The trafficking of newly synthesized Na,K-ATPase and E-cadherin is observed in polarized epithelial cells. E-cadherin’s exit from the Golgi complex is not susceptible to 19°C temperature block. Furthermore, these proteins exit the Golgi and are delivered to the basolateral cell surface in separate vascular carriers.
Abstract
Recent evidence indicates that newly synthesized membrane proteins that share the same distributions in the plasma membranes of polarized epithelial cells can pursue a variety of distinct trafficking routes as they travel from the Golgi complex to their common destination at the cell surface. In most polarized epithelial cells, both the Na,K-ATPase and E-cadherin are localized to the basolateral domains of the plasma membrane. To examine the itineraries pursued by newly synthesized Na,K-ATPase and E-cadherin in polarized MDCK epithelial cells, we used the SNAP and CLIP labeling systems to fluorescently tag temporally defined cohorts of these proteins and observe their behaviors simultaneously as they traverse the secretory pathway. These experiments reveal that E-cadherin is delivered to the cell surface substantially faster than is the Na,K-ATPase. Furthermore, the surface delivery of newly synthesized E-cadherin to the plasma membrane was not prevented by the 19°C temperature block that inhibits the trafficking of most proteins, including the Na,K-ATPase, out of the trans-Golgi network. Consistent with these distinct behaviors, populations of newly synthesized E-cadherin and Na,K-ATPase become separated from one another within the trans-Golgi network, suggesting that they are sorted into different carrier vesicles that mediate their post-Golgi trafficking.
INTRODUCTION
Polarized epithelial cells serve as barriers that separate an organism’s external environment from its internal milieu while at the same time mediating the selective import and export of substances, often against steep concentration gradients. To accomplish these physiological functions, epithelial cell plasma membranes (PM)s are segregated into distinct apical and basolateral domains, each of which harbors different subsets of membrane proteins, including adhesion molecules, receptors, ion channels, pumps, and transporters. Epithelial cells must be able to generate and maintain these polarized distributions, which are essential prerequisites of their capacity to carry out vectorial solute and fluid transport (Muth and Caplan, 2003). The lipid and protein compositions of these membrane domains are tightly regulated through the precise control of biosynthetic delivery, recycling, and degradative pathways (Gonzalez and Rodriguez-Boulan, 2009; Weisz and Rodriguez-Boulan, 2009).
During biosynthetic delivery, apical and basolateral cargoes are segregated from each other at the trans-Golgi network (TGN), where they are sorted into distinct vesicle populations for delivery to the cell surface (Mellman, 1996; Keller et al., 2001; Rodriguez-Boulan et al., 2005). Endocytic intermediates have also been shown to participate in the biosynthetic delivery of some basolateral proteins. Experiments using radioactive pulse-chase techniques demonstrated that newly synthesized transferrin receptor could be detected in endosomes before its accumulation at the basolateral PM (Futter et al., 1995). In addition, both live-cell imaging and endosomal ablation experiments revealed that the vesicular stomatitis virus G-protein (VSV-G) makes an obligate stop at recycling endosomes (REs) before its delivery to the basolateral PM (Ang et al., 2004). The list of basolateral cargoes that traffic through endosomal compartments continues to expand and includes E-cadherin and the asialoglycoprotein receptor (Leitinger et al., 1995; Desclozeaux et al., 2008). Of interest, the Na,K-ATPase appears to traffic to the cell surface in a manner that is independent of the common RE (Farr et al., 2009). Taken together, these observations suggest that sorting signals may dictate divergent trafficking behaviors at multiple stages during postbiosynthetic processing. The existence of multiple classes of sorting signals that specify distinct routes to the same ultimate destination may permit epithelial cells to respond to tissue-specific cues in defining or regulating the compositions of their plasma membranes.
In this study, we explore the biosynthetic trafficking pathways pursued by two basolateral proteins in polarized Madin–Darby canine kidney (MDCK) renal epithelial cells. E-cadherin is critically involved in the establishment and maintenance of epithelial barriers driving cell-to-cell adhesion by connecting adjacent cells through Ca2+-dependent homotypic interactions (Takeichi, 1990). E-cadherin has a single transmembrane span and interacts with β-catenin early during the course of its postsynthetic processing (Ozawa and Kemler, 1992). This interaction is essential for E-cadherin’s stable expression at the basolateral cell surface (Chen et al., 1999; Miyashita and Ozawa, 2007). The Na,K-ATPase, or sodium pump, comprises two obligate subunits. The catalytic α-subunit spans the membrane 10 times and harbors the sorting motifs that are necessary for the pump’s basolateral targeting (Muth et al., 1998; Dunbar et al., 2000). Assembly of the α-subunit with the β-subunit, which spans the membrane once, is necessary for pump to exit from the endoplasmic reticulum (ER) (Geering et al., 1989; Gottardi et al., 1993). Recent work suggested that in addition to its role in pump trafficking, the β-subunit of the Na,K-ATPase might also play a role in the maintenance of cell–cell contacts (Vagin et al., 2006, 2007; Cereijido et al., 2012).
Our previous analysis of basolateral trafficking in MDCK cells demonstrated that VSV-G and the sodium pump travel to the plasma membrane via separate pathways (Farr et al., 2009). E-cadherin uses a dileucine-based sorting motif, which differs from that used by either the VSV-G protein or the sodium pump (Miranda et al., 2002; Jenkins et al., 2013). E-cadherin surface delivery has been studied using green fluorescent protein (GFP)–tagged constructs, and it has been suggested that, like the VSV-G protein, E-cadherin travels through recycling endosomes en route to the cell surface (Desclozeaux et al., 2008). However, pools of E-cadherin have been detected in Rab11-positive compartments, suggesting that E-cadherin may pass through the apical recycling endosomes rather than the common recycling endosome used by VSV-G (Desclozeaux et al., 2008). E-cadherin’s delivery to the basolateral PM also appears to be independent of the recycling endosome adaptor component μ1B (Miranda et al., 2001b). A prominent role for clathrin in basolateral trafficking has recently emerged, as revealed through a significant loss of polarity for many basolateral proteins upon knockdown of clathrin expression (Deborde et al., 2008). The distribution of E-cadherin, however, was affected to a lesser extent than most basolateral proteins by clathrin knockdown, whereas no changes were observed for the Na,K-ATPase. In addition, knockdown of the clathrin adaptor AP-1A had no effect on the polarized distribution of the Na,K-ATPase (Gravotta et al., 2012). Of interest, it has been suggested that clathrin and the dileucine-based sorting motif function in the recycling of apically mislocalized E-cadherin rather than during biosynthesis itself (Jenkins et al., 2013). Taken together, these results suggest that E-cadherin and the Na,K-ATPase may both pursue a non–clathrin-dependent route to the basolateral PM.
We used the SNAP- and CLIP-tag labeling systems to illuminate the pathways pursued during the biosynthetic delivery of these two basolaterally destined proteins. The SNAP-tag is a modified version of the DNA repair protein 06-alkylguanine-DNA alkyltransferase, which covalently transfers the substituted benzyl group of benzylguanine (BG) to its active thiol. Temporally defined cohorts of fusion proteins that incorporate the SNAP-tag can be labeled through the use of fluorescently conjugated derivatives of benzylguanine (Keppler et al., 2004). With the recent development of the CLIP-tag, a mutated form of the SNAP-tag that uses benzylcytosine (BC) as its substrate (Gautier et al., 2008), it is possible to pulse label and detect multiple proteins simultaneously within the same cell (Figure 1). Thus this approach can be used to determine the trafficking properties of temporally defined cohorts of distinct polypeptides.
