Abstract
Introduction
The voltage gated sodium channel mutation Vgsc-1014S (kdr-east) was first reported in Kenya in 2000 and has since been observed to occur at high frequencies in the local Anopheles gambiae s.s. population. The mutation Vgsc-1014F has never been reported from An. gambiae Complex complex mosquitoes in Kenya.
Findings
Molecularly confirmed An. gambiae s.s. (hereafter An. gambiae) and An. arabiensis collected from 4 different parts of western Kenya were genotyped for kdr from 2011 to 2013. Vgsc-1014F was observed to have emerged, apparently, simultaneously in both An. gambiae and An. arabiensis in 2012. A portion of the samples were submitted for sequencing in order to confirm the Vgsc-1014F genotyping results. The resulting sequence data were deposited in GenBank (Accession numbers: KR867642-KR867651, KT758295-KT758303). A single Vgsc-1014F haplotype was observed suggesting, a common origin in both species.
Conclusion
This is the first report of Vgsc-1014F in Kenya. Based on our samples, the mutation is present in low frequencies in both An. gambiae and An. arabiensis. It is important that we start monitoring relative frequencies of the two kdr genes so that we can determine their relative importance in an area of high insecticide treated net ownership.
Keywords: Kdr, Insecticide resistance, Pyrethroids, Anopheles gambiae
Introduction
The two most widely applied vector control tools, insecticide treated nets (ITNs) and indoor residual spraying (IRS) have contributed greatly to the decline in global malaria rates [1, 2]. Pyrethroids are the most commonly used insecticides in control programs due to their low human toxicity and high efficacy against vectors [3, 4]. Previously, DDT, an organochlorine, was the most widely used insecticide for vector control with its use spread out over multiple countries for malaria control [5, 6]. The widespread use of these insecticides has likely contributed to the selection of resistance across sub-Saharan Africa [7] (http://www.irmapper.com/).
Increased resistance to pyrethroids is particularly troubling since this is the only class of insecticides approved by WHO for use on ITNs [3]. If ITNs are rendered ineffective, a surge in malaria transmission could follow [8]. Resistance to pyrethroids has been reported from multiple sites in western Kenya [9, 10] with both target site and metabolic resistance mechanisms implicated [9–15]. DDT and pyrethroids function by binding to the voltage gated sodium channels (Vgsc) on the mosquito’s neurons delaying the closing of the sodium channel; prolonging the action potential and causing repetitive neuron firing, ultimately resulting in paralysis and death [8, 16].
In Anopheles gambiae s.l., knock down resistance (kdr) is commonly caused by mutations in the Vgsc- either from leucine (TTA) to phenylalanine (TTT) or leucine to serine (TCA) [11, 17] at codon 1014. Vgsc-1014S (formerly kdr-east) was first reported in Kenya in 2000 and has been observed to occur at high frequencies in the local An. gambiae populations [10, 11]. Thus far, there has been no report of the existence of Vgsc-1014F (formerly kdr-west) in Kenya but has been reported in Uganda and Tanzania in the recent past [18, 19]. Our work demonstrates the emergence of Vgsc-1014F in western Kenya in two principal malaria vectors, An. gambiae and An. arabiensis.
Findings
Material and methods
This study was conducted in four malaria endemic districts in western Kenya with two distinct Vector control interventions: Rachuonyo and Nyando where IRS (Deltamethrin in 2011 and lambdacyhalothrin in 2012) was combined with ITNs (treated with permethrin or deltamethrin); and in Bondo and Teso where only ITNs are deployed [9]. Mosquito collections were performed annually between June and September in 2011, 2012 and 2013. Mosquito sampling, rearing and bioassays of emergent adults were conducted as described in Ochomo et al. [9].
Species identification & Vgsc genotyping
DNA was extracted from whole specimens and a PCR assay [20] was used to distinguish between An. gambiae and An. arabiensis. DNA samples were genotyped to identify the kdr genotype at amino acid position 1014 of the Vgsc using a modification of the protocol by Bass et al., [21] as described in Mathias et al., [10].
