TFP5/TP5 rescues dopaminergic neurodegeneration induced by MPTP in a mouse model of Parkinson’s disease (PD). The neuroprotective effect of TFP5/TP5 peptide is also associated with marked reduction in neuroinflammation and apoptosis. Selective inhibition of Cdk5/p25 by TFP5/TP5 peptide identifies the kinase as a potential target to reduce neurodegeneration in PD.
Abstract
Parkinson’s disease (PD) is a chronic neurodegenerative disorder characterized by the loss of dopamine neurons in the substantia nigra, decreased striatal dopamine levels, and consequent extrapyramidal motor dysfunction. Recent evidence indicates that cyclin-dependent kinase 5 (Cdk5) is inappropriately activated in several neurodegenerative conditions, including PD. To date, strategies to specifically inhibit Cdk5 hyperactivity have not been successful without affecting normal Cdk5 activity. Previously we reported that TFP5 peptide has neuroprotective effects in animal models of Alzheimer’s disease. Here we show that TFP5/TP5 selective inhibition of Cdk5/p25 hyperactivation in vivo and in vitro rescues nigrostriatal dopaminergic neurodegeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP/MPP+) in a mouse model of PD. TP5 peptide treatment also blocked dopamine depletion in the striatum and improved gait dysfunction after MPTP administration. The neuroprotective effect of TFP5/TP5 peptide is also associated with marked reduction in neuroinflammation and apoptosis. Here we show selective inhibition of Cdk5/p25 hyperactivation by TFP5/TP5 peptide, which identifies the kinase as a potential therapeutic target to reduce neurodegeneration in Parkinson’s disease.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by disabling motor abnormalities, including tremor, muscle rigidity, paucity of voluntary movements, and postural instability (Du et al., 2001). In several mammalian species, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP/MPP+) produces most of the biochemical and pathological alterations seen in PD, including the loss of dopaminergic neurons of the substantia nigra pars compacta (SNc; Smith et al., 2003). Current treatment strategies for PD consist primarily of dopamine replacement therapy with levodopa or dopamine agonists (Du et al., 2001). Although effective in the early stages of the disease, chronic dopamine replacement therapy can cause debilitating side effects. Accordingly, concerted research efforts have been focused on developing neuroprotective strategies that will halt or slow the progression of PD.
Cyclin-dependent kinase 5 (Cdk5) kinase activity was originally identified as a tau kinase implicated in Alzheimer’s disease (AD) progression and independently as a unique cell cycle kinase homologue exhibiting CDK1-like substrate specificity in brain (Zhang et al., 2012). The active kinase was found to be a heterodimer of Cdk5 bound to p25, a truncated fragment derived by calpain cleavage from p35, a larger activator. Whereas Cdk5/p35 is essential for normal development, synaptogenesis, and synaptic activity of the mammalian brain, Cdk5/p25 hyperactivity induces cell death and is associated with neuropathology. Stress-induced proteolytic conversion of p35 to p25 and Cdk5/p25 hyperactivity are associated with human AD (Nguyen et al., 2001; Cruz et al., 2003), as well as with animal models of AD, PD, amyotrophic lateral sclerosis, and other neurodegenerative disorders (Nguyen et al., 2002; Qu et al., 2007; Slevin and Krupinski, 2009; Zhang et al., 2012). Significantly, Cdk5 exhibits a critical role in MPTP-mediated neuronal toxicity in one of the best-characterized PD models (Smith et al., 2006; Qu et al., 2007). MPTP, a neurotoxin that leads to selective degeneration of the substantia nigra neurons (Levy et al., 2009), increases Cdk5 activity and triggers neuronal loss through inactivation of survival factor myocyte enhancer factor 2 (MEF2), antioxidant enzyme peroxiredoxin 2 (Prx2) and DNA damage repair enzyme apurinic/apyrimidinic endonuclease 1 (Ape1; Qu et al., 2007; Huang et al., 2010).
Because of its involvement in PD, Cdk5/p25 has been identified as a prime therapeutic target for PD. Accordingly, compounds such as roscovitine, an inhibitor targeting the ATP-binding site in Cdk5 and other cell cycle kinases, have been studied as potential therapeutic agents, but they lack specificity (Helal et al., 2004, 2009). Our approach to this problem, based on structure and kinetics of the Cdk5/p25complex, resulted in the production of several small, truncated peptides of p35 that competed with p25 binding and inhibited Cdk5 hyperactivation in vitro (Amin et al., 2002). A small peptide, P5, comprising 24 amino acids (aa), specifically inhibited Cdk5/p25 activity in cultured cortical neurons and reduced hyperphosphorylated tau and apoptosis without affecting the normal endogenous Cdk5/p35 activity or the activity of several cell cycle kinases (Zheng et al., 2005, 2010). The P5 peptide was modified as TFP5 with a transactivator of transcription (Tat) peptide conjugated at the C-terminus to facilitate passage through the blood–brain barrier and fluorescein isothiocyanate (FITC; a green fluorescent tag) attached at the N-terminus as a marker. When injected intraperitoneally into 5XFAD AD model mice, it significantly reduced Cdk5/p25 hyperactivity and hyperphosphorylated tau and rescued behavior deficits of spatial working memory and motor deficits (Shukla et al., 2013). Moreover, TFP5 also reduced toxicity in cortical neurons exposed to high glucose (Binukumar et al., 2014). We now report that intraperitoneal injection of TP5 (TFP5 without FITC) into MPTP-induced mice effectively blocks degeneration of dopamine neurons in the SNpc and almost completely prevents the loss of striatal dopamine and its metabolites. The peptide treatment also ameliorates the MPTP-induced behavioral deficits, inhibits neuroinflammation in vivo, and protects against MPP+ neurotoxicity in vitro. These results suggest that TFP5/TP5 may be effective in the treatment of Parkinson’s disease.
RESULTS
TFP5 inhibits hyperactive Cdk5/p25 in MPP+-induced mesencephalic primary cultures; a dose-dependent study
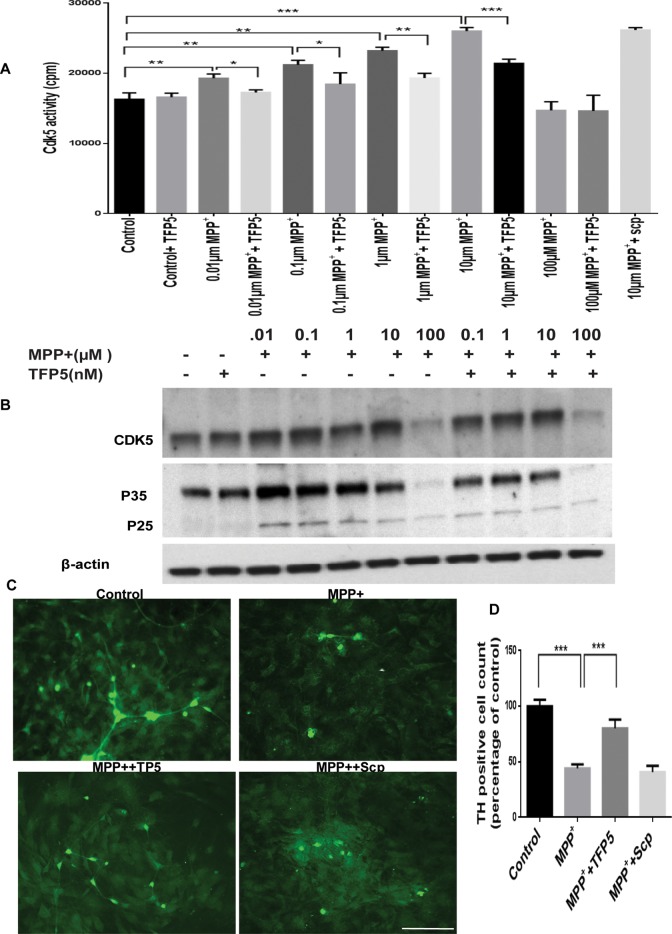
We first determined whether 24 h MPP+ incubation induces Cdk5 hyperactivation in mesencephalic primary cultures in the presence or absence of TFP5 pretreatment and coincubation. Seven days in culture (DIC) mesencephalic neuron–enriched primary cultures pretreated with TFP5 were coincubated with different micromolar concentrations of MPP+ and TFP5, followed by Cdk5 immunoprecipitation and kinase assay. From 0.01 to 10.0 μM, a concentration-dependent increase in the activity was observed in the presence of MPP+ treatment (Figure 1A); the decline at 100 μM is probably due to cell toxicity. Pretreatment and coincubation with 0.5 μM TFP5, however, was sufficient to significantly reduce the elevated activity (30%) at each concentration (Figure 1A). In Western blots of the same lysates as in Figure 1A, we see a decline of p35 expression from 0.01 to 10 μM with modest expression of p25 (Figure 1B). The kinase activities, however, are reduced at all concentrations (20–30%) by TFP5 treatment. Because 10 μM MPP+ induced maximum activity, we selected this as the measure of hyperactivity in vitro for further study. It is noteworthy that scrambled peptide did not show any inhibitory effect (Figure 1A).