FIGURE 1:
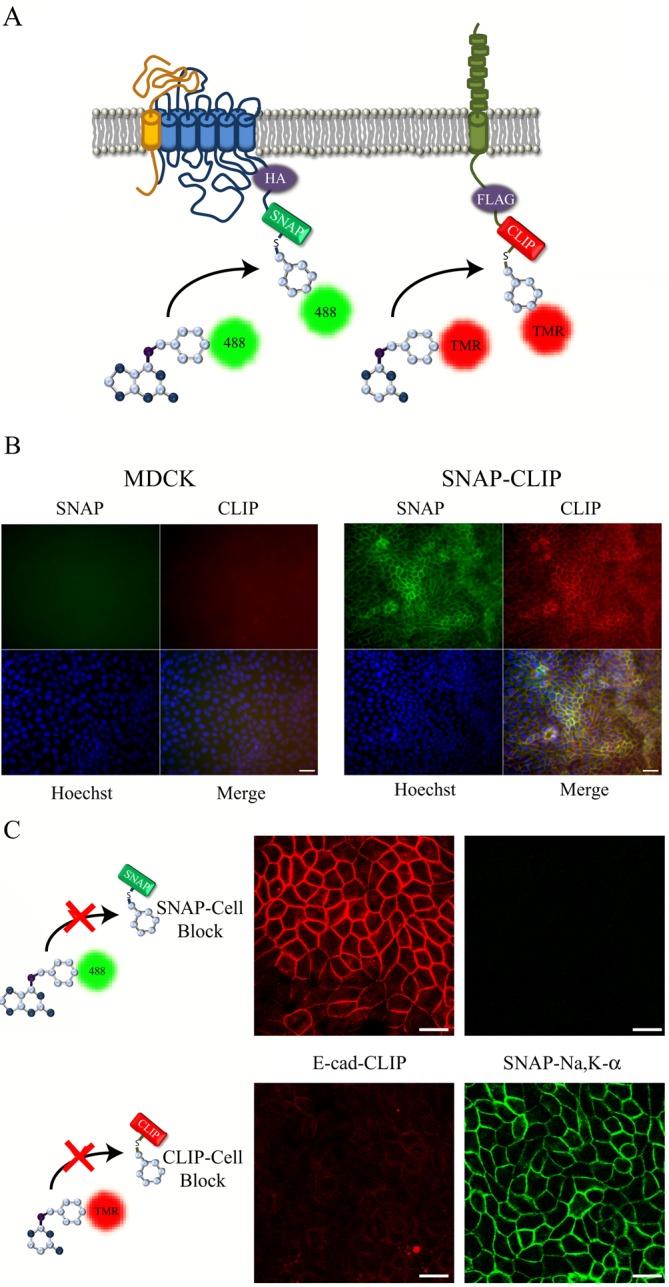
Generation of a stably transfected MDCK cell line expressing SNAP-HA–Na,K-ATPase and E-cadherin–CLIP. (A) The SNAP- and CLIP-tag labeling reaction for the N-terminally tagged Na,K-ATPase α-subunit and E-cadherin–CLIP. (B) Stably transfected cells expressing both the SNAP–Na,K-ATPase and E-cadherin–CLIP (SNAP-CLIP) or control MDCK cells were fixed and stained with CT-TMR (red), Alexa 488–SNAP (green), and Hoechst dye (blue). (C) Cells were preincubated with either BTP, to block SNAP-tag staining, or BTC, to block CLIP-tag staining, and stained as described. Bar, 20 μm.
We find that the rates of Na,K-ATPase and E-cadherin trafficking differ substantially. Surprisingly, delivery of newly synthesized E-cadherin to the plasma membrane was not susceptible to the 19°C block that inhibits protein trafficking out of the TGN (Matlin and Simons, 1983; Saraste and Kuismanen, 1984; Griffiths et al., 1989). Finally, we observed that newly synthesized E-cadherin and Na,K-ATPase become segregated from one another within the TGN, consistent with their sorting into separate carrier vesicles that pursue distinct post-Golgi trafficking itineraries.
RESULTS
Generation of a stable MDCK cell line expressing SNAP-hemagglutinin–Na,K-ATPase and E-cadherin–FLAG-CLIP
A construct was prepared encoding a chimeric protein in which the CLIP-tag was attached to the C-terminus of E-cadherin via a short linker region containing a FLAG epitope tag (Figure 1A). This construct was transfected into MDCK cells that express the SNAP-tagged sodium pump (Farr et al., 2009), and stable cell lines were selected. Western blot analysis showed robust expression of both the tagged sodium pump and E-cadherin proteins as measured using antibodies specific for the endogenous proteins, as well as antibodies directed against the respective epitope tags (Supplemental Figure S1A). We next tested whether the CLIP-tagged E-cadherin protein was detectable by fluorescence labeling and microscopy. As shown in Figure 1B, we were able to simultaneously label the sodium pump and E-cadherin using reagents that target the SNAP- and CLIP-tags (Alexa 488–BG or CT-TMR, respectively). As expected, the staining patterns for both proteins were appropriately localized at the basolateral plasma membrane, and no nonspecific labeling was detected in untransfected MDCK control cells (Figure 1B and Supplemental Figure S1B).
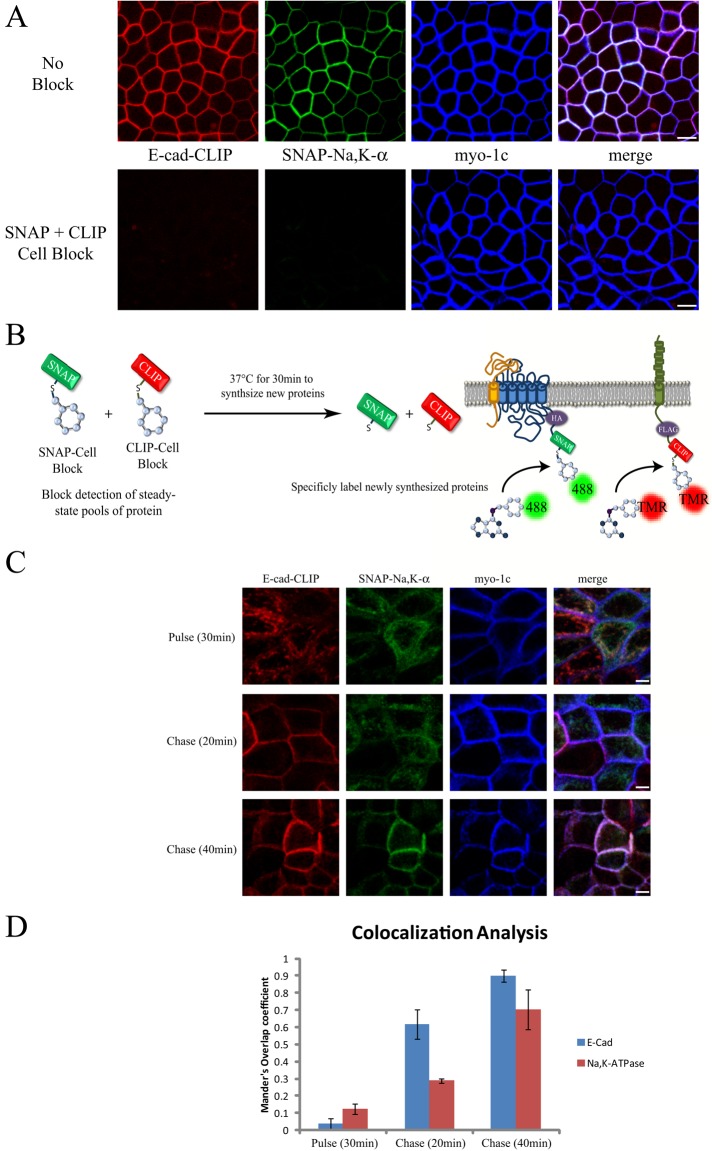
The SNAP- and CLIP-tag systems permit temporally defined cohorts of proteins to be selectively labeled and detected. This is accomplished by using membrane-permeable nonfluorescent BG and BC reagents to block covalently and irreversibly the reactive sites on previously synthesized collections of SNAP- or CLIP-tagged proteins. This blocking step prevents the fluorescence labeling and detection of the steady-state pools of the proteins of interest, allowing instead for the specific labeling and imaging of subsequently synthesized cohorts. To test the specificity and effectiveness of the blocking reagents in MDCK cells, we treated our stable cell line independently with either the SNAP or the CLIP blocking reagent for 30 min before fixation and fluorescence labeling. As shown in Figure 1C, the blocking reagents for both the SNAP- and CLIP-tags were found to be very efficient and selective, allowing us to block detection of either the sodium pump or E-cadherin. Simultaneous incubation with both SNAP- and CLIP-tag blocking reagents was also found to inhibit detection of both proteins within the same culture (Figure 2A).
FIGURE 2:
Dual pulse-chase strategy for following simultaneous trafficking of Na,K-ATPase and E-cadherin. (A) Live SNAP-CLIP cells were preincubated with nonfluorescent blocking reagents (SNAP + CLIP block) or mock treated (No Block) for 30 min at 37°C, fixed, stained as described in Figure 1, and processed for immunofluorescence with an anti–myosin 1c antibody to label the lateral plasma membrane (blue). Bar, 10 μm. (B) The dual pulse-chase strategy to specifically detect biosynthetic trafficking. (C) SNAP-CLIP cells pretreated with blockers were washed and allowed to synthesize new protein for 30 min at 37°C (pulse). After the pulse period, samples were treated with CHX and incubated at 37°C for the indicated times before fixation and labeling as described. Bar, 5 μm. (D) The fraction of protein present at the plasma membrane (as defined by staining for myo-1C) in the experiments outlined in C as assessed with Manders colocalization analysis. Data are mean ± SD from three independent experiments.