Exon sequencing of Vgsc
Previous studies in western Kenya have only reported the presence of Vgsc-1014S mutation. Therefore, in order to confirm the presence of Vgsc-1014F and to determine if was a de novo origin, a subsample of the Vgsc-1014F carriers were Sanger sequenced. Prior to sequencing, conventional PCR was used to amplify the exon 20 [22] which contains the 1014 locus]. Samples were sequenced at Centre for Genomic Research, University of Liverpool, UK and resulting sequences aligned using CodonCode aligner (http://www.codoncode.com/aligner/).
Analysis for the origin of Vgsc-1014F Mutation
Gene sequences obtained from the sequencing exons 20 and 27 were aligned using Codon Code aligner (http://www.codoncode.com/) and the contigs transferred to DnaSP (http://www.ub.edu/dnasp/) as FASTA files. The files were concatenated and then run using the PHASE algorithm in DnaSP [23]. The phased files were exported as a Phylip file to TCS, a statistical parsimony software for phylogenetic network estimation (http://darwin.uvigo.es/software/tcs.html).
Results
Frequency of Vgsc-1014S and Vgsc-1014F in the study sites from 2011 to 2013
We observed low frequencies of Vgsc-1014S in An. arabiensis,even though we had high frequencies of the same allele in An. gambiae, they were much lower than has been reported previously [10]. We saw a simultaneous appearance of Vgsc-1014F inboth An. gambiae and An. arabiensis in 2012 in all four study sites (Table 1) and thereafter compared the mean frequencies of the genes among the three years (Table 2). Of these, 19 samples (12 An. gambiae and 7 An. arabiensis) were sequenced. 3 An. gambiae and 3 An. arabiensis were confirmed to be homozygous for Vgsc-1014F with one An. arabiensis heterozygote detected. The sequences were deposited in GenBank (Accession numbers: KR867642- KR867651, KT758295-KT758303).
Table 1.
Frequency of Vgsc-1014F and Vgsc-1014S mutations in An. gambiae and An. arabiensis populations of western Kenya from 2011 to 2013
| An. arabiensis | An. gambiae | ||||||
|---|---|---|---|---|---|---|---|
| District | Year | N | Vgsc_1014S | Vgsc_1014F | N | Vgsc_1014S | Vgsc_1014F |
| Bondo | 2011 | 105 | 0.052 | 0 | 0 | ||
| 2012 | 129 | 0.031 | 0.047 | 2 | 0 | 0 | |
| 2013 | 236 | 0.008 | 0.125 | 2 | 0 | 0 | |
| Nyando | 2011 | 284 | 0.016 | 0 | 0 | ||
| 2012 | 82 | 0.012 | 0.024 | 1 | 0 | 0 | |
| 2013 | 173 | 0.055 | 0.023 | 5 | 0 | 0 | |
| Rachuonyo | 2011 | 20 | 0.05 | 0 | 0 | ||
| 2012 | 53 | 0 | 0.047 | 1 | 0 | 0 | |
| 2013 | 136 | 0.018 | 0.011 | 5 | 0 | 0 | |
| Teso | 2011 | 7 | 0 | 0 | 211 | 0.94 | 0 |
| 2012 | 4 | 0 | 0 | 189 | 0.68 | 0.054 | |
| 2013 | 60 | 0.019 | 0 | 317 | 0.85 | 0.025 | |
Table 2.
Comparison of mean frequencies of Vgsc-1014F and Vgsc-1014S mutations in An. arabiensis using ANOVA and Tukey’s test. A similar analysis could not be done for An. gambiae as only one site (Teso) had sufficient numbers of An. gambiae
| Vgsc-1014F | Vgsc-1014S | |||||||
|---|---|---|---|---|---|---|---|---|
| Year | Difference | Lower limit | Upper limit | Adjusted P-value | Difference | Lower limit | Upper limit | Adjusted P-value |
| 2011–2012 | 0.043 | −0.019 | 0.105 | 0.186 | −0.084 | −0.877 | 0.709 | 0.953 |
| 2011–2013 | 0.046 | −0.016 | 0.108 | 0.152 | −0.032 | −0.824 | 0.761 | 0.993 |
| 2012–2013 | 0.003 | −0.059 | 0.065 | 0.99 | 0.052 | 0.741 | 0.845 | 0.982 |
Only a single 1014F haplotype was observed (Fig. 1), suggesting a common origin in the species and subsequent interspecific transfer. However it should be noted that our ability to resolve different haplotypes was constrained by the low levels of diversity at this locus and our small amplicon length (478bp).
