Abstract
One quarter of all deaths worldwide each year result from infectious diseases caused by microbial pathogens. Pathogens infect and cause disease by producing virulence factors that target host cell molecules. Studying how virulence factors target host cells has revealed fundamental principles of cell biology. These include important advances in our understanding of the cytoskeleton, organelles and membrane-trafficking intermediates, signal transduction pathways, cell cycle regulators, the organelle/protein recycling machinery, and cell-death pathways. Such studies have also revealed cellular pathways crucial for the immune response. Discoveries from basic research on the cell biology of pathogenesis are actively being translated into the development of host-targeted therapies to treat infectious diseases. Thus there are many reasons for cell biologists to incorporate the study of microbial pathogens into their research programs.
INTRODUCTION
Infectious diseases cause approximately one quarter of all deaths worldwide each year (Fauci and Morens, 2012). These include the “big three”—HIV/AIDS, tuberculosis, and malaria—which account for 10% of all deaths. They also include emerging diseases such as Ebola, Middle East Respiratory Syndrome, and methicillin-resistant Staphylococcus aureus. Infections are caused by microbial pathogens from different domains of the tree of life—viruses, bacteria, or eukaryotes. All share the ability to colonize their hosts and cause pathology through their interactions with host cells.
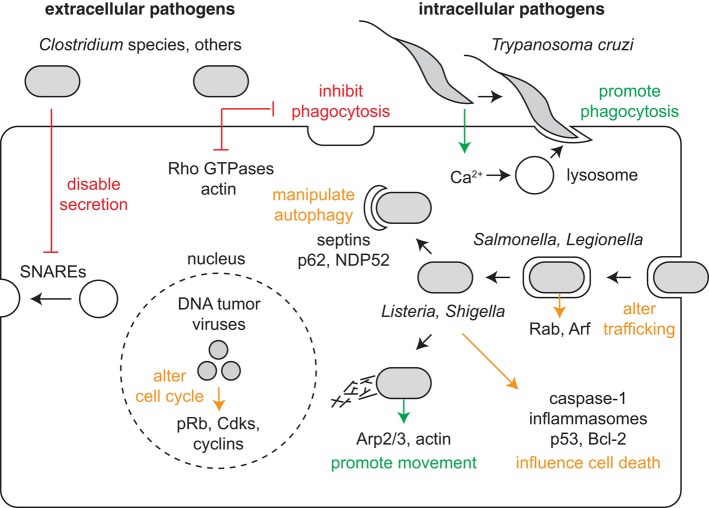
To influence host cells, each pathogen produces a distinct set of virulence factors that target specific host cell structures, pathways, and molecules. The function of virulence factors differs depending on where the pathogen establishes residence (Figure 1). Extracellular pathogens reside around or in contact with host cells but resist internalization into cells. They produce virulence factors that inhibit phagocytosis and otherwise disable elements of the immune response. Intracellular pathogens instead encourage their internalization into host cells, grow within a preferred cellular compartment or organelle, and then exit the cell to disseminate the infection. They produce virulence factors that promote phagocytosis, enable movement to their preferred compartment, manipulate membrane trafficking and autophagy to resist killing and permit growth and replication, and exit the cell to promote spread.
FIGURE 1:
Pathogen virulence factors influence cellular pathways and structures. Extracellular pathogens produce virulence factors that act at a distance or on contact with a host cell. These virulence factors inhibit cellular processes (indicated by red) including phagocytosis and secretion. In contrast, intracellular pathogens produce virulence factors that promote intimate interactions with host cells. These activate cellular processes (indicated by green), including phagocytosis, intracellular movement to a preferred compartment or organelle, and cell-to-cell spread. Virulence factors can also either activate or inactivate cellular processes (indicated by orange) to prevent microbial killing and enable growth and replication. These pathways include those involved in membrane trafficking, autophagy, cell death, and cell cycle regulation.
The cellular and molecular targets of pathogen virulence factors are the same systems studied by most cell biologists. They include: the cytoskeleton, organelles and membrane-trafficking intermediates, signal transduction pathways, cell cycle regulators, the organelle and protein recycling machinery, and cell-death pathways (Table 1). Studying the mechanisms by which virulence factors target host cells has two important impacts. First, such studies reveal crucial mechanisms of infection. Second, these studies aid in the elucidation of fundamental cellular mechanisms—for example, tyrosine kinase signaling or actin-based motility, to name a very few.
