Abstract
Depletion imposes both need and desire to drink, and potentiates the response to need-relevant cues in the environment. The present fMRI study aimed to determine which neural structures selectively increase the incentive value of need-relevant stimuli in a thirst state. Towards this end, participants were scanned twice—either in a thirst or no-thirst state—while viewing pictures of beverages and chairs. As expected, thirst led to a selective increase in self-reported pleasantness and arousal by beverages. Increased responses to beverage when compared with chair stimuli were observed in the cingulate cortex, insular cortex and the amygdala in the thirst state, which were absent in the no-thirst condition. Enhancing the incentive value of need-relevant cues in a thirst state is a key mechanism for motivating drinking behavior. Overall, distributed regions of the motive circuitry, which are also implicated in salience processing, craving and interoception, provide a dynamic body-state dependent representation of stimulus value.
Keywords: deprivation, drinking, fMRI, motivation, saliency network
Introduction
Thirst provides a powerful demonstration of motivation in action. Humans need to keep fluid levels in balance and even short periods without water endanger survival. Fluid ingestion thus provides a main biological imperative and strong motivation assures that drinking is prioritized in accordance with internal needs. Similar to other vital functional behavior systems for feeding, defense and sexual reproduction, a complex drinking/osmoregulation system controls stimulus sensitivities, motor responses and motivational conditions surrounding fluid ingestion (Timberlake et al., 2001; Watts, 2001). According to this behavior systems view, fluid depletion has motivational effects on multiple levels of the drinking system, ranging from the initiation of appetitive search behaviors to the modulation of anticipatory and consummatory responses.
In recent years, great progress has been made in identifying the neural mechanisms of thirst and the brain systems involved in osmoregulation. Numerous physiological studies have explored the interoceptive signals of dehydration and their sensing by key motivational structures such as the hypothalamus (Johnson and Thunhorst, 1997; Sternson, 2013). More recently, neuroimaging methods enabled the study of thirst- and drinking-related brain responses in humans (e.g. Denton et al., 1999b; Hallschmid et al., 2001). The majority of neuroimaging studies examined the drinking system by measuring activity changes commanded by hypertonic saline infusions, which trigger a decrease in plasma osmolality. These studies found that saline infusion led to increased brain activity in the anterior-, mid-cingulate (ACC, MCC) and insular cortices (INS; Denton et al., 1999a,b; Parsons et al., 2000; Egan et al., 2003; Farrell et al., 2008, 2006). Furthermore, activity in the ACC and posterior cingulate cortex (PCC) was correlated with both, feelings of thirst and plasma sodium concentrations, suggesting that these regions are implicated in the conscious representation of thirst (Denton et al., 1999a,b; Parsons et al., 2000; Farrell et al., 2008). A related line of research relied on water depletion to manipulate thirst and generally confirmed the findings obtained via saline infusion (Farrell et al., 2011).
Further studies explored the neural processes associated with the consummatory stage of motivated behaviors, i.e. the sensing and consuming of water. In these studies, administering small quantities of water prompted increased regional cerebral blood flow (rCBF) and blood-oxygen-level dependent (BOLD) responses in the insular cortex, especially in posterior and middle regions, as well as in the amygdala (AMY) and lateral orbitofrontal cortex (OFC; Frey and Petrides, 1999; Zald and Pardo, 2000; Wagner et al., 2006; Yang et al., 2008). Furthermore, the incentive value of water decreases during consumption, and this process plays an important role in the cessation of drinking. Possible neural correlates involve the mid-insular cortex and medial OFC, which showed a decrease in activation after the consumption of water when compared with the depleted state (de Araujo et al., 2003). Moreover, the subjective pleasantness of water is positively correlated with BOLD activity in OFC, ACC and the insular cortex (de Araujo et al., 2003; Saker et al., 2014). Of note, these regions have also been shown to be involved in motivational processes by studies on fear, food, sexual behaviors or drugs (Kober et al., 2008; van der Laan et al., 2010; Kühn and Gallinat, 2011; Sescousse et al., 2013). Overall, neural regions of the drinking system include core structures of the brain’s motive circuitry.
However, little is known about how motivation shapes brain responses during the appetitive stage of behavior and the responding to motivationally relevant stimuli (Craig, 1918; Konorski, 1967). In the case of the drinking system, the appetitive stage of motivated behavior consists of exploratory and approach behaviors that bring the organism into contact with fluid stimuli. This stage hosts a phenomenon everybody is familiar with: A glass of water looks more desirable when one is thirsty. Incentive motivation theory suggests that this effect reflects the dynamic shifting of stimulus value due to a body state of fluid depletion (Konorski, 1967; Bindra, 1978; Toates, 1986). Specifically, viewing a glass of water arouses relatively low motivation to drink in conditions of sufficient hydration. Conversely, viewing the same glass of water effectively arouses high motivation to drink in a fluid depleted organism. The significance of distinguishing between the appetitive and consummatory phases of ingestive behavior has been stressed recently. Specifically, in the food domain, appetitive behavior is cued by distinct environmental cues and acts on at least partially distinct neuroendocrine factors and neural structures when compared with consummatory behaviors (Keen-Rhinehart et al., 2013). In this vein, research focusing on neural correlates related to drinking is complemented by this study with regard to thirst-modulated processing of drinking-related stimuli.