E-cadherin trafficking to the PM is faster than that observed for the sodium pump
We next examined the biosynthesis of the sodium pump and E-cadherin in order to define their respective biosynthetic trafficking pathways. To follow simultaneously the expression and delivery of these proteins to the PM, we developed a dual pulse-chase strategy using both the SNAP- and CLIP-tag systems. For these experiments, previously synthesized proteins were first blocked as described in Figure 2A, followed by a short incubation at 37°C to permit the synthesis of new proteins with active “unblocked” tags (Figure 2B). As shown in Figure 2C, a 30-min pulse was sufficient to allow for the detection of newly synthesized sodium pump and E-cadherin. No significant colocalization of the newly synthesized proteins with myosin 1C (myo1C), a marker of the basolateral membrane, was observed after this interval (Figure 2D). Of interest, after a 20-min chase period, the entire E-cadherin signal was detected at the cell surface, whereas a substantial fraction of the sodium pump signal remained localized within cytoplasmic structures. After an additional 20 min of chase time, both newly synthesized Na,K-ATPase and E-cadherin were completely localized to the cell surface (Figure 2, C, 40 min, and D). Consistent with these data, we find that E-cadherin’s early trafficking steps occur more rapidly than do those of the Na,K-ATPase, as evidenced by the fact that the N-linked sugars attached to E-cadherin acquire endoglycosidase H (Endo H) resistance faster than do those of the glycosylated β-subunit of the Na,K-ATPase (Supplemental Figure S2).
Although the images presented in Figure 2C strongly suggest that newly synthesized E-cadherin is inserted into the lateral PM after a 20-min chase period, it is formally possible that a portion of this signal could be attributed to vesicles that are docked at the PM but have yet to fuse. To determine whether the newly synthesized E-cadherin and Na,K-ATPase proteins are indeed inserted into the basolateral plasma membrane, we combined the SNAP- and CLIP-labeling techniques with cell surface biotinylation. This experiment used the fact that the SNAP-tag is readily amenable to labeling in cell lysates (Morton et al., 2010). In this experiment, cells were incubated with the membrane-permeant SNAP- and CLIP-blocking reagents as described earlier, and the cells were then allowed to synthesize new protein for 20 min at 37°C before addition of cycloheximide (CHX) to block further protein synthesis. At each time point, cells were washed twice with ice-cold phosphate-buffered saline (PBS), lysed in Triton extraction buffer, and labeled with fluorescent reagents for SNAP and CLIP. The labeled lysates were separated by SDS–PAGE, and fluorescence signals associated with the Na,K-ATPase and E-cadherin were visualized using a fluorescent gel imaging system as described in Materials and Methods. As shown in Figure 3, we observed significant labeling of the Na,K-ATPase and E-cadherin in unblocked control lysates. Addition of membrane-permeant blocking reagents abolished this labeling, consistent with what we observe in the microscopy protocol used in the experiments depicted in Figure 2. Newly synthesized cohorts of fluorescently labeled E-cadherin and Na,K-ATPase were readily detected in lysates from cells that had been incubated for 20 min at 37°C (pulse) after the blocking step. The appearance of the higher–molecular weight precursor form of E-cadherin, which is cleaved during postbiosynthetic processing (Shore and Nelson, 1991), provided further evidence that our labeling strategy specifically detected newly synthesized cohorts (Figure 3A, Pulse). The intensity of the band corresponding to the E-cadherin precursor diminished within 20 min of CHX addition in association with a concomitant increase in the intensity of the band corresponding to mature E-cadherin (Figure 3A, chase).
FIGURE 3:
Newly synthesized E-cadherin traffics to the cell surface faster than the Na,K-ATPase. SNAP-CLIP cells were subjected to block, incubated at 37°C for 20 min to begin synthesis of new cohorts of sodium pump and E-cadherin, and then chased in medium containing 150 μg/ml CHX for the indicated times. At each time point, cultures were lysed and labeled with SNAP 782 and CLIP 647. (A) Lysates were processed by SDS–PAGE and imaged using an Odyssey Imager. (B) Samples were treated as described; however, before lysis, filter-grown cultures were subjected to cell surface biotinylation, and lysates prepared from these cells were precipitated with streptavidin–agarose to recover proteins that were exposed at the cell surface. (C) Quantification of data in B normalized relative to the highest value for E-cadherin and the Na,K-ATPase. Data represent mean (n = 3) and SEM from three independent experiments.
To detect cell surface delivery, we modified a protocol for selective cell surface biotinylation (Gottardi et al., 1995) such that we could capture newly synthesized SNAP- and CLIP-labeled molecules as they appeared at the cell surface. For this experiment, cultures were treated as described; however, we performed cell surface biotinylation before cell lysis and labeling with fluorescent reagents. We again observed complete blocking of the SNAP- and CLIP-tag signals upon addition of the membrane-permeant blocking compounds bromothenylpteridine (BTP) and bromothenylcytosine (BTC), respectively (Figure 3B, Lysates). The lysates were subjected to precipitation with streptavidin beads, and the recovered proteins were separated by SDS–PAGE and detected on a fluorescent gel imager. This protocol allowed us to determine the time points at which the newly synthesized cohorts of our proteins of interest became available to biotin labeling at the cell surface. The results of these studies, depicted in Figure 3B (Cell Surface), closely mirror the data obtained through the use of the imaging protocol used in Figure 2. E-cadherin was rapidly deployed to the cell surface, achieving from 80–100% of maximal surface expression within 20 min, whereas the quantity of Na,K-ATPase at the plasma membrane continued to increase throughout the course of 60 min of chase (Figure 3C).
Delivery of E-cadherin to the PM is not impeded by the 19°C Golgi block
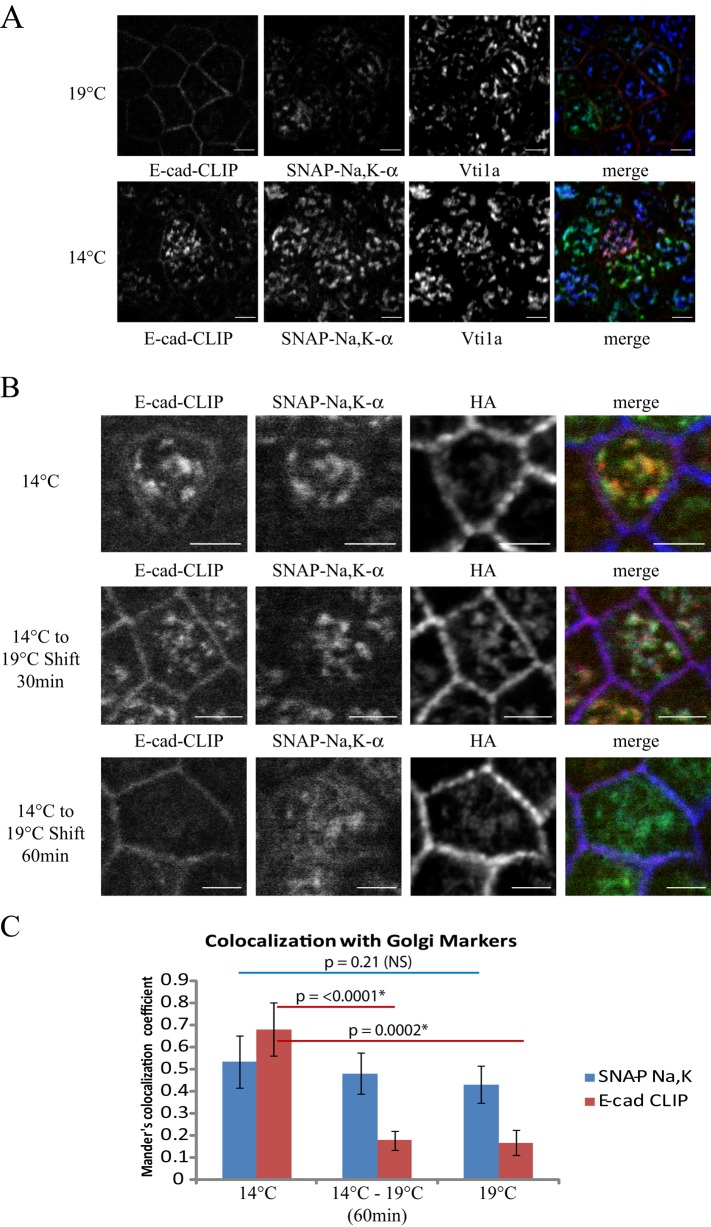
Our previous analysis of Na,K-ATPase surface delivery used the Golgi temperature block, which prevents trafficking of proteins out of the TGN (Matlin and Simons, 1983; Saraste and Kuismanen, 1984; Rindler et al., 1985). Using this protocol, we were able to synchronize release of the newly synthesized Na,K-ATPase and VSV-G proteins from the TGN, allowing us to analyze their post-Golgi trafficking steps with a high degree of temporal resolution (Farr et al., 2009). As can be seen in Figure 4A, combining our block chase protocol with a 2-h incubation at 19°C results in accumulation of the newly synthesized cohort of Na,K-ATPase within the TGN, where it colocalized with the Golgi marker Vti1a. Of interest, in the same population of cells, the newly synthesized cohort of E-cadherin was detected at the cell surface, suggesting that its trafficking is not susceptible to inhibition at 19°C. To rule out the trivial explanation that the water bath temperature might not accurately reflect the temperature of the medium bathing the cells, we directly analyzed medium temperatures using a thermocouple device and confirmed that the cells were maintained at 19°C throughout these experiments (unpublished data).