FIGURE 1:
TFP5 inhibits hyperactive Cdk5/p25 in MPP+-induced mesencephalic primary cultures. A dose-dependent study. (A) Ventral mesencephalic neuronal-enriched cultures were pretreated with TFP5 (500 nM) or scrambled peptide for 12 h and then coincubated with different concentrations of MPP+ and TFP5 for 24 h. Cdk5 was immunoprecipitated from equal amounts of lysates using C-8 antibody. Immunoprecipitates were then subjected to in vitro kinase pad assays with histone H1 as substrate. Activity, as counts/minute, was quantified from three separate experiments and summarized in the bar graphs (***p < 0.001; **p < 0.01; *p < 0.05). (B) Ventral mesencephalic neuronal-enriched cultures were treated as in A, after which SDS–PAGE and Western blots were prepared with the Cdk5 and p35 antibodies. Note p25 expression in all MPP+ lanes. The results are expressed as mean ± SEM of three independent experiments (***p < 0.001). (C) Mesencephalic tissues from E14 mouse embryos were cultured and grown on polylysine-coated cover slips. Representative images. The neuronal cultures were pretreated with TFP5 (500 nM) or scrambled peptide for 12 h and then coincubated in the presence of 10 μM concentration of MPP+ and TFP5 for 24 h. The cells were fixed and stained. (D) Numbers of TH-immunoreactive (IR) neurons. Scale bar, 100 μm (C).
Because the Cdk5/p25 inhibitor TFP5 was effective at blocking deregulated kinase activity, we further asked whether the effect can be replicated in primary dopaminergic neurons from mesencephalic cultures. Mesencephalic tissues from E14 mouse embryos were cultured and grown on polylysine-coated coverslips. The neuronal cultures were pretreated or not with TFP5 (500 nM) or scrambled peptide for 12 h and then coincubated in the presence of 10 μM concentration of MPP+ and TFP5 for 24 hr. The tyrosine hydroxylase (TH)–positive neurons in the control cells displayed a normal cell body and neurite integration, as shown in Figure 1C. After exposure to 10 μM MPP+ for 48 h, dopaminergic neurons with swollen cell bodies and fragmented neurites were observed across the field. The number of TH-positive neurons and the length of TH-positive neurites were significantly reduced (Figure 1D). In contrast, treatment with TFP5 (500 nM) effectively attenuated MPP+-induced injury of dopaminergic neurons. Scrambled peptide control at 500 nm, however, had no protective effect on these cells. Taken together, these results demonstrate that TFP5 has a neuroprotective effect in cell culture models of dopaminergic neurodegeneration.
TFP5 treatment inhibits MPP+-induced inflammation and apoptosis in mesencephalic primary cultures
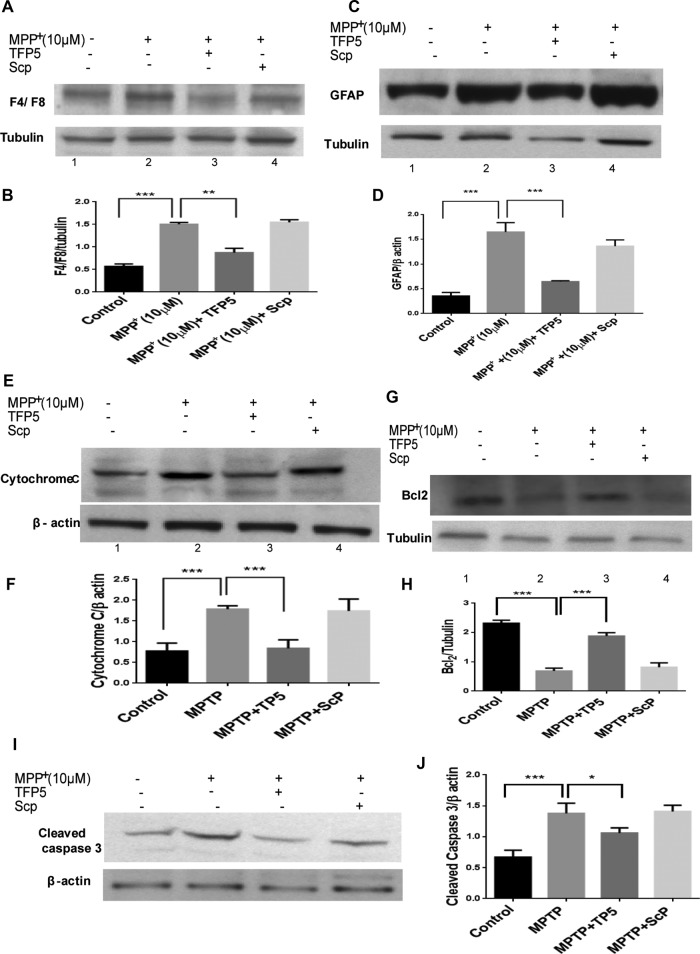
To investigate antiinflammatory effects of TFP5 against PD-related neurodegeneration, we pretreated or not primary neuron-glia cultures from mouse midbrains (∼50% glia, 50% neurons) with TFP5 (500 nM) or scrambled peptide for 12 h and then coincubated them in the presence of 10 μM concentration of MPP+ and TFP5 for 24 h. We measured the expression levels of F4/F8 (microglia marker) and glial fibrillary acid protein (GFAP; astrocyte marker; Figures 2, A and B, and C and D). It is evident that treatment with 10 μM MPP+ increased the expression of both F4/F8 and GFAP (164 and 173%) compared with the control, whereas treatment with TFP5 ameliorates these effects. Scrambled peptide had no effect compared with MPP+ treatment. Next we examined the effect of TFP5 on MPP+-induced apoptosis by measuring caspase-3 activation and cytochrome c release in the mixed cultures. Caspase-3 is an important signaling protein located downstream in the apoptosis signaling pathway. Cytochrome c release and caspase-3 activation are accepted measures of cellular apoptosis (Gentil et al., 2003). Treatment of mesencephalic primary cultures with MPP+ for 24 h resulted in robust activation of caspase-3 and cytochrome c release (Figure 2, E and F, and I and J). Pretreatment and coincubation with TFP5 significantly inhibited the increased levels of caspase-3 and cytochrome c. To confirm further the antiapoptotic function of TFP5, we determined the expression levels of Bcl-2 (an antiapoptotic factor) by Western blot. As shown in Figure 2, G and H, MPP+ treatment for 24 h significantly decreased the protein level of Bcl-2. This result is in line with previous reports (Liu et al., 2010). Treatments with TFP5 significantly increased Bcl-2 expression (Figure 2, G and H). The foregoing results indicate that TFP5 almost completely blocked the up-regulation of cytochrome c release and caspase-3 activation and decreased Bcl-2 expression. In all cases, scrambled peptide treatment had no effect. Together, these results showed that TFP5 treatment has antiapoptotic effects in mesencephalic cells in primary culture.
FIGURE 2:
TFP5 treatment inhibits MPP+-induced inflammation and apoptosis in mesencephalic primary cultures. Ventral mesencephalic cultures were pretreated with TFP5 (500 nM) or scrambled peptide for 12 h and then coincubated in the presence of 10μM MPP+ and TFP5 (500 nM) or scrambled peptide for 24 h. The cell lysates were subjected to SDS–PAGE, and Western blots were prepared with antibodies to F4/F8 (A, B), GFAP (C, D), cytochrome c, cytosol fraction (E, F), BCl2 (G, H), and caspase 3 (I, J). Bar graphs represent quantification of respective mean densities from three separate experiments in each case (***p < 0.001; **p < 0.01; *p < 0.05).
