Abstract
Background
Haemophilus parasuis (H. parasuis) can invade the body and cause systemic infection under stress conditions. Marbofloxacin has been recommended for the treatment of swine infections. However, few studies have investigated the PK/PD characteristics and PK/PD cutoff (COPD) of this drug against H. parasuis.
Results
MICs of marbofloxacin against 198 H. parasuis isolates were determined. The MIC50 and MIC90 were 2 and 8 mg/L, respectively. An in vitro dynamic PK/PD model was established to study the PK/PD relationship of marbofloxacin against H. parasuis. The PK/PD surrogate markers Cmax/MIC, Cmax/MPC (the maximum concentration divided by MIC or mutant prevention concentration (MPC)) and AUC24h/MIC, AUC24h/MPC (the area under the curve during the first 24 h divided by MIC or MPC) simulated the antimicrobial effect of marbofloxacin successfully with the R2 of 0.9928 and 0.9911, respectively. The target values of 3-log10-unit and 4-log10-unit reduction for AUC24h/MPC were 33 and 42, while the same efficacy for AUC24h/MIC were 88 and 110. The COPD deduced from Monte Carlo simulation (MCS) for marbofloxacin against H. parasuis was 0.5 mg/L. The recommended dose of marbofloxacin against H. parasuis with MIC ≤ 2 mg/L was 16 mg/kg body weight (BW).
Conclusions
The PK/PD surrogate markers AUC24h/MIC, Cmax/MIC and AUC24h/MPC, Cmax/MPC properly described the effects of marbofloxacin. Marbofloxacin can achieve the best efficacy at dosage of 16 mg/kg BW for strains with MIC values ≤ 2 mg/L, therefore, it is obligatory to know the sensitivity of the pathogen and to treat animals as early as possible. The very first COPD provide fundamental data for marbofloxacin breakpoint determination.
Keywords: Marbofloxacin, PK/PD, H. parasuis, COPD, Monte Carlo simulation
Background
Haemophilus parasuis (H. parasuis) is not only a common inhabitant bacterium of the upper respiratory tract in swine but also an etiological agent of Glässer’s disease characterized by arthritis, fibrinous polyserositis, and meningitis [1]. H. parasuis can invade the body and cause systemic infection under stress conditions, for example, weaning, transporting, and decaying of maternal immunity [2]. It can also co-infect with immunosuppressive agents, i.e., porcine reproductive and respiratory syndrome (PRRS) virus [2]. Strains of serovars 1, 5, 10, 12, 13, and 14 were highly virulent and caused death or morbidity [3]. Among all of the serovars, serovars 5 and 4 are the most prevalent among isolates reported in China [4], Denmark [5], Germany [3], and the United States [6, 7].
H. parasuis infection is often treated with sulfanilamide, quinolones, or cephalosporins. However, some isolates have developed resistance to these drugs [8]. The most important factor for the emergence and dissemination of resistance is the exposure, especially exposure to sub-optimal drug concentrations [9]. The PK/PD modelling which helps determine exposure-response relationships is of great importance in determining antimicrobial regimens administered to animals to attain appropriate effects [10]. The Fluoroquinolones and Cephalosporin of 3th and 4th generations that have been re-licensed in Europe took into account not only the classical paradigm of concentration-dependent dosage but also the PK/PD indices best descried the effects and minimized the emergence of resistances, such as AUC24h/MIC, Cmax/MIC, the percent time that drug concentrations were above the minimum inhibitory concentration (%T > MIC), AUC24h/MPC, the percent time that marbofloxacin concentrations were above the mutant prevention concentration (%T > MPC) or the mutant selection window (TMSW). Marbofloxacin, a third generation fluoroquinolone, has been developed solely for veterinary treatment. It acts as a concentration-dependent bactericidal agent against Gram-negative and gram-positive bacteria [11]. Marbofloxacin has been recommended by the European Committee and China for the treatment of swine infections with a dosage regimen of 2 mg/kg/24 h BW for three to five days. However, the PK/PD relationship of it against H. parasuis is sparse. Susceptibility breakpoint for an antimicrobial may assist in determining whether an antibacterial is potentially useful in the treatment of a bacterial infection. Knowing whether an antimicrobial is useful will promote prudent use of antimicrobial drugs. Breakpoints should be set prior to an antibacterial being used clinically or at the time of an approved use. Its setting requires integration of knowledge of the wild-type distribution of MICs, the PK/PD relationship of an antibacterial, and clinical outcomes of infections when the antibacterial is used [12]. Veterinary susceptibility breakpoints are developed by the Clinical and Laboratory Standards Institute (CLSI) subcommittee on Veterinary Antimicrobial Susceptibility Testing (VAST) [13]. At this time, however, no veterinary specific clinical breakpoints of marbofloxacin have been established for swine disease caused by H. parasuis.