FIGURE 4:
Trafficking of newly synthesized E-cadherin is not affected by the 19°C Golgi block. (A) SNAP-CLIP cells were subjected to block, incubated at 37°C for 30 min to begin synthesis of new sodium pump and E-cadherin, and placed at 19 or 14°C for 2 h to accumulate newly synthesized protein in intracellular compartments. CT-TMR is depicted in red, Alexa 488–SNAP is shown in green, and the Golgi marker Vti1a is shown in blue. Bar, 5 μm. (B) Samples were treated as described and either fixed immediately (14°C) or warmed to 19°C for the indicated times. Samples were labeled as described, and the steady-state pool of Na,K-ATPase was labeled with an antibody directed against the HA-epitope tag to illuminate the basolateral membrane (blue). Bar, 5 μm. (C) Samples treated as in B were stained with antibodies against the Golgi compartment (gm130, Vti1a, and Golgin-84), and Manders colocalization analysis was performed as in Figure 2 to assess the fraction of E-cadherin and Na,K-ATPase that colocalized with the Golgi markers under each condition. Data are representative of two similar experiments. Asterisk indicates statistical significance using unpaired Student’s t tests. NS, not significant.
The observation that E-cadherin delivery to the cell surface is not susceptible to a 19°C temperature block suggests that either it does not pass through the TGN or its trafficking through the Golgi is not dependent on those components of the trafficking machinery whose activities are inhibited by incubation at 19°C. The former hypothesis is unlikely, as it is well established that E-cadherin is core glycosylated in the ER and this glycosylation undergoes maturation in the Golgi during its postbiosynthetic processing (Shore and Nelson, 1991). In addition, live-cell imaging experiments have detected newly synthesized E-cadherin colocalized with Golgi markers (Lock and Stow, 2005). To investigate this further, we took advantage of another low-temperature blocking strategy. Incubation of cells at temperatures between 14 and 15°C has been shown to inhibit the trafficking of proteins out of the ER–Golgi intermediate compartment (ERGIC) in many cell types (Saraste and Kuismanen, 1984; Marie et al., 2009). After incubation of cultures at 14°C, we observed significant accumulation of the newly synthesized pools of both E-cadherin and the sodium pump in intercellular compartments (Figure 4A, 14°C). Of interest, our colocalization experiments demonstrated patterns of localization at 14°C that closely resemble sequestration within compartments of the Golgi complex rather than accumulation in the ERGIC. Significant overlap was observed for both the sodium pump and E-cadherin with the Golgi markers gm130, Vti1a, and Golgin-84, whereas we saw no significant overlap of newly synthesized cargoes with staining for anti-ERGIC53 (Supplemental Figure S3A) or the recycling endosome marker Rab11 (Supplemental Figure S3B).
We next used a temperature shift strategy to determine whether the 14°C accumulated proteins would travel to the cell surface when the culture temperature was increased to 19°C. As expected, the Na,K-ATPase remained sequestered in the Golgi and was blocked from reaching the PM after incubation at 19°C for up to 2 h (Figure 4B; unpublished data). E-cadherin, however, was rapidly detected at the PM within 30 min of the switch to 19°C, where it colocalized with the steady-state cell surface pool of Na,K-ATPase (Figure 4B, HA). Within 1 h of the shift to 19°C the E-cadherin signal was completely localized to the cell surface and mimicked what we observed with constant incubation at 19°C (Figure 4B). To quantitatively assess escape from the Golgi, we performed Manders colocalization analysis on cells treated as in Figure 4B and then counterstained with a mixture of antibodies to illuminate simultaneously multiple subcompartments of the Golgi complex (gm130, Vti1a, and Golgin-84). As shown in Figure 4C, quantitation of images from this experiment revealed that the Na,K-ATPase was sequestered within the Golgi under all conditions tested. E-cadherin, however, was significantly released from the Golgi complex upon shifting the cell temperature from 14 to 19°C.
Our results clearly indicate that CLIP-tagged E-cadherin is not impeded in its trafficking out of the TGN during a 19°C blockade. It is possible, however, that addition of the CLIP-tag to the C-terminus of E-cadherin might alter its behavior and the itinerary it pursues en route to the cell surface. To examine directly the biosynthetic trafficking of endogenous E-cadherin and Na,K-ATPase molecules in untransfected MDCK cells, we performed a metabolic labeling experiment in conjunction with cell surface biotinylation. For this experiment, cells were pulse labeled with [35S]methionine/cysteine for 15 min at 37°C, followed by a chase period (at the indicated temperature) in medium containing an excess of methionine and cysteine. At each time point, cells were washed with ice-cold PBS++, and cell surface biotinylation was performed before extraction of the cells in buffer containing Triton X-100. Extracts were divided equally and precipitated with antibodies directed against the sodium pump or E-cadherin. Bound proteins were liberated using 1% SDS to generate fractions corresponding to the total cellular populations of Na,K-ATPase and E-cadherin. These samples were then diluted 10-fold before precipitation with streptavidin beads to isolate the cell surface exposed pools of these proteins.
As expected from the results depicted in Figure 3, extracts from the initial pulse period contained primarily the 135-kDa precursor form of E-cadherin (Figure 5A). This protein was converted to the 120-kDa mature form of E-cadherin during the chase at 37°C, as previously reported (Shore and Nelson, 1991). Very little cleavage was detected when samples were incubated at 14°C for up to 4 h, whereas relatively slow cleavage occurs at 19°C, with full processing being completed after 3 h of incubation at this temperature. Of interest, blockade of E-cadherin processing also correlates with decreased E-cadherin degradation. At both 37 and 19°C, the amount of mature E-cadherin decreases during the intervals 30–90 min and 2–4 h, respectively, whereas this disappearance is not observed at 14°C. Although sodium pump synthesis and stability do not appear to be affected under any of the conditions tested, this experiment reveals that the processing of the sugars attached to the Na,K-ATPase β subunit to produce the fully mature 55-kDa form of the protein is inhibited at 14°C (Figure 5B).