The main goal of this study was to determine which regions of the brain’s motivational circuitry provide the dynamic and body state dependent representation of stimulus value most relevant to the organization of purposeful behavior. To examine the neural basis of motivation-dependent processing in the drinking system, we scanned participants who were either thirsty (7 h fluid-depleted) or not thirsty while they viewed pictures of beverages and chairs. We assumed that thirst selectively increases the processing of drinking-related stimuli in the anterior and PCC, insula, AMY and OFC. These neural structures were hypothesized based on previous findings regarding neural structures associated with body-state dependent regulation of consummatory behaviors towards water stimuli (de Araujo et al., 2003; Wagner et al., 2006; Saker et al., 2014) as well as studies investigating the effects of hunger on neural responses towards appetitive food stimuli (LaBar et al., 2001; Mohanty et al., 2008; Goldstone et al., 2009). A signed interaction term assured that the interaction effects were limited to voxels showing increased responding to drinking-related stimuli when thirsty rather than larger activations to chair stimuli when participants were not thirsty. Furthermore, main effects were analyzed to determine neural regions providing a state-independent representation of stimulus value and representing body state.
Materials and methods
Participants
Twenty-four participants (12 females, 1 left-handed) between 20 and 32 years of age (M = 22.9) with normal or corrected-to-normal vision participated in the study. Participants were recruited at the University of Konstanz and received either course credit or €8 per hour. The study was approved by the ethical review board of the University of Konstanz and informed consent was acquired from all participants. Participants were not included in the study when they had a history or currently suffered from psychiatric, neurological or endocrine diseases or taking medication that affects the endocrine or central nervous system.
Stimulus materials
The stimulus materials comprised pictures of beverages (n = 24) and chairs (n = 24), which were shown on a uniform gray-colored background. A pilot study (n = 12) revealed that water, apple juice and tea were regarded as the most potent thirst-quenching beverages. Accordingly, the beverage stimuli depicted glasses filled with water, apple juice and tea. None of the beverage pictures included commercial labels. Similar to previous studies on the neural correlates of object recognition (Haxby et al., 2001; Downing et al., 2006), pictures of chairs were chosen as control category. Chair pictures were selected to match the beverage pictures in terms of complexity, size and overall appearance.
Procedure
An initial screening session served to inform participants about the study, including the requirement to refrain from drinking to evoke thirst, and to check their eligibility for fMRI scanning. Participants were scanned in two sessions while being either thirsty or not thirsty. The order of these sessions was counter-balanced and ∼1 week apart. To control for variations in circadian rhythm, all sessions were scheduled at 6 pm. For the thirst session, participants had to completely refrain from drinking for 7 h. For the no-thirst session, participants were instructed to follow their normal drinking and eating habits. All participants reported that they had fully complied with these instructions.
At the start of both sessions, participants reconfirmed their informed consent and were prepared for MR scanning. Before entering the scanner, thirst and hunger were probed using rating scales. During fMRI scanning, participants viewed pictures of beverages and chairs, which were presented using a visual system (NordicNeuroLab, Bergen, Norway) positioned in front of the subject’s eyes. The 48 pictures comprising both categories were repeated three times, resulting in 144 trials. In a slow event-related paradigm, pictures were shown for 2 s, followed by a variable inter-stimulus interval (ISI) showing a white fixation cross on a black background. The ISI was exponentially distributed with a mean of 7.5 s and a range of 6–12 s (see e.g. Amaro and Barker, 2006). After obtaining a second rating of thirst and hunger, a T1-weighted structural scan was recorded. A third rating of thirst and hunger was obtained after participants left the scanner, and they additionally rated all pictures on emotional dimensions of valence and arousal. Finally, the participants were provided with as much water as desired.
Self-report data
Thirst and hunger ratings were collected using a 7-point Likert scale, ranging from not thirsty/not hungry to very thirsty/very hungry, respectively. Rating data were obtained immediately before, mid-term, and at the end of MR scanning. These data were entered into a two factorial repeated measures ANOVA with the factors ‘Body State’ (thirst vs no-thirst) and ‘Time’ (pre- vs mid-term vs post).
Participants used the Self Assessment Manikin (Bradley and Lang, 1994) to rate the stimuli on emotional dimensions of valence and arousal on a 9-point Likert scale, ranging from unpleasant over neutral to pleasant for valence and from calm to exciting for arousal. Valence and arousal ratings were entered into a two factorial repeated measures ANOVA with the factors ‘Body State’ (thirst vs no-thirst) and ‘Category’ (beverages vs chairs).
Where appropriate, the Greenhouse-Geisser procedure was used to correct for violations of sphericity. Post-hoc tests were corrected using the Bonferroni method.
MRI data acquisition and analysis
MR acquisition took place on a 1.5 Philips Intera MR system (Philips, Hamburg, Germany). For functional scanning, a T2*-weighted Fast Field Echo, Echo Planar Imaging sequence utilizing parallel scanning technique was used (SENSE; Pruessmann et al., 1999). In plane resolution was 3 × 3 mm and slice thickness was 3.5 mm (32 axial slices; no gap; FOV = 240 mm; acquisition matrix = 80 × 80; TE = 40 ms; flip angle = 90°; TR = 2500 ms). In addition, a standard T1-weighted high-resolution structural scan with 1 × 1 × 1 mm voxel resolution was obtained.
Pre-processing and statistical analysis of the functional data were conducted using SPM8 (Wellcome Department of Imaging Neuroscience, University College London, UK; Friston et al., 1994). Pre-processing steps included realignment and slice time correction for the functional images. No subject displayed head movements exceeding 3 mm or 3° on any axis and thus data from all participants were included in further analysis. Images were normalized to the MNI EPI template and resampled at 3 × 3 × 3 mm voxel size. A Gaussian spatial kernel of full width at half maximum with an 8-mm radius was used for smoothing the data.