COPD determined by MCS that considers pharmacokinetic variation in target animals and PK/PD indices assisted in the defining of susceptibility breakpoints from the perspective of exposure–response relationship [14]. This method has also been used by regulatory agencies such as the U.S. FDA and the European Medicines Agency (EMA), or relevant specialized groups such as CLSI-AST and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), in defining the susceptibility breakpoints [15].
The purpose of this investigation was to study the PK/PD relationship of marbofloxacin against H. parasuis, derive a COPD of marbofloxacin and recommend a reasonable dosage regimen. MICs of marbofloxacin against those isolates were determined. An in vitro PK/PD infection model was used to investigate marbofloxacin effects against H. parasuis strain of serotype 5, which is a highly virulent serotype and is one of the most prevalent serotypes in China. Pharmacokinetics of marbofloxacin in swine obtained from a previous study and PK/PD indices were integrated into a Monte Carlo simulation to derive a COPD. A rational regimen of marbofloxacin against H. parasuis was determined.
Methods
Animal ethics
All husbandry practices and experimental operations were performed with full consideration of animal welfare. Research ethical approval was granted by the South China Agriculture University Animal ethics committee (2014–03).
Strains and antibiotic
A strain of H. parasuis, serovar 5 (V5), kindly provided by Professor Ming Liao, College of Veterinary Medicine, South China Agricultural University, Guangzhou, Guangdong Province, China was used in the present study. V5 was marbofloxacin susceptible with a MIC of 0.015 mg/L. The other 189 isolates of H. parasuis were collected from swine in south China regions between August 2010 and July 2011. Serotypes of these isolates are not known. Tryptone soya agar (TSA) and Tryptone soya broth (Oxoid Ltd., Basingstoke, Hampshire, UK), supplemented with 2 % beta-Nicotinamide adenine dinucleotide trihydrate (NAD) (Qingdao Hope Bio-Technology Co., Ltd., Shandong, China) and 5 % new-born calf serum (Guangzhou Ruite Bio-tec Co., Ltd., Guangdong, China), were used to culture H. parasuis. Marbofloxacin was purchased from Hebei Yuanzheng Pharmaceutical Company (Hubei, China).
Susceptibility testing
Considering that there is no recommended testing method by CLSI’s VAST Subcommittee for H. parasuis, MICs were conducted in accordance with the CLSI recommendations for Actinobacillus pleuropneumoniae. [16] (Wakiec et al., 2008) The Actinobacillus pleuropneumoniae ATCC 27090 strain was used for quality control purpose. MIC50 and MIC90 were defined in the present study as the lowest concentration that inhibited the growth of 50 % and 90 % of isolates tested, respectively. The mutant prevention concentration (MPC) value was determined according to previous reports [17, 18]. Single bacteria colony from 24 h growth on TSA was grown for 12 h in TSB broth, then concentrated through centrifugation and re-suspended it in TSB to a final concentration of ~ 3 × 1010 CFU/mL. An aliquot of 500 μL samples was plated onto TSA plates containing various concentrations of marbofloxacin, and then incubated for 24 h, 36 h and 48 h for re-growth. MPCpr was defined as the lowest drug concentration that inhibits growth. A second measurement was performed using linear drug concentration increment within 20 % per sequential increase. All the determinations were carried out in triplicates.
In vitro PK/PD model
The in vitro one-compartment PK/PD infection model equipment was constructed according to previously described method with some improvements [19]. An inverted 50 mL centrifuge tube with a cellulose ester membrane (0.2-μm pore size) covering the top was placed in the central compartment to prevent bacteria from flowing out to the medium. A magnetic stir bar was placed on the bottom of the central compartment which mixed the broth and enabled the drug to fully contact the bacteria. The flow rate was 0.171 mL/min to simulate the half-life of marbofloxacin in swine as described previously [20].
In vitro time kill curves of marbofloxacin
A 12 h culture of V5 at logarithmic phase was added to the central compartment to reach a final concentration of 107 colony forming unit (cfu)/mL. An incubation period of 30 min was applied to adapt the bacteria to the new environment. Different doses of marbofloxacin or control (sterile normal saline) were administered into the central compartment, and at the same time, the peristaltic pump was turned on. Samples were obtained at time points of 0, 3, 6, 9, 12, and 24 h. 100 μL of the samples were diluted properly with sterile normal saline, aliquots of the last four diluted samples were dropped onto the TSA plates and incubated at 37 °C for 24 h. The limit of determination was 400 cfu/mL.
Pharmacokinetics and PK/PD analysis
The samples for marbofloxacin concentration determination were centrifuged at 8,000 rpm at 4°C for 10 min. The supernatant was stored at −80 °C and analyzed using High Performance Liquid Chromatography (HPLC), which had been optimized by our laboratory [21] within 1 month. All experiments were performed in duplicate on different days. The PK data were analyzed using Phoenix WinNonlin 6.0 software (Pharsight Co. Ltd.).