TFP5 inhibits MPTP-induced Cdk5/p25 hyperactivity in vivo
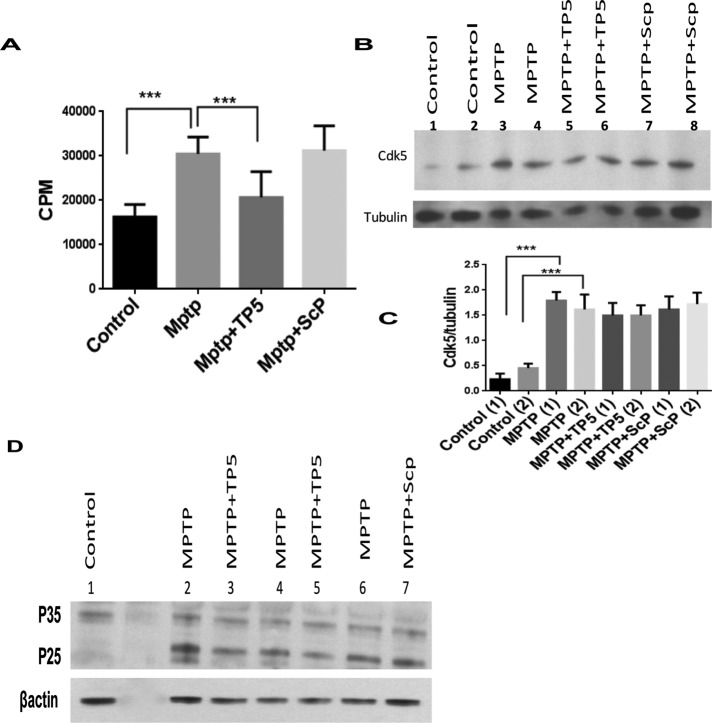
As a more effective test of the efficacy of TFP5 as a therapy for PD, an in vivo model system should be used. To determine the effect of TFP5 treatment in vivo, we adopted the four-dose MPTP mouse model of PD (Jackson-Lewis and Przedborski, 2007; for details, see Materials and Methods). For the initial sets of experiments, animals were injected intraperitoneally (ip) with a single TFP5 injection, 40 mg/kg (selection of dose based on our previous study) every day for 9 d. On day 2, TFP5-treated animals received four doses of MPTP (15 mg/kg × 4, total of 60 mg/kg per day). On day 10, the animals were killed, and Cdk5 kinase activity was measured in lysates of the SN. We found that the dose of TFP5 was inadequate; TFP5-treated animals did not show significant inhibition of Cdk5/p25-deregulated activity compared with the MPTP group (Supplemental Figure S1), Accordingly, we increased the dose to a single, 80–mg/kg ip injection every day for 9 d. We used the peptide without the FITC tag (TP5) for this higher protocol since TFP5 aggregates at higher doses (unpublished data). In this case, TP5 pretreatment produced significant inhibition of Cdk5/p25 kinase activity (Figure 3A). Hence this in vivo protocol, identified as the “standard protocol,” was selected for more extensive study. We assessed Cdk5 expression in the SN of mice treated with MPTP and TP5 and noted that Cdk5 expression increased almost threefold after MPTP treatment (lanes 3 and 4, Figure 3, B and C), but overexpression was unaffected by TP5 (lanes 5 and 6, Figure 3, B and C). This observation is consistent with the suggestion that TP5 only inhibits Cdk5 activity without affecting expression. Note that Figure 3B is a representative sample Western blot, whereas Figure 3C quantifies the results of assays of six animals per group. In Figure 3D, two representative blots of the same lysates with p35 antibody (C19), which detects both p35 and p25, show MPTP-induced p25 up-regulation (compare control lanes 1 and 5 with MPTP lanes 2 and 6, respectively). These results are consistent with the data in Figure 3A; MPTP-induced p25 expression hyperactivates Cdk5 activity in the SN, which is down-regulated after TP5 treatment. In all cases, scrambled peptide, the negative control, had no effect on activity.
FIGURE 3:
TFP5 inhibits MPTP-induced Cdk5/p25 hyperactivity in vivo. (A) To determine the effect of TFP5 treatment in vivo, we adopted the four-dose MPTP mouse model of PD. Four groups of mice, six animals per group, were treated according to the standard protocol as described for animal groups in Materials and Methods: group A, controls injected with vehicle; group B, MPTP day 2, 4× 15 mg/kg injections; group D, 9-d pretreatment with TP5 plus MPTP on day 2; group E, 9-d pretreatment with scrambled peptide (Scp) plus MPTP on day 2. All killed on day 10. (A) Ventral midbrains were dissected and homogenized in lysis buffer and centrifuged, and supernatants were collected. Protein concentrations were determined, and equal amounts of lysate protein (350 μg) were taken for immunoprecipitation with Cdk5 antibody (C-8). Immunoprecipitates were then subjected to in vitro kinase assays with histone H1 as substrate. Activity, as counts/minute, was quantified from six animals per group and summarized in a bar graph (***p < 0.001). (B, C) Lysate samples from each group were subjected to SDS–PAGE and Western immunoblotting. Membranes were probed with Cdk5 antibody and reprobed with an antibody for tubulin as a loading control. (B) Sample blot is shown. (C) Quantification from six different animals. Note that Cdk5/tubulin ratio, although elevated by MPTP treatment, is unaffected by TP5. Quantification of mean density of Cdk5 from three separate experiments (***p < 0.001). (D) Western blots were also prepared from these lysates to assess the expression of p35 and p25. Representative sample of experiments, with actin as loading control.
Dopaminergic neurons of the SN express Cdk5/p35 and are protected from cell death by TP5 after MPTP induction
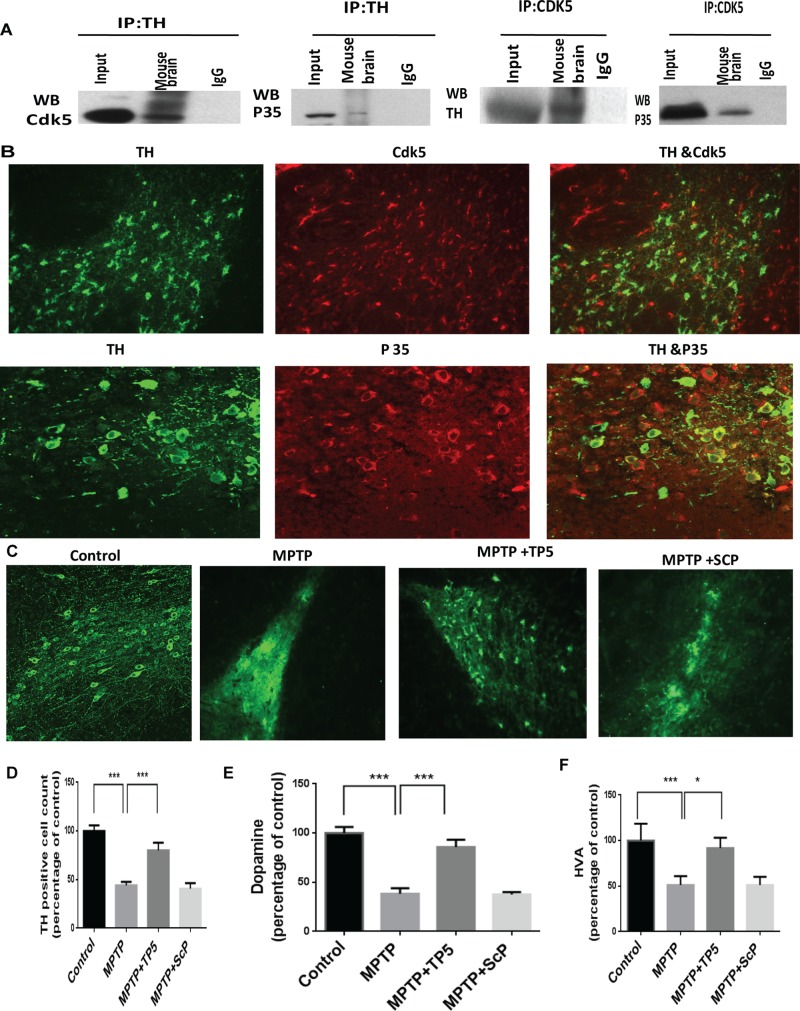
To identify the cells of the SN that express Cdk5, were carried out reciprocal immunoprecipitations with TH and Cdk5 antibodies with lysates of the SN. The results in Figure 4A show that TH coimmunoprecipitates Cdk5 and p35, whereas Cdk5 coimmunoprecipitates the expected p35 together with TH. Colocalization of CDk5/p35 with TH was also confirmed in cells of the SN in immunohistological assays of ventral brain sections (Figure 4B).
FIGURE 4:
Dopaminergic neurons of the SN express Cdk5/p35and are protected from cell death by TP5 after MPTP induction. (A) Cdk5 physically interacts and colocalizes with TH and p35. Reciprocal coimmunoprecipitation (IP; pull-down) experiments were carried out with mouse brain lysates, comparing input (10%) and Cdk5 IPs with TH IPs using antibodies to Cdk5 and TH, respectively. Both p35 and TH were pulled down with Cdk5 antibody, whereas the reciprocal pull down with TH shows both Cdk5 and p35. This indicates that dopamine neurons are expressing Cdk5/p35. (B) This association was confirmed in the immunohistochemical assay of mouse brain sections showing colocalization of Cdk5/p35 and TH with antibodies to Cdk5, TH, and p35. (C) Dopamine neurons and processes were identified by TH immunostaining of representative midbrain sections 10 d after MPTP treatment with or without TP5/Scp (80 mg/kg daily; see Materials and Methods for details). Control group (group A), MPTP-treated group (group B), MPTP plus TP5 group (group D), and MPTP + SCP group (group E). Note the marked reduction in TH-positive cell bodies and processes after MPTP administration (compare control and MPTP) and the protection by TP5. Photomicrographs are from a representative experiment repeated three times with similar results. (D) Quantification of TH-positive neurons (***p < 0.001). (E, F) TP5 prevents striatal loss of dopamine and dopamine metabolites after MPTP administration. Striatal tissues from brains of mouse groups treated as described were assayed for dopamine and HVA The levels of dopamine (E) and HVA (F) were determined with ELISA and expressed as ng/100 mg of wet tissue (n = 8; ***p < 0.001, *p < 0.05).
