012). Variables associated with institutionalization, such as comorbidity, substance abuse and IQ, may also explain some of the variability in the results, as they have been differently accounted for in different studies. Altogether, these methodological differences may explain at least partially why results concerning OFC structure in psychopathy have been inconsistent. Another possibility is that OFC alterations are specific to certain dimensions of the psychopathic personality that may not have been assessed in previous studies. Accordingly, our results suggest OFC alterations may be predominantly associated with meanness-related traits.
Finally, our whole-brain analysis revealed a trend level negative association between left amygdala volume and total psychopathy, which was consistent with our predictions and with the significant correlation between TriPM and amygdala volume (Figure 2A). However, this trend was not confirmed by the ROI regression analysis, which suggested structural amygdala alterations may be more closely associated with specific components of psychopathy.
Structural alterations associated with triarchic components of psychopathy
Boldness is thought to result from a low fear genotype. Briefly, reduced proneness to stress is a direct consequence of the defensive system’s low responsivity to threat and punishment, whereas the high social dominance and thrill-seeking features result from reward seeking motivation unrestrained from fear and anxiety (Patrick et al., 2009). We had therefore predicted this component would be associated with alterations within the fear circuitry, namely in the amygdala, insula and OFC. However, among these structures, our analyses only revealed a positive association between boldness and right insula volume. The insula has been previously implicated in defensive networks (e.g. Mobbs et al., 2009; Gramsch et al., 2014), and its connectivity during threat responding is modulated by trait anxiety (Wheelock et al., 2014). More importantly, opposite structural alterations, i.e. reduced insula volume, have been reported in pathologically anxious patients (de Wit et al., 2014; Moon et al., 2014), supporting the idea that our insula findings may reflect low anxiety features captured by the boldness construct. Our whole-brain analysis also showed an unpredicted positive association between boldness and GMV in the pons. The pons is mainly involved in basic homeostatic and motor/sensory processes, with very few studies having reported alterations as a function of personality variables (e.g. neuroticism; Indovina et al., 2014); (alexithymia; Moriguchi et al., 2007). More research is therefore needed to ascertain the potential contribution of this structure to the boldness phenotype and rule out the possibility of this result being spurious.
Our results seem to support the triarchic model’s assumption that boldness-related traits, particularly those associated with reduced stress and anxiety, may reflect specificities of the defensive system. It should be noted, however, that no associations were found between boldness and GMV in other structures of the defensive circuitry, particularly the amygdala, using either of the analytic strategies we employed. This finding does not necessarily call into question the link between boldness and defensive system responsivity, as other structures implicated in defensive responses (i.e. insula) were indeed associated with boldness in our data. Likewise, it is not incongruent with earlier reports linking psychopathy to reduced sensitivity to threat and punishment (Birbaumer et al., 2005; Rothemund et al., 2012), on the one hand, and to amygdala dysfunction (Gordon et al., 2004; Blair, 2008; Marsh et al., 2008), on the other. However, it may indicate that the traits captured by the boldness construct, albeit related to affective-motivational processes, may not have driven previous findings of amygdala aberrations in psychopathy. Our data suggest that those findings are perhaps more specifically linked to meanness and disinhibition.
Meanness is characterized by poor empathy, lack of close attachments, cruelty and exploitativeness. It is believed to result from the interplay between low dispositional fear and negative environmental influences that disrupt socialization and compromise the capacity for affiliation (Patrick et al., 2009, 2012). Contrary to boldness, present data suggest meanness may be associated with structural changes in the amygdala, with decreased GMV in the amygdala predicting higher scores in this dimension. This is consistent with previous studies specifically linking abnormal amygdala activity during socio-affective tasks to the callousness dimension of psychopathy (Han et al., 2012; Sebastian et al., 2014), which in the triarchic framework is best described by the meanness construct. Moreover, this result is in line with previous demonstrations of dissociable patterns of neural and behavioral functioning associated with boldness- and meanness-related traits in socio-affective tasks that bear on amygdala function. Briefly, it has been demonstrated that fearless dominance and cold heartedness (PPI-R dimensions purported to tap the same traits as boldness and meanness, respectively; Drislane et al., 2013) predict inverse alterations in the amplitude of the N170 to emotional facial expressions, which could reflect differential modulation of the cortical generators of this component by the amygdala (Almeida et al., 2014). Likewise, at the behavioral level, previous work has demonstrated that interpersonal distance preferences in social interactions, which are shown to be regulated by the amygdala (Kennedy et al., 2009), are associated with cold heartedness but not with fearless dominance, nor with overall psychopathy score (Vieira and Marsh, 2014). Taken together with these data, our results point to a contribution of amygdala structural abnormalities to psychopathic traits related to low empathy, reduced attachment and interpersonal deviancy, in line with previous suggestions (Blair, 2008; Marsh, 2013).
