Abstract
Purpose
To compare the effect of combined oxytocin–misoprostol versus oxytocin and misoprostol alone in reducing blood loss at cesarean delivery.
Methods
One hundred fifty patients of 18–40 years with singleton term pregnancies scheduled for cesarean section under spinal anesthesia were recruited in a prospective double-blind randomized clinical trial to one of the three following groups to receive 20 IU infusion of oxytocin (group O), 400-µg sublingual misoprostol tablets (group M) or 200-µg misoprostol plus 5 IU bolus intravenous oxytocin (group MO) after delivery. The hemoglobin level before surgery and 24 h after surgery, the need for additional oxytocic therapy, and the incidence of adverse effects were recorded.
Results
The mean blood loss during surgery was significantly lower in group MO compared to other groups (P = 0.04). Comparison of mean arterial pressure (P = 0.38) and heart rate (P = 0.23) changes during spinal anesthesia and surgery failed to reveal any statistically significant differences between all groups through repeated measure analysis.
Conclusion
The use of combined lower dose of misoprostol–oxytocin significantly reduced the amount of blood loss during and after the lower segment cesarean section compared to higher dose of oxytocin and misoprostol alone, and its use was not associated with any serious side effects.
Keywords: Misoprostol, Oxytocin postpartum hemorrhage, Oxytocin, Blood loss, Cesarean, Spinal anesthesia
Introduction
Every day, approximately 1,000 women die from preventable causes related to pregnancy and childbirth; 99 % of these deaths occur in low-resource countries, with more than half occurring in Sub-Saharan Africa and one-third occurring in South Asia [1, 2] Postpartum hemorrhage is responsible for about a third of maternal deaths. Uterine atony accounts for 70 % of primary postpartum hemorrhage and various approaches have been used for this condition [3–5]. Nowadays, the incidence of fatal PPH has decreased because of active management of third stage of labor which includes controlled cord traction, uterine fundal massage, and administration of a pharmacological uterotonic [6]. Although surgeon experience has a great role in reducing the occurrence of primary postpartum hemorrhage at cesarean section, the administration of uterotonics, sometimes in high doses, helps in preventing or stopping excessive blood loss from an atonic uterus. These include oxytocin, ergometrine, and prostaglandins. Oxytocin and ergometrine are light- and heat-sensitive and require cold-chain storage. Prostaglandins E2 and F2α are used as second- or third-line agents but they are heat-labile and too expensive for use in developing countries [7]. Misoprostol, a synthetic prostaglandin E1 analog, has been emerged to prevent and treat gastroduodenal damage induced by nonsteroidal anti-inflammatory drugs (NSAIDs) [8].
Moreover, misoprostol has been used in obstetrics and gynecology, including medication abortion, medical management of miscarriage, induction of labor, cervical ripening before surgical procedures, and the treatment of postpartum hemorrhage. Misoprostol’s advantages over other synthetic prostaglandin analogs are its low cost, long half life, heat stability, and worldwide availability [9, 10].
Oxytocin is a short amino-acid polypeptide hormone, (C43H66N12O12S2), released from the posterior lobe of the pituitary gland [11]. It is widely used to stimulate uterine contractions to accelerate labor progress [3]. Although oxytocin is the gold standard drug for prevention and treatment of PPH, it requires cool storage, sterile equipment, and trained personnel, so that routine use of oxytocin in low-resource settings may be difficult [12]. Misoprostol offers many advantages over oxytocin in such settings. It is heat-stable, costs lower, and has variable routes of administration: orally, rectally, vaginally, or sublingually. Also, it is formulated as a tablet, stable at room temperature, widely available and affordable, and does not require any special skills, equipment, or facilities for its use [9, 12]. After oral administration, the plasma concentration increases rapidly, peaks at 30 min, and then declines rapidly. The sublingual route allows fast absorption of drug and more sustained therapeutic effect than oral administration as it avoids first-pass effect. Misoprostol stimulates uterine contractions by selectively binding to myometrial prostanoid receptors, and it has a long half life and minimal adverse effects, such as gastrointestinal symptoms, shivering, pyrexia, fatigue, and headache [7]. It is reported that sublingual misoprostol (800 μg) is a safe and effective treatment for women experiencing PPH [13, 14]. Regarding high incidence of anemia in pregnant women, even a small reduction of postpartum blood loss might be clinically valuable and decreases patients’ distress. In general, a synergistic effect of two agents would allow a reduction in dose for both agents and therefore limit the side effects while improving efficacy. We hypothesized that the combined use of lower dose of oxytocin and misoprostol may decrease the blood loss after cesarean section with minimal side effect compared to oxytocin infusion and misoprostolalone..To test our hypothesis, we designed this randomized double-blind, placebo-controlled study to compare the effect of the combined use of lower dose of oxytocin and misoprostol versus oxytocin infusion and misoprostol alone to reduce blood loss at cesarean section.