TABLE 1:
Summary of cellular pathways targeted by pathogens.
| Pathway/structure | Pathogenic process | Pathogens | Host targets | References |
|---|---|---|---|---|
| Cytoskeleton | Inhibit phagocytosis | Many extracellular pathogens, e.g., Clostridium spp., Yersinia spp. | Rho proteins, actin | Lemichez and Aktories, 2013 |
| Phagocytosis | Most intracellular pathogens | Rho proteins, signaling proteins, phagocytic proteins, actin | Pizarro-Cerdá and Cossart, 2006 | |
| Movement, spread | Many pathogens, e.g., L. monocytogenes, S. flexneri, E. coli, vaccinia virus | Arp2/3 complex, signaling proteins, actin | Welch and Way, 2013 | |
| Membrane trafficking | Growth/replication | Many extracellular pathogens, e.g., C. botulinum, C. tetani; many intracellular pathogens, e.g., T. cruzi, L. pneumophila | SNARE proteins, Rab proteins, Arf proteins | Asrat et al., 2014 |
| Cell cycle | Growth/replication | Many viruses, e.g., adenovirus, human papilloma virus, SV40 | pRb, cyclins, Cdks | Bagga and Bouchard, 2014 |
| Signal transduction | Growth/replication | Many pathogens, e.g., vaccinia virus, RSV, A-MuLV | Tyrosine kinases, other kinases, GTPases, lipid modifiers | Martin, 2004 |
| Autophagy | Growth/replication | Many intracellular pathogens, e.g., S. Typhimurium, L. monocytogenes, S. flexneri, M. tuberculosis | NDP52, p62, optineurin | Mostowy, 2014; Sorbara and Girardin, 2015 |
| Cell death | Growth/replication | Many pathogens, e.g., S. Typhimurium, P. falciparum | Caspase-1, inflammasomes, p53, Bcl-2 | Guo et al., 2015 |
This table is meant to provide important and interesting examples without being comprehensive.
The study of pathogen interactions with host cells also has practical impacts on fighting infectious diseases. One is advancing our understanding of immunity. Immune cells are often the targets of pathogen virulence factors, and understanding the interactions of pathogens with immune cells enhances the development of effective immune-based therapies for infections. Another is identifying cellular molecules crucial for infection, which are then exploited as targets of drugs to treat infectious diseases.
In this Perspective, I provide concrete answers to the basic question posed in the title: Why should cell biologists study microbial pathogens? Along the way, I explore three smaller questions: What have we learned about basic cell biology from studying pathogens and their virulence factors? What has studying the cell biology of host–pathogen interactions taught us about immunity? How has identifying cellular targets of pathogens led to the development of therapeutic agents to treat infection?
WHAT HAVE WE LEARNED ABOUT BASIC CELL BIOLOGY FROM STUDYING PATHOGENS AND THEIR VIRULENCE FACTORS?
The study of how pathogens and their virulence factors impact host cells has been fertile ground for uncovering basic cell biological principles and mechanisms. Such discoveries have enhanced our understanding of the many structures, pathways, and molecules that are commonly exploited by pathogens during infection.
One area of cell biology in which the study of pathogens has enabled fundamental advances is the cytoskeletal field. Extracellular bacterial pathogens often target actin or its regulators by secreting toxins that translocate across cellular membranes to inhibit phagocytosis by immune cells (Lemichez and Aktories, 2013). An example is Clostridium botulinum, which causes the paralytic illness botulism. C. botulinum produces several secreted toxins, including C3 toxin, which enters host cells and ADP-ribosylates and inactivates the Rho family GTPase Rho (paralysis is caused by a separate toxin, as discussed below; Narumiya et al., 1988; Aktories et al., 1989). By examining the effect of microinjecting C3 toxin into cells, Alan Hall and coworkers discovered that Rho signals to promote the formation of focal adhesions and stress fibers (Chardin et al., 1989; Paterson et al., 1990; Ridley and Hall, 1992). Sadly, Alan passed away earlier this year in the prime of his career. Similar studies showed that Rho family proteins are also required for phagocytosis (Hall, 2012). Many bacteria, such as Clostridium difficile, the leading cause of hospital-acquired diarrhea, or Yersinia pestis, the causative agent of plague, inhibit phagocytosis by producing virulence factors that disable Rho, Rac, and/or Cdc42, highlighting the generality of this pathogenic strategy (Lemichez and Aktories, 2013). Bacterial toxins continue to be important tools for revealing the functions of Rho GTPases in cells.