FIGURE 5:
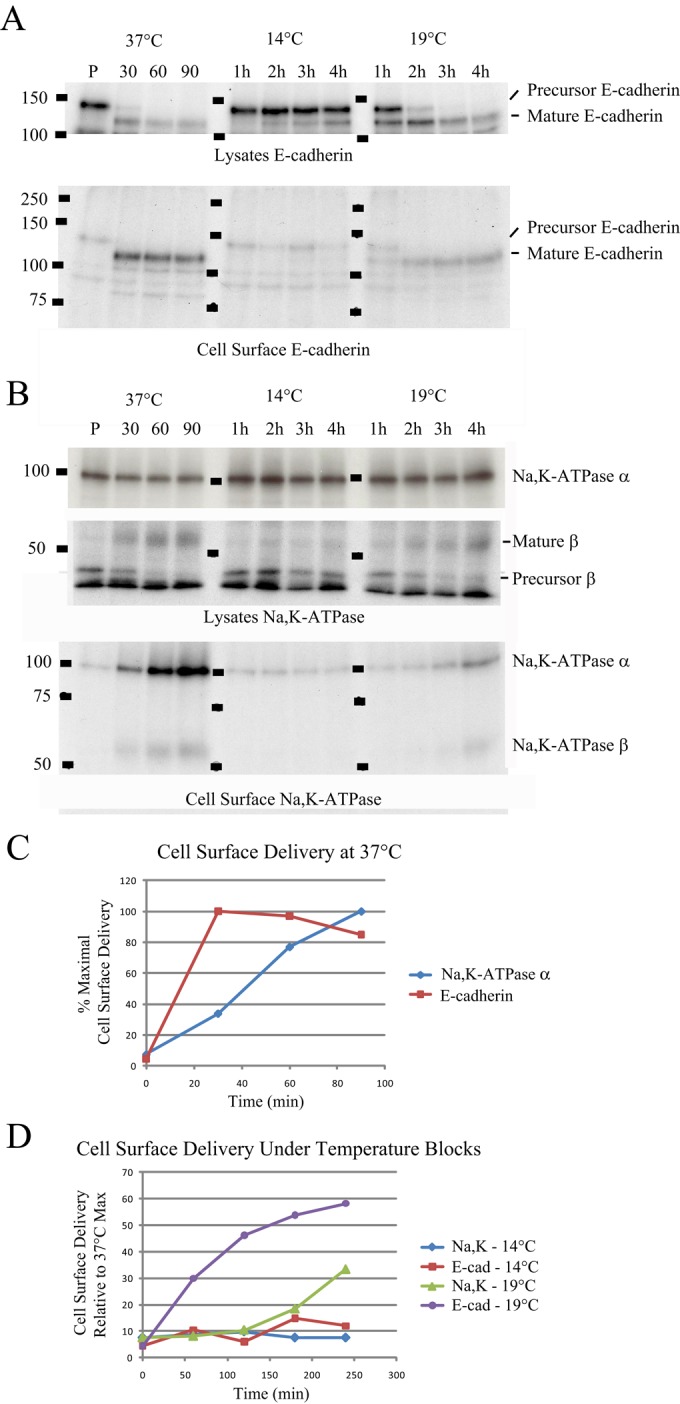
Endogenous E-cadherin is not retained in the Golgi complex during an incubation at 19°C. Cells were pulse labeled with [35S]methionine/cysteine for 15 min and chased for the indicated times at various temperatures. Samples were subjected to cell surface biotinylation and then lysed in Triton X-100 buffer. (A) Extracts were precipitated with anti–E-cadherin antibodies, washed, and eluted with 1% SDS before analysis by 8% SDS–PAGE (Lysates). Next 1% SDS eluates were diluted 10-fold, precipitated with streptavidin-agarose, and analyzed as before (Cell Surface). (B) Identical extracts from A were precipitated with antibodies directed against the sodium pump and processed as described. (C) Autoradigraphs from A and B were scanned by densitometry and normalized relative to the highest value for E-cadherin and the Na,K-ATPase. Representative data from two independent experiments. (D) Data analysis was performed as in C and normalized relative to the maximal expression obtained at 37°C.
Streptavidin precipitation of the biotinylated cell surface protein demonstrated little cell surface–exposed sodium pump or E-cadherin immediately after the pulse labeling (Figure 5, A and B). In the E-cadherin precipitates, a faint band that corresponds to the precursor form of E-cadherin was detected; however, most likely this is due to nonspecific binding of the precursor form of E-cadherin to the streptavidin beads, as the precursor form of E-cadherin has been shown not to reach the PM (McNeill et al., 1990; Shore and Nelson, 1991). After 30 min of chase, E-cadherin levels at the PM reached steady state (Figure 5, A and C), whereas the size of the surface pool of the Na,K-ATPase continued to increase out to 90 min at 37°C (Figure 5, B and C). At 14°C, both proteins were completely prevented from delivery to the PM, even out to 4 h of chase period (Figure 5D). Incubation of cultures at 19°C blocked surface delivery of the sodium pump, with 70% less sodium pump reaching the cell surface after 4 h at 19°C as compared with 90 min at 37°C. E-cadherin delivery was affected to a much lesser extent by incubating the cells at 19°C. Surface delivery of E-cadherin during the 2- to 4-h chase at 19°C reached 50–60% of the PM levels detected during chase at 37°C (Figure 5D).
The addition of brefeldin A during the 19°C block inhibits E-cadherin surface expression
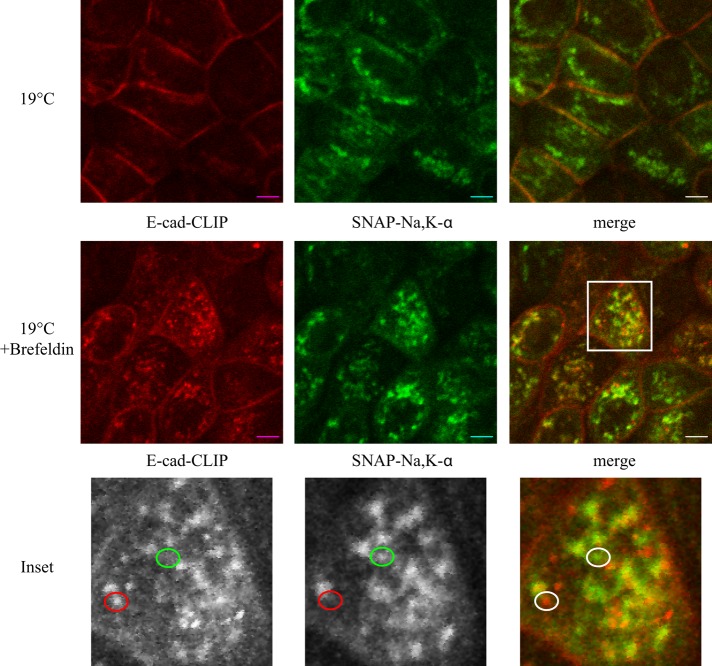
In a variety of cell types, treatment with brefeldin A (BFA) blocks the progress of newly synthesized proteins through the secretory pathway by preventing anterograde trafficking between the ER and the Golgi, thus inducing the dissolution of the cis and medial Golgi compartments and the redistribution of their intrinsic proteins to the ER. In polarized MDCK cells, however, the effects of BFA are less pronounced. BFA does not cause dissolution of the Golgi stacks but instead induces tubulation of endocytic organelles (Hunziker et al., 1991). BFA induces missorting of MDCK cell apical cargoes to the basolateral surface at low concentrations, 1–3 μg/ml, whereas it inhibits surface delivery altogether at concentrations >10 μg/ml (Low et al., 1991, 1992). In particular, E-cadherin surface delivery is blocked at BFA concentrations >3 μg/ml (Low et al., 1992). To test whether BFA could inhibit delivery of E-cadherin to the cell surface under the 19°C Golgi block conditions, we performed a 19°C pulse- chase experiment in the presence or absence of 5 μg/ml BFA. As expected from our earlier results, in the absence of BFA, E-cadherin was delivered to the cell surface at 19°C, whereas the Na,K-ATPase was blocked from exiting the TGN (Figure 6, 19°C). Of interest, in the presence of BFA, E-cadherin was retained in intracellular compartments. Closer examination revealed that under these conditions, a significant fraction, but not all, of the staining associated with newly synthesized E-cadherin was colocalized with markers of the Golgi complex. In addition, a large number of punctate structures could be detected that were exclusively stained either for newly synthesized E-cadherin or for newly synthesized sodium pump (Figure 6, inset, and Supplemental Figure S4). We were unable to identify an organelle-specific marker that colocalized with the discrete Na,K-ATPase–labeled structures. These data suggest that, under 19°C/BFA incubation conditions, at least a portion of the pools of newly synthesized E-cadherin and Na,K-ATPase resides in distinct compartments.
FIGURE 6:
The Na,K-ATPase and E-cadherin are blocked from reaching the cell surface in the presence of brefeldin A. SNAP-CLIP cells were blocked and pulsed as described in the legend to Figure 4 and incubated in the presence or absence of 5 μg/ml BFA for 2 h at 19°C. Samples were fixed and imaged as described. CT-TMR is depicted in red, and Alexa 488–SNAP is shown in green. The inset depicts areas in which the sodium pump and E-cadherin are segregated from one another. Bar, 5 μm.
DISCUSSION
We find that the trafficking of newly synthesized Na,K-ATPase and E-cadherin from the Golgi complex to the cell surface is differentially susceptible to the 19°C temperature block. Whereas incubation of cells at 19°C prevents the trafficking of the newly synthesized Na,K-ATPase from the Golgi to the basolateral plasma membrane, newly synthesized E-cadherin is able to depart the Golgi and travel to the basolateral cell surface under these conditions. Both proteins are retained in a Golgi-associated compartment when cells are incubated at 14°C, but they appear to be at least partially segregated from one another in separate subcompartments of the Golgi complex. Thus it would appear that these two proteins both pass through the Golgi complex en route to the basolateral plasma membrane, but the trafficking pathways that they pursue are subject to distinct biochemical constraints and thus involve distinct components of the cellular trafficking machinery.