Materials and Methods
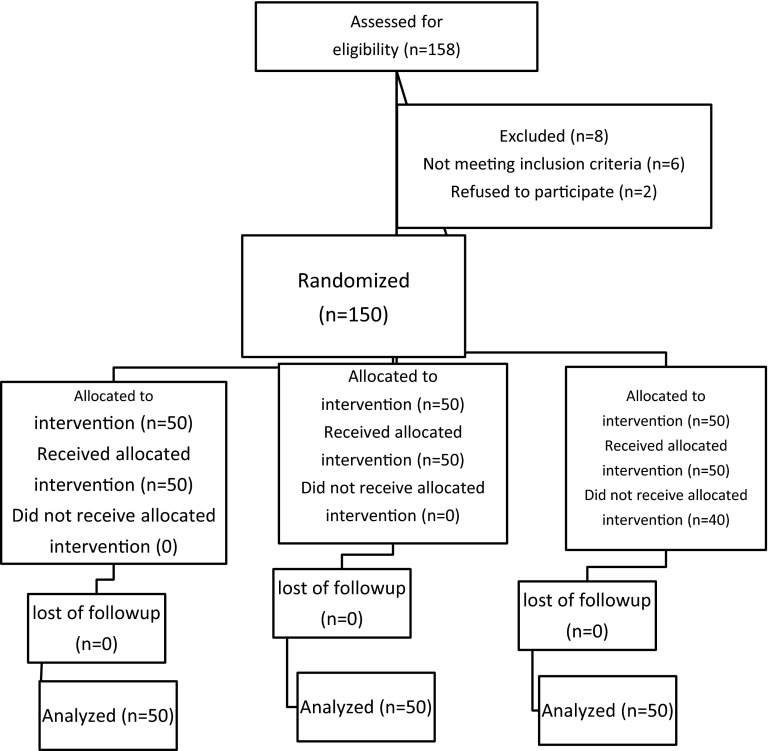
This clinical trial was registered at the United States National Institutes of Health (www.clinicaltrials.gov), with the number NCT01571323. Following obtaining informed patients consent, one hundred fifty-eight patients of 18–40 years with singleton term pregnancies undergoing elective or emergency lower segment cesarean section under spinal anesthesia were recruited in a prospective, double-blinded randomized trial. The Consolidated Standards of Reporting Trials (CONSORT) recommendations for reporting randomized, controlled clinical trials [15] were followed (Fig. 1). Exclusion criteria included women with any risk factor of postpartum hemorrhage i.e., anemia (Hb8 g%), multiple gestation, antepartum hemorrhage, poly-hydramnios, two or more previous cesarean sections and/or a history of previous rupture uterus, current or previous history of significant disease including heart disease, liver, renal disorders, or known coagulopathy.
Fig. 1.
Consort flow diagram
Patients were randomly allocated to one of the three groups of 50 each. The oxytocin group (group O) received 20 IU infusion of oxytocin in one liter Ringers lactate solution at the rate of 1,000 cc/h plus one sublingual placebo tablet soon after delivery. The misoprostol group (group M) received placebo (distilled water) in 1,000 cc Ringer lactate at the same rate plus 400-µg sublingual misoprostol tablet. The combined misoprostol–oxytocin group (group MO) received 200-µg misoprostol plus 5 IU bolus intravenous oxytocin after delivery. The main outcome measures were the determination of blood loss at cesarean section, change in hemoglobin levels, need for additional oxytocics, and drug-related side effects. The volume of blood in the suction bottle and blood-soaked sponges was measured. Hemoglobin values were determined both before surgery and 24 h following surgery. Hemodynamic variables were recorded before spinal anesthesia, 5 min spinal anesthesia, 10 and 20 min after administration of oxytocic drugs during surgery. The need for additional oxytocic therapy, operating time, need for blood transfusion, side effects of study drug, and any significant puerperal morbidity were also recorded.