The study of pathogens that mobilize actin for movement has also led to the discovery of fundamental mechanisms of cytoskeletal dynamics and regulation. Such pathogens include the bacteria Listeria monocytogenes, Shigella flexneri, and Escherichia coli, which cause food-borne illnesses, as well as vaccinia virus, which is the smallpox vaccine strain. These microbes undergo actin-based motility either within or on the surface of cells, which enables cell-to-cell spread during infection (Welch and Way, 2013). Through biochemical reconstitution of L. monocytogenes and/or S. flexneri motility, the host Arp2/3 complex and its activators (the WASP family proteins) were revealed as key actin-nucleating factors for bacterial pathogens and host cells (Welch et al., 1997, 1998), and a minimal set of proteins that is sufficient to drive actin-based motility was defined (Loisel et al., 1999). Moreover, the study of enteropathogenic/enterohemorrhagic E. coli and vaccinia virus, which induce actin assembly from outside the cell through the plasma membrane, revealed important roles for tyrosine kinase signaling and protein clustering in regulating actin assembly (Frischknecht et al., 1999; Campellone et al., 2008).
Studying pathogens has also led to fundamental advances in the field of membrane trafficking. A classic example involves the extracellular bacterial pathogens C. botulinum, mentioned above, as well as Clostridium tetani, which causes the paralytic disease tetanus. In addition to the C3 exoenzyme, C. botulinum produces botulinum toxins A–G (type A is familiarly known as Botox), and C. tetani secretes tetanus toxin. These toxins specifically cleave SNARE protein components of the vesicle fusion machinery, including VAMP, SNAP-25, and syntaxin (Link et al., 1992; Schiavo et al., 1992; Blasi et al., 1993a, b). Microinjection of nerve cells with these toxins showed that SNARE molecules are critical for neurotransmitter release via vesicle fusion with the plasma membrane (Schiavo et al., 1992; Blasi et al., 1993b). Preventing neurotransmitter release results in the paralysis caused by botulinum and tetanus toxins. In a contemporary study, it was revealed that SNARE proteins form a complex that is sufficient to mediate vesicle docking and fusion (Söllner et al., 1993). Thus bacterial toxins were used in discovering fundamental mechanisms of membrane fusion and vesicular transport.
Advances in our understanding of how membrane-trafficking pathways contribute to repairing plasma membrane wounds (Sonnemann and Bement, 2011) have also come from the study of intracellular pathogens. A classic example is Trypanosoma cruzi, a eukaryotic parasite and the causative agent of Chagas disease, which in its chronic form can cause cardiovascular and intestinal illness. As the T. cruzi parasite contacts the plasma membrane of a host cell before invasion, intracellular Ca2+ is elevated, and cellular lysosomes are recruited to the point of contact between the parasite and host cell. Surprisingly, lysosomes participate in exocytosis at the invasion site, which facilitates invasion (Tardieux et al., 1992, 1994). It was later revealed that the Ca2+-dependent fusion of lysosomes (Reddy et al., 2001) and other organelles (Shen et al., 2005) plays a key role in repairing plasma membrane wounds in uninfected cells. Along with work on T. cruzi, studying how intracellular bacterial pathogens such as Legionella pneumophila, the causative agent of Legionnaire’s disease, manipulate membrane-trafficking pathways is advancing our understanding of these pathways in uninfected cells (Asrat et al., 2014).
Cell cycle regulatory mechanisms have also been exposed through the investigation of virus interactions with host cells (Bagga and Bouchard, 2014). Classic examples involve the study of DNA tumor viruses, which include adenovirus, human papilloma virus, and SV40. These viruses rely on the host DNA replication machinery, and thus they induce cell cycle progression into S phase to favor viral DNA replication. A key discovery was that these viruses encode proteins, such as E1A from adenovirus, that bind to the tumor-suppressor protein pRb and related proteins (Whyte et al., 1988). The role of pRb as a negative regulator of cell cycle progression was subsequently revealed when it was found that E1A binding to pRb competes with and releases the bound transcription factor E2F, which turn activates the expression of cell cycle regulatory genes that promote entry into S phase (Bagchi et al., 1991; Bandara and La Thangue, 1991; Chellappan et al., 1991; Raychaudhuri et al., 1991). Other viruses target different cell cycle regulators, including Cdks and cyclins (Bagga and Bouchard, 2014), and studying how viruses manipulate host cells will continue to reveal cell cycle regulatory mechanisms.