We used the SNAP- and CLIP-tag systems to monitor protein synthesis and trafficking of two basolaterally destined proteins. These tagging systems allowed us to generate stable cell lines with minimal overexpression of the two proteins of interest and observe simultaneously the postsynthetic trafficking routes pursued by both proteins in fully polarized cells. We previously showed that adding the SNAP-tag to the NH2 terminus on the Na,K-ATPase α-subunit does not perturb this protein’s trafficking or function (Farr et al., 2009). In the present study, we find that there were no adverse effects resulting from CLIP-tag addition to the E-cadherin COOH terminus, as the steady-state localization (Supplemental Figure S1B) and binding to β-catenin (unpublished data) were not affected. Perturbations in β-catenin binding would be expected to significantly affect E-cadherin trafficking because uncoupled E-cadherin has been shown to be retained in intracellular compartments, where it is targeted for degradation (Chen et al., 1999; Miyashita and Ozawa, 2007). The kinetics of CLIP-tagged E-cadherin’s biosynthetic delivery was also not affected compared with that of the endogenous E-cadherin protein (Figures 2C and 5C). Processing of CLIP-tagged E-cadherin to the mature form occurred with similar kinetics as observed for the endogenous protein, and, as expected, only mature E-cadherin reached the cell surface (McNeill et al., 1990; Shore and Nelson, 1991). Similar to a previous report, we saw no cross-reactivity between the SNAP- and CLIP-tags for either the blocking or labeling reagents (Gautier et al., 2008), and we were able to detect newly synthesized proteins by both microscopy and fluorescent gel imaging. Thus these techniques allowed for multiplexed analysis of temporal cohorts of proteins, facilitating the delineation of the trafficking routes of two proteins simultaneously within the same cell. It is interesting to note that, although the steady-state expression of both the SNAP–Na,K-ATPase and E-cadherin–CLIP proteins appeared to be quite uniform throughout all of the cells in a clonal monolayer (Figure 1A), the rate of synthesis of both these proteins appeared to vary between neighboring cells (Figure 2C). This observation suggests that the rate of protein synthesis in a given cell might be influenced by local or stochastic factors. Further investigation is required to explore these interesting possibilities.
The kinetics of basolateral and apical protein biosynthesis and cell surface delivery has been studied extensively using metabolic labeling and selective cell surface biotinylation (Low et al., 1991; Gottardi et al., 1995). Using the SNAP- and CLIP-tag systems, our results demonstrate that the rates of biosynthetic delivery of the Na,K-ATPase and E-cadherin expressed in polarized MDCK cells differ substantially from one another. Examination of both tagged and endogenous versions of these proteins demonstrated that E-cadherin traverses the biosynthetic route to the basolateral membrane much faster than the Na,K-ATPase. Maximal E-cadherin delivery was detected within 30 min, compared with 90 min for the sodium pump (Figures 2 and 3). The kinetics of the cell surface delivery of many basolateral proteins has been measured (Le Bivic et al., 1990; Gravotta et al., 2007) and half-times typically range from 50 to 90 min. Our imaging results correlate well with previously published analyses of E-cadherin surface delivery (Shore and Nelson, 1991) and of the Na,K-ATPase (Caplan et al., 1990), where maximal surface delivery was detected at 45 and 120 min, respectively. Together these results further validate the use of the SNAP- and CLIP-tags for dual pulse-chase analysis of biosynthetic delivery.
We previously reported that the Na,K-ATPase and VSV-G exhibit strikingly different rates of transport from the Golgi to the PM (Farr et al., 2009). Our findings indicated that the Na,K-ATPase does not traverse REs en route to the cell surface, whereas VSV-G passes through the RE compartment. These data suggested that transit through the recycling endosome might slow VSV-G transport. Our finding that E-cadherin is not susceptible to the 19°C Golgi block prohibited a similar analysis of E-cadherin’s post-TGN trafficking pathway. Our imaging results, however, suggest that the step that differentiates the rates of Na,K-ATPase and E-cadherin surface delivery might occur during passage of these proteins through the endoplasmic reticulum. At the earliest time points after which recovery from the CLIP-block is detected (Figure 2, pulse), E-cadherin is already detected in discrete punctate structures reminiscent of the Golgi complex, whereas the SNAP signal associated with the newly synthesized Na,K-ATPase is found in a diffuse staining pattern reminiscent of the ER at the same time points. These observations do not permit us to conclude whether the Na,K-ATPase and E-cadherin are segregated from one another into distinct carriers at the level of the ER or they employ the same classes of carriers, and their apparent segregation is simply the consequence of distinct transport rates. It is interesting to note in this regard that the export of the newly synthesized pools of the Na,K-ATPase and E-cadherin appears to be differentially dependent on the participation of elements of the cytoskeleton. Overexpression of a construct that encodes domains of the Golgi-targeted βI spectrin in MDCK cells leads to retention of newly synthesized Na,K-ATPase in the endoplasmic reticulum, whereas the trafficking of E-cadherin appears to be unaffected (Devarajan et al., 1997).
Reduced temperature is often used as a simple means to slow biosynthetic protein trafficking and synchronize it so that distinct steps can be effectively resolved. Seminal work describing the effects of reduced temperatures on protein trafficking demonstrated that at 20°C, terminal glycosylation of viral glycoproteins can proceed, but their transit to the cell surface is inhibited at the level of the TGN (Matlin and Simons, 1983; Saraste and Kuismanen, 1984; Griffiths et al., 1989). At 15°C, many glycoproteins are sequestered at an earlier stage of delivery and remain sensitive to Endo H (Saraste et al., 1986). The 20°C block, however, is not an absolute, one-size-fits-all method for inhibiting cell surface delivery. Instead, it appears to slow transport to the PM (Saraste and Kuismanen, 1984), and the effects of the 20°C block may vary as a function of the cargo being observed. Mottet et al. (1986) saw significant differences between two Sendai virus glycoproteins (HN and F0) with regard their sensitivity to Endo H treatment, suggesting retention in the ER for HN versus the TGN for F0 at 20°C. At the other extreme, our results identify E-cadherin as a protein resistant to effects of reduced temperature Golgi blocking on its trafficking.
By applying a preincubation step at 14°C, we were able to synchronize the majority of newly synthesized sodium pump and E-cadherin signals within the Golgi complex, where they predominantly colocalized with one another (Supplemental Figure S3). This presynchronization allowed us to eliminate potential confounding effects attributable to varying rates of protein synthesis that could have complicated our analysis of the kinetics of these proteins’ surface delivery after biosynthetic recovery from the blocking incubations. Shifting the temperature to 19°C resulted in rapid delivery of E-cadherin to the cell surface, whereas the Na,K-ATPase remained sequestered within Golgi structures. The observed sequestration of the sodium pump demonstrates that the Golgi block was effective at 19°C, yet E-cadherin delivery was essentially unaffected. The molecular mechanisms that account for the fact that postbiosynthetic protein trafficking is disrupted at 15°C and 20°C remain unclear. It has been demonstrated, however, that significant morphological changes occur within the TGN during incubation at 20°C, including swelling and loss of tubule formation (Griffiths et al., 1989; Ladinsky et al., 2002). Of interest, when cells were subjected to a combined BFA/19°C block, we found that the newly synthesized cohorts of the Na,K-ATPase and E-cadherin were substantially segregated from one another in discrete structures. Our results demonstrate that reduced temperature does not uniformly inhibit the biosynthetic trafficking of every protein out of the Golgi complex and that there appears to be as-yet-unelucidated cargo-specific factors that determine a protein’s susceptibility to this manipulation. Our data further demonstrate that newly synthesized proteins bound for the basolateral PM can be sorted into separate pathways before their departure from the TGN.
MATERIALS AND METHODS
Antibodies
Antibodies used in this study are as follows: a monoclonal antibody directed against the HA epitope was obtained from Covance (Berkeley, CA); monoclonal antibodies directed against gp135 (Gravotta et al., 2007) and gp58 (Ang et al., 2003) were a kind gift from Ira Mellman (Genentech); antibodies targeted against proteins of the Golgi complex were purchased in the Golgi Sampler Kit (BD Transduction Labs, San Jose, CA); antibody directed against E-cadherin (BD Transduction Labs); and rabbit polyclonal antibody directed against myo1c (Sigma-Aldrich, St. Louis, MO). Alexa-conjugated secondary antibodies were purchased from Life Technologies (Carlsbad, CA). For Western blotting and immunoprecipitation, total Na,K-ATPase α1-subunit was detected with an antibody directed against residues 338–724 of the chicken Na+,K+ (α5; Tamkun and Fambrough, 1986), and the Na,K-ATPase β-subunit was detected with monoclonal antibody gp58 (Fullekrug et al., 2006; kind gift of I. Mellman).
