Abstract
During stem cell divisions, mitotic microtubules do more than just segregate the chromosomes. They also determine whether a cell divides virtually symmetrically or asymmetrically by establishing spindle orientation and the plane of cell division. This can be decisive for the fate of the stem cell progeny. Spindle defects have been linked to neurodevelopmental disorders, yet the role of spindle orientation for mammalian neurogenesis has remained controversial. Here we explore recent advances in understanding how the microtubule cytoskeleton influences mammalian neural stem cell division. Our focus is primarily on the role of spindle microtubules in the development of the cerebral cortex. We also highlight unique characteristics in the architecture and dynamics of cortical stem cells that are tightly linked to their mode of division. These features contribute to setting these cells apart as mitotic “rule breakers,” control how asymmetric a division is, and, we argue, are sufficient to determine the fate of the neural stem cell progeny in mammals.
INTRODUCTION
The primary task of the mitotic spindle is to separate and symmetrically distribute the chromosomes to the nascent daughter cells. However, an equally important function for reproduction, development, and tissue homeostasis is to set the cell division plane. This is especially relevant for cells in which components are distributed unevenly, such as polarized cells. The cleavage furrow then follows this plane and determines which, and by how much, components are inherited asymmetrically by the daughter cells. Cell component asymmetry can also determine asymmetry in daughter cell fate (Gonczy, 2008; Siller and Doe, 2009). A perfectly symmetric inheritance of the various cell components is quantitatively impossible, even for the most finely tuned mitotic machinery, if, for example, a component exists as a single copy only. Therefore, every cell division is most likely asymmetric to some extent. The matter may ultimately be whether the component asymmetries are sufficient to determine cell function and fate. If not, the division will become virtually symmetric in terms of cell fate.
Neural stem cells (NSCs) give a fascinating example of different levels of division asymmetry. The primary NSCs of the mammalian CNS are the polarized neuroepithelial cells, of neuroectodermal origin, from which all neurons and glial cells are derived. When cerebral cortex development initiates, the cell and molecular biology of NSCs changes. They can then drive the thickening and layering of the neocortex by proliferating, elongating along the apicobasal axis of the tissue, and generating different NSC and progenitor types, neurons and glia (Götz and Huttner, 2005). On neurogenesis onset, neuroepithelial cells gradually convert to apical radial glia (aRG). Both cell types share a similar architecture and an apical mitosis that groups them as apical progenitors (APs). The characteristics of these and other main types of NSCs have been recently reviewed (Taverna et al., 2014).
FROM MITOSIS TO MITOSIS
Microtubules play diverse roles in NSCs—for example, in nuclear translocation, intracellular transport, and cell migration (Breuss and Keays, 2014). After apical mitosis, the G1 nucleus of APs migrates basally to make space for other mitoses. When S phase is completed basally, the G2 nucleus migrates to prepare for the next apical mitosis. These interphase events are collectively known as interkinetic nuclear migration (INM) and involve both the actin and the microtubule cytoskeleton (Taverna and Huttner, 2010; Miyata et al., 2014). The somata of another type of NSCs, called basal radial glia (bRG, or outer radial glia), which originate from aRG and delaminate to undergo basal mitosis, can also exhibit bursts of migration. This occurs at the onset of mitosis and is called mitotic somal translocation (Hansen et al., 2010), a process involving the actomyosin cytoskeleton and apparently opposed by microtubules (Ostrem et al., 2014).
AN ATYPICAL MITOSIS–CYTOKINESIS Liaison
For NSCs to proliferate, their divisions must be symmetric in terms of daughter cell fate. Fate symmetry is often correlated with a high symmetry in the inheritance of cell components, especially during early cortical development. With a “vertical” division plane (see below) that cleaves proliferating APs perpendicular to the ventricular surface, both daughter cells can inherit the complete epithelial architecture, including the apical domain and basal process (Götz and Huttner, 2005; Taverna et al., 2014). This constitutes a formidable challenge for the highly elongated APs, but it is a challenge that can be met by a noncanonical order of cytokinesis onset and anaphase onset: dividing one part of the cell, the basal process, before segregating the chromosomes, and then dividing the rest of the cell.