As marbofloxacin is concentration dependent, the PK/PD index of marbofloxacin was AUC24h/MIC and Cmax/MIC [22]. The PK/PD indexes were calculated using the pharmacokinetic data and MIC value in each dose of the time–kill curve. The in vitro drug effect was quantified by changes in log10 cfu counts between 24 h and 0 h. Data were analysed using sigmoid Emax model WINNONLIN software (version 6.1; Pharsight, CA, USA) with the following equation:
Where E0 is the change in log10 cfu/mL after 24 h incubation in the control sample, compared with the initial inoculum. Emax is the difference in effect between the greatest amount of growth (as seen for the growth control, E0) and the greatest amount of kill. Ce is the AUC24h/MIC, Cmax/MIC or Cmax/MPC in the effect compartment. EC50 is the AUC24h/MIC, Cmax/MIC or Cmax/MPC value producing a 50 % reduction in bacterial counts from the initial inoculum, and N is the Hill coefficient that describes the steepness of the curve.
Monte Carlo analysis (MCS)
A 10,000-subject Monte Carlo simulation was conducted using Crystal Ball Professional V7.2.2 software based on a previous pharmacokinetic study of marbofloxacin in pigs and PK/PD target indices obtained in this study. AUC24h and Cmax were assumed to be log-normally distributed in the form of mean values and confidence intervals. The COPD is the MIC at which the probability of target attainment (PTA) equals to 90 %, which is the most commonly used standard for susceptibility breakpoints in other bacteria [12].
Dosage calculation
In order to deduce a more rational regimen, the general formula was employed to estimate dosages for different magnitudes of efficiency [23].
Where Dose is the optimal dose (mg/kg day), CL is the body clearance (L/kg day), AUC/MIC is the breakpoint marker for the desired effect, MIC90 is the MIC inhibiting 90 % of strains (mg/L), F is the bioavailability, and fu is the free drug fraction.
Results
MICs of marbofloxacin against H. parasuis were widely distributed, ranging from 0.003 mg/L to 16 mg/L (Fig. 1). A trimodal distribution was observed with peak value observed at 0.003, 0.06, and 2 mg/L, respectively. The MIC50 and MIC90 were 2 and 8 mg/L, respectively. The MPC of strain V5 was 0.04 mg/L at different time endpoints (24, 36 and 48 h), which was about 2 ~ 3 times of the MIC value (0.015 mg/L).
Fig. 1.
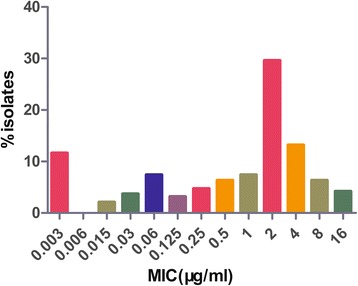
Minimum inhibitory concentrations of marbofloxacin against 199 isolates of H. parasuis
The pharmacokinetics of marbofloxacin in pigs was well simulated by this model with the relative deviation below 7 %. Time killing curves were shown in Fig. 2. The marbofloxacin inhibited H. parasuis moderately when the AUC24h/MIC was less than 13.8. The bacteria decreased rapidly within 12 h, but re-grew to 106 cfu/mL at 24 h with the AUC24h/MIC of 46. When the AUC24h/MIC was 55 and 73, H. parasuis could not be detected at 12 h, however, re-growth was observed at 24 h. When AUC24h/MIC was 92 and 110, marbofloxacin killed H. parasuis without regrowth in 24 h (4-log-unit and 5-log-unit decrease, respectively). The relationship between in vitro antimicrobial efficacy and PK/PD surrogate markers (AUC24h/MIC or Cmax/MIC) was described using the sigmoid Emax model. Both of the PK/PD surrogate markers simulated the in vitro antimicrobial effect of marbofloxacin successfully with the R2 of 0.9928 and 0.9911 respectively (Figs 3, 4). The estimated Log Emax, Log E0, EC50, and slope were shown in Tables 1 and 2 respectively. The target values of 3-log10-unit and 4-log10-unit decreases for Cmax/MIC were 6.5 and 8 while for AUC24h/MIC were 88 and 110, respectively. The same effects for surrogates Cmax/MPC and AUC24h/MPC were 2.5, 3 and 33, 42 respectively. They also simulated the in vitro antimicrobial effect of marbofloxacin successfully with the R2 of 0.9928 and 0.9911 respectively.
Fig. 2.