To investigate the neuroprotective effects of TP5 on MPTP-induced neuronal death in vivo, we treated C57BL6 mice with TP5 (80 mg/kg ip) daily for 9 d together with appropriate controls. On day 2, mice were administered MPTP (4 × 15 mg/kg, ip). Eight days later, the brains were analyzed by immunohistochemistry to quantify TH-positive neurons in the SNpc. MPTP treatment reduced the number of TH-positive neurons by 77% compared with saline-treated controls (p < 0.001; Figure 4, C and D). Mice that received daily treatments of TP5 at 80 mg/kg showed an increase of TH-positive neurons in the SNpc to 52% of control (p < 0.01 and 0.001, respectively; Figure 4D). The neuroprotective effect of TP5 was dose dependent, as a 40–mg/kg dose of TP5 failed to protect dopamine neurons from MPTP toxicity (unpublished data). Moreover, scrambled peptide did not show any dopaminergic neuroprotection compared with the MPTP group. MPTP injections also caused significant decreases (p < 0.01) in the level of dopamine and its metabolites in the striatal region of MPTP-injected mice; dopaminergic cell loss was coupled to reduced dopamine levels (Figure 4, E and F). MPTP-induced dopamine and homovanillic acid (HVA) depletion of >50% was attenuated almost to control levels in mice treated with TP5 for 9 d compared with the MPTP-injected mice and/or scrambled peptide controls. Taken together, these results suggest that TP5 can improve neurochemical deficits in the MPTP mouse model of PD.
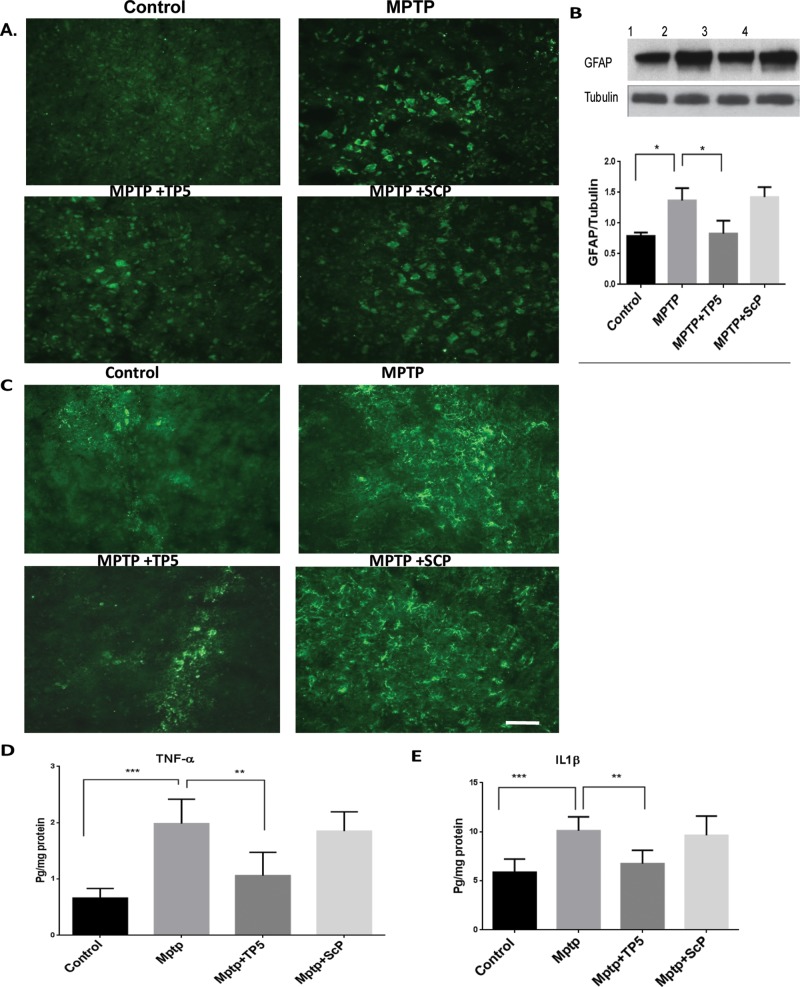
TP5 suppresses MPTP-induced astroglial and microglial activation in the SN in vivo
Microglial activation has been implicated in the propagation of SNpc neurotoxicity in multiple animal models of PD. Post-mortem analysis of idiopathic PD patients revealed strong immunoreactivity for CD68, a marker of phagocytic microglia (Croisier et al., 2005; Vroon et al., 2007). Furthermore, administration of MPTP has been reliably shown to induce this phagocytic microglia phenotype in the SNpc of mice (Vroon et al., 2007; Chung et al., 2010; Chung et al., 2011). Previous studies report in addition the presence of reactive microglia in MPTP-treated SN exhibiting nigral DA neuronal degeneration (Wu et al., 2003; Block et al., 2007). Accordingly, we investigated whether a TP5 injection regimen can inhibit MPTP-induced glial activation in the SN in vivo. Nine days after the final MPTP treatment, with or without TP5, brain tissues were processed for immunostaining using an antibody against CD11b and GFAP to detect microglial and astrocyte activation, respectively (Figure 5). Consistent with earlier reports (Wu et al., 2003), numerous GFAP-positive reactive astrocytes (Figure 5, A and B) and CD11b-positive (activated) microglia (Figure 5C) were observed in MPTP-treated SN compared with saline and scrambled peptide controls. TP5 treatment mitigated these effects of MPTP, dramatically decreasing the number of Mac-1– and GFAP-positive reactive astrocytes cells in the MPTP-treated SN. Scrambled peptide had no effects on glial activation compared with the MPTP group (Figure 5, D and H). We investigated other signs of an MPTP inflammatory response. Post-mortem analysis of human PD tissue showed that microglia are immunoreactive for multiple proinflammatory cytokines, including tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β; McGeer and McGeer, 2004). Further, mice that are genetically altered to inhibit cytokine production or are deficient in receptors for these cytokines provide neuroprotection in the SNpc after MPTP exposure (Klevenyi et al., 1999; Sriram et al., 2002). Moreover, transgenic mice lacking the inducible nitric oxide synthase (iNOS) gene are resistant to MPTP-induced neurotoxicity (Liberatore et al., 1999). Thus we examined whether MPTP-induced expression of IL-1β and TNF-α in the SN was affected by TP5. Nine days after the final MPTP injection, midbrain tissues lysates were analyzed by enzyme-linked immunosorbent assay (ELISA; Figure 5, D and E). The results showed that the levels of TNF-α protein (Figure 5D) and IL-1 β (Figure 5E) were significantly increased in the midbrain of MPTP-treated mice compared with saline controls. Treatment with TP5 inhibited these MPTP-induced effects, reducing levels of TNF-α (p < 0.001) and IL-1β (p < 0.001) ∼50%. Here, too, scrambled peptide had no effects.
FIGURE 5:
TP5 suppresses MPTP-induced astroglial and microglial activation and inflammation in the SN in vivo. (A) Sections of SN tissues obtained from the same animals as used in Figure 3 were immunostained with GFAP antibody for astrocyte and Cdllb (C) for microglia. The heightened expression of GFAP and CdIIb was observed in the MPTP group as compared with the control group, whereas the MPTP group treated with TP5 reveals a moderate staining of Cd11b and GFAP. The control group, however, shows almost negligible staining. Scale bar, 200 mm. (B) Western blots of tissue lysates from each group show a marked reduction in the expression of MPTP-induced GFAP by TP5. In addition, sandwich ELISAs of lysates from each group also show a TP5 rescue of overexpressed TNF-α (D) and IL-1β (E) in MPTP-induced inflammation (n = 8, ***p < 0.001, **p < 0.01).
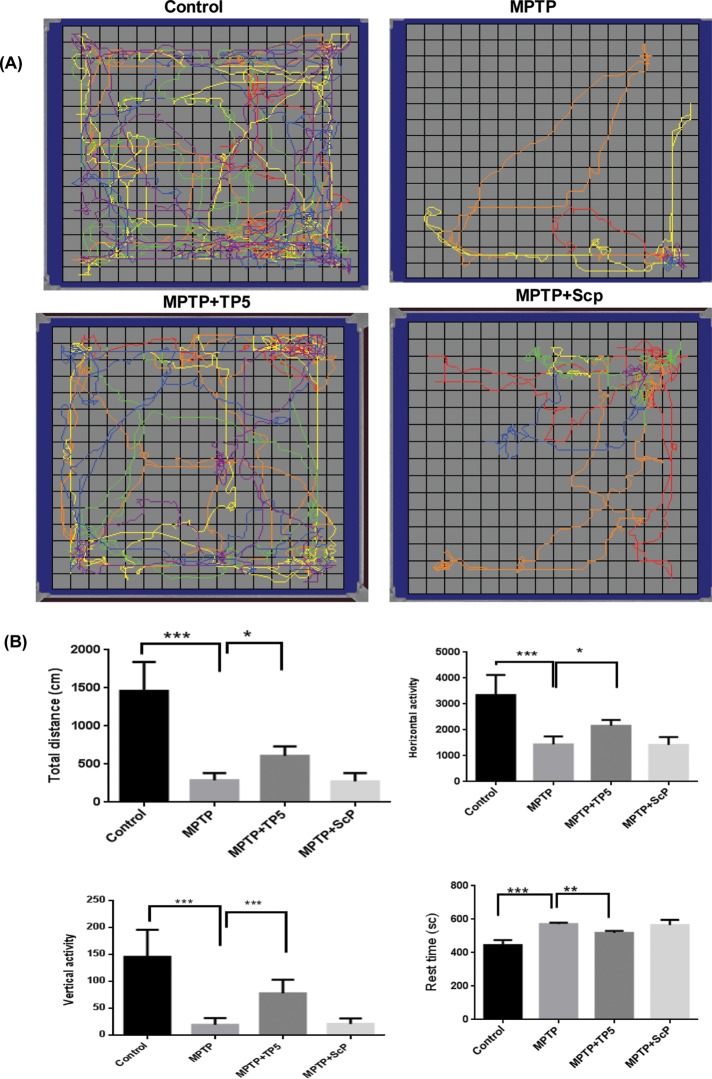
TP5 improved locomotor functions in MPTP-treated mice
We examined whether TFP5 protects against neurobehavioral deficits caused by MPTP. Using the same treatment protocol as described earlier, at 16 and 48 h after the MPTP injections on day 2, we tested mice from each treated group for locomotor activity in an open field test (Figure 6). Representative maps using Versaplot software (AccuScan, Columbus, OH) depict the locomotor activity pattern of mice over a 10-min period (Figure 6A). We observed a marked decrease in total distance traveled after MPTP treatment (85%), which was restored ∼30% after TP5 treatment (p < 0.01). Quantification of individual components of the running pattern showed different levels of recovery after TP5 treatment (Figure 6B); horizontal activity was restored 30% (p < 0.001) and vertical activity 60% (p < 0.0001), whereas the increase in rest time was only 10% (p < 0.01). We see that TP5 significantly improved MPTP-induced hypolocomotion.