Sample size estimation was based on the previous studies which reported that the mean amount of blood loss with the use of oxytocin during a CS is 600 cc, and misoprostol can reduce it by 200 cc [16, 17]. Thus, considering 90 % power and 5 % error, the sample size was determined to be 50 cases in each group. Data were analyzed using SPSS (SPSS 15.0, SPSS Inc, Chicago, II, USA). Continuous variables were tested for normal distribution by the Kolmogorov–Smirnov test. Parametric data were expressed as mean and standard deviation (SD) and analyzed by independent T test and Anova test. The effect of time on the hemodynamic parameters was analyzed using repeated measures analysis of variance test. The χ2 test was used to analyze the incidence of side effects. A P value <0.05 was considered as significant, statistically.
Results
One hundred fifty-eight patients were recruited of whom 8 excluded from the study groups due to logistical reasons or other factors violating the study protocol (Fig. 1). There were no significant differences between the three groups regarding the demographic properties (age, gestational age, and duration of surgery (Table 1). Table 2 shows the difference of volume sucked in the suction bottle after placental delivery in ml was significantly lower in MO group (234.8 ± 92.5) than M (294.4 ± 109.01) and O (285.74 ± 139.68) groups (P = 0.04). As shown in Table 2, hemoglobin decreased slightly after birth in all of three groups, but the mean decline of hemoglobin in MO group (1.1 ± 0.08 mg/dl) was smaller than that in the O group (1.38 ± 0.13 mg/dl) and in the M group (1.14 ± 0.29 mg/dl). The difference of the mean decline of hemoglobin between the three groups were statistically significant (P = 0.001). The amount of additional oxytocin requirement were not statistically significant difference among the three groups (P > 0.05).
Table 1.
Demographic data associated with the study
| M | O | MO | P value | |
|---|---|---|---|---|
| Age(years) | 27.92 ± 5.39 | 27.32 ± 4.3 | 27.42 ± 5.4 | 0.082 |
| Gestational age (weeks) | 37.68 ± 1.62 | 38.36 ± 1.72 | 37.94 ± 1.55 | 0.11 |
| Duration of surgery(min) | 38.12 ± 2.3 | 37.58 ± 1.72 | 37.1 ± 2.49 | 0.07 |
Values are presented as mean ± SD
M misoprostol, O oxytocin, MO combined misoprostol–oxytocin
Table 2.
Main outcome measures
| Groups | P value | |||
|---|---|---|---|---|
| M | MO | O | ||
| Volume sucked in the suction bottle | 294.4 ± 109.01 | 234.8 ± 92.54 | 285.74 ± 139.68 | 0.04 |
| Hemoglobin difference (g/dl) | 1.14 ± 0.29 | 1.1 ± 0.08 | 1.38 ± 0.13 | <0.001 |
| Additional oxytocin requirement | 8 (16 %) | 7(14 %) | 7(14 %) | 1 |
All data are expressed as mean ± SD except additional oxytocin requirement which was presented as number of patients (%)
M misoprostol, O oxytocin, MO combined misoprostol–oxytocin
As shown in Table 3, comparison of mean arterial pressure (MAP) (P = 0.38) and heart rate (HR) (P = 0.23) changes during spinal anesthesia and surgery failed to reveal any statistically significant differences between all groups through repeated measure analysis.
Table 3.