Single subject data were modeled with a thirst and a no-thirst session, each containing two covariates of interest representing beverage and chair condition onsets as well as covariates of no interest, including six movement parameters obtained during realignment and one covariate incorporating an overall intercept to the model. Group level random effects analysis combined all subjects’ covariates of interest into a full factorial model with the factors ‘Body State’ (thirst vs no-thirst) and ‘Category’ (beverages vs chairs). Based on our a priori hypotheses, a linear contrast with (BeveragesThirst > ChairsThirst) vs (BeveragesNo-thirst > ChairsNo-thirst) was calculated to test for the interaction of ‘Body State by Category’. A further constraint assured that the interaction examines a signed interaction, which is only sensitive for an enlarged activation of beverages over chairs during the thirst state, excluding voxels showing enlarged activation for chairs over beverages during the no-thirst state. The signed interaction test was realized through (i) a cross interaction contrast with a statistical threshold of P < 0.05 (False discovery rate (FDR) corrected at voxel level) with a voxel extent of k ≥ 15 voxels, and (ii) inclusively masking the result with the main effect of ‘beverage > chair’ at P < 0.05. To test for a reversed interaction effect, the linear contrast for (ChairsThirst > BeveragesThirst) vs (ChairsNo-thirst > BeveragesNo-thirst) was also calculated. As we did not have any a priori hypotheses, no further constraints were applied onto this contrast. Furthermore, linear contrasts were calculated for the main effects of ‘Category’ and ‘Body State’ (FDR P < 0.05, k ≥ 15 voxel).
Results
Thirst and hunger ratings
Participants were significantly more thirsty in the thirst (M = 5.9, SE = 0.15) when compared with the no-thirst (M = 2.9, SE = 0.22) condition, F(1, 23) = 193.7, P < 0.0001, partial η2 = 0.89. Furthermore, within sessions, thirst ratings varied across the three measurements, F(2, 46) = 25.6, P < 0.001, partial η2 = 0.53. Specifically, ratings of thirst were larger for the second (M = 4.7, SE = 0.17) and third (M = 4.7, SE = 0.21) when compared with the first (M = 3.8, SE = 0.15) rating at the beginning of the session, ts(23) > 5.3, P < 0.001. Finally, a significant interaction of ‘Body State’ and ‘Time’ was obtained, F(2, 46) = 6.4, P < 0.005, partial η2 = 0.22, indicating that the increase of thirst across the session was somewhat larger for the no-thirst (First: M = 2.0, SE = 0.23; Second: M = 3.3, SE = 0.24; Third: M = 3.3, SE = 0.28; ts(23) > 5.7 and 5.9, P < 0.001) than the thirst condition (First: M = 5.5, SE = 0.17; Second: M = 6.1, SE = 0.17; Third: M = 6.0, SE = 0.19; ts(23) = 3.4 and 2.6, P < 0.05.
The analysis of hunger ratings revealed a significant main effect of ‘Time’, F(2, 46) = 17.6, P < 0.001, partial η2 = 0.43, indicating that hunger ratings increased from the first (M = 2.5, SE = 0.20) over the second (M = 2.9, SE = 0.21) to the third (M = 3.2, SE = 0.25) rating, [pre- vs mid-term: t(23) = −3.8, P < 0.005; pre- vs post: t(23) = −5.0, P < 0.001; mid-term vs post: t(23) = −2.9, P < 0.05]. However, no significant main effect of ‘Body State’, F(1, 23) = 2.6, n.s., nor an interaction involving the factor ‘Body State’, F(2, 46) = 2.0, n.s., was observed.
Stimulus valence and arousal
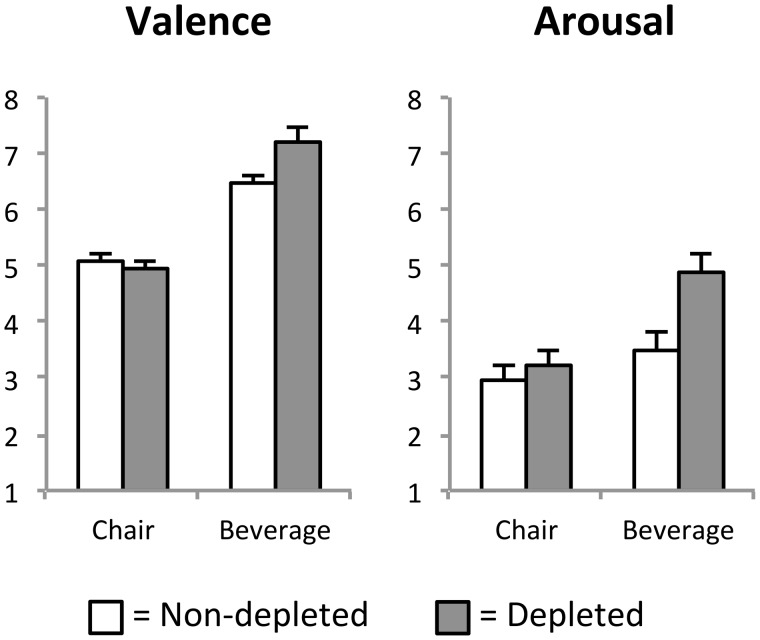
Significant main effects of ‘Category’ were obtained for valence and arousal ratings, Valence F(1, 23) = 67.2, P < 0.001, partial η2 = 0.75, Arousal F(1, 23) = 13.9, P < 0.001, partial η2 = 0.38. Specifically, beverage when compared with chair pictures were perceived as more pleasant (M = 6.8 and 5.0, SE = 0.17 and 0.13) and more arousing (M = 4.1 and 3.2, SE = 0.25 and 0.26, respectively). However, these main effects were qualified by significant interactions of ‘Body State’ × ‘Category’ for both valence and arousal ratings, Valence F(1, 23) = 9.0, P < 0.01, partial η2 = 0.28, Arousal F(1, 23) = 7.3, P < 0.05, partial η2 = 0.24. As shown in Figure 1, thirst selectively affected the evaluation of beverage pictures. When compared with the control condition, beverage pictures were perceived as more pleasant [t(23) = 2.5, P < 0.05] and arousing [t(23) = 3.8, P < 0.001] in the thirst condition. In contrast, valence and arousal ratings of chair pictures did not differ between thirst and no-thirst states, Valence: t(23) = −1.0, n.s., Arousal: t(23) = 1.4, n.s. Finally, a significant effect of ‘Body State’ was observed for arousal ratings, F(1, 23) = 14.3, P < 0.001, partial η2 = 0.38, with increased arousal ratings during a thirst when compared with a no-thirst state.