Fig. 1.
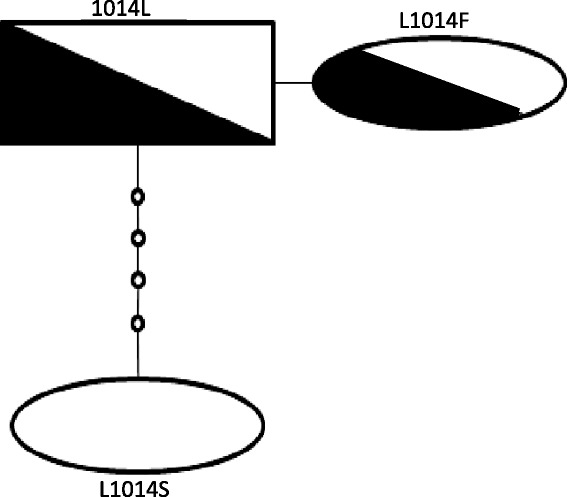
A TCS plot of the three haplotypes present in the populations assayed. White colour represents An. gambiae while black colour represents An. arabiensis
Discussion
This is the first report of Vgsc-1014F in Kenya, which appears to have emerged in both An. gambiae and An. arabiensis around 2012 and is confirmed via DNA sequencing in multiple samples. The gene has previously been observed in Uganda [18], then much later in Tanzania [19] and now in Kenya. We have developed this report to alert researchers and programmatic staff to the presence of Vgsc-1014F mutation in these two important Anopheles vectors so that they can modify their resistance marker screening procedures. It is important therefore that we start monitoring allele and genotype frequencies so that we can assess their impact in an area of high bednet ownership.
Acknowledgements
The authors acknowledge the support of Dr. David Weetman of the Vector Group, Liverpool School of Tropical Medicine, Judith Wandera, Richard Amito, Edward Esalimba, Gideon Nyansikera and the technical support of the KEMRI/CDC Malaria Branch, Centre for Global Health Research and Centre for Biotechnology, Research and Development, KEMRI and the National Malaria Control Program. We also acknowledge the support of Dr. Tessa Knox, Dr. Abraham Mnavaza and Dr. Evan Mathenge of WHO. The research is funded by the Bill and Melinda Gates Foundation through WHO (Award #54497 awarded to Dr. Charles Mbogo). We are grateful to the Director, KEMRI for the permission to publish this data.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EO, KS, NMB, LK, FA, JMV, CO, JG, MJD and CM designed and developed the study. EO, KS, BK, ER, NMB, LK, FA, JV, CO, KS, MJD and CM contributed to development of the protocol. EO, KS, ER and MJD performed gene sequencing and data analysis. EO, KS and BK performed the laboratory analysis of the samples. All authors took part in manuscript preparation, read and approved the final manuscript.
Contributor Information
Eric Ochomo, Phone: +254 723 845457, Email: eochomo@kemricdc.org.
Krishanthi Subramaniam, Email: krishanthi.subramaniam@lstmed.ac.uk.
Brigid Kemei, Email: brigidkemei@ymail.com.
Emily Rippon, Email: emily.rippon@lstmed.ac.uk.
Nabie M. Bayoh, Email: nbayoh@kemricdc.org
Luna Kamau, Email: lkamau@kemri.org.
Francis Atieli, Email: fatieli@kemri.org.
John M. Vulule, Email: vulule@yahoo.com
Collins Ouma, Email: collinouma@yahoo.com.
John Gimnig, Email: hzg1@cdc.gov.