FIGURE 6:
TP5 improved locomotor functions in MPTP-treated mice. (A) Sixteen hours after standard protocol treatment, the four groups of mice were assessed for spontaneous motor activity in a novel environment (open field) for 10 min, as described in Materials and Methods. Compared to controls, MPTP-treated mice initiated less spontaneous locomotor activity than saline control. After TP5 treatment mice, displayed a significant improvement in motor activity. (B) Individual movement components were analyzed as shown in the bar graphs. TP5-treated animals showed only a modest improvement in distance run and horizontal activity (*p < 0.05), whereas vertical activity was robustly improved (***p < 0.001). Resting time also was affected by TP5 treatment (**p < 0.01). Data are means ± SEM for 8–10 mice/group. Statistical significance was assessed by one-way repeated-measures analysis of variance with Bonferroni post hoc test.
Similar patterns of locomotor behavior were obtained in tests at 48 h (Supplemental Figure S2).
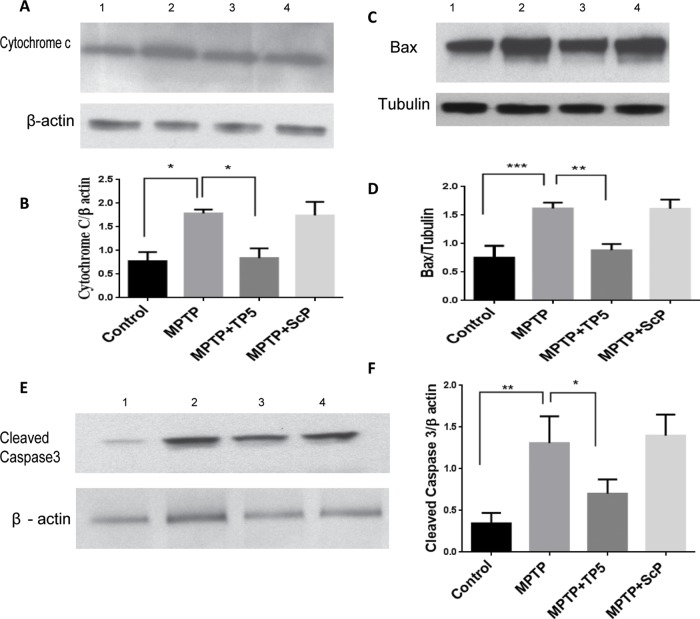
TP5 protects from chronic MPTP-induced apoptosis
The loss of nigral dopaminergic neurons in PD results from extensive apoptosis marked by up-regulation of several apoptotic proteins (Levy et al., 2009). To determine whether TP5 inhibits neuronal apoptosis induced by MPTP, we subjected groups of mice to the standard protocol. At day 10, SN tissue was dissected, lysed, and prepared for Western blots with antibodies specific for cleaved caspase-3, Bax, and cytochrome c as an assay for apoptosis. The Western blot analysis showed that MPTP-evoked PD apoptotic phenotypes were significantly 1.5- to 2-fold greater than saline and SCP control injections (Figure 7, A and C). These changes, however, were significantly attenuated by TP5 treatment, in some cases almost to baseline levels (p < 0.05). Moreover, scrambled peptide–treated mice did not show any significant alterations. Our results are consistent with our hypothesis concerning the efficacy of TP5 treatment; prolonged treatment of TP5 markedly reduces the expression of apoptotic markers in PD mice.
FIGURE 7:
TP5 protects from chronic MPTP-induced apoptosis. (A) Striatal tissue lysates from the brains of four groups of mice (as before) were prepared as Western blots to assay for the expression of key apoptotic marker molecules. A blot for cytochrome c shows a significant increase over controls after MPTP induction, which is completely reduced by TP5 treatment. (B) Quantification of A (ratio of cytochrome c to actin). (C, D) Expression levels of striatal cleaved caspase 3 and Bax (E, F) were also significantly increased in MPTP-group mice compared with controls. Here, too, treatment with TP5 significantly decreased the expression levels. Values are expressed as mean ± SEM (n = 3; ***p < 0.001; **p < 0.01, *p < 0.05).
DISCUSSION
For many years, MPTP-induced toxicity in mice has served as a useful model for PD (Mizuno et al., 1998; Przedborski and Jackson-Lewis, 1998; Schapira et al., 1998; Franco-Iborra et al., 2015). MPTP, a mitochondrial toxin, when injected into mice, is first converted to MPP+ by monoamine oxidase B and then taken up selectively into nigral dopaminergic neurons by the dopamine transporter. Here it specifically inhibits mitochondrial complex I activity, which disrupts the electron transport chain (Przedborski and Jackson-Lewis, 1998). Defective mitochondria result in reactive oxygen species overexpression, mitochondrial fusions, DNA mutations and alterations, and endoplasmic reticulum interactions, resulting in the release of mitochondrial cytochrome c into the cytoplasm, where it can complex with apoptosis-activating factor 1 (Apaf-1), caspase-9, and caspase-3, the downstream apoptosis executioner (Li et al., 1998). This results in the morphological changes associated with apoptosis, including DNA fragmentation and cytoskeletal disruption (Stennicke and Salvesen, 2000).
There are additional pathways to PD, however, leading to the death of dopamine neurons (Venderova and Park, 2012) including MPTP-induced hyperactivity of Cdk5 (Smith et al., 2003; Qu et al., 2007; Wen et al., 2014). In the study by Wen et al. (2014), for example, Cdk5 activation phosphorylates Raf kinase inhibitor protein, which undergoes autophagy, releasing the Erk/mitogen-activated protein kinase cascade to drive dopamine neurons into the S phase and cell death. Cdk5 hyperactivation has been implicated as an event associated with neurodegenerative disorders in humans and model rodents such as AD and amyotrophic lateral sclerosis (Patrick et al., 1999; Ahlijanian et al., 2000; Cruz et al., 2003; Lau and Ahlijanian, 2003; Nguyen and Julien, 2003; Noble et al., 2003; Cheung and Ip, 2004). In fact, up-regulated Cdk5 has also been reported in dopamine neurons in post-mortem studies of PD patients (Brion and Couck, 1995; Nakamura et al., 1997). Hence Cdk5 has been identified as a therapeutic target in neurodegenerative disorders (Tsai et al., 2004). Several studies have indicated that hyperactive Cdk5 results from stress-induced cleavage of p35 to p25 by calpains, the calcium-dependent proteases (Lee et al., 2000; Crews et al., 2011). P25 forms a stable hyperactive complex with Cdk5; hence a principal therapeutic target in neurodegeneration is Cdk5/p25 and not the normal Cdk5/p35. In our laboratory, we have identified a specific fragment of p35, the Cdk5 inhibitory peptide (CIP), which displayed a specific inhibitory effect on Cdk5/p25 activity in vitro without affecting “normal” Cdk5/p35 activity or other Cdks (Amin et al., 2002; Zheng et al., 2002, 2005; Shukla et al., 2013). We also showed that the selective inhibition of Cdk5/p25 hyperactivation in vivo, through overexpression of CIP in p25 transgenic mice, rescues the neurodegenerative AD pathologies caused by Cdk5/p25 hyperactivation without affecting neurodevelopment afforded by normal Cdk5/p35 activity (Shukla et al., 2013; Sundaram et al., 2013). A further fine-tuning of these studies has identified a smaller, 24-aa peptide derived by serial truncation of CIP. This 24-aa peptide (termed P5) has been shown to inhibit Cdk5/p25 activity in transfected HEK cells and primary neurons without affecting Cdk5/p35 or other Cdks (Zheng et al., 2010). P5, modified as TFP5 so as to penetrate the blood–brain barrier after intraperitoneal injections in AD model mice, inhibited abnormal Cdk5/p25 hyperactivity and significantly rescued AD pathology in 5XFAD mice (Shukla et al., 2013).