Finally, our understanding of signal transduction pathways and their contribution to diseases like cancer has also been heavily influenced by the study of viruses (Martin, 2004). Famous examples are the Rous sarcoma virus (RSV), which causes sarcomas in chickens, and Abelson murine leukemia virus (A-MuLV), which causes lymphosarcomas in mice. The capacity of RSV to transform normal cells into tumor cells was found to be associated with the viral src gene and its product v-Src (Brugge and Erikson, 1977; Weiss et al., 1977). The v-Src protein and the v-Abl protein from A-MuLV were subsequently shown to be tyrosine kinases (Hunter and Sefton, 1980; Witte et al., 1980), the first discovery of this protein class. Cellular homologues of these proteins, c-Src and c-Abl, were soon identified (Stehelin et al., 1976; Shalloway et al., 1981; Heisterkamp et al., 1982), demonstrating that viral oncogenes are derived from cellular counterparts. It was also soon recognized that the human ABL1 gene, which encodes c-Abl, participates in the Philadelphia chromosomal translocation, which is commonly associated with leukemias (de Klein et al., 1982). Thus viruses were key to demonstrating the importance of tyrosine kinases in signaling in normal and cancer cells and the roles of oncogenes in cancer. Future studies of pathogens will continue to reveal ways in which diverse signaling pathways and proteins influence normal cell physiology and disease.
WHAT CAN STUDYING THE CELL BIOLOGY OF HOST–PATHOGEN INTERACTIONS TEACH US ABOUT IMMUNITY?
Cells of the immune system are often targeted by pathogens to avoid or subvert immune defenses. Certain facets of the interaction between pathogens and immune cells lie at the interface between the fields of immunology and cell biology. The study of such areas is of increasing importance in understanding general mechanisms of pathogenesis, and may prove particularly relevant in harnessing the immune system to fight infection. In this section, I highlight emerging areas of intersection between basic cellular pathways and the innate immune response to pathogens. In the subsequent section, I discuss how studying these areas impacts the development of therapeutics to treat infectious diseases.
Autophagy has emerged as a process that is of central interest in the fields of cell biology and immunology, and research in both disciplines has synergized to reveal key ways in which autophagy impacts basic cell function and disease. Studies by cell biologists have uncovered the importance of autophagy in maintaining homeostasis during normal, stressful, or disease conditions, and have identified important molecular players in this pathway (Boya et al., 2013). Immunologists have discovered that autophagy of pathogens (also called xenophagy) is an important arm of the innate immune response that promotes intracellular pathogen sequestration in autophagosomes and their degradation in lysosomes (Huang and Brumell, 2014; Sorbara and Girardin, 2015).
An example of how studying pathogens has advanced our understanding of autophagy mechanisms involves the response to infection by the intracellular pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium), a common cause of diarrheal illness. S. Typhimurium normally resides within an endosome-like compartment called the Salmonella-containing vacuole (SCV). However, observation of the bacteria that occasionally damage the SCV and escape into the cytosol enabled the discovery of previously unknown mechanisms by which pathogens are targeted to autophagy. These include marking bacteria in the cytosol with ubiquitin (Thurston et al., 2009) and marking those in damaged SCVs with the lectin galectin-8, which recognizes glycans on damaged vacuoles (Thurston et al., 2012). Both galectin-8 and ubiquitin are then recognized by adapter proteins (including NDP52, p62, and optineurin), which recruit LC3 and initiate autophagosome formation (Thurston et al., 2009, 2012; Zheng et al., 2009; Wild et al., 2011). In addition to their roles in infection, these same pathways may be involved in targeting damaged organelles in uninfected cells (Huang and Brumell, 2014; Sorbara and Girardin, 2015) and in removing protein aggregates, for example, those associated with neurodegenerative diseases (Rubinsztein et al., 2015).