Hemodynamic changes using repeated measure analysis
| Variable | HR | MAP | ||||
|---|---|---|---|---|---|---|
| Group | MO | M | O | MO | M | O |
| 5 min before SA | 91.46 ± 17.48 | 94.63 ± 17.7 | 97.63 ± 16.37 | 84.08 ± 11.36 | 79.4 ± 16.58 | 80.76 ± 20.4 |
| After SA | 91.46 ± 16.93 | 95.2 ± 19.25 | 97.33 ± 14.1 | 79.14 ± 13.6 | 81.04 ± 15.79 | 76.76 ± 17.05 |
| 10 min after drug | 97 ± 15.57 | 93.33 ± 16.42 | 100.66 ± 1378 | 79.1 ± 12.68 | 80.23 ± 11.41 | 76.64 ± 15.49 |
| 20 min after drug | 96.56 ± 14.9 | 96.56 ± 14.9 | 101.26 ± 13.7 | 70.91 ± 13.72 | 75.67 ± 12.78 | 73.94 ± 14.7 |
| P value | 0.23 | 0.38 | ||||
SA spinal anesthesia, HR beat per minute, MAP mean arterial pressure, M misoprostol, O oxytocin, MO combined misoprostol–oxytocin
As shown in Table 4, no significant difference in terms of intraoperative and postoperative side effects including pruritus, nausea, vomiting, shivering, chest pain, and respiratory depression were found in the three groups.
Table 4.
Side effects observed in three study groups
| Side effects | Groups | P value | ||
|---|---|---|---|---|
| M | O | MO | ||
| Nausea | 11 (22 %) | 7 (14 %) | 6 (12 %) | 0.153 |
| Vomiting | 4 (8 %) | 2 (4 %) | 1 (2 %) | 0.856 |
| Dyspnea | 1 (2 %) | 0 | 1 (2 %) | 0.355 |
| Shivering | 3 (6 %) | 0 | 0 | 0.129 |
| Chest pain | 3 (6 %) | 3 (0) | 1 (6 %) | 0.770 |
| Fever | 2 (4 %) | 1 (2 %) | 2 (4 %) | 0.340 |
Data are presented as number of patients (%)
M misoprostol, O oxytocin, MO combined misoprostol–oxytocin
Discussion
This study demonstrated that the amount of blood loss during and after cesarean section in the lower dose of misoprostol–oxytocin group significantly was smaller than oxytocin and misoprostol alone, and the use of combined misoprostol–oxytocin was not associated with any serious side effects. The mean decline of hemoglobin in MO group significantly was smaller than O and M groups. We chose to administer misoprostol sublingual route to allow fast absorption of drug and more effective and sustained therapeutic effect, although other studies presented no difference (between) buccal or sublingual misoprostol inhemorrhage control [18–20].
In the present study, excessive postpartum hemorrhage (more than 500 cc) occurred in 6 cases (4 %) which was lower than what was estimated in other studies with routine uterotonic agent administration (4–6 %) [21].
Our findings also were consistent with Derman’s study, which reported that excessive hemorrhage occurred 12 % in placebo group and 4.6 % in oral misoprostol group [22].
We experienced no case with more than 1,000 cc blood loss, however, it occurred in 2 patients of Mansouri and Alsahly study [23]. Hofmeyr et al. presented no more efficacy for higher dose of misoprostol in preventing hemorrhage more than 1,000 cc through a meta-analysis [24]. However, synergistic effects of two agents would allow a reduction in dose for both agents and therefore limit the side effects while improving efficacy. Therefore, use of combined lower dose of these drugs probably lead to better control of blood loss. Maternal death rate was zero in our trial, likewise, the need to transfusion occurred gust in one case.
Totally 22 cases (14.6 %) required excess oxytocin (uterotonic) in our research which was relatively the same in all groups with no significant difference, whereas, it is mentioned as 8.3 % in Vimala et al. study [18], 30 % in Mansouri and Alsahly [23], and 23 % in Gerestenfeld and Wing [25].
Regarding side effects among the groups, although shivering, nausea, and vomiting, happened more often with misoprostol alone, as well as which are confirmed in other studies [19, 20], but in our study this difference was not statistically significant. These findings are consistent with some other researches [7, 23]. Some mentioned oxytocin- mediated hemodynamic changes in cesarean are decreasing blood pressure and peripheral vascular resistance plus increasing heart rate [26, 27].