The questions arise as to whether hyperactive Cdk5/p25 contributes to the MPTP pathology and, if so, whether treatment of these PD mice with TFP5/TP5 decreases or eliminates the iconic pathology. Indeed, our data demonstrate that TFP5/TP5 can effectively protect midbrain dopamine neurons from the toxic effects of MPTP/MPP+ in vivo and in vitro. Moreover, TP5 treatment results in a marked protective effect on the toxic depletion of dopamine and its metabolites in the striatum. Both in vivo and in vitro data show that TP5/TFP5 treatment inhibits MPTP/MPP+-induced hyperactivation of Cdk5/p25, thereby protecting the apoptotic loss of dopamine neurons. This finding, coupled with previous studies, clearly demonstrates the importance of Cdk5/p25 in models of dopaminergic neuron loss (Smith et al., 2003; Qu et al., 2007). The downstream events leading to cell death are uncertain, however. Cdk5 probably acts through multiple targets and kinase cross-talk important for neuronal survival. There are numerous proteins and substrates with which Cdk5/p35 and/or Cdk5/p25 interact (Lim et al., 2003). Some targets have been suggested in MPTP toxicity—the antioxidant enzyme Prx2 (Qu et al., 2007) and/or transcription factor MEF2 (Gong et al., 2003), apurinic/apyrimidinic endonuclease 1 (Ape1), and a protein crucial for base excision repair after DNA damage (Huang et al., 2010). The coordinated targeting of multiple Cdk5/p25 substrates may contribute to stress-induced neuronal death. Mitochondrial dysfunction triggered by MPP+ can evoke a sustained elevation of cytoplasmic calcium levels (Chen et al., 1995), which, in turn, activate calpains that may cleave p35 to p25 to form hyperactive Cdk5/p25.
The standard TP5 peptide treatment successfully induces some level of behavioral recovery. The most prominent biochemical changes in the striatum of PD patients and MPTP-treated mice are decreased levels of dopamine (Savitt et al., 2006; Jackson-Lewis and Przedborski, 2007). Such deficits in striatal dopamine in MPTP-treated mice led to a decreased latency to fall on an accelerating rotarod apparatus, reflecting diminished coordination and balance (Chung et al., 2010). TP5 treatment was found to increase striatal dopamine levels and ameliorate motor deficits in MPTP-treated mice. Accordingly, mice treated with TP5 also had significantly more TH immunoreactivity in the striatum than both MPTP- and MPTP plus scrambled peptide–treated mice. Furthermore, the depletion of TH-positive cells in the SN in response to MPTP was ameliorated by treatment with TP5. Future studies will be needed to analyze the pharmacokinetic and pharmacodynamics of TP5 by tissue selectivity, blood persistence, body clearance, potential brain localization, and distribution and also to check the possibility that the scrambled peptide and the TP5 peptide could have different proteolytic stability in vivo.
The aberrant activation of microglia and astrocytes has been shown to increase the pathophysiology associated with PD; microglia-derived proinflammatory cytokines may be another pathway in nigrostriatal DA neuronal death. Several lines of evidence point to the presence of activated glial cells expressing the proinflammatory cytokines IL-1β and TNF-α in the SN of PD patients (Nagatsu et al., 2000) and MPTP-treated mice (Zhao et al., 2007). TNF-α and IL-1β, originating from activated glia, may trigger intracellular death–related signaling pathways or participate in the induction of iNOS expression in the MPTP model (Teismann et al., 2003a, b). The present data show that TP5/TFP5 attenuates the production of both IL-1β and TNF-α in the SN of MPTP-injected mice; it also reduces microglial and astrocyte expression of CD11b and GFAP. These data suggest that treatment with TFP5/TP5 was efficacious in reducing the histological and molecular proinflammatory phenotype. This is in line with recent studies showing that selective inhibition of Cdk5/p25 hyperactivation in vivo, through overexpression or ip injection of CIP/TFP5, rescues the neuroinflammatory pathologies caused by Cdk5/p25 hyperactivation in AD model mice (Shukla et al., 2013; Sundaram et al., 2013).
Previous studies showed that mitochondrial fission is a very early invariant event that contributes to cytochrome c release and neuronal apoptosis. Using a small-molecule Cdk5 inhibitor, as well as a dominant-negative Cdk5 mutant and RNA interference knockdown experiments, Meuer et al. (2007) identified Cdk5 as an upstream signaling kinase that regulates mitochondrial fission during apoptosis of neurons. They also showed that mitochondrial fission is a modulator contributing to Cdk5-mediated neurotoxicity, thereby integrating Cdk5 into established neuronal apoptosis pathways. In our study, MPTP was found to elicit cytochrome c release and activation of caspase-3 and Bax in the SN of wild-type mice, and these events were found to be attenuated in the TP5 treatment. Studies in primary mesencephalic cultures revealed that this toxicity appears to involve, sequentially, cytochrome c release, caspase-3, and decreased levels of antiapoptotic factor Bcl-2. TFP5/TP5 treatment ameliorates all of these changes. We found that TP5 treatment in vivo resulted in significant attenuation of dopaminergic SN cell loss and striatal dopamine/HVA levels after MPTP administration. The findings reported here showing selective inhibition of Cdk5/p25 hyperactivation by TFP5/TP5 peptide identify the kinase as a potential therapeutic target to reduce neurodegeneration in Parkinson’s disease.
MATERIALS AND METHODS
Materials
P35 (C-19) polyclonal antibody, Cdk5 (C-8) polyclonal antibody, TH polyclonal antibody, and Cdk5 (J-3) monoclonal antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), all used at 1:300–500 dilutions. Antibodies to caspase-3 and cleaved caspase-3 were obtained from Cell Signaling Technology (Beverly, MA). Cdk5 inhibitor roscovitine was obtained from Biomol Research Laboratories (Plymouth, PA). TFP5 (p5 conjugated with TAT peptide and FITC), TP5 (p5 conjugated with TAT peptide), and scrambled TFP5 peptide (Scp) were synthesized by Peptide 2.0 (Chantilly, VA). Sequences used were as follows:
TFP5: FITCGGGKEAFWDRCLSVINLMSSKMLQINAYARAARRAARR.
TP5: KEAFWDRCLSVINLMSSKMLQINAYARAARRAARR.
Scp peptide: FITCGGGGGGFWDRCLSGKGKMSSKGGGINAYARAARRAARR.
Mesencephalic cell culture and treatment
The mesencephalic neuron-glia cultures were prepared from C57BL6/J mice using a modified method reported by Ossola et al. (2011). In brief, mesencephalic tissues were dissected from embryos at 15–16 d, stripped of the meninges and blood vessels, and minced. The tissues were dissociated by 0.1% trypsin digestion for 15 min at 37°C and gentle trituration. The cells were suspended in MEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1 g/l glucose, 2 mM l-glutamine, 1 mM sodium pyruvate, and 50 U/ml penicillin/streptomycin and plated at equal density of 0.6 million cells/well on 12-mm coverslips precoated with 0.1 mg/ml poly-d-lysine. Before seeding, culture vessels consisting of 6-cm dishes were coated with poly-d-lysine (50 μg/ml) at room temperature overnight. It was reported that the composition of these neuron-glia cultures was ∼11% microglia, 48% astroglia, and 41% neurons, of which 2.8–3.8% of the cells were TH-positive DA neurons (Gao et al., 2002). The neuron-enriched cultures were prepared by adding 10 mM Ara-C to mesencephalic neuron-glia cultures for 48 h at 72 h after seeding. In accord with the standard treatment protocol, cultures were pretreated or not with TFP5 (500 nM) or scrambled peptide for 12 h and then coincubated in the presence of different concentrations of MPP+ and TFP5 or scrambled peptide for 24 h.
Immunocytochemistry
The primary mesencephalic neurons were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min and processed for immunocytochemical staining. First, nonspecific sites were blocked with 2% bovine serum albumin, 0.5% Triton X-100, and 0.05% Tween 20 in PBS for 30 min at room temperature. Cells were then incubated with primary antibody such as anti-TH (1:500, mouse monoclonal) at 4°C overnight. Appropriate secondary antibodies (Alexa Fluor 488 or 594; Invitrogen) were used, followed by incubation with 10 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) for 5 min at room temperature to stain the nucleus. Cover slips containing stained cells were washed twice with PBS and mounted on poly-d-lysine–coated slides (Sigma-Aldrich). Cells were viewed under a Nikon inverted fluorescence microscope, and images were captured with a SPOT digital camera.
Immunoprecipitation and kinase assays
Kinase assays were performed as described previously, with modification (Binukumar et al., 2014). Briefly, seven DIC mesencephalic neuron-glia cultures or neuron-enriched cultures were pretreated or not with TFP5 (500 nM) or scrambled peptide for 12 h and then coincubated in the presence of different concentrations of MPP+ and TFP5 or scrambled peptide for 24 h, followed by lysis in T-PER tissue protein extraction reagent (Thermo Scientific). Cdk5 was immunoprecipitated with the polyclonal C8 antibody for 2 h at 4°C, and immunoglobulin was isolated using protein A–Sepharose beads for 2 h at 4°C. Immunoprecipitates were washed three times with lysis buffer and then once with 1× kinase buffer containing 20 mM Tris-Cl, pH 7.4, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, 10 mM MgCl2, 10 mM sodium fluoride, and 1 mM sodium orthovanadate. The samples were added to the reaction mix containing kinase buffer, 50 μM ATP, 20 g of histone H1, and 0.5 Ci of [32P]ATP and incubated at 30°C for 1 h. Reactions were halted by the addition of loading buffer, and samples were then electrophoresed on 12% SDS–PAGE gels. Histone bands were visualized by Coomassie blue staining. Gels were dried, and then autoradiographs were scanned on a PhosphorImager. Radioactive band density was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD), and statistical analysis was performed. In pad assays, 25-μl aliquots of the incubation mixture were placed on a Whatman p81 paper square, air-dried, and washed five times for 15 min each in 75 mM phosphoric acid and once in 95% ethanol. After air-drying, squares were transferred to vials containing Bio-Safe II scintillation fluid for counting.