AP basal process splitting occurs mostly before and during early neurogenesis. This starts with an actin and anillin accumulation at the basal process tip. The entire process is then divided unidirectionally to reach the apical soma, where cytokinesis will be completed. Strikingly, this extrasomal division starts before chromosome segregation, as early as prophase and proceeding during prometaphase (Kosodo et al., 2008). This may be the only known example of cleavage furrow ingression preceding karyokinesis. Of interest, cleavage of the AP soma continues in the same basal-to-apical direction (Figure 1, A and D). The mechanisms that govern the unidirectional cytokinesis of the entire epithelial architecture await discovery. Signals between mitotic microtubules and the actinomyosin cell cortex are known to play fundamental roles in guiding cytokinesis (D’Avino et al., 2015), and microtubules at the interphase/mitosis transition could have similar functions in AP basal process splitting. We speculate that motors are likely to convey process-splitting signals on microtubule tracks, and their basal deployment may depend on mitosis entry instead of chromosome segregation.
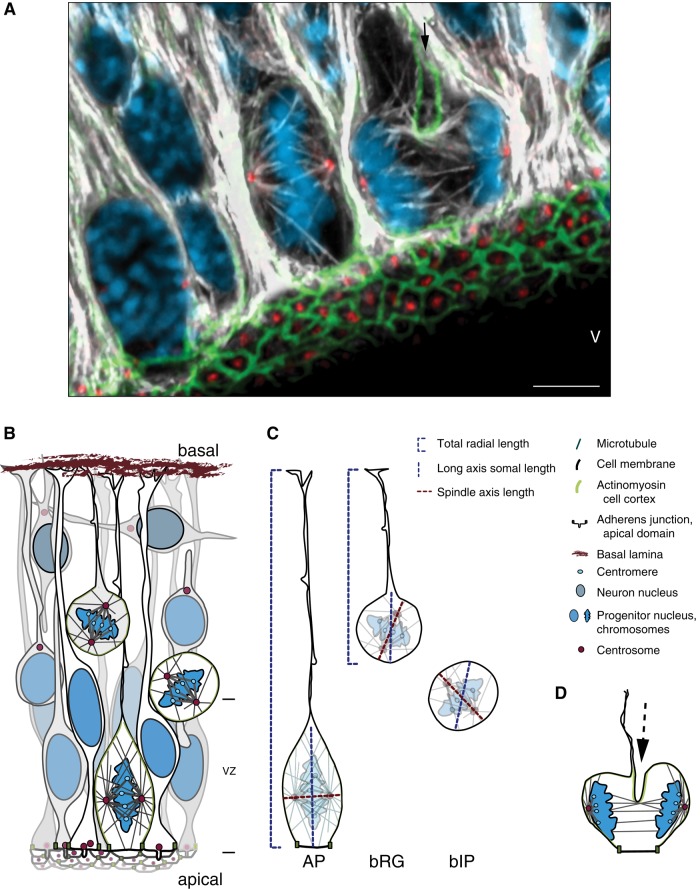
FIGURE 1:
(A) Apical progenitors (APs) dividing at the ventricular surface of the developing cortical wall. Maximum intensity projection of three 0.75-μm optical sections of E14.5 wild-type mouse dorsolateral telencephalon, showing mitotic microtubules in dividing APs. Double immunofluorescence for α- and γ-tubulin combined with 4′,6-diamidino-2-phenylindole (DAPI) and phalloidin staining; DNA in blue, microtubules in white, centrosomes and basal bodies in red, and actin in green. Long astral microtubules can be seen extending from the centrosomes to the apical and basal cell cortex in the metaphase AP. Unidirectional cleavage furrow (black arrow) ingressing in the basal-to-apical direction in the anaphase AP. Scale bar, 5 μm. V, ventricle. (B) Cartoon of major cell types and cell divisions in the dorsolateral telencephalon. APs extend processes connecting them to both the apical (ventricular) surface and the basal lamina, and they are also connected to each other via an apical adherens junction belt. In interphase, APs have a primary cilium in the apical domain, which is internalized to liberate the centrosomes for mitosis. AP cell divisions occur at the apical surface, with abundant astral microtubules maintaining a mostly horizontal spindle orientation. During neurogenesis, other progenitors derived from APs accumulate basally. In rodents, most of these are basal intermediate progenitors (bIPs), which lose both their apical and basal processes. They typically divide to produce two neurons. Also present, especially in mammals with a large neocortex, are basal radial glia (bRG), which delaminate from the apical surface but still have processes and more self-renewing capacity. The spindles of these types of basal progenitors (BPs) are oriented more variably, probably due to fewer astral microtubules. Neurons produced by all these progenitors migrate basally. Note that the cortical wall thickness is not drawn to scale, and other layers basal to the ventricular zone (VZ), including the six neuronal layers characteristic of the mammalian cerebral cortex, are not shown in detail. (C) Orientation of the spindle axis (dashed red line) compared with the longest axis of the soma (dashed blue line) and to the longest, apicobasal axis of the entire cell, including the processes (dashed blue bracket). In APs, the spindle axis is mostly oriented perpendicular, and not along, the longest cell axis, which breaks the long-axis Hertwig rule. In BPs (bRG and bIPs), which lack an apical domain, spindle orientation is more variable, and the cell somata are not as elongated, making a particular orientation of the spindle less relevant. (D) Anaphase AP undergoing cytokinesis with a unidirectional cleavage furrow, ingressing in the basal-to-apical direction (arrow; see also anaphase AP in A).