Our data showed meanness was also associated with GMV increase in the lateral OFC and striatum. This is consistent with prior research showing an association between callous-unemotional traits and OFC volume in a sample of disordered youth (Fairchild et al., 2013). It is also in line with reports of augmented caudate body volume as a function of the affective-interpersonal factor or the PCL-R (Glenn et al., 2010). Importantly, these results parallel those for total TriPM score, suggesting those findings may have been mainly driven by affective-interpersonal traits. Our data thus support the centrality of those features to the psychopathy construct and illustrate how structural alterations putatively related to core affective deficits may be found in subclinical samples.
Finally, levels of disinhibition in our sample were related to grey matter reductions within the amygdala. Disinhibition has been theorized to reflect a general propensity for impulse control problems and poor emotional regulation, which probably result from dysfunctional anterior brain systems (Patrick, 2008; Patrick et al., 2009). We had therefore predicted alterations within the OFC, ACC and striatum as a function of disinhibition. Despite the significant bivariate correlation between disinhibition and left striatum volume (Figure 4D), regression analysis showed that decreased amygdala volume was the single predictor of high scores in this component after other ROIs were accounted for. This seems at odds with the proposed etiological basis of disinhibition-related traits, although it is in agreement with previous studies demonstrating both structural (Benegal et al., 2007; Sasayama et al., 2010) and functional amygdala abnormalities (Deveney et al., 2013; Wilbertz et al., 2013; Hulvershorn et al., 2014) in populations with high levels of externalizing traits, and with recent research showing larger amygdala volumes in inhibited individuals (Clauss et al., 2014).
Methodological considerations and limitations
In this study, we combined two analytical strategies to better characterize the volumetric brain alterations related to psychopathy and its subcomponents. These strategies did not always yield comparable results, which should be interpreted in light of the specificities of each methodology. Whole-brain analyses are more spatially sensitive, enabling the study of voxel-wise effects, but may lack statistical power when more stringent multiple comparison corrections are used. This may explain why amygdala volume was shown to contribute to variation in meanness and disinhibition in the ROI regressions despite no amygdala voxels being significant in the whole-brain analysis. Most previous studies that reported alterations in amygdala volume as a function of psychopathy have relied on more powerful ROI approaches (Yang et al., 2009, 2010; Pardini et al., 2014). Only the largest structural imaging study of psychopathy to date (N = 296) has reported an association between psychopathy and amygdala concentration, but not volume, using a whole-brain analysis (Ermer et al., 2012).
It is also worth noting that, among the triarchic components, whole-brain analyses only yielded significant results using a corrected threshold for boldness. In addition to power constraints, this may have resulted from the predominance of low scorers in meanness and disinhibition in our sample, particularly compared with boldness, for which higher levels were observed.
One potential limitation of this study was that sex was not controlled for in the analysis. Although we controlled for TIV, which accounted for a large proportion of sex-related variability in GMV (r = 0.571, P < 0.001) (Ruigrok et al., 2014), there may be relevant regional interactions between sex and psychopathic traits. However, our sample size did not enable meaningful comparisons between males and females. When sex was included in the whole-brain analysis, no psychopathy-related effects were found at a corrected threshold, which may have resulted from decreasing the degrees of freedom and further limiting the power of the analysis. In the ROI analyses, however, the inclusion of sex as a covariate did not alter the direction or significance of the results. It will be important for future studies seeking to investigate the potential influence of sex in psychopathy-related effects to incorporate larger samples of male and female participants. Our confidence in the present findings is supported by the inclusion of other important potential confounds (age and IQ), which have frequently not been controlled for in previous studies (e.g. Boccardi et al., 2011) and may have driven prior results.
