Abstract
Objectives
To study correlation between ovarian reserve with biophysical markers (antral follicle count and ovarian volume) and biochemical markers (S. FSH, S. Inhibin B, and S. AMH) and use these markers to predict poor ovarian response to ovarian induction.
Methods
This is a prospective observational study. One hundred infertile women attending the Obst & Gynae Dept, KGMU were recruited. Blood samples were collected on day 2/day 3 for assessment of S. FSH, S. Inhibin B, and S. AMH and TVS were done for antral follicle count and ovarian volume. Clomephene citrate 100 mg 1OD was given from day 2 to 6, and patients were followed up with serial USG measurements. The numbers of dominant follicles (> or = 14 mm) at the time of hCG administration were counted. Patients with <3 follicles in the 1st cycle were subjected to the 2nd cycle of clomephene 100 mg 1OD from day 2 to day 6 with Inj HMG 150 IU given i.m. starting from day 8 and every alternate day until at least one leading follicle attained ≥18 mm. Development of <3 follicles at end of the 2nd cycle was considered as poor response.
Results
Univariate analyses showed that s. inhibin B presented the highest (ROCAUC = 0.862) discriminating potential for predicting poor ovarian response, In multivariate logistic regression model, the variables age, FSH, AMH, INHIBIN B, and AFC remained significant, and the resulting model showed a predicted accuracy of 84.4 %.
Conclusion
A derived multimarker computation by a logistic regression model for predicting poor ovarian response was obtained through this study. Thus, potential poor responders could be identified easily, and appropriate ovarian stimulation protocol could be devised for such pts.
Keywords: Anti-mullerian hormone, Logistic regression analysis, Poor responders, Inhibin B, Antral follicle count
Introduction
Infertility has emerged as a serious health problem in India. This has led to an increasing demand for assisted reproduction technologies. This involves ovulation induction with various stimulation protocols. Not all the patients who are subjected to ovulation induction show favorable response—some may result in poor ovarian response. The Rotterdam ESHRE/American Society for Reproductive Medicine (ASRM) sponsored PCOS consensus workshop group in 2004, where a consensus was reached on the minimal criteria to define poor ovarian response as follows:
Either advanced age more than 40 years.
Less than 3 oocytes retrieved with conventional stimulation protocol.
Abnormal ovarian reserve test (i.e., AFC < 5–7 follicles or AMH < 0.5–1.1 ng/ml.
Two episodes of poor response after maximal stimulation were sufficient to define patient as poor responder [1]. Thus, poor responders can be identified by estimation of ovarian reserve. Many hormones and ultrasound measurements have been assessed as a marker of ovarian reserve [2]. Biochemical markers identified are FSH, inhibin B, AMH, and estradiol. Biophysical markers include antral follicle count and ovarian volume [3]. Many researchers have used single as well as multiple markers to assess the ovarian reserve. So we planned this study to assess the predictive values of biophysical (antral follicle count and ovarian volume) and biochemical markers (S. FSH, S. Inhibin B, and S. AMH) in identifying poor ovarian reserve in Indian population. The objective of this study is to estimate the biochemical and biophysical markers in ovulation induction in infertile women, to analyze the correlation between these markers, and to possibly create a logistic regression model with these markers to predict poor ovarian response to stimulation.
Methods
This was a prospective observational study including 100 infertile women conducted in the Department of Obst & Gynae in collaboration with Department of Pathology, KGMU and the Central Drug Research Institute, Lucknow from June 2012 to 2013.
Inclusion Criteria Apparently healthy infertile women less than 40 years of age having ovulatory factor infertility willing to participate in the study with written informed consent. Exclusion Criteria Unwillingness to participate in the study. Patients suffering from acute infections, PID, Endometriosis, active tuberculosis, acute liver disease; and hypersensitivity to the drugs used, immunocompromised individuals, and h/o ovarian surgery. Detailed history of patients was taken and thorough examination done. All preliminary investigations including the thyroid profile and tubal patency test were done.