Animals and MPTP treatment
C57BL/6 male mice 8–10 wk old and weighing 24–28 g were housed under standard conditions: constant temperature (22 ± 1°C), humidity (relative, 30%), and a 12-h light/dark cycle. Mice were allowed free access to food and water. Use of the animals and protocol procedures were approved and supervised by Institutional Animal Care. The following groups of animals were used in most experiments:
(A) Sham/ saline group: Twenty C57BL/6 mice were injected ip with a single saline (0.9%) injection every 2 h for a total of four injections over the course of 1 d.
(B) MPTP/saline group: Twenty C57BL/6 mice were injected ip with a single MPTP injection (15 mg/kg calculated to the freebase) every 2 h for a total of four injections over the course of 1 d (total of 60 mg/kg per day).
(C) MPTP (15 mg/kg) plus TFP5 (40 mg/kg) group: Ten C57BL/6 mice were injected ip with a single TFP5 injection (40 mg/kg, injection) every day for a total of nine injections over the course of 9 d. At day 2, these mice also received four injections with MPTP (15 mg/kg, total of 60 mg/kg per day).
(D) MPTP (15 mg/kg) plus TP5 (80 mg/kg) group: Ten C57BL/6 mice were injected ip with a single TP5 (80 mg/kg, MW) every day for a total of nine injections over the course of 9 d. At day 2, mice also received four injections with MPTP (15 mg/kg, total of 60 mg/kg per day)
(E). MPTP (15 mg/kg) plus scrambled peptide (80 mg/kg) group: Ten mice were injected ip with a single scrambled peptide (80 mg/kg) every day for a total of nine injections over the course of 9 d. On the day for MPTP injection (day 2), mice also received four injections with MPTP (15 mg/kg, total of 60 mg/kg per day), similarly to the MPTP group.
Western blot analysis
Western blot analysis was performed as described previously (Binukumar et al., 2014). In brief, cells or brain tissues were lysed or homogenized in extraction buffer, respectively, and centrifuged at 10,000 × g for 5 min, and supernatants were collected and protein determined. Equal amounts of total protein (50 μg/lane) were resolved on a 4–20% SDS–polyacrylamide gel and blotted onto a polyvinylidene fluoride membrane. This membrane was incubated in blocking buffer/5% dry milk powder (wt/vol) for 1 h at room temperature, followed by incubation overnight at 4°C in primary antibodies. Primary antibodies used were as follows: Cdk5 (1:500), p35 (1:1000), TH (1:1000), cytochrome c (1:250) Bcl2 (1:200), and Bax (1:200; all Biovision), β-actin (1:10,000; Sigma-Aldrich), caspase-3 (1:250), and tubulin (1:10,000; Sigma-Aldrich). The membranes were then washed four times in Tris-buffered saline/Tween-20 (5 min each), followed by incubation in respective secondary antibody (goat anti-mouse or goat anti-rabbit immunoglobulin (L)–horseradish peroxidase conjugate at a dilution of 1:3000) for 2 h at room temperature. Western blots were analyzed using the Amersham Biosciences ECL kit following the manufacturer’s instructions (GE Healthcare).
Immunohistochemistry
Ten-micrometer cryostat sections of midbrain and substantia nigra were collected on slides and prepared for immunohistochemistry, which was performed according to standard protocols for single or double immunostaining (Wu et al., 2002). Primary antibodies were p35 (1:100), Cdk5 (1:250), TH (1:500), and GFAP (1:300; Santa Cruz Biotechnology) and Iba1 (1:1000; Wako Chemicals). Immunostaining was visualized by fluorescein and Texas red secondary antibodies (Vector Laboratories) and was examined by transmitted or confocal microscopy. TH immunostaining was carried out on striatal and midbrain sections, and the TH- and DAPI-stained SNpc neurons were counted. The striatal density of TH immunoreactivity was determined as described (Bifsha et al., 2014). To quantify dopamine neuron degeneration, cell counts were performed using ImageJ. For cell counts of degenerating neurons, TH-stained coronal sections were loaded on ImageJ; the sections spanned regular intervals (30 or 100 mm) across the rostrocaudal extent of midbrains of three mice each in the different groups. For each section, total number of TH+ cells was separately counted for SNc in both hemispheres. Values are reported as means ± 2 SD. Statistical significance was calculated using analysis of variance (Bifsha et al., 2014).
Behavioral measurements
We performed the open-field experiment to test locomotor function of mice after MPTP and TP5/scrambled peptide treatments (Fredriksson et al., 1999; Ghosh et al., 2013). An automated device (model RXYZCM-16; AccuScan, Columbus, OH) was used to measure the spontaneous activity of mice. The activity chamber was 40 × 40 × 30.5 cm and made of clear Plexiglas and covered with a Plexiglas lid with holes for ventilation. The infrared monitoring sensors were located every 2.54 cm along the perimeter (16 infrared beams along each side) and 2.5 cm above the floor. Two additional sets of 16 sensors were located 8.0 cm above the floor on opposite sides. Data were collected and analyzed by a VersaMax Analyzer (model CDA-8; AccuScan). Before any treatment, mice were placed inside the infrared monitor for 10 min daily for three consecutive days to train them. At 16 and 48 h after the last MPTP injection, open-field experiments were conducted. In the open-field experiment, mice were monitored for horizontal activity, vertical activity, total distance traveled (centimeters), total movement time (seconds), total rest time (seconds), and rearing activity over a 10-min test session. We used Versaplot and Versadat software to analyze the data among the four groups.
Dopamine and HVA
Total brain or mesencephalon was collected, and fresh 50-μl (1 μg/ml) brain lysates were detected with 50 μl of primary antibody (1 h) and 100 ml of anti-rabbit secondary antibody (30 min) at room temperature according to the manufacturer’s protocols for dopamine (Abnova) and HVA (Eagle Biosciences).
ELISA
For cytokine assays, the concentrations of TNF-α, IL-1β, and IL-6 in the mixed culture medium/brain lysates were measured with an ELISA kit (Thermo Scientific), according to the manufacturer’s instructions. Absolute concentrations were derived by comparison with a standard curve.
Statistical analysis
Data were analyzed with Prism 3.0 software (GraphPad Software, San Diego, CA). Bonferroni and Dunnett multiple comparison testing was used. Differences with *p < 0.05 were considered significant
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Programs of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
Abbreviations used:
- Cdk5
cyclin-dependent kinase 5
- IL-1β
interleukin-1β
- MEF2
myocyte enhancer factor 2
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson’s disease
- Prx2
peroxiredoxin 2
- SN
substantia nigra
- TNF-α
tumor necrosis factor α.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E15-06-0415) on September 23, 2015.