A second example highlights how the study of pathogens can reveal new pathways that influence autophagy, such as an emerging link between cytoskeletal elements and the autophagy machinery (Mostowy, 2014). In the case of L. monocytogenes, the ability of the bacterial ActA protein to recruit actin-polymerizing factors masks the bacteria from ubiquitination and the initiation of autophagosome formation (Yoshikawa et al., 2009). This suggests a potential role for actin in autophagy inhibition. In contrast, for S. flexneri, recruitment of actin promotes the subsequent recruitment of septin proteins, which cage the bacteria and are crucial for autophagy (Mostowy et al., 2010, 2011). Thus, in the case of S. flexneri, actin and septins play a stimulatory role in autophagy. Deciphering the roles of cytoskeletal elements in autophagy regulation and the innate immune response should be an active area of future investigation.
Beyond autophagy, the study of pathogens has revealed previously unappreciated pathways for regulating programmed cell death that are integral to innate immunity and inflammation. One such pathway was discovered by studying cell death induced by S. Typhimurium. It was noted that S. Typhimurium infection induced cell death in macrophages that was dependent on caspase-1 (Hersh et al., 1999). Moreover, the characteristics of death were distinct from apoptosis in key respects, including diffuse rather than compacted DNA fragmentation, and loss rather than maintenance of plasma membrane integrity (Brennan and Cookson, 2000). This mechanism of cell death was subsequently called pyroptosis, which is a proinflammatory cell death, to distinguish it from apoptosis, which is anti-inflammatory. Subsequent studies showed that caspase-1 activation involves a multiprotein complex called an inflammasome (Martinon et al., 2002), of which there are multiple varieties with different functions in innate immunity and inflammation (Guo et al., 2015). In addition to their roles in infection, inflammasomes also play an important role in metabolic and neurological diseases (Guo et al., 2015). The continued study of inflammasomes will enhance our understanding of inflammatory responses to infection and of the role of inflammation in normal cell function and disease.
HOW HAS IDENTIFYING CELLULAR TARGETS OF PATHOGENS LED TO THE DEVELOPMENT OF THERAPEUTIC AGENTS TO TREAT INFECTION?
There are two predominant approaches for combating infectious diseases. One is to stimulate the immune system to prevent or reduce the impact of infection, for example, through vaccination. Another is to reduce or eliminate an existing infection with drugs that kill the infectious agent and/or enhance the immune response. Studying the cell biology of pathogenesis is contributing to both of these therapeutic avenues.
An interesting example involves an emerging connection between autophagy and vaccine efficacy. The bacillus Calmette-Guerin (BCG) vaccine, used to combat tuberculosis caused by the intracellular pathogen Mycobacterium tuberculosis, consists of an attenuated strain of the related bacterium Mycobacterium bovis. BCG has a protective effect against meningitis and disseminated tuberculosis in children but does not prevent primary infection with M. tuberculosis or reactivation of pulmonary infection, which is the main route of spread of the disease. Thus a more effective vaccine is needed. Interestingly, augmenting autophagy with rapamycin was found to enhance presentation of a BCG antigen by antigen-presenting cells and enhance protection against M. tuberculosis infection in animal model (Jagannath et al., 2009). A similar phenomenon was observed for the yellow fever vaccine YF-17D, a live attenuated virus that, in contrast with BCG, is almost always effective in protecting against infection with the yellow fever virus. It was shown that YF-17D stimulated expression of the kinase GCN2, which in turn stimulated dendritic cells to initiate autophagy and enhanced antigen presentation to T-cells (Ravindran et al., 2014). These findings suggest that stimulating autophagy may be a general strategy for enhancing antigen presentation and vaccine efficacy.
In cases in which vaccination is not possible or fails to confer protection, antiviral, antibacterial, and antiparasitic drugs are commonly used to treat infections. These drugs generally target pathogen molecules that are both essential for pathogen growth and are distinct from host molecules, enhancing the selective toxicity of the drug for the infectious agent while minimizing side effects on the host. This raises the following question: Can host cell components that are important for pathogenesis also be effective drug targets for treating infectious diseases? The answer is yes, and it is noteworthy that targeting host molecules appears to be an emerging strategy for the development of drugs to treat infections.