Collection of samples
On the morning of day 2/3 of the menstrual cycle, 8 ml of venous blood was withdrawn from cubital vein in plain vacuutainers, centrifuged at 3,500 rpm and stored at −4 °C in the refrigerator at QMH and transported within 1 h to the pathology lab. In the lab, each sample was divided into three sets of ependorfs, labeled, and stored at a −20 °C. Analysis of sample-assay of s. FSH and s. AMH was done in a single batch using sandwich ELISA kit at the Department of Pathology, KGMU, and the third set of samples were analyzed for s. Inhibin B using human inhibin B ELISA kit.
Ultrasound assessment was done using transvaginal probe of WIPROGE Logiq5 ultrasound machine. Bilateral ovaries, uterine cavity, and endometrial thickness were assessed. Biophysical parameters of the study antral follicle count (size 2–10 mm) and ovarian volume were noted.
Ovulation induction
All the 100 patients were subjected to ovulation induction.
First cycle Ovulation induction was done with standard stimulation protocol of clomephene citrate 100 mg 1OD from day 2 to 6, and patients were followed up with serial USG measurements until at least one leading follicle attained ≥18 mm.
Second cycle Ovulation induction was done with clomephene 100 mg 1OD from day 2 to 6 with Inj HMG 150 IU given i.m. starting from day 8 and every alternate day until at least one leading follicle attained ≥18 mm.
Follow up All patients were followed up by follicular monitoring with vaginal ultrasonography starting on the 8th day of the cycle and then every other day until HCG 10,000 IU was administered as a single I.M injection to trigger ovulation when at least one leading follicle attained ≥18 mm. Number of dominant follicles (> or = 14 mm) at the time of HCG administration was counted to analyze the result of ovulation induction. Patients with three or more follicles in 1st cycle were taken in group 1. Patients with less than three follicles in 1st cycle were taken in group 2.
Patients of group 2 who did not conceive were subjected to 2nd cycle of ovulation induction. In the second-cycle ovulation, induction was done with clomephene citrate 100 mg from day 2/day 3 for 5 days with inj HMG 150 IU given i.m. on day 8, and then every alternate day until HCG 10,000 IU was administered as a single I.M injection to trigger ovulation, when at least one leading follicle attained ≥18 mm. Patients developing less than three follicles at end of second cycle were considered as poor response.
Results
Out of a total 100 patients enrolled in the study and evaluated for outcome at first cycle, 27 (27 %) showed good response and were classified as group 1; and 73 (73 %) who showed poor response were classified as group 2. Group 1 was excluded from the second-cycle observations, and hence second-cycle observations were made in 73 patients of group 2 only. Out of these 73 patients, 27 (36.9 %) turned out to be good responders (group 2A), while remaining 46 (63.1 %) were poor responders.(Group 2B).Thus, cumulative outcome of two cycles showed, a total of 54 (54 %) to be good responders and remaining 46 (46 %) to be poor responders (Table 1).
Table 1.
Association of demographic, clinical and biomarkers with pattern of response in entire study period
| S. no. | Test variable | Group 1 + 2A (n = 54) good responders | Group 2B (n = 46) poor responders | P value |
|---|---|---|---|---|
| 1. | Age(years) | 27.6 ± 4.3 | 30.4 ± 5.4 | 0.010 |
| 2. | BMI(kg/m2) | 20.82.01 | 22.8 | 0.003 |
| 3. | S.FSH(IU/l) | 6.75 ± 2.68 | 8.84.37 | 0.013 |
| 4. | S.AMH(pg/ml) | 734.5 ± 511.6 | 201.3 ± 253.1 | <0.001 |
| 5. | S.inhibin-B(pg/ml) | 55.35 ± 34.75 | 17.2 ± 25.5 | <0.001 |
| 6. | Antral follicle count | 3.75 ± 1.55 | 1.4 ± 1.7 | <0.001 |
| 7. | Ovarian volume(cm3) | 9.91 ± 3.5 | 9.30 ± 6.08 | 0.539 |
For age, S. FSH, and ovarian volume, the odds ratio were found to be above unity but were not found to be significantly associated with the outcome (p > 0.05). However, S. AMH, S. Inhibin, and AFC had Odds ratio lower than unity, and S. AMH and AFC also had a significant association with the outcome (p < 0.05). The model showed a good fit (p = 0.935) and had a predicted accuracy of 84.4 % (Table 2).