Conclusions
To our knowledge, this study represents the first attempt to identify the neuroanatomical correlates of distinct psychopathic traits using the triarchic framework in a sample of healthy adults from the community. In using a community sample, we were not only able to avoid confounds frequently associated with institutionalized samples (e.g. comorbidity, substance abuse), but also focus specifically on the neural alterations that are associated with psychopathic personality traits rather than antisocial behavior. Overall, our results support previous suggestions that affective and behavioral impairments associated with psychopathic traits result from impairments in the amygdala, striatum and OFC. Furthermore, our results were consistent with some of the prior findings obtained in clinical and criminal samples, further strengthening the idea that meaningful psychopathy-related effects may be investigated using dimensional approaches in non-clinical populations (Drislane et al., 2014). Finally, present findings may contribute to assess the validity of the triarchic model’s assumptions regarding the etiology of the psychopathy components.
More research is needed to clarify the extent to which specific sample characteristics may explain remaining discrepancies in the literature about structural brain alterations in psychopathy (e.g. community vs institutionalized), as well as to describe the nature of those alterations (e.g. volumetric vs morphological).
Funding
This work was supported by a doctoral scholarship awarded to JBV (SFRH/BD/76254/2011), and a research grant (PTDC/PSI-PCO/114953/2009) by the Foundation for Science and Technology (Fundação para a Ciência e Tecnologia—FCT; Portugal). We also appreciate the support of the Intellectual and Development Disorders Research Center (IDDRC) grant to the Center for Functional and Molecular Imaging (CFMI) at Georgetown University (5P30HD040677-13).
Disclosure
Structural scans used in this study were collected as part of a larger project on fMRI analysis of socio-economic decision-making (Vieira et al., 2014), but the analyses reported here are original and independent from those previously reported.
References
- Almeida P.R., Ferreira-Santos F., Vieira J.B., Moreira P.S., Barbosa F., Marques-Teixeira J. (2014). Dissociable effects of psychopathic traits on cortical and subcortical visual pathways during facial emotion processing: an ERP study on the N170. Psychophysiology, 51(7), 645–57. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2010). VBM tutorial. Available:http://www.fil.ion.ucl.ac.uk/∼john/misc/VBMclass10.pdf.
- Ashburner J., Friston K.J. (2005). Unified segmentation. Neuroimage, 26(3), 839–51. [DOI] [PubMed] [Google Scholar]
- Benegal V., Antony G., Venkatasubramanian G., Jayakumar P.N. (2007). Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction Biology, 12(1), 122–32. [DOI] [PubMed] [Google Scholar]
- Bertsch K., Grothe M., Prehn K., et al. (2013). Brain volumes differ between diagnostic groups of violent criminal offenders. European Archives of Psychiatry and Clinical Neuroscience, 263(7), 593–606. [DOI] [PubMed] [Google Scholar]
- Birbaumer N., Veit R., Lotze M., et al. (2005). Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry, 62(7), 799–805. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Chen G., Hommer D.W. (2012). Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biological Psychology, 89(2), 408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J. (2004). The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition, 55(1), 198–208. [DOI] [PubMed] [Google Scholar]
- Blair R.J. (2008). The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society London B Biological Sciences, 363(1503), 2557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J. (2010). Neuroimaging of psychopathy and antisocial behavior: a targeted review. Current Psychiatry Reports, 12(1), 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J. (2013). The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience, 14(11), 786–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M., Bocchetta M., Aronen H.J., et al. (2013). Atypical nucleus accumbens morphology in psychopathy: another limbic piece in the puzzle. International Journal of Law and Psychiatry, 36(2), 157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M., Frisoni G.B., Hare R.D., et al. (2011). Cortex and amygdala morphology in psychopathy. Psychiatry Research, 193(2), 85–92. [DOI] [PubMed] [Google Scholar]
- Budhani S., Richell R.A., Blair R.J. (2006). Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. Journal of Abnormal Psychology, 115(3), 552–8. [DOI] [PubMed] [Google Scholar]