Targeting host molecules has advanced the furthest in developing treatments for viral infections. Prime examples are drugs that target host cell receptor molecules and prevent viral entry. Maraviroc is a Federal Drug Administration (FDA)-approved drug that is used in combination therapy to treat HIV infection. It inhibits viral entry into cells by binding to the chemokine- and HIV-receptor CCR5 (Wood and Armour, 2005). Other inhibitors of host proteins involved in HIV infection are also in development (Arhel and Kirchhoff, 2010). Moreover, inhibitors of the cellular receptors for other viruses have been identified. A small molecule that inhibits cellular infection with Ebola virus, the causative agent of a dramatic hemorrhagic fever, works by binding to the endosomal protein NPC1 (Côté et al., 2011). In fact, the identification of this inhibitor revealed that NPC1 is a crucial factor for Ebola virus infection. A more recent study identified an engineered protein that inhibits infection with the influenza virus, which causes flu, by binding to sialic acid, which is used as a receptor for virus entry (Connaris et al., 2014). One intranasal dose of this inhibitor protected mice from an otherwise lethal dose of the 2009 pandemic H1N1 virus, while also enabling sufficient viral replication to potentially protect the animals from future infection.
Antibacterial agents that target host cell molecules are also being explored. Such agents will be particularly useful in cases for which there is no effective vaccine, and/or resistance to conventional antibiotics is common, as with M. tuberculosis. A recent fruitful approach was to screen through a library of bioactive small molecules known to target host proteins for those that restrict the growth of M. tuberculosis (Stanley et al., 2014). This identified compounds that affect several protein classes, including G protein–coupled receptors, ion channels, membrane transport proteins, kinases, and anti-inflammatories. Through this and other studies, a list can be compiled of FDA-approved drugs that target host molecules and inhibit M. tuberculosis infection, including the antidepressants fluoxetine (Prozac; Stanley et al., 2014) and nortriptyline (Sundaramurthy et al., 2013); the epidermal growth factor receptor kinase inhibitor gefitinib (Stanley et al., 2014) and Abl kinase inhibitor imatinib (Napier et al., 2011; Bruns et al., 2012); and the antiseizure medication carbamazepine (Schiebler et al., 2015). A common theme linking these drugs is that they induce autophagy, again highlighting the potential utility of autophagy-stimulating drugs in the prevention and treatment of infections (Rubinsztein et al., 2015).
Finally, targeting components of the host cell may also prove fruitful for combating infection by intracellular parasites such as Plasmodium falciparum and other Plasmodium species, which are the causative agents of malaria. During infection of host hepatocytes, Plasmodium suppresses the expression of the cell cycle regulator and proapoptotic protein p53 and increases the expression of the antiapoptotic protein Bcl-2, thus preventing host cell apoptosis (Kaushansky et al., 2013). It was recently found that counteracting the parasite’s anti-apoptotic program by treatment with the small molecule p53 activator Nutlin-3 and/or the Bcl-2 inhibitors Obatoclax or ABT-737, all of which are under study as anticancer therapeutics, delayed or prevented onset of disease caused by P. falciparum (Douglass et al., 2015). This reveals the potential therapeutic value of drugs that target host cell death molecules for treating infectious disease.
Thus it appears that developing drugs that target host components is a viable strategy to combat infectious diseases, and this may prove complementary to the traditional approach of developing antimicrobials that target pathogen proteins. Although potential drawbacks of such a strategy include the risk of toxicity to the host, potential benefits may include an increased flexibility in developing combination therapies and a reduced capacity of the pathogens to become resistant to drug treatment.
CONCLUSIONS AND FUTURE DIRECTIONS
In writing this Perspective, I hope to encourage cell biologists to study pathogens by collaborating with pathogenesis researchers or incorporating work on infectious diseases into their own research programs. Such investigations might focus on well-known pathogens or new and emerging infectious agents. The motivation may be a desire to reveal new biological principles or to better understand and treat important diseases. Regardless of the choice of pathogen or source of motivation, future studies by cell biologists will uncover new ways in which pathogens influence host cell pathways and structures, and new cell biological mechanisms that operate under normal circumstances and in various disease states. Future studies will also contribute to the development of new vaccines and drugs that target host cell proteins to prevent or treat infections. Therefore basic and translational research at the interface between microbiology, cell biology, and immunology will be an increasingly important source of information and innovation relevant to biology in general and infectious disease in particular.
Acknowledgments
I thank Rebecca Lamason and Chris Patane for helpful comments on the manuscript. Work in the Welch lab is funded by National Institutes of Health grants R01 GM059609 and R01 AI109044.
Abbreviations used:
- A-MuLV
Abelson murine leukemia virus
- BCG
bacillus Calmette-Guerin
- RSV
Rous sarcoma virus
- SCV
Salmonella-containing vacuole.