Basal process splitting is not seen in all early APs, and later in neurogenesis, when all APs are aRG that can reach hundreds of micrometers in length, basal process splitting has not been reported (Kosodo and Huttner, 2009). A possible limitation in aRG is the time available for mitosis. If basal process splitting were triggered by mitosis entry, aRG would only have the limited preanaphase time to achieve it, which could be insufficient. The remaining alternative to generate two basal lamina-contacting aRG is basal process regrowth after division. This has been observed in zebrafish, and evidence suggests that it also happens in mammals (Shitamukai and Matsuzaki, 2012). Further, in delaminated bRG, the basal process can regrow (Hansen et al., 2010; Betizeau et al., 2013). The mechanisms for basal process regrowth are unknown, but we speculate that sibling or neighboring aRG processes could serve as guides.
SPINDLE FORMATION AND ORIENTATION
When mitosis starts, interphase microtubules depolymerize for cellular reorganization and to provide tubulin subunits for mitotic microtubules. In addition, the centrosomes are liberated from each other to nucleate a bipolar spindle. In APs, this happens when the nucleus is at or near the ventricular surface. The basal process also becomes thinner (Kosodo and Huttner, 2009), likely the result of cytoplasmic flow to the soma. A core function of this flow could therefore be to provide sufficient tubulin subunits for spindle assembly, in addition to concentrating other cytoplasmic components for their subsequent distribution to the daughter cells. Some stabilized microtubules may nevertheless remain to support the basal process. NSC spindle assembly and orientation have been reviewed in detail (Lancaster and Knoblich, 2012; Shitamukai and Matsuzaki, 2012); we therefore focus on mammalian NSC particularities and some recent discoveries.
BREAKING THE HERTWIG RULE
A remarkable particularity of APs is the orientation of their mitotic spindle with respect to cell axes. Oskar Hertwig proposed more than a century ago that spindles preferentially extend along the longest axis of dividing cells (Hertwig, 1884). This results in a shorter cleavage along the orthogonal axis. Preference for a long axis also offers more longitudinal space for spindle establishment in prometaphase and requires less additional cell elongation during anaphase. This long-axis “Hertwig rule” has been broadly documented (Gillies and Cabernard, 2011). APs, however, break it. Most AP spindles remain oriented perpendicular to, rather than along, their elongated apical-basal axis through neurogenesis. Cleavage is therefore “vertical,” along the longer axis. This holds true also for the metaphase soma only, which becomes more rounded but remains longer along the apical-basal axis (Figure 1C). “Horizontal” AP cleavages—that is, conforming to the Hertwig rule—do exist; their proportion is low but increases during neurogenesis, particularly in large-brain animals (Huttner and Kosodo, 2005; Konno et al., 2008; Shitamukai et al., 2011; Shitamukai and Matsuzaki, 2012; LaMonica et al., 2013). However, this increase happens not only in horizontal but in all nonvertical cleavages.
The existence of various spindle orientations tells us that APs are not incapable of conforming to the Hertwig rule. Why, then, do they often break it to adopt a more challenging long-axis cleavage? This is most likely due to a need for the highest possible epithelial division symmetry for proliferation. Consistent with this scenario, spindle orientation becomes progressively more variable in neurogenic AP divisions, and fewer polarized components are distributed symmetrically to the daughter cells (Mora-Bermudez et al., 2014). In broad terms, the more epithelial a progenitor is after division, the higher its proliferative or self-renewing capacity seems to remain.