Martin J. Donnelly, Email: martin.donnelly@lstmed.ac.uk
Charles Mbogo, Email: cmbogo@kemri-wellcome.org.
References
- 1.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Killeen GF, Smith TA, Ferguson HM, Mshinda H, Abdulla S, Lengeler C, et al. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med. 2007;4(7):e229. doi: 10.1371/journal.pmed.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaim M, Aitio A, Nakashima N. Safety of pyrethroid treated mosquito nets. Med Vet Entomol. 2000;14:1–5. doi: 10.1046/j.1365-2915.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 4.Smolen M, Sang M, Lirff R. Hazards and Exposures Associated with DDT and Synthetic Pyrethroids Used for Vector Control. Geneva: World Wildlife Fund; 1999. pp. 1–46. [Google Scholar]
- 5.Ratovonjato J, Randrianarivelojosia M, Rakotondrainibe ME, Raharimanga V, Andrianaivolambo L, Le Goff G, et al. Entomological and parasitological impacts of indoor residual spraying with DDT, alphacypermethrin and deltamethrin in the western foothill area of Madagascar. Malar J. 2014;13:21. doi: 10.1186/1475-2875-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangena JA, Adiamoh M, D'Alessandro U, Jarju L, Jawara M, Jeffries D, et al. Alternative treatments for indoor residual spraying for malaria control in a village with pyrethroid- and DDT-resistant vectors in the Gambia. PLoS One. 2013;8(9):e74351. doi: 10.1371/journal.pone.0074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knox TB, Juma EO, Ochomo EO, Pates Jamet H, Ndungo L, Chege P, et al. An online tool for mapping insecticide resistance in major Anopheles vectors of human malaria parasites and review of resistance status for the Afrotropical region. Parasit Vectors. 2014;7:76. doi: 10.1186/1756-3305-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African Anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27(2):91–8. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Ochomo E, Bayoh NM, Kamau L, Atieli F, Vulule J, Ouma C, et al. Pyrethroid susceptibility of malaria vectors in four Districts of western Kenya. Parasit Vectors. 2014;7:310. doi: 10.1186/1756-3305-7-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathias D, Ochomo E, Atieli F, Ombok M, Bayoh N, Olang G, et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malar J. 2011;10:10. doi: 10.1186/1475-2875-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranson H, Jensen B, Vulule J, Wang X, Hemingway J, Collins F. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 12.Stump A, Atieli F, Vulule J, Besansky N. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg. 2004;70:591–6. [PubMed] [Google Scholar]
- 13.Kamau L, Agai D, Matoke D, Wachira L, Gikandi G, Vulule JM. Status of insecticide susceptibility in Anopheles gambiae sensu lato and Anopheles funestus mosquitoes from Western Kenya. J Insect Sci. 2007;8:11. doi: 10.1673/031.008.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochomo E, Bayoh MN, Brogdon WG, Brogdon JE, Gimnig JE, Ouma C, et al. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Med Vet Entomol. 2013;27:156–64. doi: 10.1111/j.1365-2915.2012.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vulule J, Beach R, Atieli F, Roberts J, Mount D, Mwangi R. Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Med Vet Entomol. 1994;8:71–5. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 16.Davies TG, Field LM, Usherwood PN, Williamson MS. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life. 2007;59(3):151–62. doi: 10.1080/15216540701352042. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Torres D, Chandre F, Williamson M, Darriet F, Berge J, Devonshire A, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in themajor malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 18.Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East andWest African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar J. 2006;5:16. doi: 10.1186/1475-2875-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, Kollo D, et al. Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock- down Magesa resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Health. 2014;19(3):331–41. doi: 10.1111/tmi.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 21.Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111. doi: 10.1186/1475-2875-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto J, Lynd A, Elissa N, Donnelly MJ, Costa C, Gentile G. Caccone A, do Rosario VE: Co-occurrence of East and West African kdr mutations suggests high levels of resistance to pyrethroid insecticides in Anopheles gambiae from Libreville. Gabon Med Vet Entomol. 2006;20(1):27–32. doi: 10.1111/j.1365-2915.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- 23.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]