Although, due to the synergistic effect of oxytocin with misoprostol on patients undergoing cesarean section under spinal anesthesia, the hemodynamic changes in the MO group were prominent, these findings were not statistically significant and unproblematic from the clinical point of view. The authors assumed that due to the use of lower dose of two drugs, these hemodynamic changes were controlled easily. Nevertheless, this is partially contrary by WHO recommendation for PPH management, which suggests that the simultaneous administration of misoprostol with treatment doses of oxytocin is not advisable [28, 29].
However, further studies in another population and larger sample size and different dosages of drugs are required.
Conclusion
Based on the data found in our study, it was concluded that administration of lower dose of misoprostol plus oxytocin significantly reduced the amount of blood loss during and after cesarean section compared to oxytocin and misoprostol when given alone, and use of them was not associated with any serious side effects.
Acknowledgments
Compliance with ethical requirements and Conflict of interest
The research proposal of this study has been reviewed by the Medical Research Ethical Committee of the Qazvin University of Medical Science and there is no conflict with ethical consideration (date: 23/June/2012). The authors of this paper have not declared any conflicts of interest.
Hamideh Pakniat
completed her Medical Doctorate (MD) at Azad University of Medical Sciences, Iran, in 1994 and Residency in Obstetrics and Gynecology at Qazvin University of Medical Sciences, Iran, in 1999. She is Assistant Professor of Qazvin University of Medical Sciences, Iran. She teaches Evidence Based Practice, supervise Residents and Students Dissertations. She received an award from the Iranian National Board of Obstetrics and Gynecology in 1999 and also a certificate and letter for the best invention in the 8th international exhibition of interventions in 2013. She has several publications in Persian and English to her credit. She has also done a film on Cesarean Section for the Ministry of Health's medical training (movie site http://www.medtube.ir).
Footnotes
Registration number: ACTRN12612000095864 and ClinicalTrials.gov Identifier: NCT01571323
Contributor Information
Hamideh Pakniat, Phone: +98-912-1822448, Phone: +98-283323637, Email: pakniat110@yahoo.com.
Marzieh Beigom Khezri, Phone: +98-912-3811009, Email: mkhezri@qums.ac.ir.
References
- 1. WHO. Maternal mortality. Fact sheet No. 348. 2010. http://www.who.int/mediacentre/factsheets/fs348/en/index.html. Accessed 4 Jan 2012.
- 2.Rushwan H. Misoprostol: an essential for medicine for managing postpartum hemorrhage in low-resource settings? Int J Gynecol Obstet. 2011;114:209–210. doi: 10.1016/j.ijgo.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham FG, Hauth JC, Leveno KJ, et al. Williams obstetrics. 23. New York: Medical publishing division, McGRAW-Hill; 2010. Chapter 35; pp. 757–760. [Google Scholar]
- 4.Nirmala K, Zainuddin AA, Ghani NA, et al. Carbetocin versus syntometrine in prevention of postpartum hemorrhage following vaginal delivery. J Obstet Gynaecol Res. 2009;35:48–54. doi: 10.1111/j.1447-0756.2008.00829.x. [DOI] [PubMed] [Google Scholar]
- 5.Prendiville WJ, Elbourne D, Mcdonald S. Active versus expectant management in the third stage of labour. In: The Cochrene library. Chichester: Wiley; 2003.
- 6.Matsubara S, Yano H, Taneichi A, et al. Uterine compression suture against impending recurrence of uterine inversion immediately after laparotomy repositioning. J Obstet Gynaecol Res. 2009;35:819–823. doi: 10.1111/j.1447-0756.2008.01011.x. [DOI] [PubMed] [Google Scholar]
- 7.Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at caesarean section in Nigeria: a randomized controlled trial. J Obstet Gynaecol Res. 2011;37(7):715–721. doi: 10.1111/j.1447-0756.2010.01399.x. [DOI] [PubMed] [Google Scholar]
- 8.Park SC, Chun HJ, Kang CD, et al. Prevention and management of non-steroidal anti-inflammatory drugs-induced small intestinal injury. World J Gastroenterol. 2011;17(42):4647–4653. doi: 10.3748/wjg.v17.i42.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen R, O’Brien BM. Uses of misoprostol in obstetrics and gynecology. Rev Obstet Gynecol. 2009;2(3):159–168. [PMC free article] [PubMed] [Google Scholar]
- 10.Tang OS, Ho PC. The pharmacokinetics and different regimens of misoprostol in early first-trimester medical abortion. Contraception. 2006;74:26–30. doi: 10.1016/j.contraception.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Speroff L, Fritz MD. Clinical gynecologic endocrinology and infertility. 7th edn. Philadelphia: Lippincott, Williams and Wilkins; 2011; Chapter 21:940–45.