Spindle orientation establishment along the short axis remains poorly understood. Recent data from chick APs show that a broad equatorial belt of the LGN, a protein known to link astral microtubules to the cell cortex together with Numa and dynein (Siller and Doe, 2009), favor a mostly horizontal spindle (Peyre et al., 2011). Consistent with this view, LGN perturbations in mouse APs result in fewer horizontal spindles (Konno et al., 2008).
Conforming to versus breaking Hertwig’s rule is less of an issue for basal progenitors. These cells have delaminated from the apical surface and divide basally, show more-variable spindle orientations, and their cell soma is not as elongated as that of APs (Figure 1C). Spindle orientation may then be less relevant, as any orientation could produce, in terms of cell structure, a symmetric division in basal intermediate progenitors and an asymmetric division in bRG.
ACHIEVING A/SYMMETRY
How predictive spindle orientation is of division a/symmetry strongly depends on spindle alignment with cell components, and minor misalignments in APs are enough to bypass the narrow apical domain (Kosodo et al., 2004). Similarly, basal process bypassing when cytokinesis runs adjacent (Figure 1D), rather than through it, can be regarded as a small misalignment that leads to asymmetric inheritance of the basal process. Alignment precision is therefore particularly decisive in APs.
How do mammalian APs control spindle and cleavage alignment to such fine levels? LGN is enriched only at the central and basal cell cortex of proliferating APs. Lack of apical anchoring sites for astrals may therefore be sufficient to prevent a vertical spindle orientation in proliferating APs (Konno et al., 2008; Shitamukai et al., 2011; Mora-Bermudez et al., 2014). When APs become neurogenic, basal LGN enrichment is also lost, with a corresponding reduction in basal astrals and an increase in spindle tilting. By contrast, the abundance of astrals reaching the central zone of the cell cortex remains stable (Mora-Bermudez et al., 2014). This shows that not all astrals have the same function. Centrally anchored astrals may thus constitute the minimal requirement for spindle assembly, whereas pole-specific astrals—basal and/or apical—could provide additional anchoring for a strictly horizontal spindle orientation. Such additional anchors seem especially important for highly dynamic growing tissues, such as the developing neocortex. Without robust anchoring, friction and collisions between cells undergoing division, INM, and delamination could also perturb spindle orientation.
IS SPINDLE ORIENTATION SUFFICIENT TO DETERMINE NSC FATE?
The causal role of spindle orientation for stem cell fate has been established for the polarized Drosophila neuroblasts and mammalian epithelia such as the skin. In these cells, orienting the spindle along the apicobasal axis, which leads to a cleavage perpendicular to this axis, results in an asymmetric differentiative division (Gillies and Cabernard, 2011). In mammalian APs, however, most differentiative divisions occur with a cleavage along the apicobasal axis, showing the situation to be less simple (Kosodo et al., 2004; Konno et al., 2008).
In the search for the basis of NSC fate determination, many studies have measured spindle orientation and reached diverse conclusions, depending on which protein was manipulated (reviewed by Lancaster and Knoblich, 2012). For example, impairment of LGN function increased bRG-like delaminated NSCs, but no dramatic effects on neuronal layer thickness were reported (Konno et al., 2008). By contrast, decreased neurogenesis was observed after depletion of Lfc, a RhoA guanine nucleotide exchange factor (Gauthier-Fisher et al., 2009), and manipulation of Inscuteable levels also affected neuronal output (Postiglione et al., 2011). In addition, most genes linked to primary microcephaly encode proteins associated with centrosomes (Bond and Woods, 2006; Gilmore and Walsh, 2013). Microcephaly proteins linked to spindle misorientation and faulty neurogenesis include Aspm (Fish et al., 2006) and Cdk5Rap2 (Buchman et al., 2010). However, the mechanisms leading to microcephaly remain unclear (Pulvers et al., 2010). In addition, a precise determination of spindle orientation can be important for matching a/symmetry of AP division to progeny fate (Juschke et al., 2014). At any rate, the spectrum of aforementioned effects makes it unlikely that spindle orientation per se is the only determinant of most phenotypes observed. Instead, perturbations of specific proteins that alter spindle orientation could have additional, pleiotropic effects that may be spindle independent.