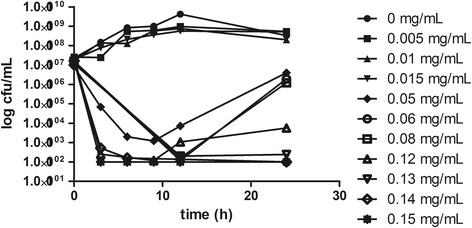
Time–kill curve of marbofloxacin against H. parasuis in the in vitro PK/PD model
Fig. 3.
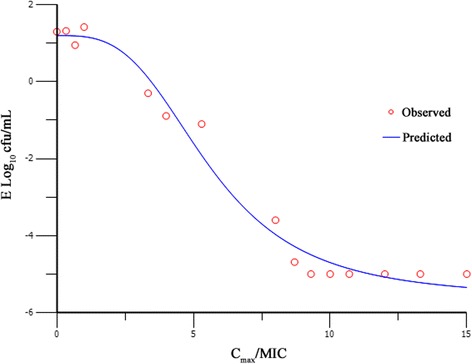
Sigmoid E max model relationships between antibacterial effect [E, log10 (cfu/mL)] and Cmax/MIC of marbofloxacin in the in vitro PK/PD model against H. parasuis with an inoculum size of 1 × 107 cfu/mL
Fig. 4.
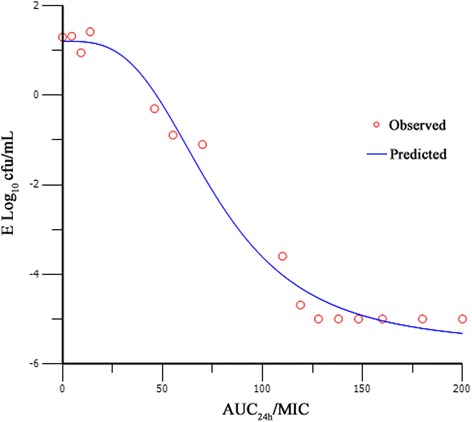
Sigmoid E max model relationships between antibacterial effect [E, log10 (cfu/mL)] and AUC24h/MIC of marbofloxacin in the in vitro PK/PD model against H. parasuis with an inoculum size of 1 × 107 cfu/mL
Table 1.
PK/PD analysis of marbofloxacin with the parameter of Cmax/MIC and Cmax/MPC against H. parasuis
| Parameter (units) | Value |
|---|---|
| Log Emax (cfu/mL) | 6.8 |
| Log E0 (cfu/mL) | 1.2 |
| Cmax/MIC EC50 | 5.6 |
| Cmax/MIC (bacteristasis) | 3 |
| Cmax/MIC (bactericidal) | 6.5 |
| Cmax/MIC (bacteria elimination) | 8 |
| Cmax/MPC (bactericidal) | 2.5 |
| Cmax/MPC (bacteria elimination) | 3 |
| Slope (N) | 3.2 |
Note: E 0 is the change in log10 cfu/mL after 24 h incubation in the control sample compared with the initial inoculum. Emax is the difference in effect between the greatest amount of growth (as seen for the growth control, E0) and the greatest amount of kill. EC50 is the Cmax/MIC value producing a 50 % reduction in bacterial counts from the initial inoculum, and N is the Hill coefficient that describes the steepness of the dose–response curve
Table 2.
PK/PD analysis of marbofloxacin with the parameter of AUC24h/MIC and AUC24h/MPC against H. parasuis
| Parameter (units) | Value |
|---|---|
| Log Emax (cfu/mL) | 6.8 |
| Log E0 (cfu/mL) | 1.2 |
| AUC24h/MIC EC50 | 76 |
| AUC24h/MIC (bacteristasis) | 47 |
| AUC24h/MIC (bactericidal) | 88 |
| AUC24h/MIC (bacteria elimination) | 110 |
| AUC24h/MPC (bactericidal) | 33 |
| AUC24h/MPC (bacteria elimination) | 42 |
| Slope (N) | 3.2 |
Note: E 0 is the change in log10 cfu/mL after 24 h incubation in the control sample compared with the initial inoculum. Emax is the difference in effect between the greatest amount of growth (as seen for the growth control, E0) and the greatest amount of kill. EC50 is the AUC24h/MIC value producing a 50 % reduction in bacterial counts from the initial inoculum, and N is the Hill coefficient that describes the steepness of the dose–response curve
As there are no PK data of marbofloxacin with a dose of 2 mg/kg BW, data of 2.5 mg/kg BW were used for MCS. In the simulation, with the PK/PD target AUC24h/MIC of 88, PTA > 90 % could only be achieved for isolates with MIC ≤ 0.125 mg/L (Fig. 5). For dosage regimen of 8 mg/kg BW with a single dose administered by IM, PTA > 90 % could be achieved for isolates with MIC ≤ 0.5 mg/L (Fig. 5). The COPD for marbofloxacin against H. parasuis was 0.5 mg/L. The recommended dose of marbofloxacin for bactericidal effect against H. parasuis was 16 mg/kg BW.