- 12.Alfirevic Z, Blum J, Walraven G, et al. Prevention of postpartum hemorrhage with misoprostol. Int J Gynecol Obstet. 2007;99:S198–S201. doi: 10.1016/j.ijgo.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labour: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375(9710):210–216. doi: 10.1016/S0140-6736(09)61924-3. [DOI] [PubMed] [Google Scholar]
- 14.Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non- inferiority trial. Lancet. 2010;375(9710):217–223. doi: 10.1016/S0140-6736(09)61923-1. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trial. BMC Med Res Methodol. 2001;1:2. doi: 10.1186/1471-2288-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vimala N, Kumar MS. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. Int J Gynaecol Obstet. 2006;92(2):106–110. doi: 10.1016/j.ijgo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Fazel MR, Samimi M, Fakharian E. A comparison of rectal misoprostol and intravenous oxytocin on hemorrhage and homeostatic changes during cesarean section. Middle East J Anesthesiol. 2013;22(1):41–46. [PubMed] [Google Scholar]
- 18.Vimala N, Mittal S, Kumar S, et al. Sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Int J Gynaecol Obstet. 2004;87(1):1–5. doi: 10.1016/j.ijgo.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Lapaire O, Schneider MC, Stotz M, et al. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean section. Int J Gynecol Obstet. 2006;95:2–7. doi: 10.1016/j.ijgo.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 20.hamm j, Russel Z, Botha T, et al. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005;192(5):1404–6. [DOI] [PubMed]
- 21.ACOG Practice Bulletin Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1047. doi: 10.1097/00006250-200610000-00046. [DOI] [PubMed] [Google Scholar]
- 22.Derman RT, Kodkany BS, Goudar SS, et al. Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet. 2006;9543:1248–1253. doi: 10.1016/S0140-6736(06)69522-6. [DOI] [PubMed] [Google Scholar]
- 23.Mansouri HA, Alsahly N. Rectal versus oral misoprostol for active management of third stage of labor: a randomized controlled trial. Arch Gynecol Obstet. 2011;283:935–939. doi: 10.1007/s00404-010-1466-5. [DOI] [PubMed] [Google Scholar]
- 24.Hofmeyr GJ, Gülmezoglu AM, Novikova N, et al. Misoprostol to prevent and (19) treat postpartum haemorrhage: a systematic review and meta-analysis of maternal deaths and dose-related effects. Bull World Health Organ. 2009;87:666–677. doi: 10.2471/BLT.08.055715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstenfeld TS, Wing DA. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum hemorrhage after vaginal delivery. Am J Obstet Gynecol. 2001;185(4):878–882. doi: 10.1067/mob.2001.117360. [DOI] [PubMed] [Google Scholar]
- 26.Langesaeter E, Rosseland LA, Stubhaug A. Hemodynamic effects of oxytocin during cesarean delivery. Int J Gynaecol Obstet. 2006;95(1):46–47. doi: 10.1016/j.ijgo.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Svanström MC, Biber B, Hanes M, et al. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008;100(5):683–689. doi: 10.1093/bja/aen071. [DOI] [PubMed] [Google Scholar]
- 28.WHO. WHO recommendations for the prevention and treatment of postpartum hemorrhage. Geneva: WHO; 2012.
- 29.El Ayadi AM, Robinson N, Geller S, et al. Advances in the Treatment of Postpartum Hemorrhage. Expert Rev of Obstet Gynecol. 2013;8(6):525–537. doi: 10.1586/17474108.2013.847622. [DOI] [Google Scholar]