- Carre J.M., Hyde L.W., Neumann C.S., Viding E., Hariri A.R. (2013). The neural signatures of distinct psychopathic traits. Society for Neuroscience, 8(2), 122–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J.A., Seay A.L., VanDerKlok R.M., et al. (2014). Structural and functional bases of inhibited temperament. Social Cognitive & Affective Neuroscience, 9(12), 2049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke D.J., Michie C. (2001). Refining the construct of psychopathy: towards a hierarchical model. Psychological Assessment, 13(2), 171–88. [PubMed] [Google Scholar]
- Cope L.M., Shane M.S., Segall J.M., et al. (2012). Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Research, 204(2–3), 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito S.A., Mechelli A., Wilke M., et al. (2009). Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain, 132(Pt 4), 843–52. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R., Hare R.D., Bramati I.E., et al. (2008). Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage, 40(3), 1202–13. [DOI] [PubMed] [Google Scholar]
- de Wit S.J., Alonso P., Schweren L., et al. (2014). Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. American Journal of Psychiatry, 171(3), 340–9. [DOI] [PubMed] [Google Scholar]
- Decety J., Chen C., Harenski C., Kiehl K.A. (2013). An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Frontiers in Human Neuroscience, 7, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveney C.M., Connolly M.E., Haring C.T., et al. (2013). Neural mechanisms of frustration in chronically irritable children. American Journal of Psychiatry, 170(10), 1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drislane L.E., Patrick C.J., Arsal G. (2013). Clarifying the content coverage of differing psychopathy inventories through reference to the triarchic psychopathy measure. Psychological Assessment, 26(2), 350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drislane L.E., Patrick C.J., Sourander A., et al. (2014). Distinct variants of extreme psychopathic individuals in society at large: evidence from a population-based sample. Personal Disorders, 5(2), 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermer E., Cope L.M., Nyalakanti P.K., Calhoun V.D., Kiehl K.A. (2012). Aberrant paralimbic gray matter in criminal psychopathy. Journal of Abnormal Psychology, 121(3), 649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermer E., Cope L.M., Nyalakanti P.K., Calhoun V.D., Kiehl K.A. (2013). Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry, 52(1), 94–103 e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G., Hagan C.C., Walsh N.D., Passamonti L., Calder A.J., Goodyer I.M. (2013). Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry, 54(1), 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, A. (2009). Discovering Statistics Using SPSS. 3rd Edition. Los Angeles: SAGE Publications.
- Frick P.J., White S.F. (2008). Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry, 49(4), 359–75. [DOI] [PubMed] [Google Scholar]
- Glenn A.L., Raine A., Yaralian P.S., Yang Y. (2010). Increased volume of the striatum in psychopathic individuals. Biological Psychiatry, 67(1), 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H.L., Baird A.A., End A. (2004). Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry, 56(7), 516–21. [DOI] [PubMed] [Google Scholar]
- Gramsch C., Kattoor J., Icenhour A., Forsting M., Schedlowski M., Gizewski E.R., Elsenbruch S. (2014). Learning pain-related fear: neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain. Neurobiological of Learning and Memory, 116, 36–45. [DOI] [PubMed] [Google Scholar]
- Gregory S., ffytche D., Simmons A., et al. (2012). The antisocial brain: psychopathy matters. Archives of General Psychiatry, 69(9), 962–72. [DOI] [PubMed] [Google Scholar]
- Gu X., Liu X., Guise K.G., Naidich T.P., Hof P.R., Fan J. (2010). Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. Journal of Neuroscience, 30(10), 3739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.R., Drislane L.E., Patrick C.J., Morano M., Lilienfeld S.O., Poythress N.G. (2014). Development and validation of triarchic construct scales from the psychopathic personality inventory. Psychological Assessment, 26(2), 447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T., Alders G.L., Greening S.G., Neufeld R.W., Mitchell D.G. (2012). Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Social Cognitive and Affective Neuroscience, 7(8), 958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare R.D. (1980). A research scale for the assessment of psychopathy in criminal populations. Personality and Individual Differences, 1, 111–9. [Google Scholar]
- Hare R.D., Harpur T.J., Hakstian A.R., Forth A.E., Hart S.D., Newman J.P. (1990). The revised psychopathy checklist: reliability and factor structure. Psychological Assessment, 2, 338–41. [Google Scholar]