Table 2.
Predictors for poor response in women (<3 oocytes) for Multivariate logistic regression model
| Test variable | Odds ratio | 95 % CI | p value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.040 | .903 | 1.198 | .583 |
| S. FSH | 1.021 | .848 | 1.229 | .827 |
| S. AMH | .998 | .996 | 1.000 | .024 |
| S. Inhibin | .976 | .953 | 1.000 | .054 |
| AFC | .596 | .413 | .862 | .006 |
| Ovarian Volume | 1.007 | .894 | 1.133 | .913 |
| Constant | 4.363 | .529 | ||
χ2 = 2.997 (df = 8); p = 0.935 (Hosmer and Lemeshow goodness of fit test) Probability of < 3 oocytes = {e4.363+0.040*Age+0.021*SFSH−0.002*SAMH−0.024*SInhibin−0.517*AFC+0.007*Ov.Vol.}/{1- e4.363+0.040*Age+0.021*SFSH−0.002*SAMH−0.024*SInhibin−0.517*AFC+0.007*Ov.Vol.}
χ2 = 4.336 (DF = 2); p = 0.11
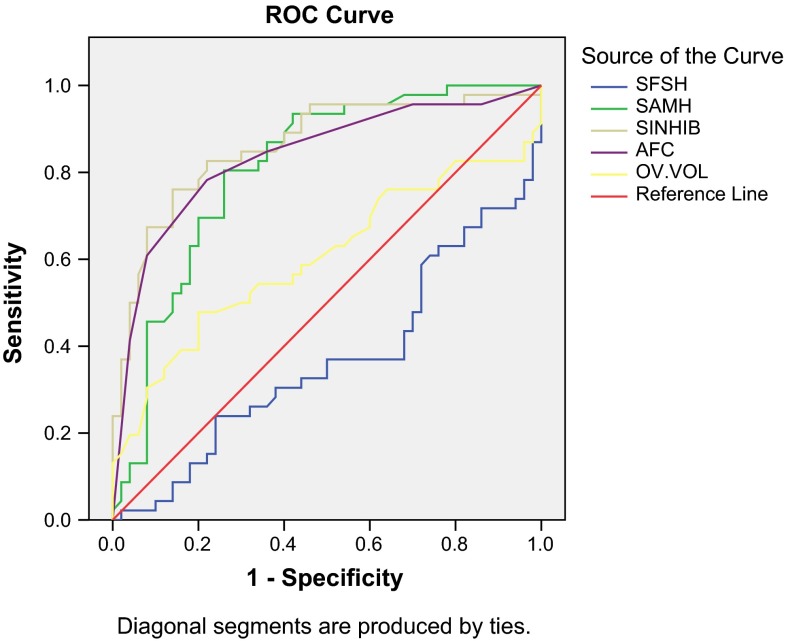
Receiver operating characteristic (ROC) curve analysis for poor outcome during entire study with S. FSH, S. AMH, S. Inhibin, AFC and Ovarian Volume as predictors revealed maximum area under curve (AUC) for S. Inhibin and minimum AUC for ovarian volume. Except for ovarian volume, the association was significant statistically for all the tested parameters. On selected cutoff values, S. AMH had maximum sensitivity (80.4 %), while S. Inhibin had maximum specificity (80 %) and ovarian volume had minimum sensitivity (58.7 %) as well as specificity (54.0 %) (Table 3; Fig. 1).
Table 3.