Footnotes
REFERENCES
- Aktories K, Braun U, Rösener S, Just I, Hall A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem Biophys Res Commun. 1989;158:209–213. doi: 10.1016/s0006-291x(89)80199-8. [DOI] [PubMed] [Google Scholar]
- Arhel N, Kirchhoff F. Host proteins involved in HIV infection: new therapeutic targets. Biochim Biophys Acta. 2010;1802:313–321. doi: 10.1016/j.bbadis.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Asrat S, de Jesús DA, Hempstead AD, Ramabhadran V, Isberg RR. Bacterial pathogen manipulation of host membrane trafficking. Annu Rev Cell Dev Biol. 2014;30:79–109. doi: 10.1146/annurev-cellbio-100913-013439. [DOI] [PubMed] [Google Scholar]
- Bagchi S, Weinmann R, Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991;65:1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- Bagga S, Bouchard MJ. Cell cycle regulation during viral infection. Methods Mol Biol. 2014;1170:165–227. doi: 10.1007/978-1-4939-0888-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara LR, La Thangue NB. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991;351:494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993a;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993b;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- Brugge JS, Erikson RL. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977;269:346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Bruns H, Stegelmann F, Fabri M, Döhner K, van Zandbergen G, Wagner M, Skinner M, Modlin RL, Stenger S. Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages. J Immunol. 2012;189:4069–4078. doi: 10.4049/jimmunol.1201538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Cheng HC, Robbins D, Siripala AD, McGhie EJ, Hayward RD, Welch MD, Rosen MK, Koronakis V, Leong JM. Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly. PLoS Pathog. 2008;4:e1000191. doi: 10.1371/journal.ppat.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P, Boquet P, Madaule P, Popoff MR, Rubin EJ, Gill DM. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Connaris H, Govorkova EA, Ligertwood Y, Dutia BM, Yang L, Tauber S, Taylor MA, Alias N, Hagan R, Nash AA, et al. Prevention of influenza by targeting host receptors using engineered proteins. Proc Natl Acad Sci USA. 2014;111:6401–6406. doi: 10.1073/pnas.1404205111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, et al. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klein A, van Kessel AG, Grosveld G, Bartram CR, Hagemeijer A, Bootsma D, Spurr NK, Heisterkamp N, Groffen J, Stephenson JR. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- Douglass AN, Kain HS, Abdullahi M, Arang N, Austin LS, Mikolajczak SA, Billman ZP, Hume JC, Murphy SC, Kappe SH, et al. Host-based prophylaxis successfully targets liver stage malaria parasites. Mol Ther. 2015;23:857–865. doi: 10.1038/mt.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366:454–461. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- Frischknecht F, Moreau V, Rottger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting JPY. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N, Groffen J, Stephenson JR, Spurr NK, Goodfellow PN, Solomon E, Carritt B, Bodmer WF. Chromosomal localization of human cellular homologues of two viral oncogenes. Nature. 1982;299:747–749. doi: 10.1038/299747a0. [DOI] [PubMed] [Google Scholar]
- Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Brumell JH. Bacteria-autophagy interplay: a battle for survival. Nat Rev Microbiol. 2014;12:101–114. doi: 10.1038/nrmicro3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- Kaushansky A, Ye AS, Austin LS, Mikolajczak SA, Vaughan AM, Camargo N, Metzger PG, Douglass AN, MacBeath G, Kappe SHI. Suppression of host p53 is critical for Plasmodium liver-stage infection. Cell Rep. 2013;3:630–637. doi: 10.1016/j.celrep.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemichez E, Aktories K. Hijacking of Rho GTPases during bacterial infection. Exp Cell Res. 2013;319:2329–2336. doi: 10.1016/j.yexcr.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Link E, Edelmann L, Chou JH, Binz T, Yamasaki S, Eisel U, Baumert M, Sudhof TC, Niemann H, Jahn R. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992;189:1017–1023. doi: 10.1016/0006-291x(92)92305-h. [DOI] [PubMed] [Google Scholar]
- Loisel TP, Boujemaa R, Pantaloni D, Carlier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- Martin GS. The road to Src. Oncogene. 2004;23:7910–7917. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Mostowy S. Multiple roles of the cytoskeleton in bacterial autophagy. PLoS Pathog. 2014;10:e1004409. doi: 10.1371/journal.ppat.1004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Bonazzi M, Hamon MA, Tham TN, Mallet A, Lelek M, Gouin E, Demangel C, Brosch R, Zimmer C, et al. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe. 2010;8:433–444. doi: 10.1016/j.chom.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Mostowy S, Sancho-Shimizu V, Hamon MA, Simeone R, Brosch R, Johansen T, Cossart P. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J Biol Chem. 2011;286:26987–26995. doi: 10.1074/jbc.M111.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier RJ, Rafi W, Cheruvu M, Powell KR, Zaunbrecher MA, Bornmann W, Salgame P, Shinnick TM, Kalman D. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe. 2011;10:475–485. doi: 10.1016/j.chom.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Sekine A, Fujiwara M. Substrate for botulinum ADP-ribosyltransferase, Gb, has an amino acid sequence homologous to a putative rho gene product. J Biol Chem. 1988;263:17255–17257. [PubMed] [Google Scholar]