Fig. 1.
Illustration of valence and arousal ratings for beverage and chair picture categories. Both valence and arousal ratings showed an interaction of ‘Body State by Category’.
fMRI data
Interaction between stimulus category and a body state of thirst
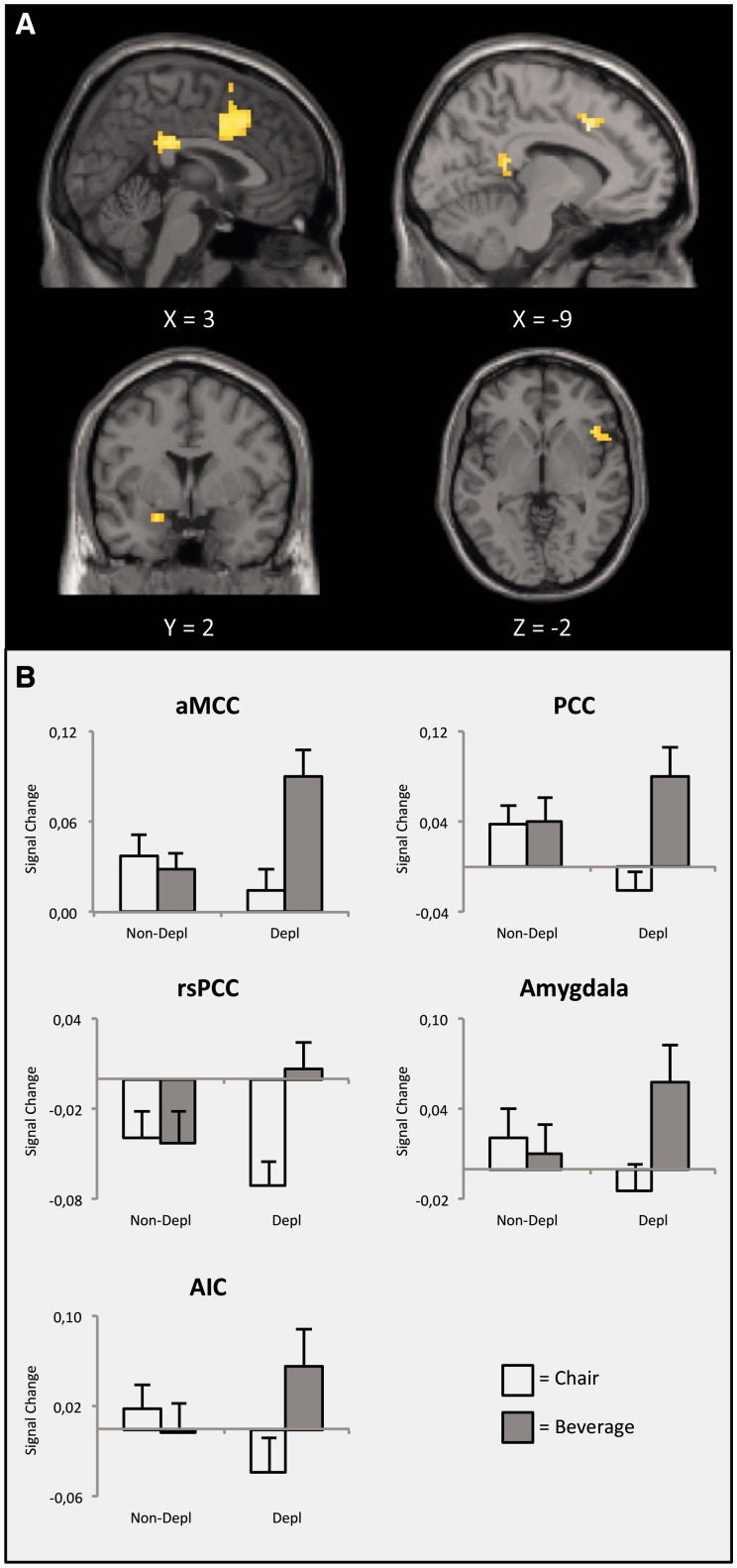
The selective effect of thirst on the processing of beverage pictures was examined by the linear cross interaction contrast (BeveragesThirst > ChairsThirst) vs (BeveragesNo-thirst > ChairsNo-thirst). With regard to the a priori hypothesis, significant interactions were observed in the anterior MCC (aMCC), PCC, AMY (left) and anterior INS (right) (see Figure 2A). As shown in Figure 2B, increased BOLD responses to beverage when compared with chair pictures were observed in these brain regions during thirst state. In contrast, there were no differences between beverage and chair categories during the no-thirst state. Additional structures showing a significant interaction included the dorsal precentral gyrus (right), ventral postcentral gyrus (right), supramarginal gyrus (left) and inferior parietal lobule (left) (see Table 1).
Fig. 2.
Sagittal, coronal, and axial sections showing the interaction of ‘Body State by Category’ in the cingulate cortex, AMY and anterior insula. Statistical maps are thresholded at P < 0.05 (FDR) with a voxel extent of k ≥ 15 (A). Extracted signal changes relative to baseline activation corresponding to the regions presented under A, illustrating an interaction effect of ‘Body State by Category’ (B). Abbreviations: aMCC, anterior middle cingulate cortex; PCC, posterior cingulate cortex; rsPCC, retro-splenial cingulate cortex; AIC, anterior insular cortex.
Table 1.