- Huettel S., Song A., McCarthy G. (2008). Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- Hulvershorn L.A., Mennes M., Castellanos F.X., et al. (2014). Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 53(3), 351–61 e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I., Riccelli R., Staab J.P., Lacquaniti F., Passamonti L. (2014). Personality traits modulate subcortical and cortical vestibular and anxiety responses to sound-evoked otolithic receptor stimulation. Journal of Psychosomatic Research, 77(5), 391–400. [DOI] [PubMed] [Google Scholar]
- Kaufman A.S. (1990). K-BIT: Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Kennedy D.P., Glascher J., Tyszka J.M., Adolphs R. (2009). Personal space regulation by the human amygdala. Nature Neuroscience, 12(10), 1226–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A. (2006). A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research, 142(2–3), 107–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Baskin-Sommers A., Zeier J., Newman J.P. (2011). Investigating the neural correlates of psychopathy: a critical review. Molecular Psychiatry, 16(8), 792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld S.O. (1998). Methodological advances and developments in the assessment of psychopathy. Behaviour Research and Therapy, 36(1), 99–125. [DOI] [PubMed] [Google Scholar]
- Lilienfeld S.O., Widows M.R. (2005). PPI-R: Psychopathic Personality Inventory—Revised. Lutz, FL: Psychological Assessment Resources. [DOI] [PubMed] [Google Scholar]
- Lockwood P.L., Sebastian C.L., McCrory E.J., et al. (2013). Association of callous traits with reduced neural response to others' pain in children with conduct problems. Current Biology, 23(10), 901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus D.K., John S.L., Edens J.F. (2004). A taxometric analysis of psychopathic personality. Journal of Abnormal Psychology, 113(4), 626–35. [DOI] [PubMed] [Google Scholar]
- Marsh A.A. (2013). What can we learn about emotion by studying psychopathy? Frontiers in Human Neuroscience, 7, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A., Cardinale E.M. (2014). When psychopathy impairs moral judgments: neural responses during judgments about causing fear. Social Cognitive & Affective Neuroscience, 9(1), 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Fowler K.A., et al. (2013). Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. Journal of Child Psychology and Psychiatry, 54(8), 900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G., et al. (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry, 165(6), 712–20. [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Stoycos S.A., Brethel-Haurwitz K.M., Robinson P., VanMeter J.W., Cardinale E.M. (2014). Neural and cognitive characteristics of extraordinary altruists. Proceedings of the National Academy of Sciences of the United States of America, 111(42), 15036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.G., Colledge E., Leonard A., Blair R.J. (2002). Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia, 40(12), 2013–22. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Marchant J.L., Hassabis D., et al. (2009). From threat to fear: the neural organization of defensive fear systems in humans. Journal of Neuroscience, 29(39), 12236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C.M., Kim G.W., Jeong G.W. (2014). Whole-brain gray matter volume abnormalities in patients with generalized anxiety disorder: voxel-based morphometry. Neuroreport, 25(3), 184–9. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y., Decety J., Ohnishi T., et al. (2007). Empathy and judging other’s pain: an fMRI study of alexithymia. Cerebral Cortex, 17(9), 2223–34. [DOI] [PubMed] [Google Scholar]
- Muller J.L., Ganssbauer S., Sommer M., et al. (2008). Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry Research, 163(3), 213–22. [DOI] [PubMed] [Google Scholar]
- Pardini D.A., Raine A., Erickson K., Loeber R. (2014). Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits, and future violence. Biological Psychiatry, 75(1), 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C.J. (1994). Emotion and psychopathy: startling new insights. Psychophysiology, 31(4), 319–30. [DOI] [PubMed] [Google Scholar]
- Patrick C.J. (2008). Psychophysiological correlates of aggression and violence: an integrative review. Philosophical Transactions of the Royal Society London B Biological Sciences, 363(1503), 2543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C.J. (2010). Operationalizing the Triarchic Conceptualization of Psychopathy: Preliminary Description of Brief Scales for Assessment of Boldness, Meanness, and Disinhibition. Florida State University. [Google Scholar]
- Patrick C.J., Bernat E.M. (2009). Neurobiology of psychopathy: a two-process theory. In: Berntson G.G., Cacioppo J.T., editors. Handbook of Neuroscience for the behavioral Sciences. New York: John Wiley & Sons. [Google Scholar]
- Patrick C.J., Drislane L.E., Strickland C.D. (2012). Conceptualizing psychopathy in triarchic terms: implications for treatment. International Journal of Forensic Mental Health, 11, 253–66. [Google Scholar]