- Paterson HF, Self AJ, Garrett MD, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerdá J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, Park Y, Jones DP, Chappert P, Davoust J, et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science. 2014;343:313–317. doi: 10.1126/science.1246829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri P, Bagchi S, Devoto SH, Kraus VB, Moran E, Nevins JR. Domains of the adenovirus E1A protein required for oncogenic activity are also required for dissociation of E2F transcription factor complexes. Genes Dev. 1991;5:1200–1211. doi: 10.1101/gad.5.7.1200. [DOI] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Bento CF, Deretic V. Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J Exp Med. 2015;212:979–990. doi: 10.1084/jem.20150956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiebler M, Brown K, Hegyi K, Newton SM, Renna M, Hepburn L, Klapholz C, Coulter S, Obregón-Henao A, Henao Tamayo M, et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol Med. 2015;7:127–139. doi: 10.15252/emmm.201404137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D, Zelenetz AD, Cooper GM. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981;24:531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Shen SS, Tucker WC, Chapman ER, Steinhardt RA. Molecular regulation of membrane resealing in 3T3 fibroblasts. J Biol Chem. 2005;280:1652–1660. doi: 10.1074/jbc.M410136200. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sonnemann KJ, Bement WM. Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annu Rev Cell Dev Biol. 2011;27:237–263. doi: 10.1146/annurev-cellbio-092910-154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara MT, Girardin SE. Emerging themes in bacterial autophagy. Curr Opin Microbiol. 2015;23:163–170. doi: 10.1016/j.mib.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Barczak AK, Silvis MR, Luo SS, Sogi K, Vokes M, Bray MA, Carpenter AE, Moore CB, Siddiqi N, et al. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 2014;10:e1003946. doi: 10.1371/journal.ppat.1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976;260:170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Sundaramurthy V, Barsacchi R, Samusik N, Marsico G, Gilleron J, Kalaidzidis I, Meyenhofer F, Bickle M, Kalaidzidis Y, Zerial M. Integration of chemical and RNAi multiparametric profiles identifies triggers of intracellular mycobacterial killing. Cell Host Microbe. 2013;13:129–142. doi: 10.1016/j.chom.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Tardieux I, Nathanson MH, Andrews NW. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic-free Ca2+ transients. J Exp Med. 1994;179:1017–1022. doi: 10.1084/jem.179.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieux I, Webster P, Ravesloot J, Boron W, Lunn JA, Heuser JE, Andrews NW. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71:1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- Thurston TLM, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol. 2009;10:1215–1221. doi: 10.1038/ni.1800. [DOI] [PubMed] [Google Scholar]
- Thurston TLM, Wandel MP, von Muhlinen N, Foeglein A, Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 2012;482:414–418. doi: 10.1038/nature10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SR, Varmus HE, Bishop JM. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977;12:983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Welch MD, Iwamatsu A, Mitchison TJ. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- Welch MD, Rosenblatt J, Skoble J, Portnoy DA, Mitchison TJ. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 1998;281:105–108. doi: 10.1126/science.281.5373.105. [DOI] [PubMed] [Google Scholar]
- Welch MD, Way M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe. 2013;14:242–255. doi: 10.1016/j.chom.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte ON, Dasgupta A, Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980;283:826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Wood A, Armour D. The discovery of the CCR5 receptor antagonist, UK-427,857, a new agent for the treatment of HIV infection and AIDS. Prog Med Chem. 2005;43:239–271. doi: 10.1016/S0079-6468(05)43007-6. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M, Mimuro H, Nakagawa I, Yanagawa T, Ishii T, et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol. 2009;11:1233–1240. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol. 2009;183:5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]