Curiously, few AP division studies have targeted the main spindle component, the microtubules. This reflects the difficulty of specifically targeting microtubule subgroups without perturbing mitotic progression. Recently this was overcome by finely titrating the concentration of small molecules specific for microtubules, such as nocodazole. With minimal concentrations, the apical-basal subset of astral microtubules was selectively targeted to manipulate spindle orientation without causing spindle-unrelated effects. This made it possible to recapitulate, or revert, the proliferation-to-neurogenesis switch by altering symmetric versus asymmetric AP divisions (Mora-Bermudez et al., 2014). Changes in only spindle orientation can therefore change mammalian NSC fate and generate BPs. Increased delamination caused by inducing nonvertical AP divisions, which give rise to bRG rather than aRG progeny, supports this conclusion (Shitamukai et al., 2011).
In addition to orientation, spindle size asymmetry also influences Drosophila neuroblast division, where the larger, more apical daughter cell inherits the larger spindle half and remains a self-renewing neuroblast (Kaltschmidt et al., 2000). Recent evidence supports spindle size asymmetry effects also in some mammalian NSCs. In mammals, however, when this type of asymmetry was observed, it was the daughter cell with the smaller spindle half that was more likely to self-renew (Delaunay et al., 2014). These similarities and differences between mammals and flies could come from differences in cell architecture and distribution of polarity components (Brand and Livesey, 2011).
CONCLUDING REMARKS
The cellular architecture of mammalian embryonic NSCs is vastly different from that of other cell types. In addition, the dynamics of NSCs through the cell cycle are tightly linked to those architectural components. It is therefore hardly surprising that their mitotic cytoskeleton also shows remarkable particularities, such as a spindle oriented along the short cell axis by microtubule subsets and cytokinesis initiating before karyokinesis in many APs. The study of these features has allowed investigators to tackle fundamental questions, and the evidence for a causal effect of spindle orientation on the fate of many mammalian NSCs seems now conclusive. This is certainly not to say that further studies will not bring much needed clarity and unexpected insights. Indeed, the molecular interplays within the vast network of spindle and division orientation molecules in NSCs are only beginning to be unraveled. Similarly, understanding their role in neurodevelopmental disorders will require extensive work. Nevertheless, the emergence and refinement of a diversity of powerful techniques, from live-tissue imaging to single-cell “omics,” ensure that our insights in this field are likely to come faster than ever.
Acknowledgments
We apologize to many colleagues whose work could not be cited owing to space limitations. We thank Elena Taverna for help with the figure and for discussions. F.M.-B. was supported by an EMBO Long Term Fellowship (ALTF 1080-2007). W.B.H. was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 655, A2; TRR 83, Tp6) and the European Research Council (250197), by the Deutsche Forschungsgemeinschaft–funded Center for Regenerative Therapies Dresden, and by the Fonds der Chemischen Industrie.
Abbreviations used:
- AP
apical progenitor
- aRG
apical radial glia
- bIP
basal intermediate progenitor
- BP
basal progenitor
- bRG
basal radial glia
- INM
interkinetic nuclear migration
- NSC
neural stem cell.
Footnotes
REFERENCES
- Betizeau M, Cortay V, Patti DD, Pfister S, Gautier E, Bellemin-Ménard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, Dehay C. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron. 2013;80:442–457. doi: 10.1016/j.neuron.2013.09.032. [DOI] [PubMed] [Google Scholar]
- Bond J, Woods CG. Cytoskeletal genes regulating brain size. Curr Opin Cell Biol. 2006;18:95–101. doi: 10.1016/j.ceb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Brand AH, Livesey FJ. Neural stem cell biology in vertebrates and invertebrates: more alike than different? Neuron. 2011;70:719–729. doi: 10.1016/j.neuron.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Breuss M, Keays DA. Microtubules and neurodevelopmental disease: the movers and the makers. Adv Exp Med Biol. 2014;800:75–96. doi: 10.1007/978-94-007-7687-6_5. [DOI] [PubMed] [Google Scholar]
- Buchman JJ, Tseng HC, Zhou Y, Frank CL, Xie Z, Tsai LH. Cdk5rap2 interacts with pericentrin to maintain the neural progenitor pool in the developing neocortex. Neuron. 2010;66:386–402. doi: 10.1016/j.neuron.2010.03.036. [DOI] [PubMed] [Google Scholar]