REFERENCES
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin ND, Albers W, Pant HC. Cyclin-dependent kinase 5 (cdk5) activation requires interaction with three domains of p35. J Neurosci Res. 2002;67:354–362. doi: 10.1002/jnr.10116. [DOI] [PubMed] [Google Scholar]
- Bifsha P, Yang J, Fisher RA, Drouin J. Rgs6 is required for adult maintenance of dopaminergic neurons in the ventral substantia nigra. PLoS Genet. 2014;10:e1004863. doi: 10.1371/journal.pgen.1004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binukumar BK, Zheng YL, Shukla V, Amin ND, Grant P, Pant HC. TFP5, a peptide derived from p35, a Cdk5 neuronal activator, rescues cortical neurons from glucose toxicity. J Alzheimers Dis. 2014;39:899–909. doi: 10.3233/JAD-131784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Brion JP, Couck AM. Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am J Pathol. 1995;147:1465–1476. [PMC free article] [PubMed] [Google Scholar]
- Chen TS, Koutsilieri E, Rausch WD. MPP+ selectively affects calcium homeostasis in mesencephalic cell cultures from embryonal C57/Bl6 mice. J Neural Transm Gen Sect. 1995;100:153–163. doi: 10.1007/BF01271538. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY. Cdk5: mediator of neuronal death and survival. Neurosci Lett. 2004;361:47–51. doi: 10.1016/j.neulet.2003.12.117. [DOI] [PubMed] [Google Scholar]
- Chung YC, Bok E, Huh SH, Park JY, Yoon SH, Kim SR, Kim YS, Maeng S, Park SH, Jin BK. Cannabinoid receptor type 1 protects nigrostriatal dopaminergic neurons against MPTP neurotoxicity by inhibiting microglial activation. J Immunol. 2011;187:6508–6517. doi: 10.4049/jimmunol.1102435. [DOI] [PubMed] [Google Scholar]
- Chung YC, Kim SR, Jin BK. Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson’s disease. J Immunol. 2010;185:1230–1237. doi: 10.4049/jimmunol.1000208. [DOI] [PubMed] [Google Scholar]
- Crews L, Patrick C, Adame A, Rockenstein E, Masliah E. Modulation of aberrant CDK5 signaling rescues impaired neurogenesis in models of Alzheimer’s disease. Cell Death Dis. 2011;2:e120. doi: 10.1038/cddis.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Iborra S, Vila M, Perier C. The Parkinson disease mitochondrial hypothesis: where are we at. Neuroscientist. 2015:1073858415574600. doi: 10.1177/1073858415574600. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Palomo T, Archer T. Effects of co-administration of anticonvulsant and putative anticonvulsive agents and sub/suprathreshold doses of L-dopa upon motor behaviour of MPTP-treated mice. J Neural Transm. 1999;106:889–909. doi: 10.1007/s007020050209. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Gentil B, Grimot F, Riva C. Commitment to apoptosis by ceramides depends on mitochondrial respiratory function, cytochrome c release and caspase-3 activation in Hep-G2 cells. Mol Cell Biochem. 2003;254:203–210. doi: 10.1023/a:1027359832177. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Saminathan H, Kanthasamy A, Anantharam V, Jin H, Sondarva G, Harischandra DS, Qian Z, Rana A, Kanthasamy AG. The peptidyl-prolyl isomerase Pin1 up-regulation and proapoptotic function in dopaminergic neurons: relevance to the pathogenesis of Parkinson disease. J Biol Chem. 2013;288:21955–21971. doi: 10.1074/jbc.M112.444224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, Blair LA, Marshall J, Mao Z. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Helal CJ, Kang Z, Lucas JC, Gant T, Ahlijanian MK, Schachter JB, Richter KE, Cook JM, Menniti FS, Kelly K, et al. Potent and cellularly active 4-aminoimidazole inhibitors of cyclin-dependent kinase 5/p25 for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett. 2009;19:5703–5707. doi: 10.1016/j.bmcl.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Helal CJ, Sanner MA, Cooper CB, Gant T, Adam M, Lucas JC, Kang Z, Kupchinsky S, Ahlijanian MK, Tate B, et al. Discovery and SAR of 2-aminothiazole inhibitors of cyclin-dependent kinase 5/p25 as a potential treatment for Alzheimer’s disease. Bioorg Med Chem Lett. 2004;14:5521–5525. doi: 10.1016/j.bmcl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Huang E, Qu D, Zhang Y, Venderova K, Haque ME, Rousseaux MW, Slack RS, Woulfe JM, Park DS. The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nat Cell Biol. 2010;12:563–571. doi: 10.1038/ncb2058. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Klevenyi P, Andreassen O, Ferrante RJ, Schleicher JR, Jr., Friedlander RM, Beal MF. Transgenic mice expressing a dominant negative mutant interleukin-1beta converting enzyme show resistance to MPTP neurotoxicity. Neuroreport. 1999;10:635–638. doi: 10.1097/00001756-199902250-00035. [DOI] [PubMed] [Google Scholar]
- Lau LF, Ahlijanian MK. Role of cdk5 in the pathogenesis of Alzheimer’s disease. Neurosignals. 2003;12:209–214. doi: 10.1159/000074622. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lim AC, Qu D, Qi RZ. Protein-protein interactions in Cdk5 regulation and function. Neurosignals. 2003;12:230–238. doi: 10.1159/000074625. [DOI] [PubMed] [Google Scholar]
- Liu WB, Zhou J, Qu Y, Li X, Lu CT, Xie KL, Sun XL, Fei Z. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem Int. 2010;57:206–215. doi: 10.1016/j.neuint.2010.05.011. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Meuer K, Suppanz IE, Lingor P, Planchamp V, Goricke B, Fichtner L, Braus GH, Dietz GP, Jakobs S, Bahr M, Weishaupt JH. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ. 2007;14:651–661. doi: 10.1038/sj.cdd.4402087. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Yoshino H, Ikebe S, Hattori N, Kobayashi T, Shimoda-Matsubayashi S, Matsumine H, Kondo T. Mitochondrial dysfunction in Parkinson’s disease. Ann Neurol. 1998;44:S99–109. doi: 10.1002/ana.410440715. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm. 2000;(Suppl):2000, 277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kawamoto Y, Nakano S, Akiguchi I, Kimura J. p35nck5a and cyclin-dependent kinase 5 colocalize in Lewy bodies of brains with Parkinson’s disease. Acta Neuropathol. 1997;94:153–157. doi: 10.1007/s004010050687. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP. Cyclin-dependent kinase 5 in amyotrophic lateral sclerosis. Neurosignals. 2003;12:215–220. doi: 10.1159/000074623. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- Nguyen KC, Rosales JL, Barboza M, Lee KY. Controversies over p25 in Alzheimer’s disease. J Alzheimers Dis. 2002;4:123–126. doi: 10.3233/jad-2002-4207. [DOI] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Ossola B, Schendzielorz N, Chen SH, Bird GS, Tuominen RK, Mannisto PT, Hong JS. Amantadine protects dopamine neurons by a dual action: reducing activation of microglia and inducing expression of GDNF in astroglia [corrected] Neuropharmacology. 2011;61:574–582. doi: 10.1016/j.neuropharm.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V. Mechanisms of MPTP toxicity. Mov Disord. 1998;13(Suppl 1):35–38. [PubMed] [Google Scholar]
- Qu D, Rashidian J, Mount MP, Aleyasin H, Parsanejad M, Lira A, Haque E, Zhang Y, Callaghan S, Daigle M, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Gu M, Taanman JW, Tabrizi SJ, Seaton T, Cleeter M, Cooper JM. Mitochondria in the etiology and pathogenesis of Parkinson’s disease. Ann Neurol. 1998;44:S89–S98. doi: 10.1002/ana.410440714. [DOI] [PubMed] [Google Scholar]
- Shukla V, Zheng YL, Mishra SK, Amin ND, Steiner J, Grant P, Kesavapany S, Pant HC. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. FASEB J. 2013;27:174–186. doi: 10.1096/fj.12-217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin M, Krupinski J. Cyclin-dependent kinase-5 targeting for ischaemic stroke. Curr Opin Pharmacol. 2009;9:119–124. doi: 10.1016/j.coph.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Smith PD, Crocker SJ, Jackson-Lewis V, Jordan-Sciutto KL, Hayley S, Mount MP, O’Hare MJ, Callaghan S, Slack RS, Przedborski S, et al. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Mount MP, Shree R, Callaghan S, Slack RS, Anisman H, Vincent I, Wang X, Mao Z, Park DS. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Caspases—controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000;1477:299–306. doi: 10.1016/s0167-4838(99)00281-2. [DOI] [PubMed] [Google Scholar]
- Sundaram JR, Poore CP, Sulaimee NH, Pareek T, Asad AB, Rajkumar R, Cheong WF, Wenk MR, Dawe GS, Chuang KH, et al. Specific inhibition of p25/Cdk5 activity by the Cdk5 inhibitory peptide reduces neurodegeneration in vivo. J Neurosci. 2013;33:334–343. doi: 10.1523/JNEUROSCI.3593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Cohen O, Choi DK, Wu DC, Marks D, Vila M, Jackson-Lewis V, Przedborski S. Pathogenic role of glial cells in Parkinson’s disease. Mov Disord. 2003a;18:121–129. doi: 10.1002/mds.10332. [DOI] [PubMed] [Google Scholar]
- Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, Przedborski S. COX-2 and neurodegeneration in Parkinson’s disease. Ann NY Acad Sci. 2003b;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Lee MS, Cruz J. Cdk5, a therapeutic target for Alzheimer’s disease. Biochim Biophys Acta. 2004;1697:137–142. doi: 10.1016/j.bbapap.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Venderova K, Park DS. Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009365. doi: 10.1101/cshperspect.a009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroon A, Drukarch B, Bol JG, Cras P, Breve JJ, Allan SM, Relton JK, Hoogland PV, Van Dam AM. Neuroinflammation in Parkinson’s patients and MPTP-treated mice is not restricted to the nigrostriatal system: microgliosis and differential expression of interleukin-1 receptors in the olfactory bulb. Exp Gerontol. 2007;42:762–771. doi: 10.1016/j.exger.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Wen Z, Shu Y, Gao C, Wang X, Qi G, Zhang P, Li M, Shi J, Tian B. CDK5-mediated phosphorylation and autophagy of RKIP regulate neuronal death in Parkinson’s disease. Neurobiol Aging. 2014;35:2870–2880. doi: 10.1016/j.neurobiolaging.2014.05.034. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu W, Szumlinski KK, Lew J. p10, the N-terminal domain of p35, protects against CDK5/p25-induced neurotoxicity. Proc Natl Acad Sci USA. 2012;109:20041–20046. doi: 10.1073/pnas.1212914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YL, Amin ND, Hu YF, Rudrabhatla P, Shukla V, Kanungo J, Kesavapany S, Grant P, Albers W, Pant HC. A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J Biol Chem. 2010;285:34202–34212. doi: 10.1074/jbc.M110.134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YL, Kesavapany S, Gravell M, Hamilton RS, Schubert M, Amin N, Albers W, Grant P, Pant HC. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 2005;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Amin ND, Albers W, Pant HC. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur J Biochem. 2002;269:4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.