ROC Analysis for Poor Outcome during overall study period
| Test result variable (s) | Area | SD errora | Asymptotic sig.b | Selected cutoff | Predicted sensitivity (%) | Predicted specificity (%) |
|---|---|---|---|---|---|---|
| SFSH | .643 | .057 | .025 | >7.10 | 63.0 | 68.0 |
| SAMH | .809 | .045 | .000 | <291.27 | 80.4 | 74.0 |
| SINHIB | .862 | .039 | .000 | <23.75 | 78.3 | 80.0 |
| AFC | .837 | .042 | .000 | <2.50 | 78.3 | 64.0 |
| OV.VOL | .603 | .060 | .083 | <9.40 | 58.7 | 54.0 |
The test result variable(s): SFSH, SAMH, SINHIB, AFC, OV.VOL has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased
aUnder the nonparametric assumption
bNull hypothesis: true area = 0.5
Fig. 1.
Receiver operating characteristic (ROC) curve
Discussion
Assisted reproduction technology has revolutionized the treatment of infertility and is being increasingly used. Attempts have been made by various researchers to determine certain markers of ovarian reserve, which could predict a successful outcome to ovulation induction in infertile women. This would be beneficial in optimizing the planned therapeutic intervention, and thus, minimize the emotional and financial strains imposed upon couples seeking fertility treatment. Through this study, these biophysical and biochemical markers had been identified, and the roles of these markers in the prediction of ovarian response to stimulation were demonstrated.
In the present study, patients with age less than 40 years were included. Maximum number of patients were aged between 26 and 30 years (n = 43) followed by those aged 20–25 years (n = 18), 31–35 years (n = 18), and >35 years (n = 10). With the increasing age, there was the decrease in the number of follicles formed. Mean age of the poor responders was found to be 30.4 ± 5.4 years as compared to that of the good responders which had a mean age of 27.6 ± 4.2 years. This difference was found to be statistically significant. Thus, age of the infertile women was found to be a significant determinant of poor ovarian response to ovarian stimulation in the present study. Similar study conducted by Tsung-Hsien Lee in 2009 on the impact of female age on ovarian reserve markers to predict the outcomes of assisted reproduction technology cycles showed that advancing age had a strong co-relation with ovarian reserve. In the present study, day 3 serum FSH concentrations were higher in poor responders as compared with the good responders. Mean serum FSH values in two good responders was 6.73 ± 2.70 IU/L, while the poor responders had the mean value of 8.88 ± 4.37 IU/L. By ROC curve analysis, the cutoff limit of 7.10 IU/L serum FSH was identified for poor ovarian response. Thus, through our study, it could be predicted that infertile women with serum FSH values >7.10 IU/L (sensitivity 67.6 % and specificity 64.0 %) are at high risk of developing poor response to ovarian stimulation. In the study conducted by Vander steeg et al. in the year 2006 to investigate the predictive value of basal FSH in subfertile women for spontaneous ongoing pregnancy, it was found that patients having S.FSH levels higher than 8 IU/L had decreased fecundity independent of female age [4]. Study conducted by Creus et al. demonstrated the cutoff value of 9.45 IU/L between canceled and non-canceled cycles of IVF: however, the sensitivity was 64.7 % [5]. In the present study, we found that serum FSH had an inverse correlation with serum Inhibin B in predicting poor ovarian response to stimulation. At the selected cutoff of serum FSH of 7.10 IU/L, the serum inhibin B value was found to be 23.75 pg/ml by ROC analysis.
In this study, the mean value of serum inhibin B was 55.4 ± 17.2 pg/ml in the good responders as compared to the poor responders in whom the mean value was 17.2 ± 15.5 pg/ml. By ROC analysis, the cutoff value of inhibin B to identify poor responders was found to be 23.75 pg/ml. Seifer et al. reported higher cycle cancelation rates and lower pregnancy rates in women with low (<45 pg/ml) day 3 serum inhibin B concentrations [6]. According to the study of Seifer et al., the value of inhibin B of 45 pg/ml was considered as a cutoff to identify poor responders.