- D’Avino PP, Giansanti MG, Petronczki M. Cytokinesis in animal cells. Cold Spring Harb Perspect Biol. 2015;7, a015834 doi: 10.1101/cshperspect.a015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay D, Cortay V, Patti D, Knoblauch K, Dehay C. Mitotic spindle asymmetry: a Wnt/PCP-regulated mechanism generating asymmetrical division in cortical precursors. Cell Rep. 2014;6:400–414. doi: 10.1016/j.celrep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- Fish JL, Kosodo Y, Enard W, Paabo S, Huttner WB. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc Natl Acad Sci USA. 2006;103:10438–10443. doi: 10.1073/pnas.0604066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier-Fisher A, Lin DC, Greeve M, Kaplan DR, Rottapel R, Miller FD. Lfc and Tctex-1 regulate the genesis of neurons from cortical precursor cells. Nat Neurosci. 2009;12:735–744. doi: 10.1038/nn.2339. [DOI] [PubMed] [Google Scholar]
- Gillies TE, Cabernard C. Cell division orientation in animals. Curr Biol. 2011;21:R599–R609. doi: 10.1016/j.cub.2011.06.055. [DOI] [PubMed] [Google Scholar]
- Gilmore EC, Walsh CA. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol. 2013;2:461–478. doi: 10.1002/wdev.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hertwig O. Das Problem der Befruchtung und der Isotropie des Eies, eine Theory der Vererbung. Jenaische Z Naturwiss. 1884;18:21–23. [Google Scholar]
- Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17:648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Juschke C, Xie Y, Postiglione MP, Knoblich JA. Analysis and modeling of mitotic spindle orientations in three dimensions. Proc Natl Acad Sci USA. 2014;111:1014–1019. doi: 10.1073/pnas.1314984111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt JA, Davidson CM, Brown NH, Brand AH. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nat Cell Biol. 2000;2:7–12. doi: 10.1038/71323. [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Huttner WB. Basal process and cell divisions of neural progenitors in the developing brain. Dev Growth Differ. 2009;51:251–261. doi: 10.1111/j.1440-169X.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Röper K, Haubensak W, Marzesco A-M, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y, Toida K, Dubreuil V, Alexandre P, Schenk J, Kiyokage E, Attardo A, Mora-Bermudez F, Arii T, Clarke JD, Huttner WB. Cytokinesis of neuroepithelial cells can divide their basal process before anaphase. EMBO J. 2008;27:3151–3163. doi: 10.1038/emboj.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonica BE, Lui JH, Hansen DV, Kriegstein AR. Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat Commun. 2013;4:1665. doi: 10.1038/ncomms2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA. Spindle orientation in mammalian cerebral cortical development. Curr Opin Neurobiol. 2012;22:737–746. doi: 10.1016/j.conb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Okamoto M, Shinoda T, Kawaguchi A. Interkinetic nuclear migration generates and opposes ventricular-zone crowding: insight into tissue mechanics. Front Cell Neurosci. 2014;8:473. doi: 10.3389/fncel.2014.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Bermudez F, Matsuzaki F, Huttner WB. Specific polar subpopulations of astral microtubules control spindle orientation and symmetric neural stem cell division. Elife. 2014;3:e02875. doi: 10.7554/eLife.02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem BE, Lui JH, Gertz CC, Kriegstein AR. Control of outer radial glial stem cell mitosis in the human brain. Cell Rep. 2014;8:656–664. doi: 10.1016/j.celrep.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre E, Jaouen F, Saadaoui M, Haren L, Merdes A, Durbec P, Morin X. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J Cell Biol. 2011;193:141–154. doi: 10.1083/jcb.201101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postiglione MP, Juschke C, Xie Y, Haas GA, Charalambous C, Knoblich JA. Mouse inscuteable induces apical-basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex. Neuron. 2011;72:269–284. doi: 10.1016/j.neuron.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvers JN, Bryk J, Fish JL, Wilsch-Bräuninger M, Arai Y, Schreier D, Naumann R, Helppi J, Habermann B, Vogt J, et al. Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline. Proc Natl Acad Sci USA. 2010;107:16595–16600. doi: 10.1073/pnas.1010494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci. 2011;31:3683–3695. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A, Matsuzaki F. Control of asymmetric cell division of mammalian neural progenitors. Dev Growth Differ. 2012;54:277–286. doi: 10.1111/j.1440-169X.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- Taverna E, Götz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- Taverna E, Huttner WB. Neural progenitor nuclei IN motion. Neuron. 2010;67:906–914. doi: 10.1016/j.neuron.2010.08.027. [DOI] [PubMed] [Google Scholar]