Regions showing a significant ‘State by Category’ interaction
| Coordinates |
||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Region | Side | BA | x | y | z | Size | PeakZ |
| 1 | aMCC | L | 24 | −3 | 14 | 37 | 244 | 5.21 |
| 2 | PCC | L / R | 23 | 0 | −28 | 28 | 60 | 4.32 |
| 3 | rsPCC | L | 29 | −6 | −43 | 16 | 21 | 4.25 |
| 4 | Anterior insula | R | 13 | 57 | 14 | −5 | 36 | 4.10 |
| 5 | AMY | L | −21 | 2 | −20 | 16 | 3.74 | |
| 6 | Ventral postcentral gyrus | R | 3 | 54 | −13 | 31 | 25 | 4.31 |
| 7 | Dorsal precentral gyrus | R | 1 | 45 | −19 | 58 | 29 | 4.12 |
| 8 | Inferior parietal lobule | L | 40 | −36 | −49 | 37 | 31 | 4.06 |
| 9 | Supramarginal gyrus | L | 40 | −63 | −31 | 28 | 48 | 4.05 |
Thresholds for activated clusters were set to P < 0.05 (FDR) with a voxel extent of k ≥ 15.
Abbreviations: aMCC, anterior middle cingulate cortex; PCC, posterior cingulate cortex; rsPCC, retro-splenial cingulate cortex; AMY, amygdala.
Testing for effects in the opposite direction (ChairsThirst > BeveragesThirst) vs (ChairsNo-thirst > BeveragesNo-thirst) did not reveal any significant effects.
Main effects of stimulus category and body state
With regard to stimulus category, beverage when compared with chair pictures elicited increased brain activation in widespread brain regions (see Table 2). Testing for the opposite effect the contrast ‘chairs > beverages’ did not reveal any significant effects.
Table 2.
Regions showing a significant ‘Category’ effect for ‘beverage vs chair’
| Coordinates |
||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Region | Side | BA | x | y | z | Size | PeakZ |
| 1 | OFC | R | 47/11 | 21 | 32 | −17 | 5732 | 5.20 |
| Inferior temporal gyrus | L | 37 | −48 | −49 | −20 | |||
| R | 37 | 51 | −46 | −23 | ||||
| Primary visual cortex | L/R | 17/18 | 0 | −76 | −20 | |||
| PCC | L/R | 23 | 0 | −31 | 40 | |||
| Caudate/VSTR | R | 12 | 5 | 4 | ||||
| DLPFC | R | 44 | 48 | 14 | 31 | |||
| ACC | L/R | 32 | 0 | 38 | 16 | |||
| MCC | L/R | 24 | 0 | 14 | 43 | |||
| Supramarginal gyrus | L | 40 | −54 | −31 | 43 | |||
| Anterior insula | R | 13 | 42 | 5 | −11 | |||
| VLPFC | R | 46 | 51 | 35 | 13 | |||
| 2 | Brainstem | L | −6 | −25 | −14 | 315 | 4.31 | |
| R | 6 | −31 | −11 | |||||
| AMY | L | −27 | −1 | −20 | ||||
| 3 | OFC | L | 47/11 | −30 | 35 | −17 | 156 | 5.84 |
| 4 | DLPFC | L | 44 | −45 | 8 | 31 | 331 | 4.62 |
| 5 | Fusiform gyrus | R | 36 | 39 | −25 | −20 | 151 | 4.19 |
| 6 | L | 36 | −39 | −16 | −29 | 29 | 3.68 | |
| 7 | Postcentral gyrus | R | 4 | 27 | −28 | 64 | 171 | 3.99 |
| 8 | L | 4 | −21 | −31 | 67 | 28 | 3.26 | |
| 9 | Supramarginal gyrus | L | 48 | −66 | −22 | 34 | 32 | 3.85 |
| 10 | R | 40 | 57 | −34 | 49 | 55 | 3.82 | |
| 11 | R | 48 | 24 | −28 | 22 | 56 | 3.66 | |
| 12 | VLPFC | L | 46 | −48 | 41 | 10 | 135 | 3.80 |
| 13 | Caudate/VSTR | L | −18 | 23 | 10 | 57 | 3.78 | |
| 14 | Anterior PFC | L | 10 | −18 | 62 | 4 | 125 | 3.74 |
| 15 | R | 10 | 18 | 65 | 19 | 21 | 3.23 | |
| 16 | AMY | R | 21 | −4 | −20 | 45 | 3.46 | |
| 17 | Precuneus | L | 7 | −3 | −58 | 58 | 148 | 3.38 |
| 18 | Anterior insula | L | 13 | −30 | 20 | 4 | 26 | 3.21 |
| 19 | L | 13 | −45 | 14 | −11 | 42 | 3.07 | |
| 20 | Inferior Occipital gyrus | R | 18 | 27 | −94 | −14 | 19 | 2.98 |
Thresholds for activated clusters were set to P < 0.05 (FDR) with a voxel extent of k ≥ 15. Conjoined clusters are listed on top of the table with the cluster peak corresponding to the first listed region.
Abbreviations: OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; MCC, middle cingulate cortex; PCC, posterior cingulate cortex; VSTR, ventral striatum; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; AMY, amygdala.
With regard to body state, neither the contrast of ‘thirst state > no-thirst state’ nor the contrast of ‘no-thirst state > thirst state’ did reveal any significant effects.
Discussion
Thirst can turn incentive stimuli, such as the sight of a glass of water, into powerful motivational magnets. This study investigated the neural correlates of such body state dependent value representations by asking participants in one experimental condition to refrain from drinking for seven hours. The neural processing of need-relevant beverage pictures and control stimuli in a thirst state was then compared with that during a no-thirst state. Reflecting body state dependent changes in incentive value, the data revealed the selective increase of neural responses towards beverage pictures during thirst in the aMCC, PCC, INS and AMY. Using drinking as important survival-related motivational behavior system, this study contributes to the decomposition of motivation processes and distinct representations of incentive value in key motivational regions (Salamone and Correa, 2012).