In this study, serum AMH has been found to be a significant predictor of poor ovarian response. The mean value of serum AMH was found to be 734.5 ± 566.4 pg/ml in good responders, while the mean value in poor responders was found to be 201 ± 253.1 pg/ml. The cutoff limit of serum AMH to identify poor responders by ROC analysis has been found to be 291.27 pg/ml. Patients having serum AMH values lower than 291.27 pg/ml (0.29 ng/ml) are at high risk of showing poor response to ovarian stimulation. This value had a high sensitivity of 80.4 % and a specificity of 74 %. An inverse correlation was found in our study between serum AMH and female age. Thus, with the increasing age, there was a decrease in the serum AMH levels. Van rooji et al. conducted a study to assess the role of AMH in identifying poor responders in IVF cycle. The cutoff limit identified by them was 0.3 ng/ml [7]. Muttukrishna et al. in 2004 postulated a cutoff limit of 0.2 ng/ml of serum AMH for poor ovarian reserve [8].
The present study showed that the AFC is a valuable test that can be used in infertile women to assess their ovarian reserve and thus chances of pregnancy. Mean value of antral follicle count was found to be higher, i.e., 3.75 ± 1.52 follicles in good responders, whereas in poor responders, the mean value of antral follicle count was found to be 1.4 ± 1.7 follicles. The cutoff limit of AFC to predict poor response to ovarian stimulation was found to be 2.5 follicles (sensitivity 78.3 % and specificity 64 %). Studies conducted by Klinkert et al. on antral follicle count in 2005 demonstrated that AFC > 5 follicles was a better predictor of ongoing pregnancy [9]. In our study, a significant correlation was found between serum AMH and antral follicle count. AFC was negatively correlated to age in our study.(r = −0.293, p value < 0.01).
Ovarian volume has been considered as a test of ovarian reserve by various authors. In our study, the mean ovarian volume in good responders was 9.91 ± 3.5 cm3, and in poor responders, the mean ovarian volume was found to be 9.30 ± 6.08 cm3. This difference was not statistically significant. Thus, ovarian volume was not found to be significantly associated with poor ovarian response to stimulation in the present study. This could be explained by the fact that most of the patients were of younger age group.(mean age of poor responders was 30.4 ± 5.4 years) Syrop et al. in their study on infertile women undergoing the first cycle of IVF concluded that total ovarian volume was a significant predictor of cycle cancelation [10].
Studies have also been conducted in the past using multiple markers of ovarian reserve to identify poor responders. In the present study, we have included six variables, viz., s.FSH, s. inhibin B, s. AMH, AFC, Ovarian volume, and female Age to develop a logistic regression model. For age, s.FSH, and ovarian volume, the odds ratio were found to be above unity but were not found to be significantly associated with outcome (p > 0.05). S.AMH, s. inhibin B, and AFC had odds ratio lower than unity, and s. AMH and AFC also had a significant association with the outcome,(P < 0.05). This model showed a predicted accuracy of 84.4 %.,However, among all the markers, serum AMH had maximum sensitivity of 80.4 %, and serum inhibin B had maximum specificity of 80 %.
Conclusion
Screening for the ovarian reserve is fundamental component of the initial infertility evaluation. An improved ascertainment of the ovarian reserve status may help one optimize the planned therapeutic intervention, and thus minimize the emotional and financial strains imposed upon couples seeking fertility treatment.
Day 3 serum FSH concentrations were significant in predicting poor ovarian reserve/response. Based on this study, it could be predicted that infertile women with serum FSH values > 7.10 IU/L are at high risk of developing poor response to ovarian stimulation. Patients having serum AMH values lower than 291.27 pg/ml (0.29 ng/ml) are at high risk of showing poor response. The cutoff value of inhibin B to identify poor responders was found to be 23.75 pg/ml. Levels of serum inhibin B lower than <23.75 pg/ml was associated with higher chances of development of poor response. The cutoff limit of AFC to predict poor response to ovarian stimulation was found to be 2.5 follicles. A derived multimarker computation for predicting poor ovarian response was obtained through this study. Through this model, potential poor responders could be identified beforehand, and thus, appropriate ovarian stimulation protocol and treatment regimens could be devised for such patients.