Fig. 5.
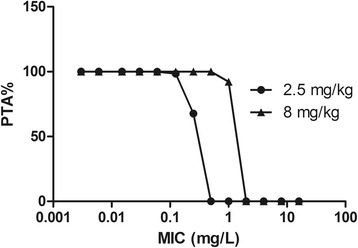
The Probability of target attainment (PTA) for different marbofloxacin doses against isolates of H. parasuis with different MICs
Discussion
Currently, a great deal of information is available on the PK/PD relationship of fluoroquinolones. The parameters Cmax/MIC and AUC24h/MIC correlate well with therapeutic outcome. According to literature, AUC24h/MIC of >125 h and Cmax/MIC of >10 was usually used as a threshold for successful therapeutic outcome of fluoroquinolones against gram negative bacteria [23]. Nevertheless, these thresholds may be different for some fluoroquinolones. The greatest influence for the differences was the immune status of the animal. Furthermore, the PK/PD indices of the same drug against different pathogens also vary. For example, the threshold of AUC24h/MIC is 46 h for bactericidal action in an ex vivo PK/PD study of marbofloxacin against Mannheimia haemolytica [24]; AUC24h/MIC ratios for no reduction, 3 log10 and 4 log10 reductions in bacterial count from the initial inoculum count were 41.9, 59.5, and 68.0 h for M. haemolytica and 48.6, 64.9, and 74.8 h for P. multocida in an ex vivo PK/PD study of marbofloxacin [25]. So it is of great importance to study the PK/PD indices of fluoroquinolones individually. Data on the PK/PD indices of marbofloxacin against H. parasuis are limited. In this study, PK/PD surrogates (Cmax/MIC, AUC24h/MIC and Cmax/MPC, AUC24h/MPC) simulated the bacterial reduction effects very well. The AUC24h/MIC ratios for no reduction, 3 log10, and 4 log10 reductions in bacterial count were 50, 88, and 110 h, while the Cmax/MIC ratios for those effects were 3.5, 6.5, and 8. The threshold value is higher than those derived from ex vivo PK/PD model. For these comparisons, it should be noted that there are significant differences between dynamic in vitro and ex vivo conditions. There is a continuous exposure to a fixed concentration of the agent for a defined duration (e.g., 24 h) in an ex vivo model, whereas a gradient of concentration in the dynamic in vitro model. The level of AUC24h/MPC (33 for 3 log10 reductions) was higher than that (12.89 for bactericidal effect) resulted from a tissue cage model of marbofloxacin against Pasteurella multocida [26]. The greatest influence for the differences might be the immune status of the animal. The value of Cmax/MPC (2.5 for bactericidal effect) was almost the same with that (Cmax/MPC > 2.2) of levofloxacin against staphylococcus aureus in a hollow fiber PK/PD model [27]. Though, both the PK/PD surrogates derived from MIC and MPC described the effect properly, PK/PD surrogates derived from MPC has been proven to be superior for fluoroquinolones over the classic PK/PD indices based on MIC for minimizing the emergence of resistances and preventing therapeutic failure [27].
Susceptibility breakpoint setting requires knowledge of the wild-type distribution of MICs, assessment of the PK/PD indices, and study of the clinical outcome of infections treated with the antibacterial. Monte Carlo simulation shows great advantage in supporting determination of the susceptibility breakpoint using drug exposure-effect relationship [14], which takes pharmacokinetic variation and PK/PD indices into consideration. For the simulation, as it had been proved that the efficacy of a single dosing regimen was better than doses administered every 24 h or 48 h of the same total amount of marbofloxacin [26], the EMA recommended dose regimen can be converted to 8 mg/kg BW with a single dose. Though both AUC24h/MIC and Cmax/MIC were PK/PD indices of fluoroquinolones, AUC24h is a much more robust estimation than the one of a single snapshot Cmax which depends on many factors such as sampling schedule. Therefore the PK/PD target was defined to be AUC24h/MIC with value of 88. The COPD of marbofloxacin against H. parasuis was 0.5 mg/L under one short dose of 8 mg/kg. This COPD value was equal to the clinical breakpoints values and the PK/PD breakpoints values of ciprofloxacin, moxifloxacin (0.5 mg/L), ofloxacin (0.5 mg/L), and levofloxacin(1 mg/L) against Haemophilus influenzae, another bacteria of Haemophilus spp in human clinic use [28]. Unfortunately, the proposed PK/PD cutoff would designate a large proportion of clinical H. parasuis isolates as resistant to the marbofloxacin. Swine infected by these isolates will probably have a low likelihood of responding to therapy.