Neural regions showing body state by picture category interactions
A related view on dynamic stimulus representations is emerging in the rapidly growing literature on the functional role of the ACC, which also responded in a body-state-dependent manner in the current study. The ACC acts as major neural site for the integration of internal and external events (Paus, 2001; Craig, 2002; 2010; Medford and Critchley, 2010). In the current dataset, regions exhibiting state-dependent responses towards beverage pictures were located in the aMCC, which a large number of previous studies associated with negative affect, pain and cognitive control, and which is regarded as an important hub for the expression of affect and the organization of goal-directed behaviors (Etkin et al., 2011; Shackman et al., 2011). Interestingly, activation of the aMCC is particularly apparent during stimulus anticipation as brought out by an array of experimental paradigms, including conflict, divided attention, response selection and threat-of-shock (Liu et al., 2011). The present data are generally consistent with these findings. However, they also add important evidence by showing that aMCC responding is not only sensitive to top-down processes, such as goals and intentions, but also modulated by ‘motivational’ interactions, i.e. the interplay of bottom-up and top-down processes during which internal factors (thirst) bias the processing of incoming stimuli on the basis of their need relevance.
Anterior and ventral regions of the PCC also showed increased activations to beverage when compared with chair pictures in thirst state. This finding may appear surprising given the partial overlap with PCC regions comprising the default mode network, which shows decreased activations during tasks that demand external stimulus processing (Raichle, 2010). However, research regarding the cue-reactivity to cocaine as well as self-reported craving in nicotine addiction showed activations in the PCC (Kühn and Gallinat, 2011). Although it is tempting to relate the present PCC findings to the urge to drink, a Neurosynth meta-analysis (Yarkoni et al., 2011) indicates that this region is also associated with recollection, self-referential processing and imagery. Furthermore, the functional role of the PCC is not yet clear and current theories incorporate broad concepts of regulation of arousal, attention, and internal/external focus (Leech and Sharp, 2013). Functionally distinct regions within the PCC may also be dynamically connected to large scale neural networks (Leech et al., 2011; Torta et al., 2013).
Furthermore, the insular cortex, another key region involved in processing salient external or internal stimuli, showed a significant interaction effect in the right anterior INS. This finding concurs with a large number of studies showing that the insula seems to act as an interface between feelings and cognitions, or as a site of integration between internal and external relevant stimuli (Craig, 2009; 2010; Menon and Uddin, 2010; Chang et al., 2013). Influential accounts of insula function have suggested a functional division, according to which the anterior insular cortex responds more to salient external input, whereas the posterior insula is more responsive to homeostatic and somatosensory processes (Craig, 2010; Menon and Uddin, 2010). A recent meta-analytic study generally provided support for these subdivisions (Chang et al., 2013). Our data add to this issue by showing that insula responses towards externally salient stimuli are modulated by internal factors. Overall, these data are consistent with the view that the insula plays a key role in the motivational integration of interoceptive and homeostatic signals with incoming external stimuli.
Saliency: a neural network perspective
Complementary to the focus on the individual structures, a brain system perspective emphasizes connectivity across large-scale neural networks. The MCC and the anterior insula are often coactivated, exhibit strong functional connectivity in the resting state, and have been suggested to form a functional network involved in salience processing (Seeley et al., 2007; Menon and Uddin, 2010). This network is thought to be involved in assessing the salience of stimuli in order to guide adaptive behavior. Specifically, the saliency network comprises several hubs, which enable autonomic response generation, access the motor system and shift processing priorities in the large scale networks implicated in working memory and attention processes (Menon and Uddin, 2010). Salience, or relevance, has been defined in this line of research fairly broadly and includes internal saliency assessments, such as interoceptive signals and feelings, as well as external saliency commanded by stimulus or task characteristics. Drinking adds to this evidence the demonstration of the integration of internal and external stimuli in structures of the saliency network. At a gross level, regions of the ACC and anterior insular cortex, consistently implicated in previous research focusing on consummatory behaviors were also observed to be relevant to the anticipatory stage of motivated behaviors. However, comparisons across studies are not sufficient to address the issue of common and distinct neural correlates across appetitive and consummatory stages of motivated behavior. As such, future studies should examine the two stages of motivated behavior simultaneously.
The present findings concur with findings studying the effects of hunger on neural responses towards food pictures. Specifically, significant effects of body state by stimulus category (food-related vs control pictures) have been observed with regard to the AMY (LaBar et al., 2001; Mohanty et al., 2008; Goldstone et al., 2009), insular cortex (Goldstone et al., 2009; Haase et al., 2009), ACC (Goldstone et al., 2009) and PCC (Mohanty et al., 2008). However, the considerable variability of findings across food-based studies presumably reflects the complexity of the food system as well as methodological differences across the studies (Schupp and Renner, 2011). Furthermore, the present findings show commonalities and differences to effects associated with craving. Specifically, meta-analysis suggests that craving is most reliably associated with ACC, AMY and ventral striatum activity (Kühn and Gallinat, 2011), although few studies directly contrasted deprived and non-deprived conditions (McClernon et al., 2005; 2009; McBride et al., 2006; David et al., 2007; Stippekohl et al., 2010). Overall, the saliency network may comprise a large scale neural network implicated in the dynamic representation of stimulus value across domains of emotion and motivation.