Constructs and cell lines
To generate the CLIP-tagged version of E-cadherin, we modified a previously described GFP-tagged E-cadherin construct (Miranda et al., 2001) that was generously provided by Jennifer Stow (University of Queensland, Brisbane, Australia). We replaced the GFP tag with the CLIP-tag (New England Biolabs, Ipswich, MA) by two-step PCR and included a FLAG tag as a linker region between the C-terminus of E-cadherin and the N-terminus of the CLIP-tag. Details of the primers used in this construction are available upon request. This construct was then transferred into pcDNA 3.1 (hygro) and stably transfected into a MDCK cell line that expresses both the SNAP-HA–Na,K-ATPase α-subunit and the rat β1 subunit. This cell line was previously described (Farr et al., 2009). All experiments were performed on polarized MDCK cells grown for 4–6 d at 37°C/5% CO2 on Transwell polycarbonate filters (Hua et al., 2006) in DMEM plus 10% fetal bovine serum (FBS).
SNAP-tag labeling and fluorescence-based pulse chase
To initiate all of the pulse-chase experiments, SNAP- or CLIP-tag activity was blocked by adding 16.8 nM BTP or 1.33 μM BTC, respectively (New England Biolabs), to complete medium (DMEM plus 10% FBS) and incubating cells at 37°C for 30 min. After the block, cells were washed three times with complete medium and either immediately fixed as described later or incubated at 37°C for the indicated time before fixation. For samples undergoing a 14–19°C blocking step, after 30 min of postblock recovery at 37°C, the cells were washed twice with CO2 Independent Medium (Life Technologies) and incubated for 2 h by suspending their growth chambers in a temperature-controlled water bath. During the last hour of this incubation, 150 μg/ml cycloheximide was added to prevent synthesis of additional new SNAP-tagged protein.
To fix the cell cultures, cells were washed twice with cold PBS (Sigma-Aldrich) supplemented with 1 mM MgCl2 and 100 μM CaCl2 (PBS++) and fixed for 1 h at 4°C in 4% paraformaldehyde. After fixation, cells were washed again in PBS++. For both fluorescent SNAP- and CLIP-tag labeling and for immunofluorescence, fixed cells were permeabilized and blocked in PBS++ containing 10% goat serum, 0.5% BSA, and 0.2% saponin for 20 min at room temperature. Next cells were incubated overnight at room temperature in blocking solution containing 0.75 μM CT-TMR and/or Alexa-488 SNAP (New England Biolabs) and the relevant primary antibodies. The next day, cells were washed three times for 10 min in PBS++ with 2% BSA and 0.2% saponin and incubated with the appropriate Alexa Fluor (Molecular Probes, Eugene, OR) secondary antibodies for 1 h as earlier, followed by an additional three washes. Cells were rinsed once in PBS++ and mounted in Vectashield (Vector Labs, Burlingame, CA).
Confocal microscopy was performed using a LSM 780 laser scanning microscope (Carl Zeiss MicroImaging, Jena, Germany) with a 63× water immersion lens (n = 1.5 at 25°C). Images were processed using LSM Image Viewer and Photoshop (Adobe Systems, San Jose, CA), version 6.0. Images are the product of eightfold line averaging, and contrast and brightness settings were chosen so that all pixels were in the linear range. Manders colocalization analysis was performed using ImageJ (National Institutes of Health, Bethesda, MD) and the “just another colocalization plugin” (Bolte and Cordelieres, 2006).
SNAP-tag labeling and biochemical pulse chase
To initiate the biochemical pulse-chase experiments, SNAP- or CLIP-tag activity was blocked by adding 16.8 nM BTP or 1.33 μM BTC, respectively (New England Biolabs), to complete medium (DMEM plus 10% FBS) and incubating cells at 37°C for 30 min. After the block, cells were washed three times with complete medium and either lysed immediately in TEN-T lysis buffer (100 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 1 mM EDTA, and complete protease inhibitors without EDTA [Roche]) or incubated at 37°C for the indicated time before lysis. After lysis, lysates were incubated with 2 μM SNAP-biotin (New England Biolabs) for 90 min at room temperature. Finally, the reaction was stopped by the addition of EDTA to a final concentration of 1 mM. Biotinylated proteins were recovered through incubation with streptavidin beads as previously described (Morton et al., 2010). The proteins recovered in the streptavidin incubation were subjected to digestion with Endo H (New England Biolabs) or protein N glycosidase F (New England Biolabs) according to standard protocols (Chow and Forte, 1993). Digested proteins were analyzed by SDS–PAGE, followed by Western blotting using antibodies directed against E-cadherin or the Na,K-ATPase β-subunit (gp58).
Fluorescent SNAP- and CLIP-tag cell surface delivery assay
Filter-grown cultures were blocked as described and allowed to synthesize new proteins for 20 min at 37°C before addition of CHX. At each time point, samples were washed twice with ice-cold PBS++ and maintained on ice. Cell surface biotinylation was performed as previously described (Gottardi et al., 1995). Briefly, cultures were washed in biotinylation buffer (10 mM triethylamine, 2 mM CaCl2, 125 mM NaCl, pH 8.9), and cell surface proteins were biotinylated using sulfo-NHS-S-S-Biotin (Thermo Fisher Scientific, Waltham, MA). After biotinylation, samples were washed three times in glycine buffer to quench residual activated biotin and twice in PBS++. For cell lysis, filters were cut out of their inserts and placed into new six-well plates. Samples were lysed in buffer containing 1% Triton X-100 and clarified by centrifugation. SNAP- and CLIP-tags were labeled by incubating the lysates with 1 μM SNAP 782 and 2 μM CLIP 647 for 1.5 h at room temperature. The labeling reaction was stopped by adding 1 mM EDTA. Biotinylated proteins were precipitated with streptavidin–agarose (Thermo Fisher Scientific) and samples analyzed by SDS–PAGE followed by Western blotting (Kimura et al., 2007). SNAP- and CLIP-labeled proteins were visualized on an Odyssey Imager (Li-Cor Biosciences, Lincoln, NE) and quantified using ImageJ.
Metabolic labeling and PM delivery assay
Filter-grown cultures were maintained at confluence for 5 d at 37°C to ensure complete polarization, washed with DMEM lacking methionine or cysteine containing 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid and 5% dialyzed FBS, and incubated for 1 h at 37°C. Cultures were then pulse labeled with medium containing 300 μCi/ml [35S]methionine/cysteine (PerkinElmer, Waltham, MA) for 15 min at 37°C and chased in medium containing a 10-fold excess of methionine and cysteine at the indicated temperatures. Before processing, samples were placed on ice and cell surface biotinylation performed as described. After biotinylation, cells were solubilized in TEN-T lysis buffer (described earlier), and protein was immunoprecipitated with antibodies directed against E-cadherin or the Na,K-ATPase. Precipitated proteins were eluted in TEN (no Triton) with 1% SDS for 20 min at room temperature. Samples were then diluted 10-fold in TEN-T, and cell surface proteins were precipitated with streptavidin-conjugated beads (Pierce). Samples were analyzed by SDS–PAGE, fixed in 10% acetic acid/20% methanol and dried before exposure to x-ray film. Images were quantified using ImageJ.
Supplementary Material
Acknowledgments
This article is dedicated with love and respect to the memory of Michael Hull, who made critically important contributions to this work and whose intellect, ability, humor, and friendship are very deeply missed. We thank Jennifer Stow for providing the plasmid-encoding E-cadherin, as well as Ivan Correa and all of the members of the Caplan laboratory for helpful discussions and support. This work was supported by American Cancer Society New England Division Shoreline Circle of Hope Postdoctoral Fellowship PF-06-243-01-CSM (G.A.F.), National Institutes of Health Training Grant 5T32GM007223-35 and National Science Foundation Graduate Research Fellowship Grant DGE-1122492 (E.H.S.), and National Institutes of Health Grants DK17433 and DK072612 (M.J.C.).
Abbreviations used:
- BC
benzylcytosine
- BG
benzylguanine
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- PM
plasma membrane
- RE
recycling endosome
- TGN
trans-Golgi network.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-09-1385) on September 30, 2015.