- Patrick C.J., Fowles D.C., Krueger R.F. (2009). Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology, 21(3), 913–38. [DOI] [PubMed] [Google Scholar]
- Rothemund Y., Ziegler S., Hermann C., et al. (2012). Fear conditioning in psychopaths: event-related potentials and peripheral measures. Biological Psychology, 90(1), 50–9. [DOI] [PubMed] [Google Scholar]
- Ruigrok A.N., Salimi-Khorshidi G., Lai M.C., et al. (2014). A meta-analysis of sex differences in human brain structure. Neuroscience & Biobehavioral Reviews, 39, 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N., Spielberg J.M., Heller W., et al. (2013). Emotion disrupts neural activity during selective attention in psychopathy. Social Cognitive and Affective Neuroscience, 8(3), 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D., Hayashida A., Yamasue H., et al. (2010). Neuroanatomical correlates of attention-deficit-hyperactivity disorder accounting for comorbid oppositional defiant disorder and conduct disorder. Psychiatry and Clinical Neurosciences, 64(4), 394–402. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., McCrory E.J., Dadds M.R., et al. (2014). Neural responses to fearful eyes in children with conduct problems and varying levels of callous-unemotional traits. Psychological Medicine, 44(1), 99–109. [DOI] [PubMed] [Google Scholar]
- Sellbom M., Phillips T.R. (2013). An examination of the triarchic conceptualization of psychopathy in incarcerated and nonincarcerated samples. Journal of Abnormal Psychology, 122(1), 208–14. [DOI] [PubMed] [Google Scholar]
- Sellbom M., Wygant D.B., Drislane L.E. (2014). Elucidating the construct validity of the psychopathic personality inventory triarchic scales. Journal of Personality Assessment, 17, 1–8. [DOI] [PubMed] [Google Scholar]
- Sheridan M.A., Fox N.A., Zeanah C.H., McLaughlin K.A., Nelson C.A., III. (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America, 109(32), 12927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T., Seymour B., O'Doherty J., Kaube H., Dolan R.J., Frith C.D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–62. [DOI] [PubMed] [Google Scholar]
- Skeem J.L., Polaschek D.L., Patrick C.J., Lilienfeld S.O. (2011). Psychopathic personality: bridging the gap between scientific evidence and public policy. Psychological Science in the Public Interest, 12(3), 95–162. [DOI] [PubMed] [Google Scholar]
- Stanley J.H., Wygant D.B., Sellbom M. (2013). Elaborating on the construct validity of the triarchic psychopathy measure in a criminal offender sample. Journal of Personality Assessment, 95(4), 343–50. [DOI] [PubMed] [Google Scholar]
- Vieira J.B., Almeida P.R., Ferreira-Santos F., Barbosa F., Marques-Teixeira J., Marsh A.A. (2014). Distinct neural activation patterns underlie economic decisions in high and low psychopathy scorers. Social Cognitive and Affective Neuroscience, 9(8), 1099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J.B., Marsh A.A. (2014). Don’t stand so close to me: psychopathy and the regulation of interpersonal distance. Frontiers in Human Neuroscience, 7, 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G.L., White S.F., Robustelli B., et al. (2013). Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. Journal of the American Academy of Child and Adolescent Psychiatry, 53(4), 456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. (2000). Simultaneous Inference for fMRI Data. Available: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Wheelock M.D., Sreenivasan K.R., Wood K.H., Ver Hoef L.W., Deshpande G., Knight D.C. (2014). Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage, 102 (Pt 2), 904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom C.S. (1977). A methodology for studying noninstitutionalized psychopaths. Journal of Consulting and Clinical Psychology, 45(4), 674–83. [PubMed] [Google Scholar]
- Wilbertz G., Trueg A., Sonuga-Barke E.J., Blechert J., Philipsen A., Tebartz van Elst L. (2013). Neural and psychophysiological markers of delay aversion in attention-deficit hyperactivity disorder. Journal of Abnormal Psychological, 122(2), 566–72. [DOI] [PubMed] [Google Scholar]
- Yang Y., Raine A., Colletti P., Toga A.W., Narr K.L. (2009). Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Molecular Psychiatry, 14(6), 561–2. [DOI] [PubMed] [Google Scholar]
- Yang Y., Raine A., Colletti P., Toga A.W., Narr K.L. (2010). Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. Journal of Abnormal Psychological, 119(3), 546–54. [DOI] [PubMed] [Google Scholar]
- Yang Y., Raine A., Lencz T., Bihrle S., LaCasse L., Colletti P. (2005). Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biological Psychiatry, 57(10), 1103–8. [DOI] [PubMed] [Google Scholar]
- Yang Y., Raine A., Narr K.L., Colletti P., Toga A.W. (2009). Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry, 66(9), 986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]