Neural regions responding to beverage stimuli
Several regions showed stronger activations to beverage when compared with chair pictures. Thus, these regions responded similarly in thirst and no-thirst state, suggesting that they represent more abstract and generalized value signals. Interestingly, the OFC and the ventral striatum were among the regions that did not show a state-depended representation of stimulus value, whereas previous findings have linked them to state-dependent representations. Specifically, studies of sensory-specific satiety show the involvement of OFC and ventral striatum contrasting the activation before and after eating a food to satiety (Small et al., 2001; Gottfried et al., 2003). Furthermore, monkey studies revealed the increased neuronal firing in OFC and ventral striatum during the anticipation of rewarding water trials (Simmons et al., 2007) and the magnitude of neuronal firing in the OFC varied with the quantity of water anticipated (Roesch and Olson, 2007). Another recent study demonstrated that rats experiencing a sodium deficit instantaneously approached a conditioned stimulus (CS) lever which they avoided in non-depleted body state with the OFC and ventral striatum were among the structures showing increased Fos activity (Robinson and Berridge, 2013). One possible explanation for the discrepancies between previous and the current findings involves the temporal organization of motivated behaviors. Specifically, neural representations of moment-to-moment value representations may vary depending on whether an animal is in an appetitive or consummatory stage (Timberlake et al., 2001). Thus, future research investigating the anticipatory and consummatory stages of drinking behavior would enable the capture of the dynamics of moment-to-moment incentive value across temporal stages of motivated behaviors.
Conclusion
At its core, incentive motivation theory holds that the power of external stimuli is calibrated dynamically based on the current body state of the organism. The present findings demonstrate that key regions of the saliency network, i.e. aMCC, PCC, anterior insular cortex and AMY, show selectively enhanced activations in response to need-relevant pictures of beverages in thirst when compared with no-thirst state. Studying the effects of thirst on the neural processing of motivational cues offers a potent model system to examine the neural correlates of moment-to-moment incentive stimulus value. Arguably, drinking represents a less complicated behavior system than eating since the body has very little means to compensate the deficit and the influence of circadian rhythms and cognitions appears less pronounced. Overall, the current study offers insights into the neural systems that implement such powerful motivational forces in the drinking system.
Funding
This work was supported in part by grants from the German Federal Ministry of Education and Research (Grant 0315671, granted to Britta Renner & Harald T. Schupp) and the German Research Foundation [Grant RE 3430, granted to Britta Renner].
Conflict of interest. None declared.
References
- Amaro E., Barker G.J. (2006). Study design in fMRI: basic principles. Brain Cognition, 60, 220–32. [DOI] [PubMed] [Google Scholar]
- Bindra D. (1978). How adaptive behavior is produced: a perceptual-motivational alternative to response-reinforcement. Behavioral and Brain Sciences, 1, 41–52. [Google Scholar]
- Bradley M.M., Lang P.J. (1994). Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25, 49–59. [DOI] [PubMed] [Google Scholar]
- Chang L.J., Yarkoni T., Khaw M.W., Sanfey A.G. (2013). Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cerebral Cortex, 23, 739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. (1918). Appetites and aversions as constituents of instincts. The Biological Bulletin, 34, 91–107. [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience, 3, 655–66. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2010). The sentient self. Brain Structure and Function, 214, 563–77. [DOI] [PubMed] [Google Scholar]
- David S.P., Munafò M.R., Johansen-Berg H., et al. (2007). Effects of acute nicotine abstinence on cue-elicited ventral striatum/nucleus accumbens activation in female cigarette smokers: a functional magnetic resonance imaging study. Brain Imaging and Behaviour, 1, 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo I.E.T., Kringelbach M.L., Rolls E.T., McGlone F. (2003). Human cortical responses to water in the mouth, and the effects of thirst. Journal of Neurophysiology, 90, 1865–76. [DOI] [PubMed] [Google Scholar]
- Denton D., Shade R., Zamarippa F., et al. (1999a). Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proceedings of the National Academy of Science of United States of America, 96, 2532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D., Shade R., Zamarippa F., et al. (1999b). Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proceedings of the National Academy of Science of United States of America, 96, 5304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing P.E., Chan A.W.-Y., Peelen M.V., Dodds C.M., Kanwisher N. (2006). Domain specificity in visual cortex. Cerebral Cortex, 16, 1453–61. [DOI] [PubMed] [Google Scholar]
- Egan G., Silk T., Zamarripa F., et al. (2003). Neural correlates of the emergence of consciousness of thirst. Proceedings of the National Academy of Science of United States of America, 100, 15241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences (Regular Edition), 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.J., Bowala T.K., Gavrilescu M., et al. (2011). Cortical activation and lamina terminalis functional connectivity during thirst and drinking in humans. The American Journal of Physiology – Regulatory Integrative and Comparitive Physiology, 301, R623–31. [DOI] [PubMed] [Google Scholar]
- Farrell M.J., Egan G.F., Zamarripa F., et al. (2006). Unique, common, and interacting cortical correlates of thirst and pain. Proceedings of the National Academy of Science of United States of America, 103, 2416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.J., Zamarripa F., Shade R., et al. (2008). Effect of aging on regional cerebral blood flow responses associated with osmotic thirst and its satiation by water drinking: a PET study. Proceedings of the National Academy of Science of United States of America, 105, 382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Petrides M. (1999). Re-examination of the human taste region: a positron emission tomography study. European Journal of Neuroscience, 11, 2985–8. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.P., Frith C.D., Frackowiak R.S.J. (1994). Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping, 2, 189–210. [Google Scholar]
- Goldstone A.P., de Hernandez C.G.P., Beaver J.D., et al. (2009). Fasting biases brain reward systems towards high-calorie foods. European Journal of Neuroscience, 30, 1625–35. [DOI] [PubMed] [Google Scholar]
- Gottfried J.A., O’Doherty J., Dolan R.J. (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301, 1104–7. [DOI] [PubMed] [Google Scholar]