Thus, in summary, this prospective study demonstrated that screening for “early ovarian aging” in women in their late twenties or early thirties using such ovarian reserve tests could provide information to them allowing them to make rational decisions about their fertility, thereby allowing those with a smaller than average follicle pool to consider early attempts to conceive, or perhaps to decide to cryopreserve oocytes or embryos for use later.
Acknowledgments
Compliance with ethical requirements and Conflict of interest
The present study was conducted in the Dept. of OB/GY in collaboration with Dept. of Pathology. This work is original and performed after taking Ethical clearance from the Institutional Ethical Committee.
Dr. S. P. Jaiswar
is working as a Professor, in the Department of Obstetrics and Gynecology, KGMU, Lucknow since 1999. She is working on Genital tuberculosis, Infertility (male and female) biomarker, and free radicals in PIH and gynecologic malignancy. She has published 35 papers in the national and 10 papers in the international journals. She received the CS Dawn prize (2009), Smt. Jagdeeswari Mishra award (2013), the Best paper presentation award in UPCON (2013), The Best paper publication award of JOGI (2011), and an award for social work from the Hanuman Samiti (2001). She participated in many national and international conferences and delivered lectures and presented research papers. She has guided many MD and PhD students. She is also a Principal Investigator in Research Projects funded by ICMR, New Delhi and UPCST, Lucknow. She was a Visiting Professor in Dharan, Nepal in 2008.
References
- 1.Elgindy EA, El-Haieg DO, El-Sebaey A, et al. Anti-Müllerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2007. [DOI] [PubMed]
- 2.Van der steeg JW et al. Prediction models in reproductive medicine.
- 3.Creus M, et al. Day 3 serum inhibin B and FSH and age as predictors of assisted reproduction treatment outcome. hum Reprod. 2000;15(11):2341–2346. doi: 10.1093/humrep/15.11.2341. [DOI] [PubMed] [Google Scholar]
- 4.Ferraretti AP, La Marca A, Fauser BCJM, et al. On behalf of the ESHRE working group on poor ovarian response definition; ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011 pp. 1–9 [DOI] [PubMed]
- 5.James P, Toner MD. Ovarian reserve, female age and the chance for successful pregnancy. Fertil Steril. 1991;55(4):784–791. [PubMed] [Google Scholar]
- 6.Johnson NP, Bagrie EM, Coomarasamy A, et al. Ovarian reserve tests for predicting fertility outcomes for assisted reproductive technology: the International Systematic Collaboration of Ovarian Reserve Evaluation protocol for a systematic review of ovarian reserve test accuracy. BJOG. 2006. [DOI] [PubMed]
- 7.Klinkert R, Broekmans FJM, Looman CWN, et al. The antral follicle count is a better marker than basal follicle-stimulating hormone for the selection of older patients with acceptable pregnancy prospects after invitro fertilization. Fertil Steril. 2005;83:811–814. doi: 10.1016/j.fertnstert.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 Inhibin B as predictive of assisted reproductive technologies outcome. Fertil Steril. 2007;88:539–546. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Muttukrishna S, Suharjono H, McGarrigle H, et al. Inhibin B, and anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? BJOG An Int J Obstet Gynaecol. 2004;111:1248–1258. doi: 10.1111/j.1471-0528.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 10.Syrop CH, Dawson JD, Husman KJ, et al. Ovarian volume may predict assisted reproductive outcomes better than follicle stimulating hormone concentration on day 3. Hum Reprod. 1999;14(7):1752–1756. doi: 10.1093/humrep/14.7.1752. [DOI] [PubMed] [Google Scholar]