Clinical dosage regimens of antimicrobial agents are traditionally determined by relating the PK of drugs in healthy animals and the in vitro antibacterial activity or the treatment outcome at given dosages in disease models or in clinical trials involving limited strains. Although these parameters can predict the potency of the drug against pathogens to a certain extent, they actually don’t provide information on the time course of antimicrobial activity. Fortunately, the relationship of PK/PD parameters and the clinical outcomes have been fully investigated and applied in optimizing drug regimens; the regimens based on PK/PD results were usually calculated by the general formula [23]. In the calculation, the PK/PD threshold, the PK parameters and MIC distribution were taken into accounted. As the fu of marbofloxacin in swine plasma was sparse, the value in dog plasma was used to substitute that. According to our best knowledge, the CL of marbofloxacin was 0.065 ± 0.011 L/kgh; the bioavailability was 90 % ± 28 %; the protein binding rate was 21.81 % ± 6.26 %; MIC50 is 2 mg/L [20, 29]. When the MIC90 was used to calculate the dose, we found the result (64 mg/kg BW) was too high to apply in clinical usage. Considering the MICs of some H. parasuis isolated in China are very high, they may exhibit resistance to marbofloxacin that cannot be treated by marbofloxacin. MIC50 was used in the calculation. the result showed that a dose of 16 mg/kg would be able to achieve bactericidal effect. This dose is much higher than the recommended dose (2 mg/kg for 3 to 5 days), and higher than that (10 mg/kg) [30] recommended for bovine respiratory disease based on the theoretical principle of single-injection, short-acting antibiotic (SISAAB), which has been primarily developed and applied to fluoroquinolones used in human medicine [31]. It seems that even a dose as high as 16 mg/kg BW cannot cure all the H. parasuis infection in China. It is well-known that fluoroquinolones can lead to cross-resistance among different members of the class [32], since they are widely used to treat respiratory diseases. It is better to check the susceptibility of pathogens before drug administration, as resistance determinants may transfer to human pathogenic bacteria, resulting in the failure of antibiotics in treatment of bacterial infection.
However, there are some limitations in our study. First, the PK/PD indices targets were based on the drug concentration at infection sites, whist drug concentration of plasma were used for MCS. Though, the penetration of marbofloxacin is good and the tissue concentration is similar to that in blood [26], concentration at infection sites should be simulated in future trials. A second limitation is that the recommended regimen is useful in just the regions of China as MIC probability distribution of a determined pathogen may vary between countries and regions and even time. Finally, our proposed COPD will need to be validated in the clinical outcome.
Conclusions
In summary, this study established an in vitro dynamic PK/PD modelling of marbofloxacin against H. parasuis. The target PK/PD values of marbofloxacin for 3-log10-unit and 4-log10-unit decreases effects were Cmax/MIC of 6.5 and 8, Cmax/MPC of 2.5 and 3, AUC24h/MIC of 88 and 110 or AUC24h/MPC of 33 and 42 respectively. The very first marbofloxacin COPD (0.5 mg/L) derived based on MCS was of great utility in marbofloxacin susceptibility test and dosing design. Marbofloxacin can have the best efficacy at dosage of 16 mg/kg BW for strains with MIC values ≤ 2 mg/L, therefore, it is obligatory to know the sensitivity of the pathogen and to treat animals as early as possible.
Acknowledgements
This study was supported by the National Science Fund for Distinguished Young Scholars (Grant # 31125026); Program for Changjiang Scholars; Innovative Research Team in University of Ministry of Education of China (Grant # IRT13063); Foundation for High-Level Talents in Higher Education of Guangdong, China; Science and Technology Program of Guangzhou, China (Grant # 12A072101642). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- H. parasuis
Haemophilus parasuis
- PK/PD
Pharmacokinetic/pharamcodynamic
- COPD
Pharmacokinetic/pharamcodynamic cutoff
- MICs
Minimal inhibitory concentrations
- Cmax/MIC
The maximum concentration divided by MIC
- UC24h/MIC
The area under the curve during the first 24 h divided by the minimum inhibitory concentration
- MPC
Mutant prevention concentration
- Cmax/MPC
The maximum concentration divided by mutant prevention concentration
- AUC24h/MPC
The area under the curve during the first 24 h divided by mutant prevention concentration
- MCS
Monte Carlo simulation
- BW
Body weight
- PRRS
Porcine reproductive and respiratory syndrome
- %T > MIC
The percent time that marbofloxacin concentrations were above the minimum inhibitory concentration
- %T > MPC
The percent time that marbofloxacin concentrations were above the mutant prevention concentration
- TMSW
The mutant selection window
- VAST
Veterinary Antimicrobial Susceptibility Testing
- EMA
European Medicines Agency
- CLSI
The Clinical and Laboratory Standards Institute
- EUCAST
The European Committee on Antimicrobial Susceptibility Testing
- V5
Serovar 5
- TSA
Tryptone soya agar
- NAD
Beta-Nicotinamide adenine dinucleotide trihydrate
- MIC50 and MIC90
MIC, inhibiting the growth of at least 50 % and 90 % of isolates in a test population
- OD
Optical density
- Cfu
Colony forming unit
Footnotes
Jian Sun and Xia Xiao contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YL conceived of the study and participated in its design and coordination and helped to draft the manuscript. JS and XX design the experiment and draft the manuscript. XX, RH, TY, YC and XF carried out the MIC determination and in vitro time kill curve studies, YL participated in the data analysis and revising the manuscript. HT and YZ carried out the bacteria isolation. All authors read and approved the final manuscript.