REFERENCES
- Ang AL, Folsch H, Koivisto UM, Papaert M, Mellman I. The Rab8 GTPase selectively regulates AP-1B–dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol. 2003;163:339–350. doi: 10.1083/jcb.200307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Caplan MJ, Forbush B, 3rd, Palade GE, Jamieson JD. Biosynthesis of the Na,K-ATPase in Madin-Darby canine kidney cells. Activation and cell surface delivery. J Biol Chem. 1990;265:3528–3534. [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L, Larre I. The Na+-K+-ATPase as self-adhesion molecule and hormone receptor. Am J Physiol Cell Physiol. 2012;302:C473–C481. doi: 10.1152/ajpcell.00083.2011. [DOI] [PubMed] [Google Scholar]
- Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol. 1999;144:687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DC, Forte JG. Characterization of the beta-subunit of the H(+)-K(+)-ATPase using an inhibitory monoclonal antibody. Am J Physiol. 1993;265:C1562–C1570. doi: 10.1152/ajpcell.1993.265.6.C1562. [DOI] [PubMed] [Google Scholar]
- Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclozeaux M, Venturato J, Wylie F, Kay J, Joseph S, Le H, Stow J. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol. 2008;295:C545–C556. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Stabach PR, De Matteis MA, Morrow JS. Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc Natl Acad Sci USA. 1997;94:10711–10716. doi: 10.1073/pnas.94.20.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar LA, Aronson P, Caplan MJ. A transmembrane segment determines the steady-state localization of an ion-transporting adenosine triphosphatase. J Cell Biol. 2000;148:769–778. doi: 10.1083/jcb.148.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr GA, Hull M, Mellman I, Caplan MJ. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol. 2009;186:269–282. doi: 10.1083/jcb.200901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullekrug J, Shevchenko A, Simons K. Identification of glycosylated marker proteins of epithelial polarity in MDCK cells by homology driven proteomics. BMC Biochem. 2006;7:8. doi: 10.1186/1471-2091-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Gautier A, Juillerat A, Heinis C, Corrêa IJ, Kindermann M, Beaufils F, Johnsson K. An engineered protein tag for multiprotein labeling in living cells. Chem Biol. 2008;15:128–136. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Geering K, Theulaz I, Verrey F, Hauptle MT, Rossier BC. A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am J Physiol. 1989;257:C851–C858. doi: 10.1152/ajpcell.1989.257.5.C851. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Rodriguez-Boulan E. Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett. 2009;583:3784–3795. doi: 10.1016/j.febslet.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Dunbar LA, Caplan MJ. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol. 1995;268:F285–F295. doi: 10.1152/ajprenal.1995.268.2.F285. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Pietrini G, Roush DL, Caplan MJ. Sorting of ion transport proteins in polarized cells. J Cell Sci Suppl. 1993;17:13–20. doi: 10.1242/jcs.1993.supplement_17.3. [DOI] [PubMed] [Google Scholar]
- Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS, Rodriguez-Boulan E. The clathrin adaptor AP-1A mediates basolateral polarity. Dev Cell. 2012;22:811–823. doi: 10.1016/j.devcel.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, Gonzalez A, Rodriguez-Boulan E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA. 2007;104:1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Fuller SD, Back R, Hollinshead M, Pfeiffer S, Simons K. The dynamic nature of the Golgi complex. J Cell Biol. 1989;108:277–297. doi: 10.1083/jcb.108.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua W, Sheff D, Toomre D, Mellman I. Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging. J Cell Biol. 2006;172:1035–1044. doi: 10.1083/jcb.200512012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Whitney JA, Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991;67:617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Vasavda C, Hostettler J, Davis JQ, Abdi K, Bennett V. E-cadherin polarity is determined by a multifunction motif mediating lateral membrane retention through ankyrin-G and apical-lateral transcytosis through clathrin. J Biol Chem. 2013;288:14018–14031. doi: 10.1074/jbc.M113.454439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Toomre D, Diaz E, White J, Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
- Keppler A, Pick H, Arrivoli C, Vogel H, Johnsson K. Labeling of fusion proteins with synthetic fluorophores in live cells. Proc Natl Acad Sci USA. 2004;101:9955–9959. doi: 10.1073/pnas.0401923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Allen PB, Nairn AC, Caplan MJ. Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase. Mol Biol Cell. 2007;18:4508–4518. doi: 10.1091/mbc.E06-08-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky MS, Wu CC, McIntosh S, McIntosh JR, Howell KE. Structure of the Golgi and distribution of reporter molecules at 20 degrees C reveals the complexity of the exit compartments. Mol Biol Cell. 2002;13:2810–2825. doi: 10.1091/mbc.01-12-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A, Quaroni A, Nichols B, Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990;111:1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B, Hille-Rehfeld A, Spiess M. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc Natl Acad Sci USA. 1995;92:10109–10113. doi: 10.1073/pnas.92.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Tang BL, Wong SH, Hong W. Selective inhibition of protein targeting to the apical domain of MDCK cells by brefeldin A. J Cell Biol. 1992;118:51–62. doi: 10.1083/jcb.118.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Wong SH, Tang BL, Tan P, Subramaniam VN, Hong W. Inhibition by brefeldin A of protein secretion from the apical cell surface of Madin-Darby canine kidney cells. J Biol Chem. 1991;266:17729–17732. [PubMed] [Google Scholar]
- Marie M, Dale HA, Sannerud R, Saraste J. The function of the intermediate compartment in pre-Golgi trafficking involves its stable connection with the centrosome. Mol Biol Cell. 2009;20:4458–4470. doi: 10.1091/mbc.E08-12-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin KS, Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983;34:233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- McNeill H, Ozawa M, Kemler R, Nelson W. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Mellman I. Membranes and sorting. Curr Opin Cell Biol. 1996;8:497–498. doi: 10.1016/s0955-0674(96)80026-3. [DOI] [PubMed] [Google Scholar]
- Miranda KC, Khromykh T, Christy P, Le TL, Gottardi CJ, Yap AS, Stow JL, Teasdale RD. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem. 2001;276:22565–22572. doi: 10.1074/jbc.M101907200. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Ozawa M. A dileucine motif in its cytoplasmic domain directs beta-catenin-uncoupled E-cadherin to the lysosome. J Cell Sci. 2007;120:4395–4406. doi: 10.1242/jcs.03489. [DOI] [PubMed] [Google Scholar]
- Morton MJ, Farr GA, Hull M, Capendeguy O, Horisberger JD, Caplan MJ. Association with {beta}-COP regulates the trafficking of the newly synthesized Na,K-ATPase. J Biol Chem. 2010;285:33737–33746. doi: 10.1074/jbc.M110.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet G, Tuffereau C, Roux L. Reduced temperature can block different glycoproteins at different steps during transport to the plasma membrane. J Gen Virol. 1986;67:2029–2035. doi: 10.1099/0022-1317-67-9-2029. [DOI] [PubMed] [Google Scholar]
- Muth TR, Caplan MJ. Transport protein trafficking in polarized cells. Annu Rev Cell Dev Biol. 2003;19:333–366. doi: 10.1146/annurev.cellbio.19.110701.161425. [DOI] [PubMed] [Google Scholar]
- Muth TR, Gottardi CJ, Roush DL, Caplan MJ. A basolateral sorting signal is encoded in the alpha-subunit of Na-K-ATPase. Am J Physiol. 1998;274:C688–C696. doi: 10.1152/ajpcell.1998.274.3.C688. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler MJ, Ivanov IE, Plesken H, Sabatini DD. Polarized delivery of viral glycoproteins to the apical and basolateral plasma membranes of Madin-Darby canine kidney cells infected with temperature-sensitive viruses. J Cell Biol. 1985;100:136–151. doi: 10.1083/jcb.100.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984;38:535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Saraste J, Palade GE, Farquhar MG. Temperature-sensitive steps in the transport of secretory proteins through the Golgi complex in exocrine pancreatic cells. Proc Natl Acad Sci USA. 1986;83:6425–6429. doi: 10.1073/pnas.83.17.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tamkun MM, Fambrough DM. The (Na+ + K+)-ATPase of chick sensory neurons. Studies on biosynthesis and intracellular transport. J Biol Chem. 1986;261:1009–1019. [PubMed] [Google Scholar]
- Vagin O, Sachs G, Tokhtaeva E. The roles of the Na,K-ATPase beta 1 subunit in pump sorting and epithelial integrity. J Bioenerg Biomembr. 2007;39:367–372. doi: 10.1007/s10863-007-9103-0. [DOI] [PubMed] [Google Scholar]
- Vagin O, Tokhtaeva E, Sachs G. The role of the beta1 subunit of the Na,K-ATPase and its glycosylation in cell-cell adhesion. J Biol Chem. 2006;281:39573–39587. doi: 10.1074/jbc.M606507200. [DOI] [PubMed] [Google Scholar]
- Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.