- Haase L., Cerf-Ducastel B., Murphy C. (2009). Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. NeuroImage, 44, 1008–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M., Mölle M., Wagner U., Fehm H.L., Born J. (2001). Drinking related direct current positive potential shift in the human EEG depends on thirst. Neuroscience Letters, 311, 173–6. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Gobbini M.I., Furey M.L., Ishai A., Schouten J.L., Pietrini P. (2001). Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science, 293, 2425–30. [DOI] [PubMed] [Google Scholar]
- Johnson A.K., Thunhorst R.L. (1997). The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Frontiers in Neuroendocrinology, 18, 292–353. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Ondek K., Schneider J.E. (2013). Neuroendocrine regulation of appetitive ingestive behavior. Frontiers in Neuroscience, 7, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., Barrett L.F., Joseph J., Bliss-Moreau E., Lindquist K., Wager T.D. (2008). Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage, 42, 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. (1967). Integrative Activity of the Brain, Chicago: University of Chicago Press. [Google Scholar]
- Kühn S., Gallinat J. (2011). Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience, 33, 1318–26. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., Gitelman D.R., Parrish T.B., Kim Y.H., Nobre A.C., Mesulam M.M. (2001). Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience, 115, 493–500. [DOI] [PubMed] [Google Scholar]
- Leech R., Kamourieh S., Beckmann C.F., Sharp D.J. (2011). Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. Journal of Neuroscience, 31, 3217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. (2013). The role of the posterior cingulate cortex in cognition and disease. Brain , 137(Pt 1), 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35, 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D., Barrett S.P., Kelly J.T., Aw A., Dagher A. (2006). Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology, 31, 2728–38. [DOI] [PubMed] [Google Scholar]
- McClernon F.J., Hiott F.B., Huettel S.A., Rose J.E. (2005). Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology, 30, 1940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon F.J., Kozink R.V., Lutz A.M., Rose J.E. (2009). 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology, 204, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N., Critchley H.D. (2010). Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function, 214, 535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214, 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A., Gitelman D.R., Small D.M., Mesulam M.M. (2008). The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cerebral Cortex, 18, 2604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L.M., Denton D., Egan G., et al. (2000). Neuroimaging evidence implicating cerebellum in support of sensory/cognitive processes associated with thirst. Proceedings of the National Academy of Science of United States of America, 97, 2332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. (2001). Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews. Neuroscience, 2, 417–24. [DOI] [PubMed] [Google Scholar]
- Pruessmann K.P., Weiger M., Scheidegger M.B., Boesiger P. (1999). SENSE: sensitivity encoding for fast MRI. Magnetic Resonance in Medicine, 42, 952–62. [PubMed] [Google Scholar]
- Raichle M.E. (2010). Two views of brain function. Trends in Cognitive Science (Regular Edition), 14, 180–90. [DOI] [PubMed] [Google Scholar]
- Robinson M.J.F., Berridge K.C. (2013). Instant transformation of learned repulsion into motivational “wanting”. Current Biology, 23, 282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch M.R., Olson C.R. (2007). Neuronal activity related to anticipated reward in frontal cortex: does it represent value or reflect motivation?. Annals of the New York Academy of Sciences, 1121, 431–46. [DOI] [PubMed] [Google Scholar]
- Saker P., Farrell M.J., Adib F.R.M., Egan G.F., McKinley M.J., Denton D.A. (2014). Regional brain responses associated with drinking water during thirst and after its satiation. Proceedings of the National Academy of Sciences, 111, 5379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J.D., Correa M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron, 76, 470–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Renner B. (2011). Handbook of Behavior, Food and Nutrition, New York, NY: Springer New York. [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G., Caldú X., Segura B., Dreher J.-C. (2013). Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37, 681–96. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews. Neuroscience, 12, 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J.M., Ravel S., Shidara M., Richmond B.J. (2007). A comparison of reward-contingent neuronal activity in monkey orbitofrontal cortex and ventral striatum: guiding actions toward rewards. Annals of the New York Academy of Sciences, 1121, 376–94. [DOI] [PubMed] [Google Scholar]
- Small D.M., Zatorre R.J., Dagher A., Evans A.C., Jones-Gotman M. (2001). Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain, 124, 1720–33. [DOI] [PubMed] [Google Scholar]
- Sternson S.M. (2013). Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron, 77, 810–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stippekohl B., Winkler M., Mucha R.F., et al. (2010). Neural responses to BEGIN- and END-stimuli of the smoking ritual in nonsmokers, nondeprived smokers, and deprived smokers. Neuropsychopharmacology, 35, 1209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W., Mowrer R., Klein S. (2001). Motivational modes in behavior systems. In: Mowrer R.R., Klein S.N.editors. Handbook of Contemporary Learning Theories, pp. 155–209, Hillsdale, NJ: Erlbaum Associaties. [Google Scholar]
- Toates F.M. (1986). Motivational Systems, Cambridge: Cambridge University Press. [Google Scholar]
- Torta D.M., Costa T., Duca S., Fox P.T., Cauda F. (2013). Parcellation of the cingulate cortex at rest and during tasks: a meta-analytic clustering and experimental study. Frontiers in Human Neuroscience, 7, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan L.N., de Ridder D.T.D., Viergever M.A., Smeets P.A.M. (2010). The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. NeuroImage, 55(1), 296–303. [DOI] [PubMed] [Google Scholar]
- Wagner A., Aizenstein H., Frank G.K., et al. (2006). Neural correlates of habituation to taste stimuli in healthy women. Psychiatry Research, 147, 57–67. [DOI] [PubMed] [Google Scholar]
- Watts A.G. (2001). Neuropeptides and the integration of motor responses to dehydration. Annual Review of Neuroscience, 24, 357–84. [DOI] [PubMed] [Google Scholar]
- Yang X.-W., Liu H.-C., Jin Z., Li K. (2008). Cortical activation induced by intraoral stimulation with water in humans with functional magnetic resonance imaging. West China Journal of Stomatology, 26, 383–6. [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., van Essen D.C., Wager T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H., Pardo J.V. (2000). Cortical activation induced by intraoral stimulation with water in humans. Chemical Senses, 25, 267–75. [DOI] [PubMed] [Google Scholar]