Contributor Information
Jian Sun, Email: jiansun@scau.edu.cn.
Xia Xiao, Email: xiami87107@sina.com.
Rui-Juan Huang, Email: 604284914@qq.com.
Tao Yang, Email: 731232633@qq.com.
Yi Chen, Email: 153787903@qq.com.
Xi Fang, Email: 290227387@qq.com.
Ting Huang, Email: 973132242@qq.com.
Yu-Feng Zhou, Email: 87647314@qq.com.
Ya-Hong Liu, Email: gale@scau.edu.cn.
References
- 1.Oliveira S, Pijoan C. Haemophilus parasuis: new trends on diagnosis, epidemiology and control. Vet Microbiol. 2004;99(1):1–12. doi: 10.1016/j.vetmic.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Macedo N, Rovira A, Oliveira S, Holtcamp A, Torremorell M. Effect of enrofloxacin in the carrier stage of Haemophilus parasuis in naturally colonized pigs. Can J Vet Res. 2014;78(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- 3.Kielstein P, Rapp-Gabrielson VJ. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J Clin Microbiol. 1992;30(4):862–865. doi: 10.1128/jcm.30.4.862-865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai X, Chen H, Blackall PJ, Yin Z, Wang L, Liu Z, et al. Serological characterization of Haemophilus parasuis isolates from China. Vet Microbiol. 2005;111(3–4):231–236. doi: 10.1016/j.vetmic.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Angen O, Svensmark B, Mittal KR. Serological characterization of Danish Haemophilus parasuis isolates. Vet Microbiol. 2004;103(3–4):255–258. doi: 10.1016/j.vetmic.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Rapp-Gabrielson VJ, Gabrielson DA. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am J Vet Res. 1992;53(5):659–664. [PubMed] [Google Scholar]
- 7.Tadjine M, Mittal KR, Bourdon S, Gottschalk M. Development of a new serological test for serotyping Haemophilus parasuis isolates and determination of their prevalence in North America. J Clin Microbiol. 2004;42(2):839–840. doi: 10.1128/JCM.42.2.839-840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SS, Sun J, Liao XP, Liu BT, Li LL, Li L, et al. Co-location of the erm(T) gene and blaROB-1 gene on a small plasmid in Haemophilus parasuis of pig origin. J Antimicrob Chemother. 2013;68(8):1930–1932. doi: 10.1093/jac/dkt112. [DOI] [PubMed] [Google Scholar]
- 9.Lees, P., Svendsen, O., Wiuff, C., 2008. Strategies to minimize the impact of antimicrobial treatment on the selection of resistant bacteria. In: Guardabassi, L., Jensen, L.B., Kruse, H. (Eds.), Guide to Antimicrobial Use in Animals. Blackwell Publishing, pp. 77–101 (Chapter 6)
- 10.Papich MG. Pharmacokinetic-pharmacodynamic (PK-PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Vet Microbiol. 2014;171(3–4):480–486. doi: 10.1016/j.vetmic.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Martinez M, McDermott P, Walker R. Pharmacology of the fluoroquinolones: a perspective for the use in domestic animals. Vet J. 2006;172(1):10–28. doi: 10.1016/j.tvjl.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Turnidge J, Paterson DL. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev. 2007;20(3):391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rey JF, Laffont CM, Croubels S, De Backer P, Zemirline C, Bousquet E, et al. Use of Monte Carlo simulation to determine pharmacodynamic cutoffs of amoxicillin to establish a breakpoint for antimicrobial susceptibility testing in pigs. Am J Vet Res. 2014;75(2):124–131. doi: 10.2460/ajvr.75.2.124. [DOI] [PubMed] [Google Scholar]
- 14.Mueller M, de la Pena A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob Agents Chemother. 2004;48(2):369–377. doi: 10.1128/AAC.48.2.369-377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrose PG. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the society of infectious diseases pharmacists. Pharmacotherapy. 2006;26(1):129–134. doi: 10.1592/phco.2006.26.1.129. [DOI] [PubMed] [Google Scholar]
- 16.Wakiec R, Gabriel I, Prasad R, Becker JM, Payne JW, Milewski S. Enhanced susceptibility to antifungal oligopeptides in yeast strains overexpressing ABC multidrug efflux pumps. Antimicrob Agents Chemother. 2008;52(11):4057–4063. doi: 10.1128/AAC.01648-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferran AA, Toutain PL, Bousquet-Melou A. Impact of early versus later fluoroquinolone treatment on the clinical; microbiological and resistance outcomes in a mouse-lung model of Pasteurella multocida infection. Vet Microbiol. 2011;148(2–4):292–297. doi: 10.1016/j.vetmic.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campion JJ, McNamara PJ, Evans ME. Evolution of ciprofloxacin-resistant Staphylococcus aureus in in vitro pharmacokinetic environments. Antimicrob Agents Chemother. 2004;48(12):4733–4744. doi: 10.1128/AAC.48.12.4733-4744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao X, Sun J, Chen Y, Huang RJ, Huang T, Qiao GG, et al. In vitro dynamic pharmacokinetic/pharmacodynamic(PK/PD) modeling and PK/PD cutoff of cefquinome against Haemophilus parasuis. BMC Vet Res. 2015;11(1):33. doi: 10.1186/s12917-015-0343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider M, Paulin A, Dron F, Woehrle F. Pharmacokinetics of marbofloxacin in pigs after intravenous and intramuscular administration of a single dose of 8 mg/kg: dose proportionality, influence of the age of the animals and urinary elimination. J Vet Pharmacol Ther. 2014;37(6):523–30. doi: 10.1111/jvp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan Q, Wang J, Yang F, Ding H, Liang C, Lv Z, et al. Pharmacokinetic/pharmacodynamic relationship of marbofloxacin against Pasteurella multocida in a tissue-cage model in yellow cattle. J Vet Pharmacol Ther. 2013;37(3):222–30. doi: 10.1111/jvp.12078. [DOI] [PubMed] [Google Scholar]
- 22.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother. 2005;55(5):601–607. doi: 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]
- 23.Toutain PL, del Castillo JR, Bousquet-Melou A. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res Vet Sci. 2002;73(2):105–114. doi: 10.1016/S0034-5288(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 24.Aliabadi FS, Lees P. Pharmacokinetics and pharmacokinetic/pharmacodynamic integration of marbofloxacin in calf serum, exudate and transudate. J Vet Pharmacol Ther. 2002;25(3):161–174. doi: 10.1046/j.1365-2885.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- 25.Potter T, Illambas J, Pelligand L, Rycroft A, Lees P. Pharmacokinetic and pharmacodynamic integration and modelling of marbofloxacin in calves for Mannheimia haemolytica and Pasteurella multocida. Vet J. 2013;195(1):53–58. doi: 10.1016/j.tvjl.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Cao C, Qu Y, Sun M, Qiu Z, Huang X, Huai B, et al. In vivo antimicrobial activity of marbofloxacin against Pasteurella multocida in a tissue cage model in calves. Front Microbiol. 2015;6:759. doi: 10.3389/fmicb.2015.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang B, Bai N, Cai Y, Wang R, Drlica K, Zhao X. Mutant prevention concentration-based pharmacokinetic/pharmacodynamic indices as dosing targets for suppressing the enrichment of levofloxacin-resistant subpopulations of Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55(5):2409–2412. doi: 10.1128/AAC.00975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Committee on Antimicrobial Susceptibility Testing [http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf]
- 29.Bidgood TL, Papich MG. Plasma and interstitial fluid pharmacokinetics of enrofloxacin, its metabolite ciprofloxacin, and marbofloxacin after oral administration and a constant rate intravenous infusion in dogs. J Vet Pharmacol Ther. 2005;28(4):329–341. doi: 10.1111/j.1365-2885.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 30.Vilalta C, Galofre N, Aragon V, Perez de Rozas AM, Fraile L. Effect of marbofloxacin on Haemophilus parasuis nasal carriage. Vet Microbiol. 2012;159(1-2):123–129. doi: 10.1016/j.vetmic.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Croisier D, Etienne M, Piroth L, Bergoin E, Lequeu C, Portier H, Chavanet P. In vivo pharmacodynamic efficacy of gatifloxacin against Streptococcus pneumoniae in an experimental model of pneumonia: impact of the low levels of fluoroquinolone resistance on the enrichment of resistant mutants. J Antimicrob Chemother. 2004;54(3):640–647. doi: 10.1093/jac/dkh393. [DOI] [PubMed] [Google Scholar]
- 32.Garau J. Update on cross-resistance of fluoroquinolones. Int J Clin Pract Suppl. 2000;115:94–98. [PubMed] [Google Scholar]


